Abstract
The compelling evidence linking small size at birth with later cardiovascular disease has renewed and amplified scientific and clinical interests into the determinants of fetal growth. It is accepted that genes and nutrition control fetal growth; however, prior to this study, it had been impossible to isolate the effect of increases and decreases in fetal oxygenation on the regulation of prenatal growth. We investigated the role of oxygen in the control of fetal growth in the chicken because in contrast to mammals, the effects on the fetus of changes in oxygenation could be isolated, by assessing them directly without alteration to the maternal or placental physiology or maternal nutrition during development. The data show that incubation at high altitude of fertilized eggs laid by sea level hens markedly restricted fetal growth. Incubation at high altitude of fertilized eggs laid by high altitude hens also restricted fetal growth, but to a lesser extent compared to eggs laid by sea level hens. By contrast, incubation at sea level of fertilized eggs laid by high altitude hens not only restored, but enhanced, fetal growth relative to sea level controls. Incubation at high altitude of sea level eggs with oxygen supplementation completely prevented the high altitude-induced fetal growth restriction. Thus, fetal oxygenation, independent of maternal nutrition during development, has a predominant role in the control of fetal growth. Further, prolonged high altitude residence confers protection against the deleterious effects of hypoxia on fetal growth.
It is generally accepted that hypoxia during pregnancy decreases birth weight; however, it has been difficult to demonstrate or isolate this effect because all human conditions or experimental animal models that induce fetal hypoxia are also accompanied by changes in nutrient delivery. For instance, elegant studies in mammalian animals have shown that maternal chronic hypoxia during pregnancy can lead to slow, disproportionate fetal growth (De Grauw et al. 1986; Jacobs et al. 1988). However, whether these effects are due to fetal under-oxygenation or fetal under-nutrition is uncertain as chronic experimental hypoxia also reduces maternal food intake (De Grauw et al. 1986). In humans, fetal hypoxia occurs most commonly under physiological conditions during the hypobaric hypoxia of pregnancy at high altitude (Moore, 1990), or under pathological conditions during pregnancy complicated by placental insufficiency (Baschat, 2004). Several investigators have reported reduced birth weight and asymmetric growth restriction in babies with increasing altitude (e.g. Lichty et al. 1957; McClung, 1969; Haas et al. 1980; Moore et al. 1998; Giussani et al. 2001; Zamudio et al. 2007). However, because most high altitude populations are also impoverished, and because placental insufficiency decreases nutrient as well as oxygen transfer to the baby, the extent to which the reduction in fetal growth under these conditions is governed by fetal under-nutrition or under-oxygenation, again, remains uncertain. By using the chick embryo as an animal model, this study could isolate the direct effects on the fetus of developmental hypoxia due to high altitude for the first time, completely independent of changes in maternal nutrition and of the physiology of the mother or the placenta. The study tested the hypothesis that fetal oxygenation, independent of maternal nutrition during development, has a predominant role in the control of fetal growth by investigating the effects of incubation at high altitude of fertilized eggs laid by sea level hens. In addition, the experiment could be done the other way around, by developing at sea level fertilized eggs laid by high altitude hens, to assess whether the hypoxia-induced effects on fetal growth could be reverted, something that is difficult if not impossible to assess in human populations. Finally, to discount the possibility that high altitude-induced fetal growth restriction is due to hypobaria rather than hypoxia, the effects of incubation at high altitude with oxygen supplementation of fertilized eggs laid by sea level hens was also investigated.
Methods
The study was done in Bolivia, in the high altitude city of La Paz (3600 m, 494 mmHg, approximate ambient dry 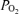 100 mmHg) and the sea level city of Santa Cruz (420 m, 760 mmHg, approximate ambient dry
100 mmHg) and the sea level city of Santa Cruz (420 m, 760 mmHg, approximate ambient dry 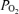 160 mmHg). Fertilized eggs were obtained from Black Leghorn chickens that had been reared at the sea level city of Santa Cruz or at high altitude city of La Paz for at least six generations. Fertilized eggs from sea level hens, laid at sea level, were randomly divided and incubated either at sea level (SLSL, n = 45) or high altitude (SLHA, n = 60). Eggs from high altitude hens, laid at high altitude, were randomly divided and incubated either at high altitude (HAHA, n = 70) or sea level (HASL, n = 50). SLHA embryos were also incubated with oxygen supplementation (SLHA + O2, n = 42) at rates to maintain sea level oxygen partial pressures according to Dalton's law (see West, 2004).
