Abstract
Mixed findings regarding the effects of whole-body heat stress on central blood volume have been reported. This study evaluated the hypothesis that heat stress reduces central blood volume and alters blood volume distribution. Ten healthy experimental and seven healthy time control (i.e. non-heat stressed) subjects participated in this protocol. Changes in regional blood volume during heat stress and time control were estimated using technetium-99m labelled autologous red blood cells and gamma camera imaging. Whole-body heating increased internal temperature (≥ 1.0°C), cutaneous vascular conductance (approximately fivefold), and heart rate (52 ± 2 to 93 ± 4 beats min−1), while reducing central venous pressure (5.5 ± 07 to 0.2 ± 0.6 mmHg) accompanied by minor decreases in mean arterial pressure (all P < 0.05). The heat stress reduced the blood volume of the heart (18 ± 2%), heart plus central vasculature (17 ± 2%), thorax (14 ± 2%), inferior vena cava (23 ± 2%) and liver (23 ± 2%) (all P≤ 0.005 relative to time control subjects). Radionuclide multiple-gated acquisition assessment revealed that heat stress did not significantly change left ventricular end-diastolic volume, while ventricular end-systolic volume was reduced by 24 ± 6% of pre-heat stress levels (P < 0.001 relative to time control subjects). Thus, heat stress increased left ventricular ejection fraction from 60 ± 1% to 68 ± 2% (P= 0.02). We conclude that heat stress shifts blood volume from thoracic and splanchnic regions presumably to aid in heat dissipation, while simultaneously increasing heart rate and ejection fraction.
Whole-body heating has the potential to cause significant cardiovascular stress that, in healthy individuals, may be second only to the cardiovascular stress associated with exercise. In order to facilitate heat exchange, substantial increases in cutaneous vascular conductance occur during heat stress. Such a vasodilatory response can lead to large increases in systemic vascular conductance, which would greatly reduce arterial blood pressure if not compensated for by an elevation in cardiac output and/or a decrease in vascular conductance of non-cutaneous beds. However, blood pressure is typically well maintained, even during pronounced heating, due to the capability of cardiac output to more than double in supine hyperthermic subjects (Rowell et al. 1969) coupled with reductions in vascular conductance in non-cutaneous beds (Rowell et al. 1971).
A reduction in central venous pressure (CVP) occurs almost immediately with the onset of passive heating (Rowell et al. 1969; Johnson & Proppe, 1996; Minson et al. 1998; Crandall et al. 1999; Peters et al. 2000; Wilson et al. 2007) and, depending on the magnitude of the heat stress, CVP may approach 0 mmHg. The mechanism responsible for this reduction in CVP may be a combination of elevated cardiac output and a redistribution of blood from the central circulation to the cutaneous circulation (Johnson & Proppe, 1996). A reduction in central blood volume due to heat stress has been hypothesized (Müller, 1905; Glaser et al. 1950; Eisalo, 1956; Koroxenidis et al. 1961; Frayser et al. 1966). In contrast to those findings, Rowell et al. (1969) reported a slight increase in central blood volume during whole-body heat stress when indexed from mean transit time of a dye injected into the right atrium and sampled at the aortic arch. More recently, it was observed that heat stress did not change central blood volume as indexed by thoracic impedance (Cui et al. 2004), whereas in another study (Cai et al. 2000b), using a less severe heat stress, an increase in thoracic impedance was observed, which is indicative of reduced central blood volume. Given the disparity of these results, the first objective of this study was to test the hypothesis that whole-body heat stress reduces central blood volume, as measured by gamma camera imaging of technetium-99m (99mTc)-labelled autologous red blood cells. Additionally, this technology was used to assess the effects of heat stress on changes in blood volume distribution in other regions of the body. Given the effects of heat stress in decreasing ventricular filling pressures (Wilson et al. 2007) and hypothesized changes in central blood volume, the second objective of this study was to test the hypothesis that whole-body heat stress alters left ventricular end-systolic (LVESV) and end-diastolic (LVEDV) volumes, based on multiple-gated acquisition (MUGA) imaging determinants of these variables.
