Abstract
The release of hormones and neurotransmitters by regulated exocytosis requires the delivery of secretory vesicles to the plasma membrane, where they dock and become primed for fusion with the plasma membrane. Upon stimulation a fusion pore is formed through which cargo molecules diffuse out of the vesicle lumen into the extracellular space. After the cargo release the fusion pore either closes (kiss-and-run, transient exocytosis), fluctuates between an open and a closed state (for short times, fusion pore flickering, or for rather longer periods, ‘pulsing pore’) or expands irreversibly (full fusion exocytosis). In almost all secretory cells spontaneous secretion of vesicle cargo can be detected in the absence of stimulation. Spontaneous and stimulated exocytosis were thought to exhibit similar properties at elementary level, differing only in the probability of occurrence. However, recent studies indicate that spontaneous exocytosis differs from the stimulated one in many respects, therefore opening questions about the physiological role of spontaneous exocytosis. In this report we address the elementary properties of spontaneous and stimulated peptidergic vesicle discharge which appears to be modulated by fusion pore conductance (diameter) and fusion pore gating.
Over the recent past, a remarkable progress has been made in our understanding of the molecular mechanisms underlying regulated release of hormones and neurotransmitters from secretory vesicles of neuroendocrine and neuronal cells. The secretory process consists of delivery of vesicles to the plasma membrane, where they dock and become primed for fusion with the plasma membrane (exocytosis) in response to a physiological stimulus, such as an increase in free intracellular calcium. Upon stimulation the fusion pore is formed, an aqueous channel between the vesicle and the plasma membrane, through which cargo molecules diffuse out of the vesicle lumen to the cell exterior. Once formed, the fusion pore either closes, to allow the vesicle to be reused in the next round of exocytosis (kiss-and-run exocytosis) (Ceccarelli et al. 1973), or it fully widens leading to the complete merge of vesicle membrane with the plasma membrane (full fusion exocytosis) (Heuser & Reese, 1973). The fusion pore can also fluctuate between an open and a closed state in the subsecond time domain (fusion pore flickering) before full fusion (Fernandez et al. 1984) or can retain the transient nature of opening and closing for several tens of minutes or longer, sometimes exhibiting remarkable rhythmicity (the ‘pulsing pore’) as was observed in the anterior pituitary cells (Stenovec et al. 2004; Vardjan et al. 2007).
In almost all secretory cell types, including neurons, basal secretion of vesicle cargo can be detected in the absence of cell stimulation (Katz, 1969). Spontaneous release events were assumed to occur with low probability (Murthy & Stevens, 1999). Therefore these events were in the past largely unstudied because of their infrequent occurrence and the belief that they exhibit similar properties at the elementary level to the stimulated events (Katz, 1969). However, many new observations using new methodological approaches revealed that spontaneous and stimulated vesicle exocytosis differ in many respects, thereby opening questions about the physiological role of spontaneous exocytosis.
In this report, we present an overview of the key findings that have lead to the emerging paradigm that the molecular basis of spontaneous and stimulated vesicle release differs. First, we will dwell on the elementary properties of spontaneous and stimulated release from a single peptidergic vesicle. We will question whether the rate of cargo release from a single vesicle during spontaneous exocytosis is regulated by the fusion pore kinetics, highlighting the special form of exocytosis, the pulsing fusion pore (Stenovec et al. 2004). Second, we will consider fusion pore events with a narrow diameter, which appears to be the characteristic of spontaneous peptidergic vesicle fusion. At the end we will question whether the mode of spontaneous single peptidergic vesicle exocytosis can be modulated by a stimulus.