160 mmHg). Fertilized eggs were obtained from Black Leghorn chickens that had been reared at the sea level city of Santa Cruz or at high altitude city of La Paz for at least six generations. Fertilized eggs from sea level hens, laid at sea level, were randomly divided and incubated either at sea level (SLSL, n = 45) or high altitude (SLHA, n = 60). Eggs from high altitude hens, laid at high altitude, were randomly divided and incubated either at high altitude (HAHA, n = 70) or sea level (HASL, n = 50). SLHA embryos were also incubated with oxygen supplementation (SLHA + O2, n = 42) at rates to maintain sea level oxygen partial pressures according to Dalton's law (see West, 2004).
Egg storage is commonly practiced in the artificial incubation of domestic birds. If the storage temperature for freshly laid chicken eggs is kept below the physiological zero (25–27°C), dormancy of the embryo can be maintained and fertile eggs can be stored for 1–3 weeks (Butler, 1991). In this study, fertilized eggs from any one group were accumulated, maintained and transported over 2–3 days at 14°C to arrest and synchronize development, prior to incubation. Eggs were weighed prior to incubation. All incubations (Polyhatch, Brinsea Products Ltd, Sandford, UK) were carried under conditions to optimize development, with controlled temperature (38°C), humidity (60%) and appropriate egg rotation. On day 20, out of the 21 day incubation period, the egg was weighed, the air cell was exposed and chorio-allantoic venous blood was drawn into a 1 ml syringe for analysis of 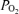 (ABL 500; Radiometer, Copenhagen, Denmark) and haematocrit, whenever possible in duplicate (Hawksley centrifuge). Following killing by spinal transection, the fetus was removed from the eggshell and weighed. Head diameter and body length (crown rump length) were measured with a digital micrometer. The fetal brain was dissected and weighed.
(ABL 500; Radiometer, Copenhagen, Denmark) and haematocrit, whenever possible in duplicate (Hawksley centrifuge). Following killing by spinal transection, the fetus was removed from the eggshell and weighed. Head diameter and body length (crown rump length) were measured with a digital micrometer. The fetal brain was dissected and weighed.
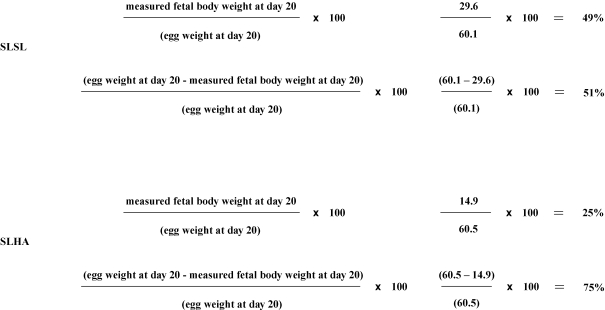
Growth efficiency was calculated as the ratio of the ‘measured fetal weight at day 20’ and the product of ‘the egg weight at day 20’ minus ‘the weight of the egg once the embryo had been removed at day 20’. An example, for a SLSL and a SLHA embryo is given in Scheme 1.
Scheme 1.
To calculate the partitioning of the resource, both the ‘measured fetal weight at day 20’ and ‘egg weight at day 20 – the measured fetal weight at day 20’ were expressed as a percentage of the egg weight at day 20 and plotted in histogram format. An example for the same SLSL and SLHA embryos as above is given in Scheme 2.
Scheme 2.
All procedures were approved by the local ethics committee (Consejo Tecnico) of the Bolivian Institute for High Altitude Biology (IBBA), Universidad Mayor de San Andrés, La Paz, Bolvia. All variables were analysed for normality of distribution and then expressed as means ± s.e.m. Comparisons between groups were assessed statistically using One-way ANOVA with an appropriate post hoc test (SigmaStat; Systat Software Inc., San Jose, CA, USA). Differences in mortality were compared using a contingency table and assessed by the chi-square (χ2) test. For all comparisons, statistical significance was accepted when P < 0.05.
