Abstract
Bulk endocytosis is the process by which nerve terminals retrieve large amounts of synaptic vesicle membrane during periods of strong stimulation intensity. The process is rapidly activated and is most probably calcium dependent in a similar manner to synaptic vesicle exocytosis. This article briefly summarizes the current knowledge of bulk endocytosis with respect to its activation, kinetics and molecular mechanism. It also presents recent data from our laboratory showing that the dephosphorylation of a group of endocytosis proteins called the dephosphins by the Ca2+-dependent protein phosphatase calcineurin is key to the activity-dependent stimulation of the process. Possible downstream effectors of calcineurin are discussed such as the large GTPase dynamin I and its phosphorylation-dependent interaction partner syndapin I.
Multiple synaptic vesicle retrieval pathways in central nerve terminals
The retrieval of synaptic vesicle (SV) membrane after exocytosis is essential for the maintenance of synaptic transmission in central nervous system synapses. Multiple routes of membrane retrieval have been described (Fig. 1), the best characterized being clathrin-dependent endocytosis (Murthy & De Camilli, 2003; Royle & Lagnado, 2003; Conner & Schmid, 2003), the most controversial being ‘kiss-and-run’ (Harata et al. 2006) and the most enigmatic being ‘bulk’ endocytosis (Royle & Lagnado, 2003). Bulk endocytosis differs from the other two modes of membrane retrieval in that it retrieves membrane for greater than one vesicle (the other two retrieve single SVs). It does so by invaginating large areas of presynaptic membrane from which SVs can be generated over time. These large endosomes can remain attached to the plasma membrane for a considerable length of time, while constantly allowing SVs to bud from them (Takei et al. 1996; Gad et al. 1998).
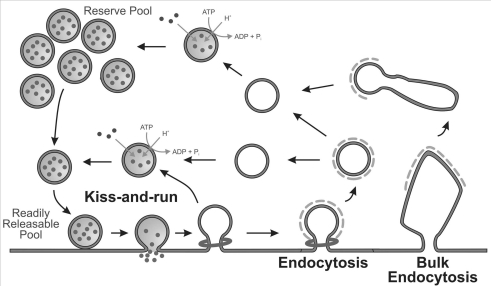
Figure 1. Multiple synaptic vesicle retrieval pathways in central nerve terminals.
Three different mechanisms are propsed to retrieve synaptic vesicle (SV) membrane after exocytosis in nerve terminals. Kiss-and-run is a mechanism where the SV never fully fuses with the plasma membrane and retrieves intact. Classical clathrin-dependent endocytosis involves the invagination of a single clathrin-coated bud from the plasma membrane before its fission and uncoating. Bulk endocytosis is the process where large areas of nerve terminal membrane are invaginated to produce endosomes from which SVs can bud.
Bulk endocytosis has been reported in various different neuronal systems, including classical small central nerve terminals (Takei et al. 1996; Marxen et al. 1999; Leenders et al. 2002), atypical large central nerve terminals such as retinal biopolar neurones (Holt et al. 2003; Paillart et al. 2003) and the calyx of Held (de Lange et al. 2003), frog and snake neuromuscular junctions (Richards et al. 2000, 2004; Teng & Wilkinson, 2000; Teng et al. 2007) and lamprey reticulospinal synapses (Gad et al. 1998). In almost all instances bulk endocytosis is activated by strong stimulation protocols, which lead to the suggestion that it is purely an emergency mechanism that controls nerve terminal surface area during times of intense activity (Royle & Lagnado, 2003). However it is of key importance to understand this recycling pathway, since bulk endocytosis will also be activated during physiological processes such as long-term potentiation and synaptic integration and also during pathological processes such as epiliptogenic burst firing. Thus bulk endocytosis will contribute to the ability of the nerve terminal to respond to intense stimuli and it is vital that this mechanism is understood.
