Abstract
5′AMP-activated protein kinase (AMPK) exists as a heterotrimer comprising a catalytic α subunit and regulatory β and γ subunits. The AMPK system is activated under conditions of cellular stress, indicated by an increase in the AMP/ATP ratio, as observed, e.g. in muscles during contractile activity. AMPK was originally thought to be activated only by local intracellular mechanisms. However, recently it has become apparent that AMPK in mammals is also regulated by humoral substances, e.g. catecholamines. We studied whether humoral factors released during exercise regulate AMPK activity in contracting and resting muscles as well as in abdominal subcutaneous adipose tissue in humans. In resting leg muscle and adipose tissue the AMPK activity was not up-regulated by humoral factors during one-legged knee extensor exercise even when arm cranking exercise, inducing a ∼20-fold increase in plasma catecholamine level, was added simultaneously. In exercising leg muscle the AMPK activity was increased by one-legged knee extensor exercise eliciting a whole body respiratory load of only 30% 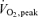 but was not further increased by adding arm cranking exercise. In conclusion, during exercise with combined leg kicking and arm cranking, the AMPK activity in human skeletal muscle is restricted to contracting muscle without influence of marked increased catecholamine levels. Also, with this type of exercise the catecholamines or other humoral factors do not seem to be physiological regulators of AMPK in the subcutaneous adipose tissue.
but was not further increased by adding arm cranking exercise. In conclusion, during exercise with combined leg kicking and arm cranking, the AMPK activity in human skeletal muscle is restricted to contracting muscle without influence of marked increased catecholamine levels. Also, with this type of exercise the catecholamines or other humoral factors do not seem to be physiological regulators of AMPK in the subcutaneous adipose tissue.
5′AMP-activated protein kinase (AMPK) exists as a heterotrimer comprising a catalytic α subunit and regulatory β and γ subunits. The AMPK system is activated under conditions of cellular stress, as seen, e.g. in skeletal muscle during contractile activity. AMPK is activated both allosterically, by an increase in the AMP/ATP ratio (Ferrer et al. 1985; Carling et al. 1989) and covalently via phosphorylation on Thr172 of the α subunit (Hawley et al. 1996; Crute et al. 1998; Stein et al. 2000) by AMPK kinases (AMPKK), e.g. LKB1 and CaMKKα/β (Hawley et al. 2003; Woods et al. 2003; Shaw et al. 2004; Sakamoto et al. 2005; Hawley et al. 2005; Hurley et al. 2005; Jensen et al. 2007b). Historically the AMPK pathway was viewed primarily as being regulated by local intracellular mechanisms. However, recently it has become apparent that AMPK in mammals is also regulated by humoral substances, e.g. the adipokines, in various kinds of tissues (Kahn et al. 2005). For example adiponectin, leptin and IL-6 have been shown to increase AMPK activity in rat skeletal muscles (Tomas et al. 2002; Minokoshi et al. 2002; Kelly et al. 2004). Adiponectin and IL-6 have also been shown to stimulate AMPK activity in adipocytes (Yamauchi et al. 2002; Wu et al. 2003; Kelly et al. 2004). The relationship between AMPK and IL-6 is also evident from the decreased basal AMPK activities in skeletal muscle and adipose tissue from IL-6 knock-out mice (Kelly et al. 2004). In contrast to adiponectin and leptin, the IL-6 plasma concentration increases during exercise (Steensberg et al. 2000; Kraemer et al. 2002; Kraemer et al. 2003; MacDonald et al. 2003). Thus a causal relationship between IL-6 and AMPK activity during exercise is possible. However, the observation of a similar relative increase in the AMPK activity of skeletal muscles and adipose tissue during exercise in IL-6 knock-out and wild-type mice does not support this view (Kelly et al. 2004). Several other studies in rodents have also observed an exercise related AMPK activation in adipose tissue (Park et al. 2002; Ruderman et al. 2003; Koh et al. 2007) and recently increased AMPK activity was reported in human adipose tissue after exercise (Watt et al. 2006). It is well known that the level of catecholamines in the blood increases during exercise (Galbo et al. 1975; Christensen & Galbo, 1983) and both in vitro incubation studies and in vivo studies with these substances as well as with other α and β-adrenergic agonists have reported increased AMPK activation in both rodent skeletal muscles (Minokoshi et al. 2002; Ruderman et al. 2003) and adipose tissue (Koh et al. 2007) and in cell cultures from these tissues (Hutchinson & Bengtsson, 2006; Yin et al. 2003). These findings indicate that catecholamines could be potential candidates as humoral AMPK activators in both human skeletal muscles and adipose tissue during exercise.
We hypothesized that an exercise protocol inducing high levels of catecholamines would increase the AMPK activity in adipose tissue and resting muscles and potentiate the AMPK activation in contracting muscles. Thus, in the present study AMPK regulation in resting and exercising muscles and abdominal subcutaneous adipose tissue was studied during one-legged knee extensor exercise with and without simultaneous arm cranking exercise, eliciting exercise periods with high and low catecholamine levels, respectively.
Methods
Subjects
Eleven young (25 ± 1 years, 82.8 ± 1.2 kg) healthy, moderately trained men gave their informed consent to participate in the study, which was approved by the Copenhagen Ethics Committee (reg. no. 01-010/03) and conformed to the Declaration of Helsinki II (1996). Two weeks before the experiments, peak pulmonary oxygen uptake  was determined during an incremental cycle ergometer test (
was determined during an incremental cycle ergometer test (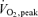 = 53 ± 1 ml kg−1 min−1). Peak work load (PWL) of the knee extensors was determined in a modified Krogh bicycle ergometer that allows dynamic contractions of only the knee extensors (Andersen et al. 1985). In addition, a determination of the work load eliciting the maximal O2 uptake (2.38 ± 0.08 l min−1) during arm cranking on an arm ergometer cycle was conducted before the experimental day.
= 53 ± 1 ml kg−1 min−1). Peak work load (PWL) of the knee extensors was determined in a modified Krogh bicycle ergometer that allows dynamic contractions of only the knee extensors (Andersen et al. 1985). In addition, a determination of the work load eliciting the maximal O2 uptake (2.38 ± 0.08 l min−1) during arm cranking on an arm ergometer cycle was conducted before the experimental day.
