Abstract
Vesicle fusion is a ubiquitous biological process involved in general membrane trafficking and a variety of specialized events, for example release of neurotransmitters and hormones, sperm acrosome exocytosis, plasma membrane repair and neurite outgrowth. Many vesicle fusion events have long been known to be activated by phospholipases and products of their activity, such as polyunsaturated arachidonic acid. Polyunsaturated fatty acids (PUFAs) have been proposed to have a number of multiple effectors, including ion channels and the cytoskeleton, but the precise mechanism of PUFA action is still unclear. It was recently reported that omega-3 and omega-6 PUFAs can act on syntaxin, a plasma membrane protein directly involved in vesicle fusion. In this review, we will discuss the role of this new mode of PUFA action in exocytosis.
Exocytosis is a cellular event where cargo-laden vesicles fuse with the plasma membrane. Vesicle fusion is involved in various biological processes including cell growth, plasma membrane repair, axonal branching, recycling of plasma membrane transporters, and release of soluble signalling molecules such as neurotransmitters or hormones into the extracellular space. It is well established that phospholipases A2, C and D, which metabolize membrane phospholipids, are somehow involved in vesicle fusion (Vitale et al. 2001; Brown et al. 2003; Rossetto et al. 2006). One class of phospholipid components, long-chain polyunsaturated fatty acids (PUFAs), has emerged as being particularly important for exocytosis. However, the mechanisms of action of PUFAs in the regulation of vesicle fusion are not well understood. They have been proposed to modulate ion channel function and to perturb cytoskeleton (Honore et al. 1994; Lesage et al. 2000; Mignen & Shuttleworth, 2000; Neco et al. 2003).
Recently, PUFAs were shown to act on syntaxin, a plasma membrane protein directly involved in fusion of vesicles with the plasma membrane (Rickman & Davletov, 2005; Darios & Davletov, 2006; Connell et al. 2007). Syntaxin belongs to the soluble NSF-attachment receptor (SNARE) protein family responsible for intracellular membrane fusion throughout the cell. A prototypical set of fusion proteins involved in neurotransmitter release consists of the plasma membrane syntaxin 1 together with SNAP-25 (synaptosome-associated protein of 25 kDa) and vesicular protein synaptobrevin (Rizo & Sudhof, 2002). The three proteins form a slightly twisted four-helical bundle between two approaching membranes (Sutton et al. 1998), probably initiating the fusion event. Since the action of PUFAs on ion channels and cytoskeleton has been discussed elsewhere (Nakamura et al. 2001; Neco et al. 2003), we will focus here on the role of PUFA-releasing enzymes and fatty acid signalling to promote activation of SNARE proteins in vesicle fusion.
PUFAs and neuronal function
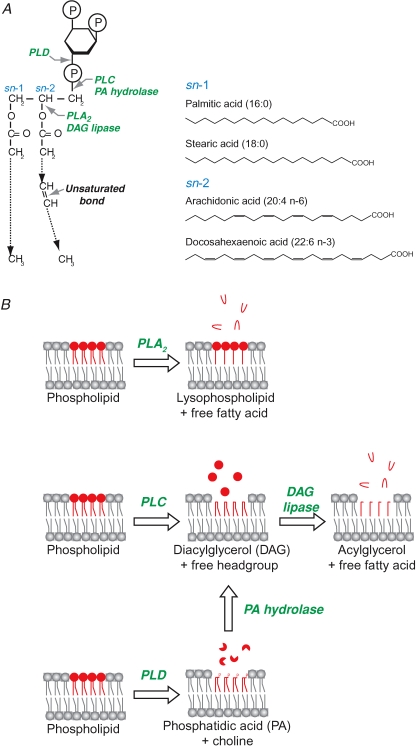
The membrane bilayers in which SNARE proteins reside are composed of several phospholipid species as well as sphingolipids and cholesterol. A typical phospholipid structure and the sites of phospholipase action are shown in Fig. 1A. Phospholipids often contain PUFAs such as arachidonic or docosahexaenoic acids in the sn-2 position, whereas the sn-1 position is normally occupied by saturated fatty acids.
Figure 1. Alternative pathways leading to the release of mobile polyunsaturated fatty acids from the lipid bilayer into the cytosol.
