Abstract
Skeletal muscle often shows a delayed force recovery after fatiguing stimulation, especially at low stimulation frequencies. In this study we focus on the role of reactive oxygen species (ROS) in this fatigue-induced prolonged low-frequency force depression. Intact, single muscle fibres were dissected from flexor digitorum brevis (FDB) muscles of rats and wild-type and superoxide dismutase 2 (SOD2) overexpressing mice. Force and myoplasmic free [Ca2+] ([Ca2+]i) were measured. Fibres were stimulated at different frequencies before and 30 min after fatigue induced by repeated tetani. The results show a marked force decrease at low stimulation frequencies 30 min after fatiguing stimulation in all fibres. This decrease was associated with reduced tetanic [Ca2+]i in wild-type mouse fibres, whereas rat fibres and mouse SOD2 overexpressing fibres instead displayed a decreased myofibrillar Ca2+ sensitivity. The SOD activity was ∼50% lower in wild-type mouse than in rat FDB muscles. Myoplasmic ROS increased during repeated tetanic stimulation in rat fibres but not in wild-type mouse fibres. The decreased Ca2+ sensitivity in rat fibres could be partially reversed by application of the reducing agent dithiothreitol, whereas the decrease in tetanic [Ca2+]i in wild-type mouse fibres was not affected by dithiothreitol or the antioxidant N-acetylcysteine. In conclusion, we describe two different causes of fatigue-induced prolonged low-frequency force depression, which correlate to differences in SOD activity and ROS metabolism. These findings may have clinical implications since ROS-mediated impairments in myofibrillar function can be counteracted by reductants and antioxidants, whereas changes in SR Ca2+ handling appear more resistant to interventions.
Intense or prolonged activation of skeletal muscle leads to impaired contractile function, that is, fatigue develops (Fitts, 1994; Allen et al. 1995; Allen et al. 2007). Restoration of the fatigue-induced force decrease can be a very slow process, in some instances requiring days for full recovery. Edwards et al. (1977) originally described a type of delayed force recovery in human muscles that was particularly marked at low stimulation frequencies, i.e. the firing frequencies of human motor units during movements requiring low to moderate forces (Marsden et al. 1971). This delayed force recovery was named low-frequency fatigue and has been observed in numerous human muscles and also in various animal muscle preparations (Jones, 1996; Westerblad et al. 2000; Keeton & Binder-Macleod, 2006). Unfortunately, the meaning of the term low-frequency fatigue has become uncertain because it is now also frequently used to describe fatigue induced by low frequency stimulation. We will therefore use ‘prolonged low-frequency force depression (PLFFD)’ to describe the phenomenon originally described by Edwards et al. (1977).
PLFFD is implicated in various neuromuscular disorders, causing an increased sense of effort that can seriously limit the physical performance (Keeton & Binder-Macleod, 2006). Moreover, PLFFD has been demonstrated after exhaustive respiratory work in the diaphragm of healthy subjects and hence it may cause respiratory failure (Polkey & Moxham, 2001). The mechanism(s) underlying PLFFD is not clear but prolonged elevation of Ca2+ and increased production of reactive oxygen species (ROS) have been suggested (Chin & Allen, 1996; Favero, 1999; Westerblad et al. 2000).
Decreased force production in skeletal muscle can in principle be due to: (a) impaired ability of cross-bridges to generate force, (b) decreased myofibrillar Ca2+ sensitivity, and/or (c) reduced Ca2+ release from the sarcoplasmic reticulum (SR) (Allen et al. 1995). Impaired cross-bridge force generation will lead to an equal reduction of force production at all stimulation frequencies, and thus mechanism (a) cannot cause PLFFD. Conversely, both decreased myofibrillar Ca2+ sensitivity and reduced SR Ca2+ release (mechanisms (b) and (c)) can have a larger effect at low frequencies. This is because of the non-linear force–Ca2+ relationship, which is flat at high but steep at low stimulation frequencies. Thus, changes in the free myoplasmic [Ca2+] ([Ca2+]i) or the Ca2+ sensitivity have little impact on force at high frequencies but a large effect at low frequencies (Jones, 1996; Westerblad et al. 2000; Allen et al. 2007).