Methods
Ten male subjects participated in the experimental portion of this study (i.e. heat stress). An additional seven male subjects served as non-heat-stress time control subjects because of the potential for redistribution of blood volume associated with prolonged exposure to the supine position. Subject characteristics were: age, 29 ± 5 years; height, 182 ± 6 cm; weight, 80 ± 11 kg for the experimental subjects and age, 32 ± 5 years; height, 184 ± 9 cm; weight, 78 ± 11 kg for the control subjects (mean ± s.d.). Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. Subjects were informed of the purpose and risks of this study, approved by the Ethical Committee of Copenhagen, K22-090/04, before providing their consent. All procedures were performed in accordance with the Declaration of Helsinki.
Instrumentation of experimental subjects
Each subject swallowed a telemetry pill for the measurements of intestinal temperature (HQ Inc, Palmetto, FL, USA). The subjects were also instrumented for the measurement of mean skin temperature from the weighted average of six thermocouples (Taylor et al. 1989) attached to the skin. Each subject was placed in a tube-lined suit that covered the entire body surface with the exception of the hands, feet, head, and the forearm from which skin blood flow was assessed. CVP was measured upon cannulation of a vein in the arm (typically the basilic vein), with the catheter advanced to the superior vena cava. Correct placement was identified via the pressure waveform. Arterial pressure was obtained following cannulation of the brachial or radial artery of the non-dominant arm. Both catheters were connected to fluid-filled pressure transducers that were zeroed to atmospheric pressure 5 cm below (i.e. dorsal) the subject's supra-sternal notch. Mean values of these pressures were obtained by integration of the respective waveforms via data analysis software (Acknowledge, Biopac, CA, USA). Heart rate was quantified via R-wave detection of the subject's ECG and forearm skin blood flow was indexed via laser–Doppler flowmetry (Perimed, Sweden).
The effects of heat stress on thoracic impedance is unclear (Cai et al. 2000b; Cui et al. 2004). Hence, comparisons were made between thoracic impedance and scintigraphy indices of changes in central blood volume during heat stress. Similarly, leg impedance was measured during heat stress to compare responses between this technique and scintigraphy of a peripheral vascular bed. Leg and thoracic admittance (inverse of impedance) were calculated based upon the impedance of 200 μA of current at 1.5 and 100 kHz (C-Guard, Danmeter, Denmark). The skin was thoroughly cleaned with alcohol prior to electrode placement. For thoracic admittance, paired electrodes were placed on the right sternocleidomastoid muscle and on the upper left rib at the mid-axillary line (Cai et al. 2000a). For leg admittance, paired electrodes were placed on the greater trochanter as well as on the medial epicondyle (Perko et al. 1993, 1995).
Instrumentation of control subjects
Non-heat stress subjects were employed as time controls for the experimental subjects. These subjects were also placed in the tube-lined suit; however, throughout the protocol normothermic (34°C) water perfused this suit. Given the absence of a heat stress, thermal and haemodynamic variables were not obtained from each of these subjects. For these time-control subjects only scintigraphy-based data were analysed.
Blood pool scintigraphy
In vitro99mTc-labelled autologous red blood cells were injected into the subject and these cells distribute throughout the circulation over the course of 5–10 min. Using a gamma camera, the number of counts within a region of interest (ROI) is proportional to the blood volume within that region, after accounting for changes in haematocrit (discussed below). In vitro isotope labelling of autologous red blood cells previously drawn was performed in both subject groups using 800 MBq of 99mTc (Ultra-Tag RBC, Mallinckrodt, TycoHealthcare, USA). This in vitro labelling technique is stable over a number of hours. During in vitro labelling of red blood cells, requiring approximately 45 min, the subject rested supine on the gamma camera table. Approximately 15 min after reinjection of the labelled red cells, a scan was performed at 12 cm min−1 from the head to just below the knee (defined as a whole-body scan) with a dual-headed gamma camera (Skylight, Philips Medical Systems) with the detectors positioned in anterior and posterior views. A second whole-body scan was performed following a rise in core temperature of ∼1.0°C (typically about 90 min after injection of labelled red blood cells). The position of the subject was unchanged throughout the protocol. Data were acquired in a 512 by 1024 matrix and stored in a dedicated acquisition station (Pegasys, ADAC, Milpitas, CA, USA). Data were subsequently analysed using a computer workstation (eNTEGRA, General Electric, Milwaukee, WI, USA). The anterior and posterior projections were combined by calculation of the geometric mean of opposite views. This calculation is necessary because it compensates for counting efficiency of the labelled red blood cells if they are located superficially or deep in the body, and if they are distributed in the anterior or posterior direction. Attenuation compensation was applied as outlined by Sorenson (1984) because without this compensation labelled red blood cells redistributed to body parts with smaller thickness (e.g. arms) will be counted with higher efficiency relative to blood redistributed to thicker body regions (e.g. torso).
