Abstract
Human papillomavirus (HPV) type 11 is strictly trophic for epithelial cells and induces benign condylomata of the external genitalia and also causes laryngeal papillomas. Primary keratinocytes are the appropriate hosts for studies of HPV gene regulation, but they are not frequently used, owing to difficulties in culturing and low transfection efficiencies. By modifying a Polybrene transfection procedure, we achieved consistently high transfection efficiencies in primary human foreskin keratinocytes and characterized the HPV type 11 enhancer in the context of the homologous E6 promoter. Contrary to previous studies with immortalized human cervical carcinoma C-33A cells, constitutive enhancer element II in the upstream regulatory region conferred no enhancer activity and did not abrogate repression by the homologous E2 protein. Rather, repression was strong, ranging from 5.6- to 20-fold for the various enhancer deletion mutations. By deletion analysis, a strong enhancer that included three nuclear factor 1 sites and one nuclear factor 1-associated factor-binding site was localized to a 45-bp region within constitutive enhancer element I, and it showed some degree of tissue specificity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard B. A., Bailly C., Lenoir M. C., Darmon M., Thierry F., Yaniv M. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J Virol. 1989 Oct;63(10):4317–4324. doi: 10.1128/jvi.63.10.4317-4324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer-Finkler E., Stoler M. H., Girardi F., Pfister H. Cell differentiation-related gene expression of human papillomavirus 33. Med Microbiol Immunol. 1990;179(4):185–192. doi: 10.1007/BF00195249. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Reddel R. R., Quanrud M., Yang K., Farrell M. P., Harris C. C. Strontium phosphate transfection of human cells in primary culture: stable expression of the simian virus 40 large-T-antigen gene in primary human bronchial epithelial cells. Mol Cell Biol. 1987 May;7(5):2031–2034. doi: 10.1128/mcb.7.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. M., Broker T. R., Chow L. T. An E1M--E2C fusion protein encoded by human papillomavirus type 11 is asequence-specific transcription repressor. J Virol. 1991 Jun;65(6):3317–3329. doi: 10.1128/jvi.65.6.3317-3329.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M. T., Broker T. R., Chow L. T. Identification of a novel constitutive enhancer element and an associated binding protein: implications for human papillomavirus type 11 enhancer regulation. J Virol. 1989 Jul;63(7):2967–2976. doi: 10.1128/jvi.63.7.2967-2976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M. T., Hirochika R., Hirochika H., Broker T. R., Chow L. T. Regulation of human papillomavirus type 11 enhancer and E6 promoter by activating and repressing proteins from the E2 open reading frame: functional and biochemical studies. J Virol. 1988 Aug;62(8):2994–3002. doi: 10.1128/jvi.62.8.2994-3002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T., Apt D., Gloss B., Isa M., Bernard H. U. The enhancer of human papillomavirus type 16: binding sites for the ubiquitous transcription factors oct-1, NFA, TEF-2, NF1, and AP-1 participate in epithelial cell-specific transcription. J Virol. 1991 Nov;65(11):5933–5943. doi: 10.1128/jvi.65.11.5933-5943.