Results
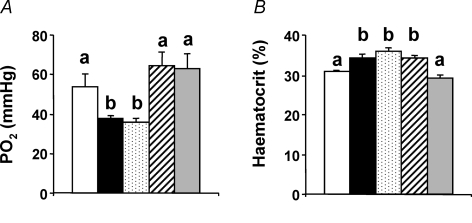
The data show that incubations at high altitude increased embryonic mortality relative to incubations at sea level, but this effect was reduced in HAHA embryos. The altitude-induced increase in mortality in the SLHA group was prevented by oxygen supplementation (mortality: SLSL = 28.8%, SLHA = 66.6%; HAHA = 52.8%; HASL = 28.1%; SLHA + O2= 30.2%; P < 0.05, χ2 test). Surviving chick embryos incubated at high altitude were hypoxic relative to chick embryos incubated at sea level, with the exception of those with oxygen supplementation, in which sea level oxygen partial pressure was maintained (Fig. 1A). Relative to SLSL and SLHA + O2 chick embryos, haematocrit was elevated in the SLHA, HAHA and HASL groups (P < 0.05), despite the latter group being incubated at sea level (Fig. 1B).
Figure 1. Partial pressure of oxygen and haematocrit.
Values are means +s.e.m. for chorioallantoic venous blood taken from sea level chick embryos incubated either at sea level (SLSL, open bar, n = 9) or high altitude (SLHA, filled bar, n = 12), high altitude embryos incubated at high altitude (HAHA, stippled bar, n = 10) or sea level (HASL, hatched bar, n = 7), and from sea level chick embryos incubated at high altitude with oxygen supplementation (SLHA + O2, grey bar, n = 12). Different letters are significantly different by one-way ANOVA with Student–Newman–Keuls test (P < 0.05).
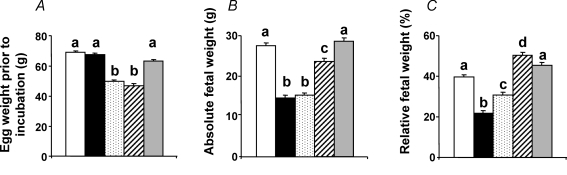
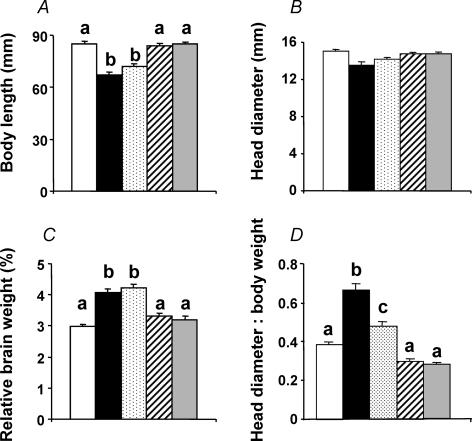
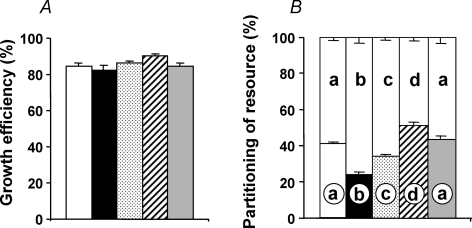
Prior to incubation, fertilized eggs from high altitude hens were lighter than fertilized eggs from sea level hens (Fig. 2A). At the end of the incubation period, in terms of absolute weight, chick embryos incubated at high altitude showed pronounced growth restriction, relative to chick embryos incubated at sea level (P < 0.05), with the exception of the SLHA + O2 group, in which the growth restriction was completely prevented (Fig. 2B). When embryonic weight was expressed as a percentage of the egg mass prior to incubation, to account for the initial differences in weights between high altitude and sea level eggs, fetal growth was restricted by 45.2% in the SLHA group and by 22.2% in the HAHA group relative to SLSL controls (Fig. 2C). Incubation at sea level of eggs laid by hens at high altitude not only restored growth, but these embryos grew heavier than the SLSL group. The embryonic growth restriction of incubations at high altitude was asymmetric as head growth was spared at the expense of body length (Fig. 3A and B). Hence, the relative brain weight, expressed as a percentage of the fetal body mass, was elevated in embryo groups, which had suffered body growth restriction during incubations at high altitude (Fig. 3C). The ratio of the head diameter to body weight was elevated in chick embryos incubated at high altitude. However, the increase in this ratio was markedly attenuated in the HAHA chicks relative to the SLHA group (Fig. 3D). Oxygen supplementation completely prevented the high altitude-induced asymmetry in fetal growth (Figs 2 and 3). Calculation of growth efficiency and the partitioning of the resource showed that reductions in oxygen availability during incubations at high altitude did not affect how well the resource is converted into fetal body mass (growth efficiency) (Fig. 4A). Rather, growth restriction at high altitude was due to a reduction in fetal resource uptake (Fig. 4B).