Given that heat stress increases haematocrit, coupled with the isotope label specifically binding to red blood cells, gamma camera-derived red blood cell counts will not accurately represent changes in blood volume within a ROI if not corrected for changes in haematocrit. This correction was performed by dividing the number of radioactive counts within a ROI by haematocrit obtained at the time of the scan (e.g. normothermia or heat stress) and multiplying that value by 100. For example, if during normothermia the number of radioactive counts within a ROI was 335 000 and haematocrit was 45%, then the value used for the relative blood volume assessment for that thermal condition would be 744 444. If during heat stress the number of radioactive counts within this ROI decreased to 280 000 and haematocrit increased to 48%, then the relative blood volume assessment for that ROI would be 583 333. Such a condition would reflect an ∼22% reduction in blood volume within that ROI by the heat stress.
To evaluate the effects of heat stress on R-wave-gated LVEDV, LVESV and ejection fraction, multiple-gated acquisition (MUGA) was performed. The anterior detector of the gamma camera was positioned in the left anterior oblique position. R-wave-gated scans were obtained for an accumulative duration of 6 min in both normothermic and heat-stressed conditions at a rate of 16 frames per R–R interval in a 64 by 64 matrix. Five millilitres of blood was drawn in the middle of each MUGA and was later counted for radioactivity upon completion of gamma camera imaging using the same gamma detector as that used for the MUGA. Relative LVEDV and LVESVs were identified from background- corrected ventricular ROI counts and from the aforementioned blood sample counts (Hojlund-Carlsen et al. 1984), corrected for haematocrit. Absolute ventricular volumes, requiring additional scans to account for attenuation of the counts due to potential changes in heart position with heating, were not determined since changes in volume (i.e. counts) due to heat stress for each individual was the primary measure of interest. Left ventricular ejection fraction was calculated as: (LVEDV – LVESV)/LVEDV × 100.
Protocol
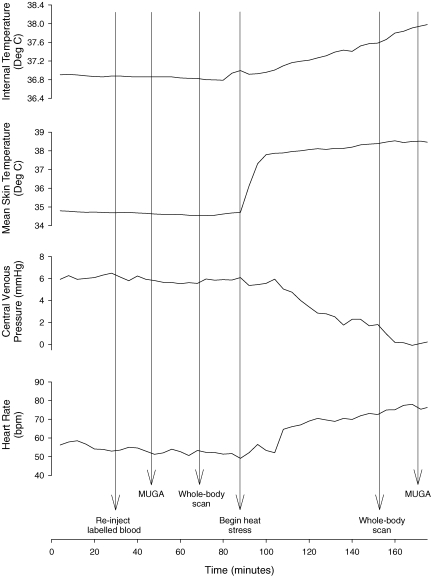
An approximate time line of when the indicated procedures were performed is illustrated in Fig. 1. Baseline (i.e. pre-heat stress) whole-body scans were performed as well as a 6 min MUGA scan of the heart, while thermoneutral water (i.e. 34°C) was perfused through the tube-lined suit. For the experimental subjects, upon completion of baseline data collection, the temperature of the water perfusing the suit was elevated to 46–48°C with a goal of increasing internal temperature ≥ 1.0°C. Upon achievement of the desired elevation in internal temperature, each experimental subject underwent another whole-body scan followed by MUGA assessment. Arterial and central venous (from the CVP catheter) blood was drawn from the respective catheters while subjects were normothermic and at the end of the heat stress. Blood samples were analysed within 10 min of being drawn via a blood gas analyser (ABL 510 Radiometer, Denmark). These values were temperature corrected based upon internal temperature measured from the telemetric pill. For the control subjects, the temperature of the water was not changed following baseline data collection, such that 34°C was perfused throughout the entire protocol. The second whole-body scan and MUGA assessment were performed at the same mean duration following the normothermic scans relative to the experimental subjects.
Figure 1. Responses to whole-body heating from a subject and times when the listed procedures were performed.
Given the differences in the time necessary to heat each subject to achieve the desired increase in internal temperature, the time line for this subject only approximates the time line for other subjects. MUGA, multi-gated acquisition.