Experimental procedure
On the experimental day the subjects arrived in the morning after an overnight fast. A venous catheter was inserted in the forearm for blood sampling. After 60 min of rest a resting blood sample was obtained and a needle biopsy from the vastus lateralis muscle in both legs and a subcutaneous fat tissue biopsy from the abdominal region were obtained under local anaesthesia (lidocaine without adrenaline, Xylocaine, Astra-Zenica, Sweden). In the muscle an incision was made for each individual biopsy (3 incisions in each leg). The incisions were separated by ∼5 cm.
The fat biopsies were obtained from two incisions ∼5–10 cm lateral to both sides of the umbilicus. One of the incisions was used for both the first (pre-exercise) and the third biopsy (post-exercise B). The biopsy needle for these biopsies was pointing lateral-cranial and lateral-caudal respectively. The second incision was used for the second biopsy only.
The subjects then performed one-legged knee extensor exercise for 40 min in a modified Krogh bicycle ergometer with an intensity of 80% PWL (50 ± 2 W). The working leg was randomized in relation to dominant and non-dominant leg. After 20 min of one-legged knee extensor exercise, arm cranking exercise was added at an intensity eliciting 80% of the maximal O2 uptake (87 ± 2 W) obtained during the arm cranking test. During the first 20 min of exercise (period A), the pulmonary oxygen uptake was ∼30% 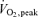 , and adding arm exercise in the last 20 min (period B) elicited a pulmonary uptake of ∼60%
, and adding arm exercise in the last 20 min (period B) elicited a pulmonary uptake of ∼60% 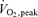 . Muscle biopsies from the working and non working leg as well as fat biopsies were obtained in the mentioned order after 20 min and 40 min of exercise. The biopsy from the exercising leg was taken immediately after termination of the exercise period (within 20 s) and biopsies from the resting leg and adipose tissue were taken within 1–1.5 min after exercise termination. Blood samples were drawn after 10, 20, 30 and 40 min of exercise from the venous catheter and the pulmonary O2 uptake was measured once during period A (15 min) and period B (35 min). In addition, surface electromyography (EMG) activity in a subset of experiments (n = 6) was recorded during the experiment on both legs in order to evaluate muscle activity in the legs. After 15–20 min of rest, three maximal knee extensions (MVC) were performed to obtain maximum EMG values for EMG normalization. Finally, EMG was recorded during rest in supine position.
. Muscle biopsies from the working and non working leg as well as fat biopsies were obtained in the mentioned order after 20 min and 40 min of exercise. The biopsy from the exercising leg was taken immediately after termination of the exercise period (within 20 s) and biopsies from the resting leg and adipose tissue were taken within 1–1.5 min after exercise termination. Blood samples were drawn after 10, 20, 30 and 40 min of exercise from the venous catheter and the pulmonary O2 uptake was measured once during period A (15 min) and period B (35 min). In addition, surface electromyography (EMG) activity in a subset of experiments (n = 6) was recorded during the experiment on both legs in order to evaluate muscle activity in the legs. After 15–20 min of rest, three maximal knee extensions (MVC) were performed to obtain maximum EMG values for EMG normalization. Finally, EMG was recorded during rest in supine position.
The above is a description of the main study. Furthermore, a control study was conducted with a subset of subjects (n = 7) from the main study. The control study was identical to the main study except that no arm exercise was performed. The control study was used to correct for a possible time-dependent regulation of parameters in the last 20 min of exercise in the main study in which arm cranking exercise was performed.
EMG recording
Four channels of bipolar surface EMG were recorded (sampling frequency 1024 Hz) from the vastus lateralis muscle, using Ag–AgCl electrodes (Type 720-01-K, Ambu, Ballerup, Denmark). The electrodes were placed proximal and distal to the biopsy insertion site on the resting leg and on the exercising leg. EMG was recorded during exercise period A and B separately. EMGmax was calculated as the largest 1 s root mean square value obtained during the MVC contractions. Root mean amplitudes of the EMG recorded during the knee extension exercise was calculated and expressed as percentage EMGmax. EMG during rest was subtracted before calculation of the relative EMG activity. For each leg data are presented as average values of the proximal and the distal recording.
Pulmonary gas analysis
During the pre-experimental tests as well as on the experimental day, expired air was colleted in Douglas bags and analysed for content of O2 by use of a Servomex paramagnetic analyser (Applied Electrochemistry Inc., Sunnyvale, CA, USA) and for content of CO2 by a Beckman infrared CO2 analyser (Beckman Instruments Inc., Fullerton, CA, USA). Total expired air was determined using a chain compensated gasometer (Warren E Collins Inc., Braintree, MA, USA)
Analysis of blood and plasma metabolites, substrates and hormones
Plasma adrenaline, noradrenaline and insulin concentrations were analysed by means of radioimmunoassay kits (High sensitive 2-Cat RIA, Labor Diagnostika Nord GmbH & Co., Germany and Insulin RIA DSL-1600, Diagnostic Systems Laboratories Inc., Webster, TX, USA). The blood content of glucose and lactate were measured by an ABL 615 analyser (Radiometer, Copenhagen, Denmark).
Muscle and fat tissue handling
The muscle and fat biopsies obtained were frozen in liquid nitrogen within 20 s and stored at −80°C. Frozen muscle biopsy specimens were freeze dried and dissected free of visible fat, blood and connective tissue before further analysis were performed.
Muscle glycogen
Glycogen content was determined as glycosyl units after acid hydrolysis of freeze dried dissected muscle biopsy specimens (Lowry & Passonneau, 1972).
Muscle nucleotides, cAMP, creatine (Cr) and phosphocreatine (PCr)
Freeze dried and dissected muscle biopsy specimens were extracted with perchloric acid and neutralized and analysed for nucleotides. The ATP, ADP and AMP content were determined by reverse-phase HPLC according to a previously described method (Tullson et al. 1990). The cAMP content was determined by use of a commercial kit (cyclic AMP (H3) assay system (Amersham Bioscience, UK). The Cr and PCr content was measured fluormetrically, as previously described (Lowry & Passonneau, 1972).