A, a typical phospholipid consists of saturated palmitic acid (C16), or more commonly stearic acid (C18), attached to the sn-1 position of the glycerol backbone, while unsaturated omega-6 arachidonic acid (C20: 4) or omega-3 docosahexaenoic acid (C22: 6) occupy its sn-2 position. The glycerol backbone also carries a hydrophilic headgroup shown here as a phosphorylated inositol; other common headgroups are choline, ethanolamine and serine. Phospholipase sites of action are shown by grey arrows. Note the presence of four and six unsaturated double bonds in arachidonic acid and docosahexaenoic acid, respectively. B, hydrolysis of the phospholipid molecule by phospholipase A2 (PLA2) produces lysophospholipid which is preferentially retained in the membrane and more soluble arachidonic acid (red hairpin) which, following diffusion into the cytosol, is either metabolized into different eicosanoid products or incorporated back in the sn-2 position of phospholipids (not shown). Phospholipase C (PLC) acts on the lipid bilayer to release soluble headgroup (red circles) leaving diacylglycerol (DAG) in the membrane. Further action of a DAG lipase results in the release of PUFAs into the cytosol. Monoacylated glycerol remains in the membrane due to straight saturated stearic or palmitic fatty acids. Phospholipase D (PLD) releases the headgroup (preferentially choline) of the phospholipid molecule producing hydrophobic phosphatidic acid which remains in membrane. Phosphatidic acid can in turn be metabolized into DAG by phospatidic acid (PA) hydrolase.
Two types of PUFAs, omega-6 and omega-3, are essential for vertebrates as they cannot be formed de novo and need to be ingested. Omega-6 arachidonic and omega-3 docosahexaenoic acid are major building blocks of cellular membranes (Svennerholm, 1968). PUFAs possess favourable biophysical properties such as flexibility and solubility, which promote membrane fluidity. In addition, when released through phospholipase action, they directly take part in regulation of many cellular processes and are also converted into eicosanoids, including prostaglandins (Brash, 2001). Of note, some of these metabolites were reported to activate vesicle fusion, whereas others inhibit it (Bazan et al. 2002).
The importance of PUFAs for neuronal function is well known (Wainwright, 2002). Mutations in PUFA-related enzymes cause mental retardation in humans (Meloni et al. 2002), and diets deficient in essential PUFAs are associated with deficits in infant brain function (Wainwright, 2002). In addition, mutations in an enzyme involved in PUFA production cause neuronal impairment in the C. elegans model organism, which can be rescued by external application of arachidonic or docosahexaenoic acid (Lesa et al. 2003). There was some uncertainty regarding the effect of arachidonic acid on catecholamine secretion (Frye & Holz, 1984; Morgan & Burgoyne, 1990) but a recent study demonstrated arachidonic acid-induced up-regulation of secretion in both permeabilized and intact cell models (Latham et al. 2007). These data together suggest that PUFAs or their metabolites are essential for exocytosis. Interestingly, PUFA-rich diets affect expression of only few genes; amongst them is the syntaxin-binding protein Munc18, suggesting a possible link with SNARE proteins (Barcelo-Coblijn et al. 2003).
PUFA-releasing enzymes and exocytosis
Phospholipase A2s (PLA2s) are a group of enzymes which catalyse the breakdown of phospholipids. They release fatty acids from the sn-2 position of the phospholipid molecule (Fig. 1A) and are postulated to be involved in vesicle fusion in many biological systems, including neurotransmitter release, insulin secretion, sperm acrosome exocytosis and neurite outgrowth (Freeman et al. 1990; Wolf et al. 1991; Nakamura, 1993; Roldan & Fragio, 1993; Tsukada et al. 1994; Almeida et al. 1999; Brown et al. 2003; Juhl et al. 2003). PLA2-mediated phospholipid hydrolysis generates free unsaturated fatty acids and lysophospholipids (Fig. 1B). Unsaturated fatty acids have intrinsically high rates of dissociation from membranes and, in contrast to lysophospholipids, can diffuse into the cytosol to intracellular sites of action without any transporter molecules (Tanford, 1973; Balsinde et al. 2002). It has been shown that PLA2 acts to ‘prime’ fusion machinery on the plasma membrane, suggesting up-regulation of SNAREs or SNARE-associated molecules (Karli et al. 1990). Interestingly, this priming of vesicle fusion is achieved through the production of arachidonic acid and not lysophospholipid (Karli et al. 1990).