Data analysis
Thermal and haemodynamic data were recorded at 200 Hz via a 16 bit A/D converter (Biopac, Santa Barbara, CA, USA). Data were reduced to average responses during a 3 min period prior to heat stress and at the last 3 min of the heat stress.
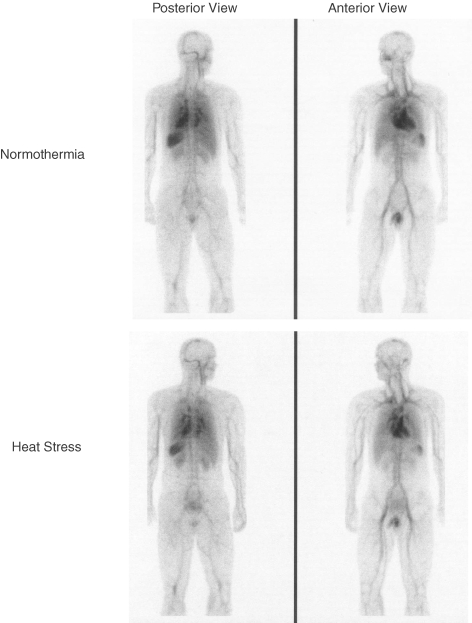
For the whole-body scan (head to knee level), radioactive counts were obtained from the following ROIs: thorax, each lung, heart, heart + central vasculature (comprised of the heart and the thoracic components of the inferior and superior vena cava, the pulmonary artery, and the ascending aorta), liver, spleen and inferior vena cava (from the distal board of the liver to the bifurcation of the abdominal aorta); see Fig. 2. Due the potential for overlap from other tissues/organs, in some of the aforementioned ROIs the entire organ was not traced but rather the portion of the organ not affected by other tissues or organs was traced. The ROIs were copied from the normothermic assessment to ensure that the sample sizes (i.e. regional blood volume areas) were identical throughout the study. After adjusting the radioactive counts within a ROI for haematocrit, to obtain an index of blood volume from red cell volume, the effects of heat stress on changes in regional blood volumes were assessed as:
Figure 2. Scintigraphic images of the scanned area in normothermia (upper panels) and heat stress (lower panels) from one subject.
Data are analysed from higher resolution images than those displayed.
This formula was also used for the control subjects but using counts from the baseline scan relative to the time control scan, rather than normothermia and heat stress, respectively.
The effects of heat stress and time control on changes in end-diastolic and end-systolic volumes were calculated in the same manner as described above. MUGA has the capability of providing very reproducible ejection fraction values from the difference in counts between end-diastole and end-systole. This assessment was performed to identify the effects of heat stress on this index of systolic cardiac function.
Statistical analysis
The effect of the perturbation (i.e. heat stress and time control) on the relative (i.e. percentage) change in regional blood volume, including cardiac volumes, was statistically evaluated via an unpaired t test between groups. Thus, a statistical difference between experimental and control values indicates that the change in blood volume within the specified ROI was due to the heat stress, as opposed to the subject remaining supine under normothermic conditions for the same duration. Given that MUGA provides an absolute ejection fraction, these values were statistically evaluated via a 2-way ANOVA with main factors of group (experimental and control groups) and perturbation (i.e. pre versus post condition) followed by a post hoc analysis. Pearson product moment correlation was used to assess the strength of the association between changes in thoracic admittance and changes in CVP, thoracic blood volume, and heart + central vasculature blood volume due to the heat stress. In addition, this test was used to assess the effects of the heat stress on the association between changes in leg admittance and changes in thigh blood volume. Data are reported as mean ± s.e.m. The level of significance was set at P≤ 0.05.
Results
Temperature and haemodynamic responses to whole-body heating are shown in Table 1. The heat stress caused expected changes in these variables including a significant reduction in CVP of ∼5 mmHg. There was no effect of heat stress on thoracic or leg admittance (P= 0.24 and 0.29, respectively). Heat stress reduced PaCO2, while pH, haematocrit and central venous oxygen saturation were increased (Table 1).
Table 1.