Muscle and fat tissue preparation
For studies of muscle and fat tissue enzyme activity and phosphorylation, muscle biopsies (freeze dried and dissected) and fat biopsies were homogenized (1: 40 dw/vol and 1: 6 ww/vol, respectively) in a buffer containing: 50 mm Hepes, 20 mm sodium pyrophosphate, 20 mmβ-glycerophosphate, 10 mm NaF, 2 mm Na3VO4, 2 mm EDTA, 1.7 mm P-40, 10% glycerol (v/v), 2 mm PMSF, 1 mm MgCl2, 1 mm CaCl2, 150 mm NaCl and complete EDTA-free protease inhibitor cocktail tablets (Roche Dioagnostics, Denmark). Muscle homogenates were rotated end over end at 4°C for 60 min, after which they were centrifuged for 30 min at 15 000 g and the supernatants were harvested as the muscle lysate. Fat homogenates were rotated end over at 4°C for 60 min, after which they were centrifuged 40 min at 10 000 g. The infranatant, the layer between the pellet and the top layer of fat, was harvested as the fat lysate. Total protein content in the muscle and fat lysates was determined by the bicinchoninic acid method (Pierce Biotechnology, Inc., Rockford, IL, USA)
AMPK activity
α-Isoform-specific AMPK activity was measured on immunoprecipitates from 200 μg of muscle lysate protein using an anti-α1-AMPK and anti α2-AMPK antibody (provided by Prof. D. G. Hardie, University of Dundee, UK) (Wojtaszewski et al. 2000). At saturating AMP concentrations (200 μm), P81 filter paper-based kinase assay was performed using SAMS peptide (HMRSAMSGLHLVKRR) as substrate (100 μm) as previously described (Wojtaszewski et al. 2000)
AMPK and acetyl CoA-carboxylase protein content and phosphorylation
Muscle and fat lysate proteins were subjected to SDS-PAGE (7.5 or 10% polyacrylamide gels) and Western blotting. The AMPK α protein content in muscle and adipose was evaluated using an anti-α-pan AMPK antibody (Upstate Biotechnology, Inc., Lake Placid, NY, USA) and an α1-AMPK antibody (provided by D. G. Hardie, University of Dundee, UK), respectively. The phosphorylation of the AMPK α subunits (Thr172) and acetyl CoA-carboxylase (ACC) (ACC1 Ser80 and ACC2 Ser221) were evaluated by Western blotting using phospho-specific antibodies (Cell Signaling Technology, Inc., Beverly, MA, USA and Upstate Biotechnology, respectively). The ACC phosphospecific antibody is raised against a peptide corresponding to the sequence in rat ACC1 containing the Ser79 phosphorylation site, but the antibody also recognized the human ACC1 and ACC2 when phosphorylated most likely at the corresponding Ser80 and Ser221, respectively (Abu-Elheiga et al. 1997). Immunoreactive bands were visualized with enhanced chemiluminescense (ECL Plus Western blotting, Amersham Biosciences, UK) and detected and quantified with the use of a coupled device image sensor and 1D software (Image Station 440CF, Kodak, USA).
Statistics
Statistical evaluation was performed by two-way ANOVA with repeated measures for data obtained from muscle and one-way ANOVA with repeated measures for data obtained from blood and fat tissue. When ANOVA revealed significant differences, a post hoc test was used to correct for multiple comparison (Student–Newman–Keuls method). Differences between groups were considered statistically significant when P < 0.05. All data are expressed as means ± s.e.m.
Results
Blood and plasma substrates, metabolites and hormones
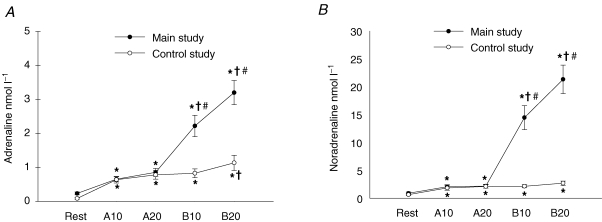
The blood glucose concentration in the main study increased during the first 10 min of exercise period B (P < 0.05) compared to rest and increased further during the last 10 min of period B (P < 0.001) (Table 1). There were no significant changes in the glucose level during the exercise periods in the control study (Table 1). There was a moderate increase in the blood lactate concentration during exercise period A (P < 0.05) and a further and much larger increase during period B in the main study (P < 0.001) (Table 1). There was also a moderate increase during period A (P < 0.001) in the control study but no further increase occurred during period B (Table 1). The plasma insulin levels did not change during the exercise periods either in the main or the control study (Table 1). The plasma adrenaline and noradrenaline concentrations increased moderately during period A compared to rest in the main study (P < 0.001) (Table 1). During the first 10 min of period B, there was a large and significant increase in both the adrenaline (P < 0.001) and noradrenaline (P < 0.001) levels (∼10- and 15-fold, respectively) and during the last 10 min of period B the levels increased further (P < 0.05 and 0.001, respectively) (Fig. 1A and B). There was a significant but moderate increase in both the adrenaline (∼5-fold) and noradrenaline (∼150%) concentrations during exercise period A in the control study (P < 0.001) (Table 1). At the end of exercise period B the adrenaline concentration further increased slightly (∼80%, P < 0.05) while there was no further increase in the noradrenaline level (Fig. 1A and B).
Table 1.
Blood glucose, insulin and lactate concentrations
| Exercise A | Exercise B | ||||
|---|---|---|---|---|---|
| Rest | 10 min | 20 min | 10 min | 20 min | |
| Glucose (mm) | |||||
| Main study | 4.9 ± 0.1 | 5.2 ± 0.2 | 5.0 ± 0.1 | 5.4 ± 0.1* | 5.9 ± 0.2*† |
| Control study | 5.0 ± 0.1 | 5.0 ± 0.1 | 5.0 ± 0.1 | 5.0 ± 0.2 | 4.9 ± 0.1 |
| Insulin (μU ml−1) | |||||
| Main study | 5.7 ± 0.6 | 7.5 ± 0.9 | 7.7 ± 0.9 | 5.8 ± 1.0 | 6.0 ± 0.9 |
| Control study | 4.1 ± 0.4 | 4.8 ± 1.1 | 5.9 ± 1.2 | 3.6 ± 0.4 | 5.8 ± 1.1 |
| Lactate (mm) | |||||
| Main study | 1.0 ± 0.1 | 2.7 ± 0.3* | 2.8 ± 0.3* | 11.4 ± 0.7*† | 13.7 ± 0.7*† |
| Control study | 0.9 ± 0.1 | 2.0 ± 0.2* | 2.1 ± 0.2* | 1.8 ± 0.2* | 1.5 ± 0.2* |
Blood glucose, insulin and lactate concentrations at rest, after 10 and 20 min of exercise in period A and after 10 and 20 min in exercise period B in the main (n = 11) and control study (n = 7).