Spontaneous versus stimulated vesicle exocytosis
Membrane fusion is considered energetically unfavourable. The repulsive electrostatic forces between two closely apposed phospholipid bilayers (< 2 nm) in an aqueous environment are significant, and these need to be overcome in order to reach the metastable transition states that leads to fusion (Kozlov & Markin, 1983). The energy barrier for fusion may be reduced by proteins, such as the SNARE (soluble N-ethylmaleimide fusion protein attachment protein receptor) proteins which have been identified to mediate spontaneous and stimulated fusion of the vesicle and the plasma membrane (reviewed in Jahn & Scheller, 2006). Deletions of the vesicle SNARE protein synaptobrevin (Deitcher et al. 1998; Schoch et al. 2001) and the target membrane SNARE protein SNAP-25 (Washbourne et al. 2002) affect spontaneous and stimulated synaptic vesicle fusion, but to different degrees. Recent structure and function studies of synaptobrevin showed that the sequences of synaptobrevin required for spontaneous and stimulated synaptic vesicle fusion differ (Deák et al. 2006). Furthermore, although both tetanus toxin and botulinum toxin D cleave synaptobrevin, tetanus toxin treatment blocked the evoked, but not the spontaneous release, whereas botulinum toxin D blocked both forms of release in crayfish neuromuscular junction (Hua et al. 1998). These results indicate that vesicles undergoing spontaneous and stimulated fusion use distinct SNARE components.
In stimulated exocytosis the release of vesicle cargo is evoked by massive Ca2+ influx from the cell exterior and the subsequent binding of Ca2+ to the vesicle SNARE proteins synaptotagmin-1 or -2 (Geppert et al. 1994; Kreft et al. 2003; Pang et al. 2006) which, on the contrary, both restrict spontaneous release (Pang et al. 2006). It is widely thought that in spontaneous exocytosis Ca2+ is provided from spontaneous localized Ca2+ transients (Llano et al. 2000; Angleson & Betz, 2001; Gonzalez-Iglesias et al. 2006). However, the frequency of spontaneous events could also be increased by Ca2+-independent mechanisms (Kijima & Tanabe, 1988). Ca2+-independent synaptic vesicle protein synaptotagmin-12 which is controlled by cAMP-dependent processes, has been recently identified as a selective modulator of spontaneous synaptic vesicle exocytosis (Maximov et al. 2007), indicating that spontaneous and stimulated release are differentially regulated by Ca2+ in some systems.
Furthermore, Sara et al. (2005) have shown that the spontaneous synaptic vesicle pool recycles independently of the activity-dependent pool, although Groemer & Klingauf (2007) recently provided evidence that synaptic vesicles recycling spontaneously and during activity belong to the same pool. Priming mechanisms that prepare synaptic vesicles for fusion, which are targeted by phorbol esters, were found to be different for the spontaneous and stimulated forms of synaptic vesicle fusion (Virmani et al. 2005). Moreover, genetic mutations in protein Rab5, which is critical for vesicle trafficking through early endosomes, have shown that spontaneous and stimulated synaptic vesicle release probably operate through distinct vesicle trafficking pathways (Wucherpfennig et al. 2003). Recently Wasser et al. (2007) reported that synaptic cholesterol probably balances spontaneous and stimulated neurotransmitter release, by hindering spontaneous and sustaining evoked exo-/endocytosis. Spontaneous and stimulated exocytosis differ also in the elementary properties of single vesicle fusion (Stenovec et al. 2004; Vardjan et al. 2007), which will be discussed in more detail in the following sections.
Slower cargo release from spontaneous versus stimulated peptidergic vesicles
Recent studies have shown that the rate (Albillos et al. 1997; Stenovec et al. 2004) and the amount of vesicle cargo release (Angleson et al. 1999) are controlled at the level of an individual secretory vesicle after the fusion pore opening (postfusion regulation of release; Rahamimoff & Fernandez, 1997). These advances have been made possible as a result of new techniques, which permit the monitoring of unitary exocytotic events. Capacitance measurements provide a method for monitoring single vesicle exocytosis since it reports changes in the net increase in cell surface area, which are reflecting vesicle fusion and fission (Neher & Marty, 1982). Amperometry is another powerful method for monitoring single-vesicle release in real-time. It is based on the electrochemical detection of released molecules with a suitable oxidation potential from a single vesicle (Wightman et al. 1991; Chow et al. 1992). Furthermore, optical methods using different fluorescent markers (styryl dyes, fluorescence-tagged proteins) have been developed to track the exo-/endocytosis of an individual vesicle (Betz & Bewick, 1992; Miesenböck et al. 1998; Shaner et al. 2005).