Temperature, haemodynamic and blood values prior to, and at the end of, heating
| Pre-heat stress | End heat stress | P value | |
|---|---|---|---|
| Intestinal temperature (°C) | 36.90 ± 0.08 | 38.22 ± 0.08 | < 0.001 |
| Mean skin temperature (°C) | 34.3 ± 0.2 | 38.3 ± 0.2 | < 0.001 |
| Mean arterial blood pressure (mmHg) | 87 ± 2 | 81 ± 2 | 0.001 |
| Central venous pressure (mmHg) | 5.5 ± 0.7 | 0.2 ± 0.6 | < 0.001 |
| Heart rate (beats min−1) | 53 ± 2 | 93 ± 4 | < 0.001 |
| Skin blood flow (flux units) | 23 ± 4 | 83 ± 7 | < 0.001 |
| Thoracic admittance (S) | 38.8 ± 3 | 39.1 ± 3 | 0.24 |
| Leg admittance (S) | 42.6 ± 3 | 42.9 ± 4 | 0.29 |
| Arterial pH | 7.403 ± 0.003 | 7.450 ± 0.020 | 0.032 |
| PaCO2 (mmHg) | 39.9 ± 0.7 | 35.7 ± 2.1 | 0.045 |
| PaO2 (mmHg) | 94.9 ± 5.4 | 101.0 ± 4.5 | 0.18 |
| Haematocrit (%) | 43.7 ± 1.2 | 47.4 ± 1.2 | < 0.001 |
| Central venous oxygen saturation (%) | 75.8 ± 2.0 | 84.0 ± 2.5 | 0.015 |
PaCO2, pressure of arterial carbon dioxide; PaO2, pressure of arterial oxygen.
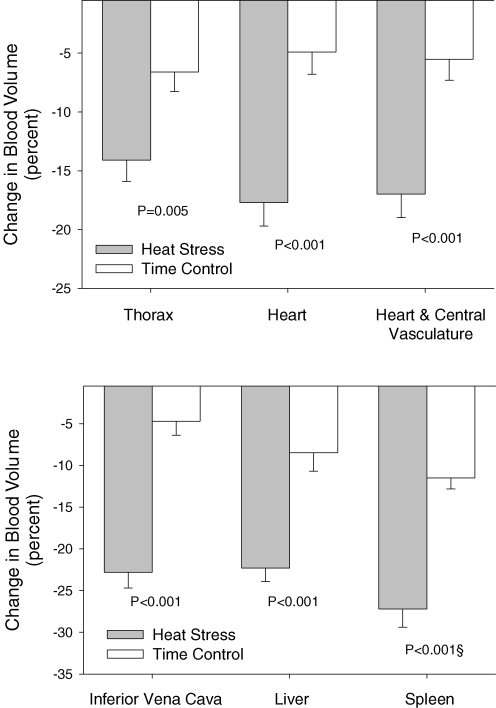
Changes in regional blood volumes due to heat stress and time control are depicted in Fig. 3. Heating caused significant reductions in whole-body scanning-derived blood volume of the heart (18 ± 2%), heart + central vasculature (19 ± 2%), and inferior vena cava (23 ± 2%) relative to control subjects. There was no difference in lung blood volumes between groups, although thoracic blood volume was reduced 14 ± 2% (P= 0.005) by heating. Heat stress caused a 22 ± 2% (P < 0.001) reduction in liver and a 27 ± 2% (P < 0.001) reduction in spleen blood volumes. In one control subject, in the absence of heat stress, spleen blood volume decreased 43% relative to pre-time-control volume. No technical rationale could be identified to explain this response, which was greater than 5 standard deviations from the mean. Similar outlier responses were not observed from other regions of interest from this subject. For this reason data from this subject were excluded from the spleen analysis. However, even with the inclusion of this subject's data, significant differences remain between heat stress and time control groups for spleen blood volume (P= 0.015), although the mean reduction in spleen blood volume for the time control group increased to 16 ± 4%. Finally, heat stress did not significantly alter thigh blood volume.
Figure 3. Percentage change in blood volume from the indicated regions between experimental (i.e. heat stressed) and time control subjects.
In each of the indicated regions heat stress significantly reduced blood volume relative to the time control trials. §Outlier data from a time control subject was removed for this analysis due to this subject exhibiting an unexplained pronounced reduction in spleen blood volume that was greater than 5 standard deviations from the mean. See comments in Results regarding the implications of removal of this subject's data point.