Significant difference from rest (P < 0.05)
significant difference from the preceding value (P < 0.05). Values are means ± s.e.m.
Figure 1. Plasma adrenaline (A) and noradrenaline (B) concentrations (nmol l−1) at rest and after 10 min (A10) and 20 min (A20) of exercise in period A and 10 min (B10) and 20 min (B20) in exercise period B.
Filled symbols show results from the main study (n = 11) and open symbols from the control study (n = 7). *Significant difference from rest (P < 0.05); †significant difference from the preceding value (P < 0.05); #significant difference from the control study. Values are means ± s.e.m.
Muscle surface electromygraphy activity
We used EMG measurement as an indication of the muscular activity both in the resting and exercising leg. As intended and expected there was only a minimal EMG activity in the knee extensor muscles of the resting leg (∼1% of EMGmax) indicating that these muscles indeed were resting during exercise. Even more important, there was no significant difference in the EMG activity in the resting leg between exercise period A and B. The last point is crucial if a potential difference in the AMPK activation in the resting leg between period A and B should be ascribed to only a humoral mediated regulation.
Muscle cAMP content
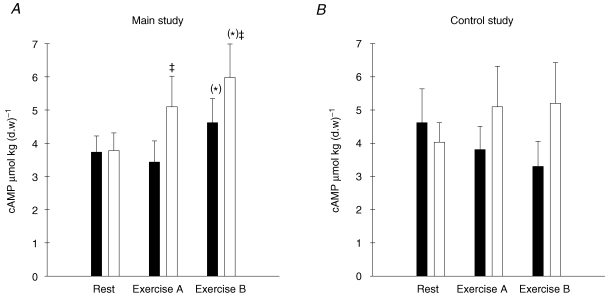
The cAMP concentration increased in the main study after exercise period B (P = 0.057) and the increase was higher in the exercising leg compared with the resting leg (P < 0.05) (Fig. 2A). There were no significant changes in the cAMP concentration during the control study (Fig. 2B)
Figure 2. Muscle cAMP concentration (μmol (kg d.w.)−1) in muscle tissue from the main study (A, n = 11) and the control study (B, n = 7).
Filled bars show values for the resting leg and open bars show values for the exercising leg at rest and after exercise period A and B. (*)P = 0.057; ‡significant difference between the working and resting leg (P < 0.05).
Muscle glycogen, creatine, creatinephosphate and nucleotides
The glycogen concentration decreased in the muscles of the working leg after exercise period A in both the main (P < 0.001) and the control study (P < 0.001). The glycogen levels were further decreased in the working leg after period B in both the main and control study (P < 0.05) (Table 2). There were no significant changes in the glycogen concentrations of the muscles in the resting legs, either in the main or the control study (Table 2). The levels of creatine (Cr) and phosphocreatine (CrP) in the working leg decreased and increased, respectively, after exercise period A in the main study (P < 0.05) (Table 2) and the pattern of changes looked the same in the control study although the differences did not reach significance (P = 0.088 and 0.078, respectively) (Table 2). There were no further changes after exercise period B in the exercising leg either in the main or in the control study (Table 2). The CrP/(Cr + CrP) ratio was decreased after exercise period A in the main study (P < 0001) and there were no further changes after exercise period B (Table 2). The pattern looked the same in the control study although the changes did not reach significance (P = 0.076) (Table 2). There were no significant changes in the Cr or CrP concentrations or the CrP/(Cr + CrP) ratio of the resting legs, either in the main or the control study (Table 2). Muscle adenosine nucleotide (AMP, ADP and ATP) levels were not different between the two legs and did not change during either exercise period A or B (Table 2).
Table 2.
Muscle glycogen, phosphocreatine (CrP) and creatine (Cr) and nucleotide concentrations:
| Rest | Exercise A | Exercise B | |
|---|---|---|---|
| Glycogen (mmol (kg d.w.)−1) | |||
| Exercising leg in main study | 524 ± 23‡ | 386 ± 21*‡ | 335 ± 29*†‡ |
| Resting leg in main study | 470 ± 25 | 459 ± 21 | 477 ± 26 |
| Exercising leg in control study | 498 ± 21 | 357 ± 7*‡ | 283 ± 24*†‡ |
| Resting leg in control study | 471 ± 27 | 455 ± 21 | 477 ± 26 |
| Phosphocreatine (CrP) (mmol (kg d.w.)−1) | |||
| Exercising leg in main study | 64 ± 4‡ | 53 ± 4*‡ | 51 ± 3* |
| Resting leg in main study | 56 ± 2 | 61 ± 4 | 57 ± 3 |
| Exercising leg in control study | 59 ± 2 | 46 ± 2 | 54 ± 4 |
| Resting leg in control study | 62 ± 4 | 53 ± 4 | 57 ± 4 |
| Creatine (Cr) (mmol (kg d.w.)−1) | |||
| Exercising leg in main study | 58 ± 4 | 69 ± 4*‡ | 77 ± 4*‡ |
| Resting leg in main study | 60 ± 4 | 57 ± 3 | 67 ± 4 |
| Exercising leg in control study | 57 ± 7 | 73 ± 4 | 67 ± 4 |
| Resting leg in control study | 57 ± 5 | 58 ± 6 | 60 ± 4 |
| CrP/(Cr + CrP) ratio | |||
| Exercising leg in main study | 0.87 ± 0.08 | 1.26 ± 0.12*‡ | 1.50 ± 0.15*‡ |
| Resting leg in main study | 1.00 ± 0.07 | 0.91 ± 0.07 | 0.96 ± 0.08 |
| Exercising leg in control study | 1.02 ± 0.11 | 1.71 ± 0.13 | 1.35 ± 0.13 |
| Resting leg in control study | 1.00 ± 0.12 | 1.19 ± 0.17 | 1.16 ± 0.14 |
| ATP (mmol (kg d.w.)−1) | |||
| Exercising leg in main study | 26.0 ± 0.5 | 25.5 ± 0.7 | 26.5 ± 0.5 |
| Resting leg in main study | 25.1 ± 0.6 | 25.8 ± 0.6 | 25.4 ± 0.6 |
| Exercising leg in control study | 26.1 ± 0.5 | 24.3 ± 0.8 | 25.1 ± 0.7 |
| Resting leg in control study | 26.3 ± 0.6 | 25.0 ± 1.0 | 24.6 ± 1.3 |
| ADP mmol (kg (d.w))-1 | |||
| Exercising leg in main study | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.1 |
| Resting leg in main study | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.2 ± 0.1 |
| Exercising leg in control study | 3.2 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 |
| Resting leg in control study | 3.3 ± 0.1 | 3.1 ± 0.16 | 3.0 ± 0.1 |
| AMP (mmol (kg d.w.)−1) | |||
| Exercising leg in main study | 0.25 ± 0.03 | 0.26 ± 0.04 | 0.22 ± 0.03 |
| Resting leg in main study | 0.21 ± 0.02 | 0.24 ± 0.03 | 0.22 ± 0.03 |
| Exercising leg in control study | 0.22 ± 0.04 | 0.26 ± 0.03 | 0.28 ± 0.04 |
| Resting leg in control study | 0.28 ± 0.05 | 0.24 ± 0.03 | 0.24 ± 0.05 |
| AMP/ATP ratio | |||
| Exercising leg in main study | 1.12 ± 0.13 | 1.06 ± 0.19 | 0.97 ± 0.12 |
| Resting leg in main study | 1.00 ± 0.11 | 1.09 ± 0.12 | 1.03 ± 0.12 |
| Exercising leg in control study | 0.82 ± 0.14 | 0.99 ± 0.13 | 1.08 ± 0.16 |
| Resting leg in control study | 1.00 ± 0.17 | 0.90 ± 0.14 | 0.92 ± 0.20 |
Muscle glycogen, phosphocreatine (CrP),creatine (Cr) concentrations, CrP/(Cr + CrP) ratios and ATP, ADP, AMP concentrations and AMP/ATP ratios in exercising and resting leg muscles at rest and after 20 min of exercise in period A and B in the main study (n = 11) and the control study (n = 7).