Properties of bulk endocytosis
Bulk endocytosis was initially thought to be a slow process, since endosomal intermediates have been shown to remain attached to the plasma membrane for long periods after stimulation (Takei et al. 1996; Gad et al. 1998). However the formation of these endocytic vacuoles is very fast, with bulk endosomes observed within 1–2 s of strong nerve terminal stimulation (Marxen et al. 1999; Leenders et al. 2002; Teng et al. 2007). This suggests that bulk endocytosis is a triggered event, most likely to be activated by the same Ca2+ stimulus that evokes SV exocytosis. Bulk endocytosis has been previously suggested to be activated by the accumulation of SV membrane in the plasma membrane; however, the speed of bulk endosome formation argues against this. Thus bulk endocytosis is rapidly triggered and possibly activated by Ca2+ influx, suggesting a requirement for a Ca2+ sensor in the process.
Bulk endocytosis was first demonstrated in central nerve terminals using electron microscopy (Takei et al. 1996), and this has remained an important tool in observing the process. However, these studies are labour intensive and do not provide real time information on the process. Since the turn of the decade, bulk endocytosis has also been able to be visualized using fluorescent methods, opening up new possibilities to investigate its activation and molecular mechanism. The first studies were performed on the frog neuromuscular junction, where it was shown that the lipid-binding styryl dye FM1-43 could selectively label bulk endocytosis when compared with its more hydrophilic counterpart FM2-10 (Richards et al. 2000). The proposed reason for this disproportionate labelling was that FM2-10 was washed out of the bulk endosomes that were still connected to the plasma membrane, whereas the more hydrophobic FM1-43 could not be removed. The selective labelling of bulk endosomes was confirmed by photoconversion of the dyes and examination of nerve terminals with electron microscopy (Richards et al. 2000). In addition to styryl dyes, large molecular weight dextrans tagged with fluorescent molecules have also been used to demonstrate the presence and kinetics of bulk endocytosis in motor nerve terminals (Holt et al. 2003; Teng et al. 2007). These fluid phase markers are too large to be accumulated inside single SVs, and therefore they selectively label the bulk endocytosis pathway.
These flourescent studies have allowed the traffic of SVs generated by bulk endocytosis to be followed in real time. It transpires that the primary route for these SVs is to replenish the reserve pool of SVs. This conclusion was first drawn in the frog neuromuscular junction, since SVs labelled by FM1-43 during strong stimulation could not immediately undergo exocytosis, whereas those SVs labelled with FM2-10 could (Richards et al. 2000). The FM1-43-labelled SVs could eventually be released, but only after a delay of approximately 10–15 min. The most obvious explanation for this time lag is that this is the time required to generate new SVs from bulk endosomes. Recently our group has shown that SVs derived from bulk endocytosis also replenish the reserve SV pool in central nerve terminals (Evans & Cousin, 2007). In these studies, a sustained component of exocytosis was observed that was attributable to the reserve pool when FM1-43 was loaded into SVs using a strong stimulus, but was absent when FM2-10 was loaded under identical conditions. Thus SVs generated by bulk endocytosis are unable to be immediately used and rejoin the SV recycling pool in the reserve pool.
Since the kinetics of bulk endocytosis and fate of SVs derived from this pathway are now known, it is perhaps surprising that very little is understood about the molecular mechanism of the process itself. While it is thought that clathrin-dependent endocytosis is responsible for SV budding from bulk endosomes (Takei et al. 1996), the molecules that activate and mediate the invagination and fission of the membrane are relatively unknown. Bulk endocytosis has been linked to the process of macropinocytosis in non-neuronal cells where membrane protrusions gather large amounts of the fluid phase in a ‘cell drinking’ mechanism (Holt et al. 2003; Teng et al. 2007). Macropinocytosis is dependent on the activation of Rho family GTPases which stimulate the actin-driven formation of these protrusions (Conner & Schmid, 2003). Interestingly in both the frog neuromuscular junction and in retinal bipolar neurones, disruption of actin function with pharmacological agents resulted in an inhibition of bulk endocytosis (Holt et al. 2003; Richards et al. 2004). In addition, bulk endocytosis was also retarded by inhibition of phosphatidylinositol 3-kinase activity (Holt et al. 2003; Richards et al. 2004), suggesting a link between this signalling cascade and the actin dynamics required for the process. Thus a requirement for some molecules in bulk endocytosis has been identified, with actin dynamics and rearrangement a major factor in the process.