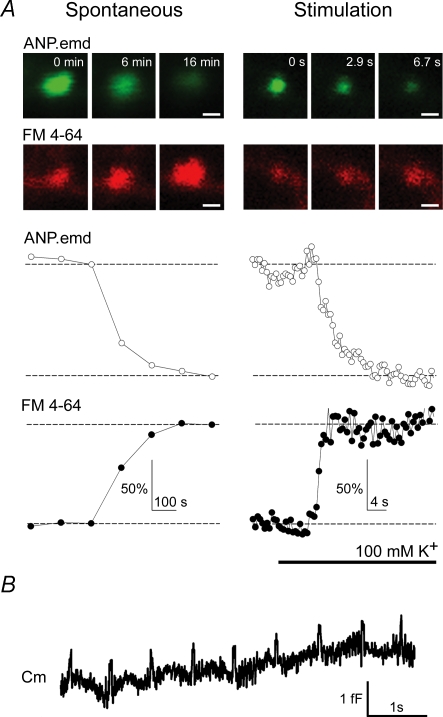
Optical studies of unitary exocytotic events in lactotrophs, prolactin-secreting cells of anterior pituitary, revealed that stimulated hormone discharge from a single vesicle is some 10–20 times faster than spontaneous hormone discharge (Stenovec et al. 2004). In these studies, the vesicle cargo release of a fluorescently tagged peptide (ANP.emd), similar in size to the hormone prolactin, was simultaneously monitored by vesicle loading with the FM 4-64 styryl dye. Stimulation resulted in dye loading and hormone release within seconds. In contrast, in 50% of spontaneously releasing vesicles, the hormone release and the FM 4-64 loading were slow (∼3 min), indicating differences in the fusion pore properties in resting and stimulated conditions (Fig. 1A; Stenovec et al. 2004).
Figure 1. Slow spontaneous secretory peptide release and the periodic transient fusion pore openings.
A, fluorescent peptide discharge (ANP.emd) and FM 4-64 dye loading into prolactin vesicles in spontaneous or stimulated conditions. Top panels show individual vesicle images recorded in different time frames separately for the ANP.emd (green) and FM 4-64 (red) fluorescence. Traces below show the time course of relative fluorescence intensity changes of the two fluorescent probes. Note the differences in time scaling for spontaneous (minutes) and stimulated (seconds) releasing vesicles. Note that the time course of loading and unloading is similar (synchronous) in spontaneously active vesicles whereas it is different in stimulated vesicles. Scale bar represents 0.5 μm. Modified from Stenovec et al. (2004) with the permission of The Federation of American Societies for Experimental Biology. B, periodic transient fusion pore openings (‘the pulsing pore’) of a single vesicle in resting pituitary cells recorded by patch clamp capacitance measurements. Note the pulsing behaviour of the fusion pore with fusion pore dwell-time of around 50 ms.
Peptide hormone release can be limited or completely prevented by the transient mode of vesicle fusion (kiss-and-run) because of the relatively large molecular size and consequent low diffusional mobility of peptide molecules (Barg et al. 2002; Tsuboi & Rutter, 2003; Obermüller et al. 2005). Membrane capacitance measurements of resting lactotrophs revealed regular repetitive transient fusion pore openings (‘the pulsing pore’) lasting for as long as 760 s (Stenovec et al. 2004; Vardjan et al. 2007). The duration of single transient events at rest was measured to be very fast (∼50 ms) (Fig. 1B; Stenovec et al. 2004; Vardjan et al. 2007), similar to resting synapses of calyx of Held (Sun et al. 2002). Based on the regular repetitive fusion pore openings and slow, but synchronous, loading and unloading of fluorescent probes in lactotrophs, a model was proposed. It predicts that the slow probe exchange through the fusion pore in resting lactotrophs (Fig. 1A) may be constrained kinetically by a long-lasting regular fusion pore gating (Stenovec et al. 2004).