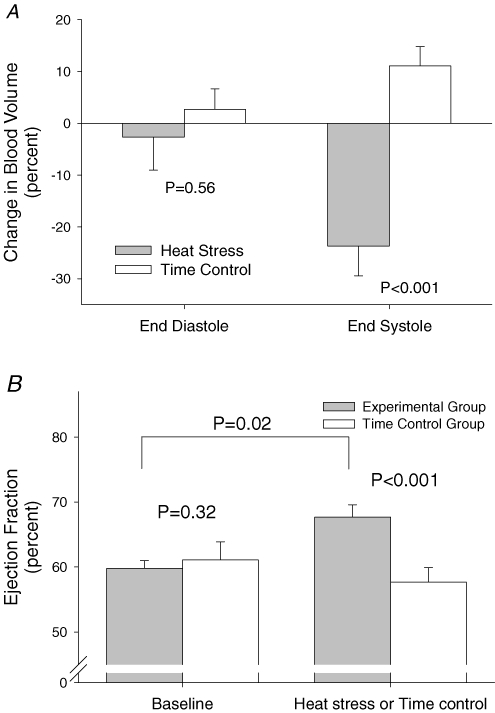
Despite pronounced reductions in CVP and whole-body scanning-derived blood volumes of the heart and heart plus central vessels, a 3 ± 6% reduction in MUGA-derived LVEDV was not different from a 2 ± 4% increase in this value in the control subjects (P= 0.29; Fig. 4). However, heating reduced LVESV by 24 ± 5%, resulting in an increase in left ventricular ejection fraction from 60 ± 1 to 68 ± 2% (P < 0.01), without a significant change in ejection fraction in the control group (Fig. 4).
Figure 4. Effects of heat stress on left ventricular end-diastolic and end-systolic volumes, as well as ejection fraction.
A, percentage change in left ventricular end-diastolic and end-systolic blood volumes as a result of whole-body heat stress and time control conditions derived from multi-gated acquisition (MUGA). Heat stress did not significantly alter left ventricular end-diastolic volume whereas left ventricular end-systolic volume was significantly reduced. B, effects of heat stress and time control conditions on left ventricular ejection fraction. There was no difference in ejection fraction between the groups prior to the heat stress or time control periods. Whole-body heating significantly increased left ventricular ejection, while this value was unchanged at the end of the time control period for this group.
There were no significant correlations between the heat stress-induced change in thoracic admittance and the change in thoracic blood volume, heart + central vasculature blood volume, or CVP. Likewise, there was no significant correlation between the change in leg admittance and the change in leg blood volume during heating.
Discussion
These results clearly demonstrate that whole-body heat stress, of the magnitude imposed on the present subjects, decreases central blood volume as indexed from the assessment of changes in thoracic blood volume as well as the volume of blood in the heart and central vascular structures. Furthermore blood volume to liver and spleen was also reduced, indicative of a shift of blood from the central circulation to the peripheral circulation; although these changes occurred in the absence of a significant increase in thigh blood volume by the heat stress.
Müller (1905) was the first to address the question of the effects of heat stress on central blood volume. He investigated this question by horizontally suspending subjects on multiple scales and identifying changes in weight of different regions (i.e. head, chest, arms, legs, etc.) during a heat stress. He reported that this perturbation moved fluid from the centre of the body to the periphery, suggesting that heat stress reduces trunk blood volume while increasing limb blood volume. Such an assessment was likely to have been very challenging given the impact of movement on the identified readings. Glaser and coworkers (Glaser, 1949; Glaser et al. 1950) also addressed this question by identifying changes in ‘radiographic shadow’ of the liver, lungs and heart via X-ray following a relatively minor heat stress (elevated oral temperature of ∼0.2°C). They reported that heating decreased the radiographic shadows of the liver and lungs (indicating decreases in blood volume in these areas), without changing the heart image, and concluded that these responses were due to changes in blood volume. Subsequent studies have used dye dilution technology to assess the effects of heat stress on central blood volume (Eisalo, 1956; Koroxenidis et al. 1961; Rowell et al. 1969), resulting in mixed findings, including reports of heat stress-induced increases in central blood volume (Rowell et al. 1969). These conflicting results are probably due to the influence of differing injection and sampling site, resulting in potential artifact in the calculation of mean transit time due to changes in blood volume of the vasculature between the injection and sampling sites relative to the right atria and the ascending aorta (Rowell et al. 1969; Johnson & Proppe, 1996). Consistent with Müller's conclusion (Müller, 1905), the present findings, with gamma camera imaging of in vitro labelled red blood cells, demonstrate that heat stress shifts blood volume to the periphery, as indexed by significant decreases in blood volume of organs in the central and splanchnic circulatory bed.