Figure 2. Fetal growth following high and lowland incubations.
Values are means +s.e.m. for the egg weight prior to incubation (A), the absolute fetal weight at the end of the incubation period (B) and the fetal weight at the end of the incubation period expressed as a percentage of the initial egg mass (C). SLSL (open bar, n = 31), SLHA (filled bar, n = 19), HAHA (stippled bar, n = 33), HASL (hatched bar, n = 30), and SLHA + O2 (grey bar, n = 26). Different letters are significantly different by one-way ANOVA with Student–Newman–Keuls or Dunn's tests, as appropriate (P < 0.05).
Figure 3. Symmetry of fetal growth following high and lowland incubations.
Values are means +s.e.m. for the body length (A), the head diameter (B), the brain weight expressed as a percentage of the fetal body weight (C) and the ratio of the head diameter to body weight (D). SLSL (open bar, n = 31), SLHA (filled bar, n = 19), HAHA (stippled bar, n = 33), HASL (hatched bar, n = 30), and SLHA + O2 (grey bar, n = 26). Different letters are significantly different by one-way ANOVA with Dunn's test (P < 0.05).
Figure 4. Use of resource for fetal growth during high and lowland incubations.
Values are mean +s.e.m. for the growth efficiency (A) and the partitioning of the resource (B), both expressed as a percentage. SLSL (open bar, n = 31), SLHA (filled bar, n = 19), HAHA (stippled bar, n = 33), HASL (hatched bar, n = 30), and SLHA + O2 (grey bar, n = 26). Values for partitioning of resource (mean +s.e.m.) are expressed as a histogram on top of the corresponding group. Different letters are significantly different by one-way ANOVA + Student–Newman–Keuls or Dunn's tests, as appropriate (P < 0.05). For calculations with examples, see Methods.
Discussion
The data show that development of chick embryos at high altitude induced fetal hypoxaemia and led to an increase in fetal haematocrit. Whilst several studies have reported an increase in packed red cell mass in the umbilical blood of human infants following gestation at high altitude (Ballew & Haas, 1986; Buys de Jorge et al. 1988; Leibson et al. 1989; Niermeyer et al. 1995; Ramirez-Cardich et al. 2004), this is the first direct demonstration that the partial pressure of oxygen is actually reduced in the fetal blood during development at high altitude. The data also show that incubation at high altitude of fertilized eggs laid by sea level hens restricted fetal growth, in similar fashion to restriction of fetal growth in the chick embryo by isobaric hypoxia (Miller et al. 2002). Incubation at high altitude of fertilized eggs laid by high altitude hens also restricted fetal growth, but to a lesser extent compared to eggs laid by sea level hens. By contrast, incubation at sea level of fertilized eggs laid by high altitude hens not only restored, but enhanced, fetal growth relative to sea level controls. Incubation at high altitude of sea level eggs with oxygen supplementation completely prevented the high altitude-induced fetal growth restriction.