Recent amperometric studies have shown that fusion pore flickering can limit dopamine release from synaptic terminals in neurons (Staal et al. 2004). This indicates that fusion pore gating may also be involved in the regulation of small chemical transmitter release in synapses despite the relatively fast diffusional mobility of small chemical transmitters (Alvarez de Toledo et al. 1993).
Narrow fusion pore in resting peptidergic vesicles
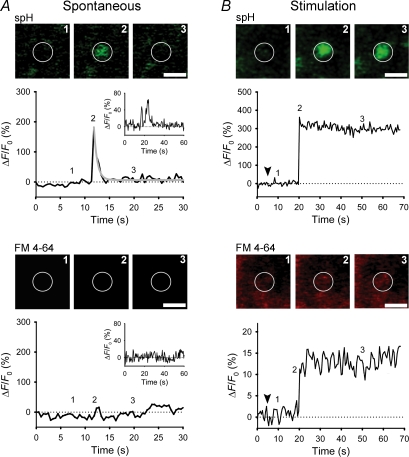
In addition to kinetic constraints, slow spontaneous release of peptides may also reflect a relatively narrow fusion pore of spontaneously releasing vesicles. The permeation of FM 4-64 dye and Hepes molecules through spontaneously forming fusion pores was studied in lactotroph vesicles expressing synaptopHluorin (spH; Vardjan et al. 2007), a pH-dependent fluorescent fusion marker (Miesenböck et al. 1998). Confocal imaging showed that half of the spontaneous exocytotic events exhibited fusion pore openings associated with a change in spH fluorescence (Fig. 2), indicating permeation of protons, but an impermeability to FM 4-64 (Fig. 2A, lower panel; molecular diameter ∼0.9 nm) and Hepes (molecular diameter ∼0.5 nm) molecules. Together with the results obtained by membrane capacitance measurements of fusion pore diameter (Spruce et al. 1990; Vardjan et al. 2007) these findings indicate an open fusion pore diameter in resting peptidergic vesicles of < 0.5 nm, much smaller than the size of neuropeptides stored in these vesicles (prolactin molecular diameter ∼5.2 nm; Vardjan et al. 2007), indicating that exocytosis without release of vesicle cargo may occur before delivery of the stimulus. Probes used in the earlier fusion pore permeability studies in resting secretory cells (FM-dyes, horseradish peroxidase, antibodies) were of relatively large size > 0.9 nm (Malgaroli et al. 1995; Ryan et al. 1997; Sara et al. 2005), leading to the underestimation of fusion pore diameter and the extent of spontaneous fusion.
Figure 2. Subnanometer fusion pores in spontaneous peptidergic vesicles.
A and B, sequential images of a single vesicle and changes in spH and FM 4-64 fluorescence at the vesicle site at rest (A) and after stimulation (B). Exocytosis resulted in a rapid increase in spH fluorescence, followed by a rapid exponential decline (grey line; spontaneous) or a persistent elevation (after stimulation). Insets in A show a repetitive spontaneous exocytotic event. Note that stimulation triggered loading of FM 4-64 dye into vesicles probably due to larger pore diameter. Numbers on plots correspond to times when images were recorded. White circles indicate sites of exocytic events. Scale bar represents 1 μm. Arrowheads indicate the onset of stimulation. Modified from Vardjan et al. (2007) with the permission of The Society for Neuroscience.
Interestingly, in stimulated lactotrophs, > 70% of exocytotic events exhibited a larger, FM 4-64-permeable pore (> 0.9 nm; Fig. 2B, lower panel) consistent with previous fusion pore permeation studies (Barg et al. 2002; Takahashi et al. 2002; Tsuboi & Rutter, 2003; Fulop et al. 2005). In capacitance measurements, stimulation decreased the fraction of events with lowest measurable fusion pore diameter of 3.2 nm from 25% to 2%, reflecting much wider pore diameters upon stimulation and probably a massive vesicle cargo discharge in the majority of these events (Vardjan et al. 2007). Similarly, stimulation led to fusion pore expansion in chromaffin cells, therefore improving the efficiency of release of small classical transmitters (Elhamdani et al. 2001; Fulop et al. 2005).