The splanchnic circulation comprises the vasculature from the large and small intestines, pancreas, spleen, stomach and liver. Rowell et al. (1970, 1971) and Minson et al. (1999) showed clear reductions in splanchnic blood flow, assessed by plasma indocyanine green dye clearance, during whole-body heating that was due primarily to increases in splanchnic vascular resistance. This increase in splanchnic vascular resistance would cause a decrease in splanchnic blood volume, by shifting to a lower point on the splanchnic compliance curve, resulting in a displacement volume of blood out of the splanchnic bed that ‘partially compensates’ for a reduction in central blood volume (Rowell, 1986). Although radionuclide imaging techniques, such as that used in the present study, cannot accurately assess blood volume of the entire splanchnic circulation, this approach can provide relative changes in blood volume of major organs of the splanchnic bed such as the liver and spleen. Of the measured organs/structures, the largest relative changes in blood volume occurred in this region with a 22% reduction in liver blood volume and a 27% reduction in spleen blood volume with heating.
Prior reports of sustained or elevated stroke volume during heat stress (Rowell et al. 1969; Rowell, 1986; Johnson & Proppe, 1996; Wilson et al. 2007), coupled with reductions in cardiac filling pressure as indexed by CVP (Rowell et al. 1969; Minson et al. 1998; Crandall et al. 1999; Peters et al. 2000) or pulmonary capillary wedge pressure (Wilson et al. 2007), indicate that heat stress increases cardiac contractility. Consistent with this hypothesis, heat stress increased ejection fraction by ∼13%. Although ejection fraction is an imperfect index of cardiac contractility, this finding provides some insight into the effects of heat stress on cardiac function.
Despite pronounced reductions in central venous and left ventricular filling pressure during passive heat stress, LVEDV was unchanged between thermal conditions. This observation is perplexing. It may be that heat stress improves diastolic function resulting in greater diastolic filling for a given filling pressure. In contrast to LVEDV, LVESV was reduced during heat stress, probably due primarily to increases in cardiac contractility resulting in the aforementioned increases in ejection fraction.
Thoracic impedance has been used to assess the effects of heating on central blood volume, resulting in mixed findings (Cai et al. 2000b; Cui et al. 2004). Thoracic admittance (inverse of impedance) was not significantly affected by the heat stress. It is possible that the pronounced sweating that occurred in all subjects obscured changes in admittance by providing an alternate current pathway to counter expected reductions in admittance due to decreases in thoracic blood volume. Given this lack of a clear change in thoracic and leg admittance with heating, there was no significant correlation between electrical admittance in these regions relative to changes in the respective blood volumes or CVP.
With the planar imaging set-up, it was not possible to detect differences in blood volume between a ROI (i.e. the heart, spleen, etc.) relative to blood volume of the skin directly over that region. Thus, as skin blood volume increases due to the heat stress, the assessment of a change in blood volume in the organ, represented by the ROI, below that area of skin may be influenced by changes in skin blood volume. The potential influence that change in skin blood volume may have on these readings depends on the ratio of volume of blood in the ROI relative to the volume of blood in skin over that region. Given the minimal thickness of the skin (< 0.1–1.5 mm) when compared to the volume of the structure assessed under the skin (i.e. liver, heart, etc.), it is unlikely that relatively small changes in skin blood volume with heating affected the calculated changes in blood volume of the ROIs evaluated.
With the exception of ejection fraction, reported values reflect a percentage change in volume of a region relative to the preheating baseline. It should be emphasized that if blood volume prior to heat stress of a particular region is small, then even a large percentage change in volume will reflect a small absolute change in volume. Therefore, equivalent percentage changes in blood volume between ROIs presented in Fig. 3 should not be interpreted as similar absolute changes in blood volume.
Conclusion
The present data demonstrate that whole-body heating reduces central blood volume (i.e. thorax, heart and heart plus central vessels) as well as left ventricular systolic blood volume. The blood volumes in organs within the splanchnic circulation were likewise reduced by whole-body heating. These findings are consistent with the hypothesis that heat stress shifts blood from central organs to the periphery, presumably the skin and large vessels draining the skin, which is necessary for appropriate thermoregulation. Finally, the present data reveal that heat stress increases cardiac performance as indexed by a significant increase in ejection fraction.