Mounting evidence also implicates phospholipase C (PLC) in exocytosis (Hammond et al. 2006). PLC releases the soluble headgroup from membrane phospholipids, leaving diacylglycerol (DAG) anchored in the membrane by its two hydrophobic carbon chains (Fig. 1B). The PLC inhibitor U73122 potently blocks synaptic vesicle exocytosis in many systems, even in the case of the most powerful secretagogue known – latrotoxin from the black widow spider (Davletov et al. 1998). The presence of DAG is necessary for activation of protein kinase C and Munc13, both of which are important positive regulators of vesicle fusion (Rhee et al. 2002). DAG can be further metabolized by DAG lipase, releasing arachidonic acid from the phospholipid sn-2 position and leaving monoacylated glycerol in the lipid bilayer (Brash, 2001). Interestingly, a DAG lipase inhibitor can block both neurite outgrowth and insulin release (Meiri et al. 1998; Guenifi et al. 2001). Recently it has been shown that a DAG lipase-like protein is obligatory for neurotransmitter release in the Drosophila genetic model (Huang et al. 2006). Genetic tests revealed a synergistic interaction between syntaxin and this DAG lipase-like protein, suggesting that the latter molecule could be directly involved in activation of SNARE function in synaptic vesicle fusion (Huang et al. 2006).
Finally, phospholipase D (PLD) has also been involved in the induction of vesicle fusion, at a late step of exocytosis (Vitale et al. 2001). PLD hydrolyses phosphatidylcholine into the choline headgroup and phosphatidic acid, which can in turn be hydrolysed into DAG by phosphatidic acid phosphohydrolase (Fig. 1B). PLD is required for the formation of certain SNARE complexes in yeast (Coluccio et al. 2004). It appears that in the case of PLD, early metabolite – phosphatidic acid – may play a role in exocytosis (Metz & Dunlop, 1990; Humeau et al. 2001).
Action of PUFAs on syntaxin
Snake neurotoxins that disrupt synaptic function contain PLA2s (Rossetto et al. 2006) and their secretogogue action appears to be blocked by application of a SNARE-cleaving botulinum toxin suggesting that PLA2s act upstream of SNARE assembly (Tse et al. 1982; Prasarnpun et al. 2004). We recently reported immediate effects of arachidonic acid on the SNARE fusion machinery (Rickman & Davletov, 2005; Darios & Davletov, 2006; Connell et al. 2007). Addition of arachidonic acid to synaptic membranes or membrane treatment with PLA2s was sufficient to potentiate SNARE complex formation (Rickman & Davletov, 2005; Darios & Davletov, 2006). It is notable that prostaglandins are ineffective in syntaxin activation (Connell et al. 2007), suggesting that it is PUFAs themselves that promote vesicle fusion.
Analysis of individual SNARE components revealed that it is syntaxin that is sensitive to the presence of two major PUFAs enriched in brain, arachidonic and docosahexaenoic acid (Darios & Davletov, 2006; Connell et al. 2007). A strong correlation was observed between changes in the structural properties of syntaxin and neuronal growth providing mechanistic insights into PUFA action in brain development (Darios & Davletov, 2006). Although this was demonstrated for syntaxin 3, which mediates vesicle fusion during neuronal growth, it is now clear that syntaxin 1, the major brain isoform, is also sensitive to arachidonic acid (Connell et al. 2007; Latham et al. 2007) suggesting a conserved mechanism of regulation of the SNARE fusion machinery.
Specificity of PUFA actions
Polyunsaturated fatty acids exhibit high specificity in up-regulation of syntaxin for entry into the SNARE complex, with saturated fatty acids being ineffective (Rickman & Davletov, 2005; Darios & Davletov, 2006; Connell et al. 2007). Since saturated fatty acids form micelles at lower concentrations than PUFAs (Tanford, 1973), micelle formation per se is not likely to underlie syntaxin activation. Furthermore, the ability of PUFAs to form micelles is inhibited in the presence of syntaxin (Connell et al. 2007). Half-maximal effective concentration for arachidonic acid activation of syntaxin was reported to be around 50 μm (Rickman & Davletov, 2005). Is such concentration achievable within the cellular environment? A high percentage of phospholipid molecules carry esterified PUFA meaning that, inside the tightly packed lipid bilayer, PUFA concentration will be in a molar range. Local phospholipase action on phospholipid membranes is likely to result in a transiently high concentration of arachidonic acid at the membrane where syntaxin resides, before arachidonic acid diffuses deeper into the cytosol (Brash, 2001). Direct measurements in endocrine insulin-secreting cells demonstrated that, upon stimulation of exocytosis, global arachidonic acid concentration can reach 50–75 μm (Wolf et al. 1991). Furthermore, a very high turnover of arachidonic acid has been demonstrated in isolated nerve terminals and neuronal growth cones, which are enriched in PLA2 (Negre-Aminou & Pfenninger, 1993). The small volume of these specialized neuronal compartments, containing the SNARE fusion machinery, suggests that syntaxins can encounter transiently high PUFA concentrations that would be sufficient to stimulate SNARE interactions.