Significant difference from rest (P < 0.05)
significant difference from the preceding value (P < 0.05)
significant difference between the working and resting leg (P < 0.05). Values are means ± s.e.m.
The α1 and α2 AMPK activities in muscle tissue
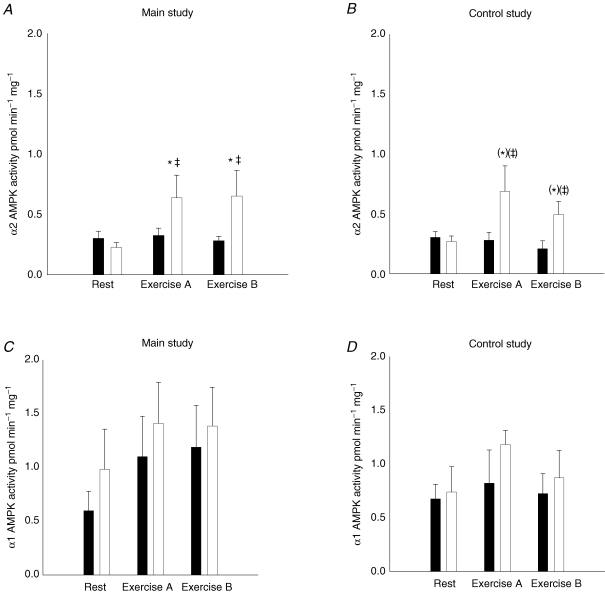
In the main study, the α2 associated AMPK activity increased ∼2-fold in the muscle of the working leg after exercise period A compared to rest (P < 0.05), but there was no further increase after period B (Fig. 3A). The α2 associated AMPK activity in the resting leg was not different from rest after either exercise period A or B in the main study (Fig. 3A). The average values of the α2 associated AMPK activity were ∼1- to 2-fold higher in the muscle of the working leg after the exercise periods compared to rest in the control study (P = 0.06) (Fig. 3B).
Figure 3. α2 and α1 associated AMPK activity in muscle tissue lysates from the main study (A and C, n = 11) and the control study (B and D, n = 7).
Filled bars show values for the resting leg and open bars show values for the exercising leg at rest and after exercise period A and B. Values are related to the resting leg at rest. *Significant difference from rest (P < 0.05); (*)P = 0.06; ‡ significant difference between the working and resting leg (P < 0.05); (‡)P = 0.06. Values are means ± s.e.m.
For the resting leg the α2 associated AMPK activity was unchanged after both exercise period A and B compared to rest in the control study (Fig. 3B). The α1 associated AMPK activity in both the working and resting leg was unchanged after exercise compared to before exercise in both the main and control study, and was not different between legs (Fig. 3C and D).
α-AMPK protein content and α-AMPK phosphorylation (Thr172) in muscle tissue
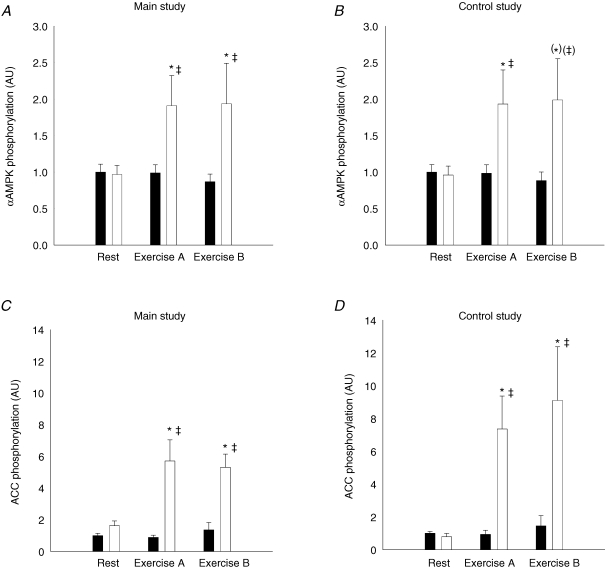
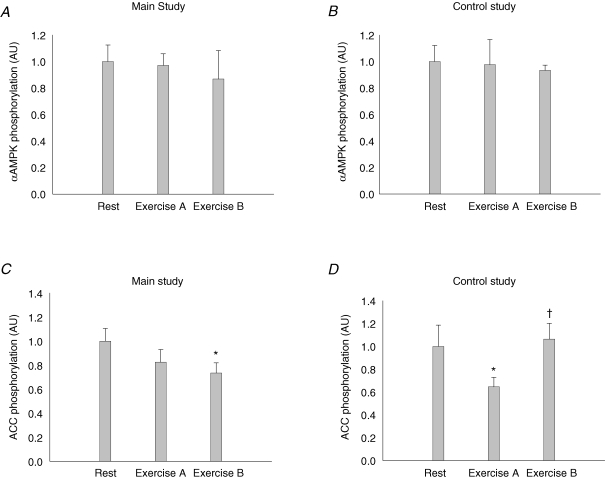
In both the main study and the control study, the α-AMPK protein content was the same in the resting and working leg muscle in the resting situation and there were no changes after the exercise periods (data not shown). The α-AMPK phosphorylation relative to α-AMPK protein content increased significantly in the muscle of the working leg after exercise period A in both the main (P < 0.05) and the control study (P < 0.05). There was no further increase in the phosphorylation level after exercise period B in either of the two studies. There were no significant changes in the α-AMPK phosphorylation after exercise in the muscle of the resting leg either in the main or the control study (Fig. 4A and B, and Fig. 6A).