Spontaneous fusion pore diameter and kinetics is modulated by a stimulus
Whether the release of vesicle cargo from neurons and neuroendocrine cells involves full fusion or the cargo is released by a transient (kiss-and-run) interaction of vesicle with the plasma membrane is still an open issue (LoGiudice & Matthews, 2006). Transient and full fusion exocytosis were reported to coexist in some systems and it has been suggested that switching between the transient to full fusion mode due to increased cell stimulation can result in the modulation of the amount of cargo release from a single vesicle (reviewed in Harata et al. 2006). However, this does not seem to be the case in the peptidergic lactotroph vesicles, where the transient exocytosis appears to be the predominant form of exocytosis in spontaneous and stimulated vesicles, where fusion pore properties of the same vesicle were monitored under resting conditions and also after stimulation (Vardjan et al. 2007).
In these optical studies on lactotrophs expressing spH, 65% of all spontaneous events were transient, some of them occurred repeatedly presumably due to fusion pore pulsing (Fig. 2A, upper panel; Vardjan et al. 2007). In 35% of all events, spH fluorescence persisted after fusion for at least 100 s. In contrast to spontaneous events, the majority of 100 mm KCl-stimulated events were persistent (> 93%; Fig. 2B, upper panel). In persistent events FM 4-64 loading was fourfold slower than in transient events, suggesting that the spH signal may persist because of a rapidly flickering fusion pore (Stenovec et al. 2004; Staal et al. 2004) and not because of a long fusion pore lifetime (Ohara-Imaizumi et al. 2002). This was confirmed by capacitance measurements, which have shown that transient fusion pore events with an estimated mean burst duration of > 100 s are the predominant mode of spontaneous and stimulated exocytosis in lactotrophs (Stenovec et al. 2004; Vardjan et al. 2007). The effect of fusion pore closure is poorly reflected in the spH measurements due to reacidification, which is rather slow (τ= 4–5 s; Atluri & Ryan, 2006) in comparison to the fusion pore closure observed as an off-step in capacitance measurements.
Capacitance measurements of spontaneous and stimulated events also revealed that transient vesicle fusion occurred four times more frequently after stimulation with a twofold longer fusion pore dwell-time and a wider pore diameter (Vardjan et al. 2007) that were confirmed also with the permeation studies of molecules through the fusion pore (Fig. 2). Stimulus thus prolongs the effective open time of the transiently opened fusion pore and expands its initial resting subnanometer diameter enabling hormone secretion without full fusion (Fig. 3; Vardjan et al. 2007). This is in contrast to previous studies on chromaffin cells where low levels of stimulation triggered kiss-and-run exocytosis, whereas with stronger stimulation the predominant mode of exocytosis was full fusion (Fulop et al. 2005; Elhamdani et al. 2006).
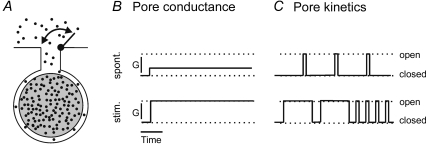
Figure 3. Modes of vesicle content discharge as a function of fusion pore properties before and after stimulation.
A, diagram shows a docked vesicle, the lumen of which is transiently connected to the extracellular medium by an open fusion pore. The fusion pore gates may reversibly close to hinder or stop the release of hormone molecules (filled dots) from the dense core (grey body) of the vesicle. B and C, the transport of molecules through the fusion pore is the function of fusion pore conductance (G, diameter; B) and/or fusion pore kinetics (C). A wider fusion pore (higher conductance) and/or faster frequency of fusion pore openings with longer fusion pore dwell times (faster pore-opening kinetics) leads to a faster release of peptides from vesicles in stimulated events. Modified from Stenovec et al. (2004) with the permission of The Federation of American Societies for Experimental Biology.