Putative mode of action of PUFAs on the syntaxin molecule
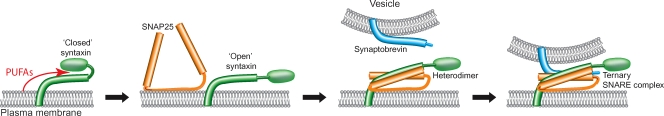
How does arachidonic acid promote SNARE interactions? The syntaxin structure is conserved throughout evolution (Munson et al. 2000; Rizo & Sudhof, 2002) and consists of a membrane anchor, SNARE motif and an autonomously folded N-terminal domain (Fig. 2). This latter N-terminal domain folds back over the helical SNARE motif thereby inhibiting SNARE complex assembly (Rizo & Sudhof, 2002), either partially (e.g. syntaxins 1 and 2) or completely (e.g. syntaxins 3 and 4) (Connell et al. 2007). An essential cytosolic protein, Munc18, is the major binding partner of syntaxins in brain, and the syntaxin 1–Munc18 complex has been crystallised. In this structure, Munc18 acts as a ‘clamp’ to hold syntaxin 1 in a closed, inactive conformation (Misura et al. 2000). Remarkably, arachidonic acid is able to activate syntaxins even in tight association with Munc18, allowing syntaxin engagement of its SNARE partners without abolition of native syntaxin–Munc18 association (Rickman & Davletov, 2005; Connell et al. 2007). It is possible that flexible unsaturated fatty acids can penetrate into hydrophobic grooves between the amphipathic syntaxin helices, producing a conformational change and promoting SNARE complex formation. A similar mode of interaction has been suggested for several fatty acid-binding proteins (Hamilton, 2004). Structural studies, e.g. a nuclear magnetic resonance approach, are now required to provide an atomic level description of the dynamic interaction between arachidonic acid and syntaxin.
Figure 2. Action of polyunsaturated fatty acids on syntaxin.
Syntaxin present in a ‘closed conformation’ is a target for PUFAs. They can induce a conformational change, leading to an ‘open’ conformation of the syntaxin and allowing interaction with SNAP-25. Upon this interaction, SNAP-25 acquires its coiled α-helical structure. A subsequent association of the heterodimer with vesicular synaptobrevin leads to formation of the ternary SNARE complex which promotes vesicle fusion.
Conclusion
Although phospholipases and polyunsaturated fatty acids have been known for several decades to play a crucial role in exocytosis, we are still lacking a complete picture of their mode of action. Together with modulation of the ion channel and cytoskeleton functions, PUFAs can act on proteins that are pivotal in vesicle fusion – syntaxins. However, phospholipases not only release PUFAs, but are responsible for the production of other lipid molecules (lysophopholipids, DAG, acylglyerol, phosphatidic acid, see Fig. 1) which may also take part in vesicle fusion (Rhee et al. 2002; Rigoni et al. 2005; Wang et al. 2006). The recent discovery that a ceramidase and a protein closely resembling a diacylglycerol lipase (DAG lipase) are essential for vesicle fusion in Drosophila (Rohrbough et al. 2004; Huang et al. 2006) further highlights the fact that various lipids play critical roles in exocytosis. Furthermore, multiple phospholipase isoforms are present in the body, varying both in distribution and regulatory mechanisms. It still remains to be determined exactly which phospholipase isoforms assist the specific vesicle fusion events, underlying the need for more targeted genetic and pharmacological investigations. Although syntaxins have emerged as likely mediators of PUFA action in exocytosis, we cannot rule out that some of the newly identified proteins essential for vesicle docking, priming, fusion and membrane retrieval may also be sensitive to the products of local lipid metabolism.
Acknowledgments
We thank Jo Westmoreland for help with illustrations.