Figure 4. αAMPK protein phosphorylation and ACCβ protein phosphorylation relative to αAMPK protein and ACCβ protein content, respectively, in muscle tissue lysates from the main study (A and C, n = 11) and the control study (B and D, n = 7).
Filled bars show values for the resting leg and open bars show values for the exercising leg at rest and after exercise period A and B. Values are related to the resting leg at rest. *Significant difference from rest (P < 0.05); (*)P = 0.057; ‡significant difference between the working and resting leg (P < 0.05); (‡)P = 0.057. Values are means ± s.e.m.
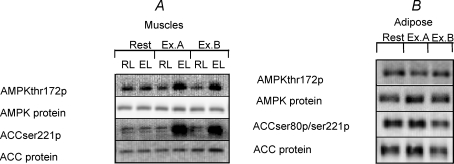
Figure 6. Representative immunoblots of protein expression and phosphorylation of invested proteins in the muscle (A) and adipose tissue (B) lysates.
The muscle lysate immuno-blots are depicted with the resting (RL) and exercising (EL) leg at rest and after exercise period A (Ex A) and B (Ex.B). The adipose lysate blots are depicted at rest and after exercise A (Ex.A) and B (Ex.B), respectively.
ACCβ protein content and phosphorylation (Ser221) in muscle tissue
At rest, the ACCβ protein content was the same in the resting and working leg muscles, and there were no changes after either of the exercise periods in the main or the control study (data not shown). The ACCβ phosphorylation relative to ACCβ protein content increased only in the muscle of the working leg after exercise period A compared to rest (P < 0.001), and there was no further increase after period B either in the main or the control study (Fig. 4C and D, and Fig. 6A).
α-AMPK protein content and α-AMPK phosphorylation (Thr172) in abdominal subcutaneous adipose tissue
The α-AMPK protein content in the abdominal subcutaneous adipose tissue was not affected by the exercise protocols in the main or the control study (data not shown). In the same way the α-AMPK phosphorylation relative to the α-AMPK protein content was the same after the exercise periods compared to rest in both the main and the control study (Fig. 5A and B, and Fig. 6B).
Figure 5. A–D, αAMPK protein and ACC protein phosphorylation relative to αAMPK and ACC protein content, respectively, in adipose tissue lysates from the main study (A and C, n = 10) and the control study (B and D, n = 7) at rest and after exercise period A and B.
*Significant difference from rest (P < 0.05); †significant difference from the preceding value (P < 0.05).
ACC protein content and phosphorylation (Ser 80/221) in abdominal subcutaneous adipose tissue
The ACC protein content in the abdominal subcutaneous adipose tissue was the same in the resting situation and after the exercise protocols in both the main and the control study (data not shown). The ACC phosphorylation was unchanged after the exercise periods in both the main and control study (data not shown), but when the ACC phosphorylation data were analysed after normalization to the ACC protein content there was a significant decrease after exercise period B in the main study (Fig. 5C) and after exercise period A in the control study (Figs. 5D and 6B).
Discussion
In the present study we investigated whether humoral mediated AMPK activation takes places in human skeletal muscle and abdominal subcutaneous adipose tissue during exercise inducing low and high catecholamine levels, respectively. In contrast to our hypothesis, we did not observe any changes in AMPK activity either in resting muscles or in adipose tissue despite large exercise induced increases in both the adrenaline and noradrenaline plasma levels.
The one-legged knee extensor exercise protocol allows for a high local exercise load concomitant with only a moderate stress load at the whole body level. One-legged knee extensor exercise therefore also elicits a low catecholamine response. The level of AMPK activation in the muscle of the contracting leg was independent of whether the arm cranking exercise was performed simultaneously. As the addition of the arm cranking exercise elicited an increase in the respiratory load to 60% of 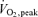 and a 15- to 23-fold increase in the plasma catecholamine concentrations compared to rest, these observations suggest that the AMPK regulation in human skeletal muscle during this kind of exercise is restricted to local, contraction-dependent mechanisms. A specific local regulation is also supported by the fact that the AMPK activity was increased in the exercising leg after 20 min of exercise with a whole body respiratory load of only 30%
and a 15- to 23-fold increase in the plasma catecholamine concentrations compared to rest, these observations suggest that the AMPK regulation in human skeletal muscle during this kind of exercise is restricted to local, contraction-dependent mechanisms. A specific local regulation is also supported by the fact that the AMPK activity was increased in the exercising leg after 20 min of exercise with a whole body respiratory load of only 30% 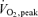 but with a local muscle specific intensity corresponding to 80% of the knee extensors peak work load. A local regulation is also confirmed by the unchanged AMPK activity in the non-exercising muscle. Thus, the present study, for the first time, strongly suggests that during exercise of a moderate intensity in man, humoral activation of AMPK does not take place in muscle or adipose tissue but activation of AMPK is a local process restricted to the working muscle.
but with a local muscle specific intensity corresponding to 80% of the knee extensors peak work load. A local regulation is also confirmed by the unchanged AMPK activity in the non-exercising muscle. Thus, the present study, for the first time, strongly suggests that during exercise of a moderate intensity in man, humoral activation of AMPK does not take place in muscle or adipose tissue but activation of AMPK is a local process restricted to the working muscle.