The dephosphins are activity-dependent triggers for bulk endocytosis
Bulk endocytosis is activated by strong stimulation and is likely to be immediately triggered by the same Ca2+ stimulus that activates the exocytosis machinery. Thus the Ca2+ sensor for bulk endocytosis must be a Ca2+-binding protein which activates certain endocytosis proteins during strong, but not mild, nerve terminal stimulation. One protein that fulfils all of these criteria is the Ca2+-dependent protein phosphatase calcineurin. Calcineurin is activated by Ca2+ influx in nerve terminals and dephosphorylates the set of endocytosis proteins called the dephosphins (Cousin & Robinson, 2001). The dephosphins are grouped together by their ability to be dephosphorylated by calcineurin on stimulation, and by the fact that they are all essential for SV endocytosis. The dephosphins are; the large GTPase dynamin I, the adaptor protein AP180, and the accessory proteins amphiphysin I/II, synaptojanin, epsin, esp15 and phosphatidylinositol phosphate kinase type Iγ (PIPKIγ). After their stimulus-dependent dephosphorylation, the dephosphins are rephosphorylated by their respective protein kinases, such as cyclin-dependent kinase 5 (cdk5), which rephosphorylates dynamin I, synaptojanin and PIPKIγin vivo (Tan et al. 2003; Lee et al. 2005).
We have identified both the dephosphorylation of the dephosphins and their subsequent rephosphorylation as essential events in bulk endocytosis (Evans & Cousin, 2007). The evidence for this is as follows: (i) inhibition of either calcineurin or cdk5 by either pharmacological antagonists or overexpression of dominant negative constructs in primary neuronal culture arrested the uptake of FM1-43 but not FM2-10 during strong stimulation; (ii) inhibition of cdk5 blocked the uptake of the fluid-phase marker horseradish peroxidase (HRP) into plasma-membrane-generated endosomes, but not single SVs, during strong stimulation; (iii) inhibition of cdk5 had no effect on either FM1-43 loading or HRP labelling if nerve terminals were challenged with a mild stimulus; and finally (iv) the sustained component of exocytosis observed when SVs were labelled with FM1-43 was abolished when either calcineurin or cdk5 activity was inhibited during the dye-loading phase (Evans & Cousin, 2007).
These findings place some or all of the dephosphins as the key mediators of bulk endocytosis, with their dephosphorylation by calcineurin as the activity-dependent trigger for the process. There is ample evidence that calcineurin can fulfil the temporal requirements of this role, since the dephosphorylation by calcineurin of the dephosphins is synchronous and rapid (<1 s; Robinson et al. 1994). Furthermore the intracellular free Ca2+ increase required to maximally dephosphorylate the dephosphins in nerve terminals (approximately 1 μm;Sihra et al. 1992) correlates well with the predicted increase in intracelluar free Ca2+ during strong stimulation. However, the key test as to whether calcineurin is the activity-dependent Ca2+ sensor for bulk endocytosis is whether it is able to dephosphorylate its substrates only at stimulation frequencies at which bulk endocytosis is observed. Unpublished experiments recently performed in our laboratory confirm that this is the case. In these experiments no dephosphorylation of the calcineurin substrate dynamin I was observed at mild stimulation frequencies, but a robust dephosphorylation was seen with increasing stimulation frequency in our neuronal cultures. Thus we propose that the activity-dependent dephosphorylation of the dephosphins is the trigger for bulk endocytosis, and that some if not all of the dephosphins are involved in the process.