The data obtained from the chick embryo in the current study resembles that presented in an epidemiological study of human populations in Bolivia (Giussani et al. 2001). In that study, birth weight records were obtained from term pregnancies in La Paz and Santa Cruz, especially from obstetric hospitals attended by wealthy or impoverished mothers. The data revealed pronounced asymmetric growth restriction in babies born at high altitude relative to sea level. When lowland babies born from mothers with high or low economic status were compared, birth weight was significantly reduced in low versus high income groups, but this difference was not as pronounced as the effect on birth weight of high altitude alone. Additional data also showed that highland babies from poor families did not have the greatest reduction in birth weight, as one would have expected. Rather, counter-intuitively, these babies were actually heavier than highland babies born from families with a high socio-economic status. The apparent conundrum was easily explained by assessing the ancestry of the families. In that study, the low socio-economic group of La Paz contained a high percentage (92%) of women from Amerindian origin with Aymara indian paternal and maternal surnames. In contrast, the high socio-economic group of La Paz contained a high European admixture. The findings of that study support the observations of Moore (1990) and Haas et al. (1980), who suggested that fetal growth restriction at altitude is correlated with the duration of high altitude residence: the longest resident population experiencing the least decline in birth weight with altitude, while the shortest historical residence groups, the greatest decline. Asymmetric growth restriction has been attributed, in part, to the sustained redistribution of the fetal cardiac output, to maintain oxygenation of essential circulations, such as those perfusing the brain, at the expense of the peripheral vascular beds (Akalin-Sel & Campbell, 1992; Giussani et al. 1993). Interestingly, a report by Martyn et al. (1996) suggested that, in human babies, an increase in the ratio of the head circumference to birth weight is associated with increased stroke in adulthood. Data in the present manuscript show that incubation of chick embryos at high altitude also produced an elevated ratio of the head diameter to body weight, and that this effect was also reduced in embryos from high altitude hens. Combined, our experiments in the chick embryo and observations in human babies in Bolivia therefore suggest that oxygen deprivation, independent of maternal nutrition during development, has a predominant role in the control of fetal growth. Further, prolonged high altitude residence confers protection against the effects of hypoxia on fetal mortality, fetal growth and the developing cardiovascular system. The overwhelming similarities between our findings in human babies and chick embryos make a serious case for the applicability of experiments with chicken eggs to model human conditions.
The physiology underlying the protection conferred by prolonged high altitude residence against the deleterious effects of hypoxia on fetal development remains unknown. In humans, a comparison of blood flow in Tibetan and Han Chinese pregnant women living at high altitude showed a greater redistribution of blood flow from the common iliac artery to the uterine artery in Tibetan than Han women. Thus, delivery of uteroplacental oxygen was increased and heavier babies were noted, despite lower arterial oxygen content in Tibetan women (Moore et al. 2001). A recent study by Zamudio et al. (2007) tested the hypothesis that greater maternal uteroplacental oxygen delivery would also explain increased human fetal growth at altitude in Andeans versus Europeans in Bolivia. They concluded that genetically mediated differences in maternal oxygen delivery did not explain the Andean advantage. Rather, the mechanism underlying this protection likely resides within the feto-placental unit. The present findings in the chick embryo confirm this expectation since protection conferred by prolonged high altitude residence could also express itself directly at the fetal, rather than the maternal or uteroplacental, levels. One possibility may be adaptations that enhance fetal arterial oxygen saturation. Whilst this has not been shown in the unborn child, an interesting study by (Beall, 2000) identified an autosomal major gene that confers higher resting oxygen saturation in sedentary Tibetan natives. In addition, Velarde et al. (1991) have also reported that Peruvian high altitude chickens have evolved a high haemoglobin-oxygen affinity, a finding suggested to be a genetic response to high altitude hypoxia. In our study, the highland chickens had lived at high altitude only for at least six generations. Whether this is sufficient time for physiological adaptations to high altitude to become genetically determined is questionable. However, the possibility exists that high altitude hypoxia may confer protection via epigenetic mechanisms, alterations in gene regulation that occur without a change in DNA sequence. Epigenetic marks such as CpG methylation and histone tail modifications have been shown to be affected by environmental conditions (Jirtle & Skinner, 2007), and because epigenetic marks are heritable (see Godfrey et al. 2007), gene expression and physiological responses can be altered in a comparatively shorter time frame, from one generation to the other, and for years to come.