In mouse flexor digitorum brevis (FDB) fibres, PLFFD was observed after fatigue induced by repeated tetanic stimulation and the force depression was associated with decreased [Ca2+]i during tetani, whereas myofibrillar Ca2+ sensitivity was unchanged (Westerblad et al. 1993). In contrast, in the present study we demonstrate a similar decrease in force production at low frequencies after fatigue in rat FDB fibres, which is associated with decreased myofibrillar Ca2+ sensitivity. Since low concentrations of hydrogen peroxide has large effects on myofibrillar Ca2+ sensitivity (Andrade et al. 2001), we hypothesize that the different causes of PLFFD (i.e. decreased SR Ca2+ release versus reduced myofibrillar Ca2+ sensitivity) are related to differences in ROS metabolism and the activity of superoxide dismutase (SOD), which converts superoxide to hydrogen peroxide. We test the hypothesis by measuring SOD activity and fatigue-induced changes in myoplasmic ROS levels in mouse and rat muscle fibres. In addition, we study muscle fibres from a transgenic mouse that overexpresses the mitochondrial Mn2+-dependent SOD isoform (SOD2 or MnSOD) (Silva et al. 2005). Muscles of these SOD2 overexpressing mice display increased SOD activity and decreased superoxide levels (Silva et al. 2005).
Methods
Animals
Three- to five-month-old male Wistar or Sprague–Dawley rats were anaesthetized with 2.5% halothane in 30% O2–70% N2O and killed (Lunde et al. 2001). SOD2 overexpressing mice and wild-type (WT) littermates were generated as described earlier and experiments were performed on 2-month-old male mice of the PAC817 clone (Silva et al. 2005). Adult male NMRI mice were used for measurements of SOD activity and ROS production as well as in experiments with the reductant dithiothreitol (DTT) and the antioxidant N-acetylcysteine (NAC). Mice were killed by rapid neck disarticulation. All experiments were approved by the Stockholm North local ethical committee.
Muscle fibre dissection, mounting and solution
Intact single muscle fibres were isolated from the flexor digitorum brevis (FDB) muscles as previously described (Lännergren & Westerblad, 1987; Lunde et al. 2001). In a few experiments on rat FDB muscles, small bundles of three to four fibres were used because of small diameters of single fibres (∼10 μm compared to normally > 30 μm); the results from these bundles were indistinguishable from those of single fibres. The isolated FDB fibre was mounted in a stimulation chamber between an Akers 801 force transducer and an adjustable holder and the length was adjusted to that giving maximal tetanic force. The fibre was superfused with a Tyrode solution (mm): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24.0; EDTA, 0.1; glucose, 5.5. Fetal calf serum (0.2%) was added to the solution to improve cell survival. The solution was bubbled with 5% CO2–95% O2, which gives an extracellular pH of 7.4. Experiments were performed at room temperature (∼24°C).
Force and [Ca2+]i measurements
The fibre was stimulated by supramaximum current pulses (duration 0.5 ms) delivered via two platinum plate electrodes. [Ca2+]i was measured with the fluorescent Ca2+ indicator indo-1 (Invitrogen/Molecular Probes), which was microinjected into the isolated fibre (or loaded in the membrane-permeant AM form in a few experiments where small bundles of fibres were used (see above)). The fibre was allowed to rest for at least 1 h after being injected and fibres that then displayed a marked reduction of the tetanic force were discarded. The mean fluorescence of indo-1 at rest or during tetanic contractions was measured and converted to [Ca2+]i using an intracellularly established calibration curve (Andrade et al. 1998). Tetanic force was measured as the mean over 100 ms where force was maximal.