References
- Almeida T, Cunha RA, Ribeiro JA. Facilitation by arachidonic acid of acetylcholine release from the rat hippocampus. Brain Res. 1999;826:104–111. doi: 10.1016/s0006-8993(99)01267-6. [DOI] [PubMed] [Google Scholar]
- Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Kitajka K, Puskas LG, Hogyes E, Zvara A, Hackler L, Jr, Farkas T. Gene expression and molecular composition of phospholipids in rat brain in relation to dietary n-6 to n-3 fatty acid ratio. Biochim Biophys Acta. 2003;1632:72–79. doi: 10.1016/s1388-1981(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Tu B, Rodriguez de Turco EB. What synaptic lipid signaling tells us about seizure-induced damage and epileptogenesis. Prog Brain Res. 2002;135:175–185. doi: 10.1016/S0079-6123(02)35017-9. [DOI] [PubMed] [Google Scholar]
- Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- Coluccio A, Malzone M, Neiman AM. Genetic evidence of a role for membrane lipid composition in the regulation of soluble NEM-sensitive factor receptor function in Saccharomyces cerevisiae. Genetics. 2004;166:89–97. doi: 10.1534/genetics.166.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell E, Darios F, Broersen K, Gatsby N, Peak-Chew SY, Rickman C, Davletov B. Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep. 2007;8:414–419. doi: 10.1038/sj.embor.7400935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Meunier FA, Ashton AC, Matsushita H, Hirst WD, Lelianova VG, Wilkin GP, Dolly JO, Ushkaryov YA. Vesicle exocytosis stimulated by alpha-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+ EMBO J. 1998;17:3909–3920. doi: 10.1093/emboj/17.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EJ, Terrian DM, Dorman RV. Presynaptic facilitation of glutamate release from isolated hippocampal mossy fiber nerve endings by arachidonic acid. Neurochem Res. 1990;15:743–750. doi: 10.1007/BF00973656. [DOI] [PubMed] [Google Scholar]
- Frye RA, Holz RW. The relationship between arachidonic acid release and catecholamine secretion from cultured bovine adrenal chromaffin cells. J Neurochem. 1984;43:146–150. doi: 10.1111/j.1471-4159.1984.tb06690.x. [DOI] [PubMed] [Google Scholar]
- Guenifi A, Simonsson E, Karlsson S, Ahren B, Abdel-Halim SM. Carbachol restores insulin release in diabetic GK rat islets by mechanisms largely involving hydrolysis of diacylglycerol and direct interaction with the exocytotic machinery. Pancreas. 2001;22:164–171. doi: 10.1097/00006676-200103000-00009. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Prog Lipid Res. 2004;43:177–199. doi: 10.1016/j.plipres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G. Elimination of plasma membrane phosphatidylinositol (4,5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci. 2006;119:2084–2094. doi: 10.1242/jcs.02912. [DOI] [PubMed] [Google Scholar]
- Honore E, Barhanin J, Attali B, Lesage F, Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 1994;91:1937–1941. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FD, Woodruff E, Mohrmann R, Broadie K. Rolling blackout is required for synaptic vesicle exocytosis. J Neurosci. 2006;26:2369–2379. doi: 10.1523/JNEUROSCI.3770-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Vitale N, Chasserot-Golaz S, Dupont J-L, Du G, Frohman MA, Bader M-F, Poulain B. A role for phospholipase D1 in neurotransmitter release. Proc Natl Acad Sci U S A. 2001;98:15300–15305. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl K, Hoy M, Olsen HL, Bokvist K, Efanov AM, Hoffmann EK, Gromada J. cPLA2α-evoked formation of arachidonic acid and lysophospholipids is required for exocytosis in mouse pancreatic β-cells. Am J Physiol Endocrinol Metab. 2003;285:E73–E81. doi: 10.1152/ajpendo.00086.2003. [DOI] [PubMed] [Google Scholar]
- Karli UO, Schafer T, Burger MM. Fusion of neurotransmitter vesicles with target membrane is calcium independent in a cell-free system. Proc Natl Acad Sci U S A. 1990;87:5912–5915. doi: 10.1073/pnas.87.15.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham CF, Osborne SL, Cryle MJ, Meunier FA. Arachidonic acid potentiates exocytosis and allows neuronal SNARE complex to interact with Munc18a. J Neurochem. 2007;100:1543–1554. doi: 10.1111/j.1471-4159.2006.04286.x. [DOI] [PubMed] [Google Scholar]
- Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J Cell Sci. 2003;116:4965–4975. doi: 10.1242/jcs.00918. [DOI] [PubMed] [Google Scholar]