All these observations are consistent with the regulation of vesicle discharge mechanisms operating after fusion. Current knowledge of regulatory mechanisms on the postfusion stage through the regulation of fusion pore diameter and/or fusion pore open duration is still very limited (Rahamimoff & Fernandez, 1997). The important role of the intracellular Ca2+ (Fernández-Chacón & Alvarez de Toledo, 1995; Hartmann & Lindau, 1995), protein phosphorylation (Scepek et al. 1998), complexin (Archer et al. 2002), synaptotagmin (Wang et al. 2001) and syntaxin (Han et al. 2004) has been pointed out. Fusion pore dynamics is important, as any modulation of release processes could have an impact on specific physiological functions of resting and stimulated cells.
Conclusions
In summary (Fig. 3), a single peptidergic vesicle may undergo unproductive exocytosis in a resting cell. The release of vesicle cargo may be restrained kinetically and/or due to a narrow fusion pore. Under stimulation, the preformed fusion pore may retain the transient (kiss-and-run) nature, but with a longer dwell time, increased frequency of reopening and an increased fusion pore diameter, which facilitates the vesicle cargo release.
Acknowledgments
We thank Ms Sonja Grilc for continuous support. This work was supported by grants P3310 381, Z37476 from the Ministry of Higher Education, Sciences and Technology of the Republic of Slovenia, NS36665 from the National Institutes of Health, EC grant no. QLG32001-04, EC support DECG CLG3-CT-2001-02004, and EC GROWBETA QLG1-CT-2001-02233.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernández-Chacón R, Fernández J. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Angleson J, Betz W. Intraterminal Ca2+ and spontaneous transmitter release at the frog neuromuscular junction. J Neurophysiol. 2001;85:287–294. doi: 10.1152/jn.2001.85.1.287. [DOI] [PubMed] [Google Scholar]
- Angleson J, Cochilla A, Kilic G, Nussinovitch I, Betz W. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Archer D, Graham M, Burgoyne R. Complexin regulates the closure of the fusion pore during regulated vesicle exocytosis. J Biol Chem. 2002;277:18249–18252. doi: 10.1074/jbc.C200166200. [DOI] [PubMed] [Google Scholar]
- Atluri P, Ryan T. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S, Olofsson C, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renström E, Rorsman P. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Betz W, Bewick G. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut W, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Deák F, Shin OH, Kavalali ET, Südhof T. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher D, Ueda A, Stewart B, Burgess R, Kidokoro Y, Schwarz T. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Artalejo C. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamdani A, Palfrey H, Artalejo C. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Neher E, Gomperts B. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Alvarez de Toledo G. Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS Lett. 1995;363:221–225. doi: 10.1016/0014-5793(95)00319-5. [DOI] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer R, Li C, Rosahl T, Stevens C, Südhof T. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Jiang Y, Tomić M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS. Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol. 2006;20:2231–2246. doi: 10.1210/me.2005-0363. [DOI] [PubMed] [Google Scholar]
- Groemer TW, Klingauf J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci. 2007;10:145–147. doi: 10.1038/nn1831. [DOI] [PubMed] [Google Scholar]
- Han X, Wang C, Bai J, Chapman E, Jackson M. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. doi: 10.1126/science.1095801. [DOI] [PubMed] [Google Scholar]
- Harata N, Aravanis A, Tsien R. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Lindau M. A novel Ca2+-dependent step in exocytosis subsequent to vesicle fusion. FEBS Lett. 1995;363:217–220. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- Heuser J, Reese T. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S, Raciborska D, Trimble W, Charlton M. Different VAMP/synaptobrevin complexes for spontaneous and evoked transmitter release at the crayfish neuromuscular junction. J Neurophysiol. 1998;80:3233–3246. doi: 10.1152/jn.1998.80.6.3233. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller R. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool, UK: Liverpool University Press; 1969. [Google Scholar]