Stimulation protocol
The force–frequency and [Ca2+]i–frequency relationships under control conditions were established by producing tetani at different frequencies (15–100 Hz for rat fibres and 20–100 Hz for mouse fibres) at 1 min intervals. The fibre was then fatigued by repeated 70 Hz tetanic contractions. Muscle fibres of the different animal models used in this study display differences in fatigue resistance. To induce a similar extent of fatigue, we therefore used slightly different stimulation protocols for the different models. Rat fibres were stimulated by 350 ms tetani given at 1 s intervals until force was reduced to ∼40% of the original (Lunde et al. 2001). Fibres from NMRI mice were fatigued by 350 ms tetani initially given at 4 s intervals and stimulation continued until force was decreased to ∼40% (Westerblad & Allen, 1991). Fibres from SOD2 overexpressing mice and WT littermates were fatigued by a fixed stimulation scheme consisting of 50 tetani (300 ms duration) given at 2 s intervals. The force–frequency and [Ca2+]i–frequency relationships were again established (as described above) 30 min after the end of fatiguing stimulation. The myofibrillar Ca2+ sensitivity was assessed by measuring the [Ca2+]i required to produce 50% of the maximal tetanic force (Ca50) in force–[Ca2+]i plots produced under control conditions and after 30 min of recovery, respectively.
In some experiments, rat and mouse fibres were exposed to the reductant DTT (0.5 mm) after performing the 30 min recovery test contractions. Changes in [Ca2+]i and force were then followed by giving 30 Hz tetani at 1 min intervals for 5 min and thereafter at 5 min intervals.
SOD2 activity
Rat and WT mouse FDB muscles were homogenized with 20 mm Hepes buffer, pH 7.2, containing 210 mm mannitol, 70 mm sucrose, 1 mm EGTA and 0.05% Triton X-100 (40 μl mg−1 wet wt). The homogenates were centrifuged at 1400 g for 5 min at 4°C. The resulting supernatant was removed and stored at −80°C for later analyses. Protein concentration in the supernatant was determined using the Bradford assay (Bio-Rad), using bovine serum albumin as standard. SOD activities were measured with a kit based on the inhibition of the reduction of tetrazolium salt induced by superoxide production (Cayman Chemical Co., Ann Arbor, MI, USA, lot number 706002). SOD2 was determined by inhibition of the copper–zinc-dependent SOD (SOD1) with 1 mm sodium cyanide. One unit (U) of SOD activity was defined as the amount of enzyme required to inhibit 50% of tetrazolium salt reduction induced by superoxide production.
ROS measurements
ROS production during fatiguing stimulation (350 ms, 70 Hz tetani given at 2 s intervals) was measured in enzymatically isolated FDB fibres (Liu et al. 1997) with 5-(and 6-)chloromethyl-2′,7′-dichlorodihydrofluorescein (CM-H2DCFH), which is oxidized to a fluorescent product (CM-H2DCF) when exposed to ROS (Murrant & Reid, 2001). The isolated fibres were exposed to 10 μm of the diacetate form of CM-H2DCFH for 45 min at room temperature. After entering the cell, esterases cleave the diacetate moiety and the indicator is trapped inside the cell. We chose this indicator because it is distributed in the cytosol and although it is non-specific and detects various ROS and also reactive nitrogen species, it is not directly oxidized by superoxide (Zhu et al. 1994; Vanden Hoek et al. 1997; Murrant & Reid, 2001).
The fluorescence of CM-H2DCF was measured with a Bio-Rad MRC 1024 confocal unit attached to a Nikon Diaphot 200 inverted microscope with a ×20 objective lens (NA 0.75). The indicator was excited with 488 nm light while recording the emitted light collected through a 522 nm long-pass filter. Confocal images were obtained at rest and at regular intervals during fatigue; the stimulation was briefly (< 5 s) paused when images were acquired. After 5 min of recovery, fibres were exposed to 1 mm hydrogen peroxide and confocal images were acquired at 1 min intervals. Data are presented relative to the fluorescence immediately before the onset of fatiguing stimulation.
Western blotting
Protein extraction and Western blotting were performed as previously described (Ekstrand et al. 2004). Primary antibodies (anti-human SOD2, Biosite Inc., San Diego, CA, USA) and secondary horseradish peroxidase-conjugated antibodies were used at a dilution of 1 : 2000.
Statistical analyses
Data are presented as means ± s.e.m. Statistical difference between two groups was established with paired or unpaired Student's t test as appropriate. Two-way repeated measures ANOVA was used when comparing repeated measurements in two groups and when this showed a significant difference, the Bonferroni post hoc test was performed. P < 0.05 was regarded as statistically significant.