Acknowledgments
The investigators express gratitude to the technical staff at the Department of Clinical Physiology, Nuclear Medicine & PET at Rigshospitalet, Copenhagen, Denmark for their services and professionalism in assisting with this study. This study was funded in part by the National Heart, Lung, and Blood Institute (HL61388, HL67422, and HL84072), Aase & Ejnar Danielsens Fond, and the Danish Medical Research Council.
References
- Cai Y, Holm S, Jenstrup M, Stromstad M, Eigtved A, Warberg J, Hojgaard L, Friberg L, Secher NH. Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol. 2000a;89:1569–1576. doi: 10.1152/jappl.2000.89.4.1569. [DOI] [PubMed] [Google Scholar]
- Cai Y, Jenstrup M, Ide K, Perko M, Secher NH. Influence of temperature on the distribution of blood in humans as assessed by electrical impedance. Eur J Appl Physiol. 2000b;81:443–448. doi: 10.1007/s004210050066. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol. 1999;86:605–610. doi: 10.1152/jappl.1999.86.2.605. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol. 2004;96:2103–2108. doi: 10.1152/japplphysiol.00717.2003. [DOI] [PubMed] [Google Scholar]
- Eisalo A. Effects of the Finnish sauna on circulation. Ann Med Exp Biol Fenn. 1956;4(Suppl.):7–96. [Google Scholar]
- Frayser R, Ross JC, Levin HS, Messer JV, Pines J. Effects of increased environmental temperature on pulmonary diffusing capacity. J Appl Physiol. 1966;21:147–150. doi: 10.1152/jappl.1966.21.1.147. [DOI] [PubMed] [Google Scholar]
- Glaser EM. The effect of cooling and warming on the vital capacity, forearm and hand volume, and skin temperature of man. J Physiol. 1949;109:421–429. doi: 10.1113/jphysiol.1949.sp004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser EM, Berridge FR, Prior KM. Effects of heat and cold on the distribution of blood within the human body. Clin Sci. 1950;9:181–187. [PubMed] [Google Scholar]
- Hojlund-Carlsen PF, Marving J, Rasmussen S, Haunso S, Pedersen JF. Accuracy of absolute left ventricular volumes and cardiac output determined by radionuclide cardiography. Int J Cardiol. 1984;6:505–525. doi: 10.1016/0167-5273(84)90331-0. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Blatteis C, Fregly M, editors. Handbook of Physiology: Adaptations to the Environment. Bethesda, MD, USA: American Physiological Society; 1996. pp. 215–243. [Google Scholar]
- Koroxenidis GT, Shepherd JT, Marshall RJ. Cardiovascular response to acute heat stress. J Appl Physiol. 1961;16:869–872. doi: 10.1152/jappl.1961.16.5.869. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol. 1999;276:R203–R212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Müller O. Uber die Blutverteilung im menschichen Korper unter dem Einfluss thermischer Reize. Arch Klin Med. 1905;82:547–585. [Google Scholar]
- Perko G, Payne G, Secher NH. An indifference point for electrical impedance in humans. Acta Physiol Scand. 1993;148:125–129. doi: 10.1111/j.1748-1716.1993.tb09541.x. [DOI] [PubMed] [Google Scholar]
- Perko G, Tilgreen R, Secher NH. The venous pump does not affect the indifference point for electrical impedance in humans. Eur J Appl Physiol Occup Physiol. 1995;72:179–182. doi: 10.1007/BF00964135. [DOI] [PubMed] [Google Scholar]
- Peters JK, Nishiyasu T, Mack GW. Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol. 2000;88:1756–1764. doi: 10.1152/jappl.2000.88.5.1756. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation Regulation During Physical Stress. New York: Oxford University Press; 1986. Thermal stress; pp. 174–212. [Google Scholar]
- Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol. 1970;28:415–420. doi: 10.1152/jappl.1970.28.4.415. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man – role of falling blood pressure. J Appl Physiol. 1971;31:864–869. doi: 10.1152/jappl.1971.31.6.864. [DOI] [PubMed] [Google Scholar]
- Sorenson J. Quantitative measurement of radiation in vivo by whole body counting. In: Hine G, Sorenson J, editors. Instrumentation in Nuclear Medicine. New York: Academic Press; 1984. pp. 311–348. [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effect of heat and cold stress on central vascular pressures during orthostatic stress in humans. J Physiol. 2007 doi: 10.1113/jphysiol.2007.137901. DOI 10.1113/jphysiol.2007.137901. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]