To give further insight into the physiological mechanisms by which high altitude hypoxia depresses fetal growth, and the protection against this effect in highland chickens, we asked two further questions: do alterations in oxygen availability during development affect how well the resource is used by the fetus? Or does oxygen availability affect how well the resource is converted into fetal body mass? Calculation of growth efficiency and the partitioning of the resource in all five groups of chick embryos revealed that high altitude hypoxia did not affect growth efficiency, but it had a marked effect on fetal resource uptake or resource utilization for tissue accretion. For instance, incubation at high altitude of embryos from sea level hens had the greatest depressive effect on fetal growth because these embryos had the least uptake of resource. By contrast, despite development under similar conditions of fetal hypoxia, fetal resource uptake was greater in HAHA compared to SLHA groups. The mechanism by which development under hypoxic conditions reduces resource utilization by the fetus remains unresolved, but it is likely to be due to the depressive effects of oxygen deprivation on ATP synthesis and/or due to the effects of hypoxia on endocrine factors important in the regulation of fetal growth, such as insulin, thyroxine, cortisol and insulin-like growth factors (IGFs; see Fowden, 1995). Elucidation of changes in fetal nutrient utilization imposed by high altitude hypoxic pregnancy in humans and other mammals would require calculation of maternal glucose delivery and umbilical glucose uptake. In experimental mammalian models, such as sheep, this is highly technical and requires the chronic implantation of catheters into the umbilical vein, fetal dorsal aorta and caudal vena cava, and into the uterine ovarian vein and the maternal aorta with simultaneous blood sampling during experimentation (Comline & Silver, 1972). The simplicity with which answers to similar questions can be obtained in the chick embryo again highlights the excellence and applicability of this animal model.
A final component of the data presented in this study shows that when fertilized eggs laid by high altitude hens were incubated at sea level, the resulting embryos not only recovered their growth, but they grew heavier than sea level controls. The haematocrit data reveal that this group of embryos retained an increased oxygen carrying capacity despite incubation at sea level. This suggests that embryos from high altitude hens incubated at sea level had a greater oxygen content than sea level controls, further supporting a role for oxygen in the control of fetal growth. The mechanism by which elevated haematocrit levels are maintained in the absence of a hypoxic stimulus is unknown, but the data may reflect an adaptive response, transmitted by the mother to the oocyte prior to egg laying, predictive of fetal development in a hypoxic environment. Another example of a maternal predictive adaptive response (Gluckman & Hanson, 2004) is that of the meadow vole, in which the photoperiodic history of the dam prior to conception, rather than the perinatal thermal environment, can better determine the offspring's coat thickness at birth (Lee & Zucker, 1988). Alternatively, the maintained elevated haematocrit in HASL embryos may highlight that genetic or epigenetic control of factors determining oxygen carrying capacity is regulated very early on in the developmental process of the oocyte by the available oxygen concentration at that time.
In conclusion, we have isolated the effects of alterations in fetal oxygenation on fetal growth. Oxygen, independent of maternal nutritional factors, has a predominant role in the control of fetal growth and prolonged high altitude residence confers protection against the effects of hypoxia on fetal development. This discovery calls for the attention of this aspect of growth regulation not only in women undergoing pregnancy at high altitude, but also in sea level human pregnancies complicated with reduced oxygen delivery to the fetus, such as during placental insufficiency and pre-eclampsia (Many et al. 1996; Baschat, 2004).
Acknowledgments
We thank Dr Wilma Tellez, Mr Armando Rodriguez, Mrs Martha Aguilar, Mrs Loyola Riveros, Mr Wilmar Velasquez and Mr Didi Maquera at IBBA, La Paz and Dr Ginella, Dr Nioshi and Dr Roca at CENETROP, Santa Cruz for their help with these studies. Financial support was provided by The British Heart Foundation and The Royal Society.