Perspectives
The identification of calcineurin as the activity-dependent trigger for bulk endocytosis raises some provocative concepts. For example, it suggests that some if not all of the dephosphins perform roles in both clathrin-dependent and bulk endocytosis. This should not be too surprising since a molecule such as dynamin I will be required for membrane fission in both processes. Dynamin I already has an identified phosphorylation-dependent binding partner, which is the endocytosis protein syndapin (Anggono et al. 2006). Dynamin I also has a phosphorylation-independent interaction with amphiphysin (Tan et al. 2003; Anggono et al. 2006) and this interaction is essential for clathrin-dependent endocytosis (Grabs et al. 1997; Wigge et al. 1997). Thus dynamin I has two major interactions: one which is phosphorylation independent (i.e. will occur regardless of stimulation intensity) and is essential for clathrin-dependent endocytosis (amphiphysin), and the other which is phosphorylation-dependent (i.e. will occur only during strong stimulation) and is therefore implicated in bulk endocytosis (syndapin). Syndapin is an excellent candidate for a bulk endocytosis effector since is it heavily linked to the control of actin polymerization through its well characterized interaction with nucleating protein N-WASP (Kessels & Qualmann, 2002). Since actin dynamics are essential for bulk endocytosis, this places the dynamin–syndapin interaction as the key event in the process. In addition, the central region of syndapin has a series of NPF amino acid repeats that interact with a family of proteins that mediate budding from endosomes (Braun et al. 2005), possibly indicating a role in SV budding from bulk endosomes. Finally syndapin has an F-BAR domain at its N terminus (Itoh et al. 2005; Tsujita et al. 2006), and this is similar to the N-BAR domain found on both amphiphysin and endophilin (Peter et al. 2004). This domain can bind and tubulate lipid both in vitro and in vivo and may facilitate the invagination and possibly fission of the bulk endosome. Therefore, the phosphorylation-dependent recruitment of syndapin by the activity-dependent dephosphorylation of dynamin I may be the key molecular event in bulk endocytosis, a process which is central to nerve terminal function.
Acknowledgments
This work was supported by The Wellcome Trust (Ref: GR070569) and Epilepsy Research UK.
References
- Anggono V, Smillie KJ, Graham ME, Valova VA, Cousin MA, Robinson PJ. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat Neurosci. 2006;9:752–760. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Pinyol R, Dahlhaus R, Koch D, Fonarev P, Grant BD, Kessels MM, Qualmann B. EHD proteins associate with syndapin I and II and such interactions play a crucial role in endosomal recycling. Mol Biol Cell. 2005;16:3642–3658. doi: 10.1091/mbc.E05-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27:401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- Harata NC, Aravanis AM, Tsien RW. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Kessels MM, Qualmann B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 2002;21:6083–6094. doi: 10.1093/emboj/cdf604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange RP, de Roos AD, Borst JG. Two modes of vesicle recycling in the rat calyx of Held. J Neurosci. 2003;23:10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Voronov S, Letinic K, Nairn AC, Di Paolo G, De Camilli P. Regulation of the interaction between PIPKIγ and talin by proline-directed protein kinases. J Cell Biol. 2005;168:789–799. doi: 10.1083/jcb.200409028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Scholten G, de Lange RP, Lopes Da Silva FH, Ghijsen WE. Sequential changes in synaptic vesicle pools and endosome-like organelles during depolarization near the active zone of central nerve terminals. Neuroscience. 2002;109:195–206. doi: 10.1016/s0306-4522(01)00450-x. [DOI] [PubMed] [Google Scholar]
- Marxen M, Volknandt W, Zimmermann H. Endocytic vacuoles formed following a short pulse of K+-stimulation contain a plethora of presynaptic membrane proteins. Neuroscience. 1999;94:985–996. doi: 10.1016/s0306-4522(99)00351-6. [DOI] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Richards DA, Rizzoli SO, Betz WJ. Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction. J Physiol. 2004;557:77–91. doi: 10.1113/jphysiol.2004.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Liu JP, Powell KA, Fykse EM, Südhof TC. Phosphorylation of dynamin I and synaptic vesicle recycling. Trends Neurosci. 1994;17:348–353. doi: 10.1016/0166-2236(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Endocytosis at the synaptic terminal. J Physiol. 2003;553:345–355. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihra TS, Bogonez E, Nicholls DG. Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release. J Biol Chem. 1992;267:1983–1989. [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nature Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Teng H, Lin MY, Wilkinson RS. Macroendocytosis and endosome processing in snake motor boutons. J Physiol. 2007 doi: 10.1113/jphysiol.2007.130989. 10.1113/jphysiol.2007.130989 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J Neurosci. 2000;20:7986–7993. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol. 2006;172:269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P, Vallis Y, McMahon HT. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr Biol. 1997;7:554–560. doi: 10.1016/s0960-9822(06)00254-5. [DOI] [PubMed] [Google Scholar]