- Kijima H, Tanabe N. Calcium-independent increase of transmitter release at frog end-plate by trinitrobenzene sulphonic acid. J Physiol. 1988;403:135–149. doi: 10.1113/jphysiol.1988.sp017243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M, Markin V. [Possible mechanism of membrane fusion] Biofizika. 1983;28:242–247. [PubMed] [Google Scholar]
- Kreft M, Kuster V, Grilc S, Rupnik M, Milisav I, Zorec R. Synaptotagmin I increases the probability of vesicle fusion at low [Ca2+] in pituitary cells. Am J Physiol Cell Physiol. 2003;284:C547–C554. doi: 10.1152/ajpcell.00333.2002. [DOI] [PubMed] [Google Scholar]
- Llano I, González J, Caputo C, Lai F, Blayney L, Tan Y, Marty A. Presynaptic calcium stores underlie largeamplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- LoGiudice L, Matthews G. The synaptic vesicle cycle: is kissing overrated? Neuron. 2006;51:676–677. doi: 10.1016/j.neuron.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Malgaroli A, Ting A, Wendland B, Bergamaschi A, Villa A, Tsien R, Scheller R. Presynaptic component of long-term potentiation visualized at individual hippocampal synapses. Science. 1995;268:1624–1628. doi: 10.1126/science.7777862. [DOI] [PubMed] [Google Scholar]
- Maximov A, Shin O, Liu X, Südhof T. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis D, Rothman J. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Murthy V, Stevens C. Reversal of synaptic vesicle docking at central synapses. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermüller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Katsuta H, Ishida H, Nagamatsu S. Monitoring of exocytosis and endocytosis of insulin secretory granules in the pancreatic beta-cell line MIN6 using pH-sensitive green fluorescent protein (pHluorin) and confocal laser microscopy. Biochem J. 2002;363:73–80. doi: 10.1042/0264-6021:3630073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Sun J, Rizo J, Maximov A, Südhof T. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006;25:2039–2050. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R, Fernandez J. Pre- and postfusion regulation of transmitter release. Neuron. 1997;18:17–27. doi: 10.1016/s0896-6273(01)80043-x. [DOI] [PubMed] [Google Scholar]
- Ryan T, Reuter H, Smith S. Optical detection of a quantal presynaptic membrane turnover. Nature. 1997;388:478–482. doi: 10.1038/41335. [DOI] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deák F, Liu X, Kavalali E. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Scepek S, Coorssen J, Lindau M. Fusion pore expansion in horse eosinophils is modulated by Ca2+ and protein kinase C via distinct mechanisms. EMBO J. 1998;17:4340–4345. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Deák F, Königstorfer A, Mozhayeva M, Sara Y, Südhof T, Kavalali E. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Shaner N, Steinbach P, Tsien R. A guide to choosing fluorescent proteins. Nat Meth. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- Spruce A, Breckenridge L, Lee A, Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990;4:643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Staal R, Mosharov E, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Poberaj I, Betz W, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. FASEB J. 2004;18:1270–1272. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- Sun J, Wu X, Wu L. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Rutter G. Multiple forms of ‘kiss-and-run’ exocytosis revealed by evanescent wave microscopy. Curr Biol. 2003;13:563–567. doi: 10.1016/s0960-9822(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani T, Ertunc M, Sara Y, Mozhayeva M, Kavalali E. Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling. J Neurosci. 2005;25:10922–10929. doi: 10.1523/JNEUROSCI.3766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Grishanin R, Earles C, Chang P, Martin T, Chapman E, Jackson M. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson P, Carta M, Costa E, Mathews J, Lopez-Benditó G, Molnár Z, Becher M, Valenzuela C, Partridge L, Wilson M. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wasser C, Ertunc M, Liu X, Kavalali E. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R, Jankowski J, Kennedy R, Kawagoe K, Schroeder T, Leszczyszyn D, Near J, Diliberto EJ, Viveros O. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Bräuninger M, González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]