Several observations in rodent muscle suggest that circulating catecholamines can activate AMPK (Minokoshi et al. 2002; Ruderman et al. 2003; Hutchinson & Bengtsson, 2006). Thus, incubation of rat soleus muscle, with the β-adrenergic agonist isoproternol increases AMPK activity (Ruderman et al. 2003), whereas in the soleus muscle of the mouse AMPK activation by the α-adrenoreceptor agonist phenylephrine was reported. (Minokoshi et al. 2002). Similarily, in rat L6 skeletal muscle cells, noradrenaline and α- but not β-adrenoceptor agonists increased AMPK activity, suggesting that only α1-adrenoceptor stimulation induces AMPK activation (Hutchinson & Bengtsson, 2006). It was speculated that the α1-adrenoceptor protein Gq signalling related calcium release could be coupled to CaMKK signalling. This kinase has been described as an upstream AMPK kinase in several tissues including skeletal muscle (Hardie et al. 2006; Rose et al. 2006; Jensen et al. 2007a,b).
As has been observed previously (Chasiotis et al. 1983a,b; Greer et al. 2000) cAMP levels during exercise were moderately increased in both exercised and rested muscles but only when plasma catecholamine levels were markedly elevated by arm cranking. This indicates that muscle was in fact affected by the high plasma catecholamine levels. Thus, the results from the present study question the physiological relevance of AMPK regulation by catecholamines in human skeletal muscles when such robust increases in catecholamine levels as we observed in the present study do not result in AMPK activation. Still, the present study cannot be generalized to all kinds of exercise – for example exercise of longer duration.
In the adipose tissue we observed unchanged AMPK phosphorylation during exercise. Also phosphorylation of the endogenous AMPK substrate, ACC, did not increase, supporting the absence of an increase in AMPK activity. In contrast to our finding, a recent human study showed an exercise induced increase in AMPK phosphorylation (∼1-fold) in subcutaneous adipose tissue (Watt et al. 2006) but no significant increase in ACC phosphorylation. Our combined leg kicking and arm cranking exercise protocol in fact induced around a 50% higher plasma adrenaline concentration compared to the study by Watt and coworkers. In that study the exercise bout consisted of ergometer cycling for a period of 90 min demanding 60% of 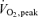 . This is identical to the relative work load in the present study during the last 20 min with the combination of one-legged knee extensor exercise and arm ergometer cycling. So besides the exercise period there is no obvious reason for these differences, at least from the point of view that the catecholamines should be the humoral regulatory factor of AMPK activity in adipose tissue. We cannot exclude the possibility that the absence of a decline in plasma insulin concentration during exercise in the present study (in contrast to the previous study by Watt et al. 2006) could have influenced the AMPK activity. For example insulin in vitro has been shown to reduce the AMPK activity in cultures of 3T3-L1 adipocytes, Fao hepatoma cells (Witters & Kemp, 1992; Yin et al. 2003) and in rodent heart muscle (Beauloye et al. 2001; Kovacic et al. 2003). Insulin has also been reported to reduce AMPK activation by forskolin (an activator of adenylyl cyclase) in cultered 3T3-L1 adipocytes (Yin et al. 2003). In addition, a recent human study reported a decreased AMPK activity in skeletal muscles after an euglycaemic hyperinsulinaemic clamp (Bandyopadhyay et al. 2006). On the other hand, studies in human skeletal muscle as well as in isolated epididymal fat and skeletal muscles from rats question that insulin causes inhibition of the AMPK activity (Moule & Denton, 1998; Hayashi et al. 2000; Hojlund et al. 2004). In addition, it has not been shown that a decrease below fasting insulin levels, as in the study by Watt et al. (2006), stimulates AMPK activity. Thus, further studies are needed to evaluate the role of plasma insulin in the regulation of AMPK. We cannot exclude the possibility that the 1–1.5 min delay from exercise termination to biopsy influenced AMPK activity in adipose tissue; however, we do not find it likely that this is the main explanation for the discrepancy between our findings and previous studies. With a half-life around 1–3 min, the levels of catecholamines will still be significantly increased at the time when the fat biopsy was obtained and plasma adrenaline concentrations at the end of the exercise period in the present study were around 50% higher relative to the level obtained in the previous study by Watt et al. (2006). Furthermore, Ruderman et al. (2003) reported that AMPK activity in rat adipose tissue and exercised muscles was still increased 30 min post-exercise compared to rest.
. This is identical to the relative work load in the present study during the last 20 min with the combination of one-legged knee extensor exercise and arm ergometer cycling. So besides the exercise period there is no obvious reason for these differences, at least from the point of view that the catecholamines should be the humoral regulatory factor of AMPK activity in adipose tissue. We cannot exclude the possibility that the absence of a decline in plasma insulin concentration during exercise in the present study (in contrast to the previous study by Watt et al. 2006) could have influenced the AMPK activity. For example insulin in vitro has been shown to reduce the AMPK activity in cultures of 3T3-L1 adipocytes, Fao hepatoma cells (Witters & Kemp, 1992; Yin et al. 2003) and in rodent heart muscle (Beauloye et al. 2001; Kovacic et al. 2003). Insulin has also been reported to reduce AMPK activation by forskolin (an activator of adenylyl cyclase) in cultered 3T3-L1 adipocytes (Yin et al. 2003). In addition, a recent human study reported a decreased AMPK activity in skeletal muscles after an euglycaemic hyperinsulinaemic clamp (Bandyopadhyay et al. 2006). On the other hand, studies in human skeletal muscle as well as in isolated epididymal fat and skeletal muscles from rats question that insulin causes inhibition of the AMPK activity (Moule & Denton, 1998; Hayashi et al. 2000; Hojlund et al. 2004). In addition, it has not been shown that a decrease below fasting insulin levels, as in the study by Watt et al. (2006), stimulates AMPK activity. Thus, further studies are needed to evaluate the role of plasma insulin in the regulation of AMPK. We cannot exclude the possibility that the 1–1.5 min delay from exercise termination to biopsy influenced AMPK activity in adipose tissue; however, we do not find it likely that this is the main explanation for the discrepancy between our findings and previous studies. With a half-life around 1–3 min, the levels of catecholamines will still be significantly increased at the time when the fat biopsy was obtained and plasma adrenaline concentrations at the end of the exercise period in the present study were around 50% higher relative to the level obtained in the previous study by Watt et al. (2006). Furthermore, Ruderman et al. (2003) reported that AMPK activity in rat adipose tissue and exercised muscles was still increased 30 min post-exercise compared to rest.