References
- Akalin-Sel T, Campbell S. Understanding the pathophysiology of intra-uterine growth retardation: the role of the ‘lower limb reflex’ in redistribution of blood flow. Eur J Obstet Gynecol Reprod Biol. 1992;46:79–86. doi: 10.1016/0028-2243(92)90250-3. [DOI] [PubMed] [Google Scholar]
- Ballew C, Haas JD. Hematologic evidence of fetal hypoxia among newborn infants at high altitude in Bolivia. Am J Obstet Gynecol. 1986;155:166–169. doi: 10.1016/0002-9378(86)90104-3. [DOI] [PubMed] [Google Scholar]
- Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111:1031–1041. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- Beall CM. Tibetan and Andean patterns of adaptation to high-altitude hypoxia. Hum Biol. 2000;72:201–228. [PubMed] [Google Scholar]
- Butler DE. Egg handling and storage at the farm and hatchery. In: Tullet SD, editor. Avian Incubation. London: Butterworth; 1991. pp. 195–203. [Google Scholar]
- Buys de Jorge MC, Contrini MA, Miranda C, Carrera C, Torrejon I, Martin B, Scaro JL. Sangre (Barc) 1988;33:97–101. [PubMed] [Google Scholar]
- Comline RS, Silver M. The composition of foetal and maternal blood during parturition in the ewe. J Physiol. 1972;222:233–256. doi: 10.1113/jphysiol.1972.sp009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grauw TJ, Myers R, Scott WJ. Fetal growth in rats from different levels of hypoxia. Biol Neonate. 1986;49:85–89. doi: 10.1159/000242515. [DOI] [PubMed] [Google Scholar]
- Fowden AL. Endocrine regulation of fetal growth. Reprod Fertil Dev. 1995;7:351–363. doi: 10.1071/rd9950351. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude vs. economic status on birth weight and body shape at birth. Ped Res. 2001;49:490–494. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Lillycrop KA, Burdge GC, Gluckamn PD, Hanson MA. Ped Res. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- Haas JD, Frongillo EF, Stepcik C, Beard J, Hurtado L. Altitude, ethnic and sex differences in birthweight and length in Bolivia. Hum Biol. 1980;52:459–477. [PubMed] [Google Scholar]
- Jacobs R, Robinson JS, Owens JA, Falconer J, Webster MED. The effect of prolonged hypobaric hypoxia on growth of fetal sheep. J Dev Physiol. 1988;10:97–112. [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Zucker I. Vole infant development is influenced perinatally by maternal photoperiodic history. Am J Physiol Regul Integr Comp Physiol. 1988;255:R831–R838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- Leibson C, Brown M, Thibodeau S, Stevenson D, Vreman H, Cohen R, Clemons G, Callen W, Moore LG. Neonatal hyperbilirubinemia at high altitude. Am J Dis Child. 1989;143:983–987. doi: 10.1001/archpedi.1989.02150200145036. [DOI] [PubMed] [Google Scholar]
- Lichty JA, Ting RY, Bruns P, Dyer E. Studies of babies born at high altitude. 1. Relation of altitude to birth weight. Am J Dis Child. 1957;93:666–669. doi: 10.1001/archpedi.1957.02060040668009. [DOI] [PubMed] [Google Scholar]
- Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174:288–291. doi: 10.1016/s0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Barker DJP, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- McClung J. Effects of High Altitude on Human Birth. Cambridge, MA: Harvard University Press; 1969. [Google Scholar]
- Miller SL, Green LR, Peebles DM, Hanson MA, Blanco CE. Effects of chronic hypoxia and protein malnutrition on growth in the developing chick. Am J Obstet Gynecol. 2002;186:261–267. doi: 10.1067/mob.2002.119629. [DOI] [PubMed] [Google Scholar]
- Moore LG. Maternal O2 transport and fetal growth in Colorado, Peru and Tibet high-altitude residents. Am J Hum Biol. 1990;2:627–637. doi: 10.1002/ajhb.1310020606. [DOI] [PubMed] [Google Scholar]
- Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;27:25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Am J Phys Anthropol. 2001;114:42–53. doi: 10.1002/1096-8644(200101)114:1<42::AID-AJPA1004>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Niermeyer S, Yang P, Shanmina, Drolkar, Zhuang J, Moore LG. Arterial oxygen saturation in Tibetan and Han infants born in Lhasa, Tibet. N Engl J Med. 1995;333:1248–1252. doi: 10.1056/NEJM199511093331903. [DOI] [PubMed] [Google Scholar]
- Ramirez-Cardich ME, Saito M, Gilman RH, Escate LE, Strouse JJ, Kabrhel C, Johnson C, Galchen R, Bautista CT. Effect of maternal anemia at high altitude on infant hematocrit and oxygenation. Am J Trop Med Hyg. 2004;70:420–424. [PubMed] [Google Scholar]
- Velarde FL, Espinoza D, Monge C, de Muizon C. A genetic response to high altitude hypoxia: high hemoglobin-oxygen affinity in chicken (Gallus gallus) from the Peruvian Andes. C R Acad Sci III. 1991;313:401–406. [PubMed] [Google Scholar]
- West JB. Respiratory Physiology. The Essentials. 7. Baltimore: Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582:883–895. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]