- Lesage F, Maingret F, Lazdunski M. Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mechano-sensitive K+ channel. FEBS Lett. 2000;471:137–140. doi: 10.1016/s0014-5793(00)01388-0. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Saffell JL, Walsh FS, Doherty P. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J Neurosci. 1998;18:10429–10437. doi: 10.1523/JNEUROSCI.18-24-10429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni I, Muscettola M, Raynaud M, Longo I, Bruttini M, Moizard MP, et al. FACL4, encoding fatty acid-CoA ligase 4, is mutated in nonspecific X-linked mental retardation. Nat Genet. 2002;30:436–440. doi: 10.1038/ng857. [DOI] [PubMed] [Google Scholar]
- Metz SA, Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J. 1990;270:427–435. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. I (ARC), a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Morgan A, Burgoyne RD. Relationship between arachidonic acid release and Ca2+-dependent exocytosis in digitonin-permeabilized bovine adrenal chromaffin cells. Biochem J. 1990;271:571–574. doi: 10.1042/bj2710571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M, Chen X, Cocina AE, Schultz SM, Hughson FM. Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- Nakamura MT, Cho HP, Xu J, Tang Z, Clarke SD. Metabolism and functions of highly unsaturated fatty acids: an update. Lipids. 2001;36:961–964. doi: 10.1007/s11745-001-0806-5. [DOI] [PubMed] [Google Scholar]
- Nakamura S. Involvement of phospholipase A2 in axonal regeneration of brain noradrenergic neurones. Neuroreport. 1993;4:371–374. doi: 10.1097/00001756-199304000-00007. [DOI] [PubMed] [Google Scholar]
- Neco P, Rossetto O, Gil A, Montecucco C, Gutierrez LM. Taipoxin induces F-actin fragmentation and enhances release of catecholamines in bovine chromaffin cells. J Neurochem. 2003;85:329–337. doi: 10.1046/j.1471-4159.2003.01682.x. [DOI] [PubMed] [Google Scholar]
- Negre-Aminou P, Pfenninger KH. Arachidonic acid turnover and phospholipase A2 activity in neuronal growth cones. J Neurochem. 1993;60:1126–1136. doi: 10.1111/j.1471-4159.1993.tb03263.x. [DOI] [PubMed] [Google Scholar]
- Prasarnpun S, Walsh J, Harris JB. β-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction. Neuropharmacology. 2004;47:304–314. doi: 10.1016/j.neuropharm.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. β phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Rickman C, Davletov B. Arachidonic acid allows SNARE complex formation in the presence of Munc18. Chem Biol. 2005;12:545–553. doi: 10.1016/j.chembiol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Rushton E, Palanker L, Woodruff E, Matthies HJ, Acharya U, Acharya JK, Broadie K. Ceramidase regulates synaptic vesicle exocytosis and trafficking. J Neurosci. 2004;24:7789–7803. doi: 10.1523/JNEUROSCI.1146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan ER, Fragio C. Phospholipase A2 activation and subsequent exocytosis in the Ca2+/ionophore-induced acrosome reaction of ram spermatozoa. J Biol Chem. 1993;268:13962–13970. [PubMed] [Google Scholar]
- Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C. Presynaptic enzymatic neurotoxins. J Neurochem. 2006;97:1534–1545. doi: 10.1111/j.1471-4159.2006.03965.x. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9:570–579. [PubMed] [Google Scholar]
- Tanford C. The Hydrophobic Effect. New York: John Wiley and Sons; 1973. [Google Scholar]
- Tse CK, Dolly JO, Hambleton P, Wray D, Melling J. Preparation and characterisation of homogeneous neurotoxin type A from Clostridium botulinum. Its inhibitory action on neuronal release of acetylcholine in the absence and presence of β-bungarotoxin. Eur J Biochem. 1982;122:493–500. [PubMed] [Google Scholar]
- Tsukada Y, Chiba K, Yamazaki M, Mohri T. Inhibition of the nerve growth factor-induced neurite outgrowth by specific tyrosine kinase and phospholipase inhibitors. Biol Pharm Bull. 1994;17:370–375. doi: 10.1248/bpb.17.370. [DOI] [PubMed] [Google Scholar]
- Vitale N, Caumont AS, Chasserot-Golaz SG, Wu S, Sciorra VA, Morris AJ, Frohman MA, Bader MF. Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 2001;20:2424–2434. doi: 10.1093/emboj/20.10.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Wolf BA, Pasquale SM, Turk J. Free fatty acid accumulation in secretagogue-stimulated pancreatic islets and effects of arachidonate on depolarization-induced insulin secretion. Biochemistry. 1991;30:6372–6379. doi: 10.1021/bi00240a004. [DOI] [PubMed] [Google Scholar]