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T., Chan W. K., Bernard H. U. Transcriptional activation of human papillomavirus 16 by nuclear factor I, AP1, steroid receptors and a possibly novel transcription factor, PVF: a model for the composition of genital papillomavirus enhancers. Nucleic Acids Res. 1990 Feb 11;18(3):465–470. doi: 10.1093/nar/18.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe T. P., Alderborn A., Anderson R. D., Parkkinen S., Bergman P., Haugen T. H., Pettersson U., Turek L. P. Transcriptional activation of the human papillomavirus-16 P97 promoter by an 88-nucleotide enhancer containing distinct cell-dependent and AP-1-responsive modules. New Biol. 1990 May;2(5):450–463. [PubMed] [Google Scholar]
- Cripe T. P., Haugen T. H., Turk J. P., Tabatabai F., Schmid P. G., 3rd, Dürst M., Gissmann L., Roman A., Turek L. P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987 Dec 1;6(12):3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental C., Kanno M., Nomiyama H., Chambon P. Cooperativity and hierarchical levels of functional organization in the SV40 enhancer. Cell. 1988 Sep 23;54(7):943–953. doi: 10.1016/0092-8674(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Carranca A., Thierry F., Yaniv M. Interplay of viral and cellular proteins along the long control region of human papillomavirus type 18. J Virol. 1988 Nov;62(11):4321–4330. doi: 10.1128/jvi.62.11.4321-4330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gius D., Grossman S., Bedell M. A., Laimins L. A. Inducible and constitutive enhancer domains in the noncoding region of human papillomavirus type 18. J Virol. 1988 Mar;62(3):665–672. doi: 10.1128/jvi.62.3.665-672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Chong T., Bernard H. U. Numerous nuclear proteins bind the long control region of human papillomavirus type 16: a subset of 6 of 23 DNase I-protected segments coincides with the location of the cell-type-specific enhancer. J Virol. 1989 Mar;63(3):1142–1152. doi: 10.1128/jvi.63.3.1142-1152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Yeo-Gloss M., Meisterenst M., Rogge L., Winnacker E. L., Bernard H. U. Clusters of nuclear factor I binding sites identify enhancers of several papillomaviruses but alone are not sufficient for enhancer function. Nucleic Acids Res. 1989 May 11;17(9):3519–3533. doi: 10.1093/nar/17.9.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
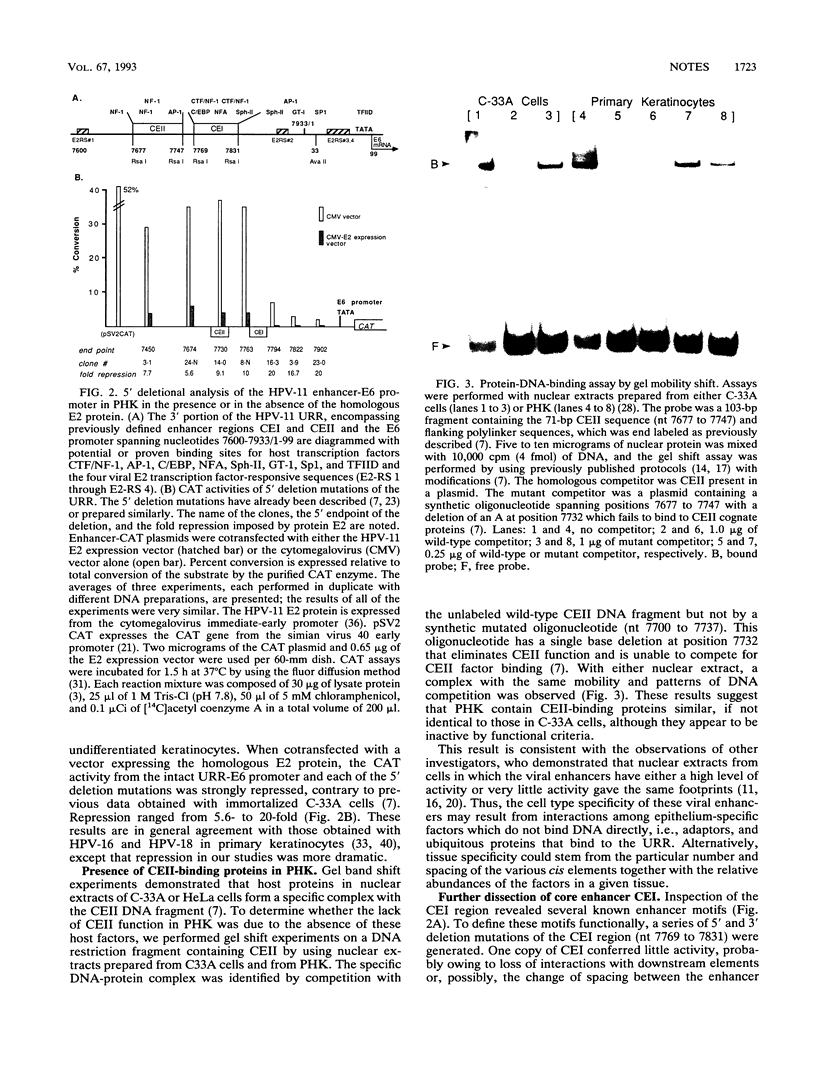
- Hirochika H., Hirochika R., Broker T. R., Chow L. T. Functional mapping of the human papillomavirus type 11 transcriptional enhancer and its interaction with the trans-acting E2 proteins. Genes Dev. 1988 Jan;2(1):54–67. doi: 10.1101/gad.2.1.54. [DOI] [PubMed] [Google Scholar]
- Ishiji T., Lace M. J., Parkkinen S., Anderson R. D., Haugen T. H., Cripe T. P., Xiao J. H., Davidson I., Chambon P., Turek L. P. Transcriptional enhancer factor (TEF)-1 and its cell-specific co-activator activate human papillomavirus-16 E6 and E7 oncogene transcription in keratinocytes and cervical carcinoma cells. EMBO J. 1992 Jun;11(6):2271–2281. doi: 10.1002/j.1460-2075.1992.tb05286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C. K., Connolly D., Blumenberg M. Comparison of methods for transfection of human epidermal keratinocytes. J Invest Dermatol. 1991 Dec;97(6):969–973. doi: 10.1111/1523-1747.ep12491889. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. I., Taichman L. B. Transient expression of a transfected gene in cultured epidermal keratinocytes: implications for future studies. J Invest Dermatol. 1989 Feb;92(2):267–271. doi: 10.1111/1523-1747.ep12276837. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Bindereif A., Green M. R. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988 Mar-Apr;5(2):22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Mack D. H., Laimins L. A. A keratinocyte-specific transcription factor, KRF-1, interacts with AP-1 to activate expression of human papillomavirus type 18 in squamous epithelial cells. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9102–9106. doi: 10.1073/pnas.88.20.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A. A., Romanczuk H., Howley P. M. The papillomavirus E2 regulatory proteins. J Biol Chem. 1991 Oct 5;266(28):18411–18414. [PubMed] [Google Scholar]
- Romanczuk H., Howley P. M. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3159–3163. doi: 10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk H., Thierry F., Howley P. M. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J Virol. 1990 Jun;64(6):2849–2859. doi: 10.1128/jvi.64.6.2849-2859.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa R., Dostatni N., Yaniv M. Control of papillomavirus gene expression. Biochim Biophys Acta. 1990 Jun 1;1032(1):19–37. doi: 10.1016/0304-419x(90)90010-x. [DOI] [PubMed] [Google Scholar]
- Sowden M., Harrison S., Ashfield R., Kingsman A. J., Kingsman S. M. Multiple cooperative interactions constrain BPV-1 E2 dependent activation of transcription. Nucleic Acids Res. 1989 Apr 25;17(8):2959–2972. doi: 10.1093/nar/17.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler M. H., Rhodes C. R., Whitbeck A., Wolinsky S. M., Chow L. T., Broker T. R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992 Feb;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Wolinsky S. M., Whitbeck A., Broker T. R., Chow L. T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989 Sep;172(1):331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Swift F. V., Bhat K., Younghusband H. B., Hamada H. Characterization of a cell type-specific enhancer found in the human papilloma virus type 18 genome. EMBO J. 1987 May;6(5):1339–1344. doi: 10.1002/j.1460-2075.1987.tb02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Howley P. M. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 1991 Jan;3(1):90–100. [PubMed] [Google Scholar]
- Thierry F., Spyrou G., Yaniv M., Howley P. Two AP1 sites binding JunB are essential for human papillomavirus type 18 transcription in keratinocytes. J Virol. 1992 Jun;66(6):3740–3748. doi: 10.1128/jvi.66.6.3740-3748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987 Nov;6(11):3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao M. C., Walthall B. J., Ham R. G. Clonal growth of normal human epidermal keratinocytes in a defined medium. J Cell Physiol. 1982 Feb;110(2):219–229. doi: 10.1002/jcp.1041100217. [DOI] [PubMed] [Google Scholar]
- Warden D., Thorne H. V. The infectivity of polyoma virus DNA for mouse embryo cells in the presence of diethylaminoethyl-dextran. J Gen Virol. 1968 Dec;3(3):371–377. doi: 10.1099/0022-1317-3-3-371. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Harris C. C. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]