A recent exercise study by Koh et al. (2007) in rats supports a causal relation between AMPK activation in epididymal fat tissue and adrenaline during exercise. In accordance with earlier studies in rats, they observed an increased AMPK activation in epididymal fat after exercise (Park et al. 2002; Ruderman et al. 2003; Koh et al. 2007). Furthermore, they substantiated adrenaline as the primary mediator of AMPK activation because β-adrenergic blockade during exercise resulted in complete blunting of exercise induced AMPK activation. In contrast to the observations in humans (Watt et al. 2006), the activation of AMPK in the epididymal fat was transient and maximal after only 15 min of exercise and fully reversed after 60 min. However, it must be emphasized that comparisons between species and different kinds of adipose tissue should be made cautiously in relation to the catecholamine signalling. Hence, in the adipose tissue the β-adrenoceptor expression is greater in rat compared with human, while it is opposite with regard to the α2 receptor (Lafontan & Berlan, 1993; Stallknecht, 2004). In addition, epididymal adipose tissue has more β-adrenoreceptors compared with subcutaneous adipose tissue, while the latter has more α2 receptors (Lafontan & Berlan, 1993; Stallknecht, 2004). Since the β receptors are coupled to Gs proteins (increase c-AMP formation) while the α2 receptors are coupled to the Gi protein (inhibit c-AMP formation) it is likely that the species and the adipose tissue source have effects on the intracellular catecholamine signalling pathway related to cAMP formation and downstream signalling (Lafontan & Berlan, 1993).
In conclusion, the present study has shown an absence of humoral mediated AMPK activation in resting and exercising skeletal muscles despite a robust increase in the plasma catecholamines levels, questioning the physiological relevance of AMPK activation by catecholamines in human skeletal muscle. Also, although catecholamines under some conditions may regulate AMPK activity in subcutaneous fat depots in humans, markedly increased catecholamine levels during exercise are not always sufficient to elicit such a response.
Acknowledgments
We thank Prof. D. Grahame Hardie, Dundee University, Scotland, for the kind donation of valuable tools for this study. Betina Bolmgren, Karina Olsen, Winnie Taagerup and Marianne Pilegaard are acknowledged for skilled technical assistance. The study was supported by grants from the Danish Diabetes Association, the Danish Medical and Natural Science Research Council, the Novo Nordisk Foundation, the Lundbeck foundation, the Copenhagen Muscle Research Centre, and an Integrated Project from the European Union (contract LSHM-CT-2004-005272). J.F.P.W. was supported by a Hallas Møller fellowship from the Novo Nordisk Foundation.
References
- Abu-Elheiga L, Almarza-Ortega DB, Baldini A, Wakil SJ. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J Biol Chem. 1997;272:10669–10677. doi: 10.1074/jbc.272.16.10669. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186:129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- Chasiotis D, Brandt R, Harris RC, Hultman E. Effects of beta-blockade on glycogen metabolism in human subjects during exercise. Am J Physiol Endocrinol Metab. 1983a;245:E166–E170. doi: 10.1152/ajpendo.1983.245.2.E166. [DOI] [PubMed] [Google Scholar]
- Chasiotis D, Harris RC, Hultman E. The cyclic-AMP concentration in plasma and in muscle in response to exercise and beta-blockade in man. Acta Physiol Scand. 1983b;117:293–298. doi: 10.1111/j.1748-1716.1983.tb07209.x. [DOI] [PubMed] [Google Scholar]
- Christensen NJ, Galbo H. Sympathetic nervous activity during exercise. Annu Rev Physiol. 1983;45:139–153. doi: 10.1146/annurev.ph.45.030183.001035. [DOI] [PubMed] [Google Scholar]
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the α1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- Ferrer A, Caelles C, Massot N, Hegardt FG. Activation of rat liver cytosolic 3-hydroxy-3-methylglutaryl coenzyme A reductase kinase by adenosine 5′-monophosphate. Biochem Biophys Res Commun. 1985;132:497–504. doi: 10.1016/0006-291x(85)91161-1. [DOI] [PubMed] [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975;38:70–76. doi: 10.1152/jappl.1975.38.1.70. [DOI] [PubMed] [Google Scholar]
- Greer F, Friars D, Graham TE. Comparison of caffeine and theophylline ingestion: exercise metabolism and endurance. J Appl Physiol. 2000;89:1837–1844. doi: 10.1152/jappl.2000.89.5.1837. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase – development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;286:E239–E244. doi: 10.1152/ajpendo.00326.2003. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: mediation by α1-adrenoceptors causing glucose uptake. Diabetes. 2006;55:682–690. doi: 10.2337/diabetes.55.03.06.db05-0901. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA. Caffeine-induced Ca2+ release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007a;293:E286–E292. doi: 10.1152/ajpendo.00693.2006. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007b;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ. Epinephrine is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J. 2007;403:473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Aboudehen KS, Carruth AK, Durand RT, Acevedo EO, Hebert EP, Johnson LG, Castracane VD. Adiponectin responses to continuous and progressively intense intermittent exercise. Med Sports Exerc. 2003;35:1320–1325. doi: 10.1249/01.MSS.0000079072.23998.F3. [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Chu H, Castracane VD. Leptin and exercise. Exp Biol Med (Maywood) 2002;227:701–708. doi: 10.1177/153537020222700903. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057–1091. [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- MacDonald C, Wojtaszewski JF, Pedersen BK, Kiens B, Richter EA. Interleukin-6 release from human skeletal muscle during exercise: relation to AMPK activity. J Appl Physiol. 2003;95:2273–2277. doi: 10.1152/japplphysiol.00242.2003. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889–903. doi: 10.1113/jphysiol.2006.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Park H, Kaushik VK, Dean D, Constant S, Prentki M, Saha AK. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol Scand. 2003;178:435–442. doi: 10.1046/j.1365-201X.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame HD, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallknecht B. Influence of physical training on adipose tissue metabolism – with special focus on effects of insulin and epinephrine. Dan Med Bull. 2004;51:1–33. [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund PB. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullson PC, Whitlock DM, Terjung RL. Adenine nucleotide degradation in slow-twitch red muscle. Am J Physiol Cell Physiol. 1990;258:C258–C265. doi: 10.1152/ajpcell.1990.258.2.C258. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–2867. [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]