Results
Prolonged low-frequency force depression in rat muscle fibres
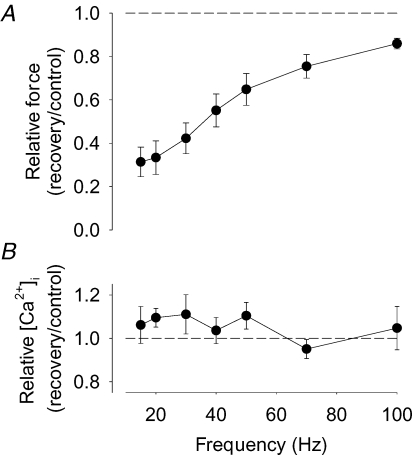
Rat FDB fibres (n = 11) were fatigued by repeated tetanic stimulation until force was decreased to 40% of the control, which required 151 ± 16 tetani. The force–frequency relationship was assessed before and 30 min after the end of fatiguing stimulation and showed a major loss of force at low stimulation frequencies (∼70% decrease at 15 Hz), whereas the force reduction was limited at higher frequencies (∼15% decrease at 100 Hz) (Fig. 1A). Tetanic [Ca2+]i, on the other hand, was not significantly decreased at any stimulation frequency (Fig. 1B), which means that the decreased force at low stimulation frequencies must be caused by changes in the force–[Ca2+]i relationship. Accordingly, Ca50 was 0.46 ± 0.03 μm under control conditions, and 30 min after the end of fatiguing stimulation it was significantly increased by 0.24 ± 0.04 μm (P < 0.001; n = 9). In addition, force at saturating [Ca2+]i was slightly decreased to 92 ± 2% of the control (P < 0.01) 30 min after fatiguing stimulation. Thus, the marked force depression at low stimulation frequencies after fatigue in rat FDB fibres was mainly due to decreased myofibrillar Ca2+ sensitivity.
Figure 1. The fatigue-induced prolonged low-frequency force depression in rat FDB fibres is due to decreased myofibrillar Ca2+ sensitivity.
Mean data (± s.e.m.) of the relative change in tetanic force (A; n = 11) and [Ca2+]i (B; n = 9). Relative changes were calculated as ratio 30 min after (recovery) to before (control) fatiguing stimulation; dashed lines indicate no change.
SOD activity and ROS production in mouse and rat muscle
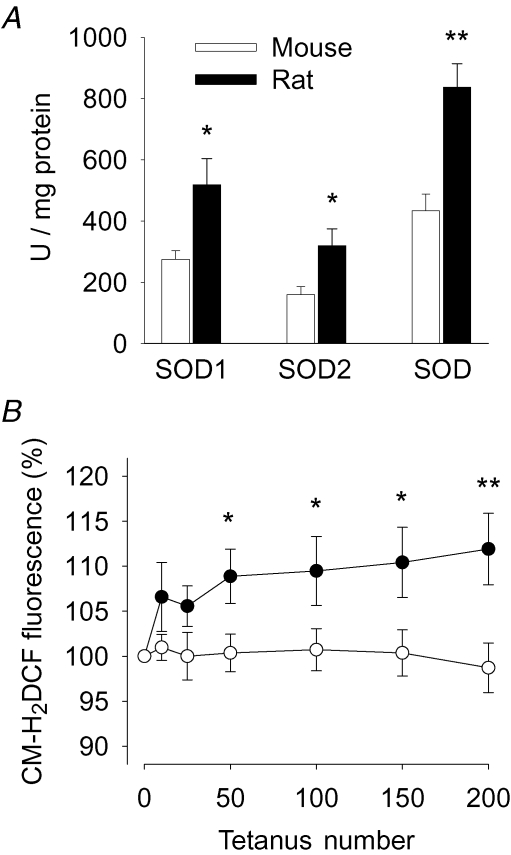
The mechanism underlying PLFFD in rat fibres (i.e. reduced myofibrillar Ca2+ sensitivity) differed from that previously observed in WT mouse fibres, where PLFFD was caused by decreased tetanic [Ca2+]i (Westerblad et al. 1993). This difference might be related to differences in ROS metabolism. Therefore, SOD activities were assayed in homogenates from WT mouse and rat FDB muscles. The results show about 50% lower activities of SOD1, SOD2, and total SOD in mouse as compared to rat muscle (Fig. 2A).
Figure 2. SOD activities and the fatigue-induced increase in myoplasmic ROS concentration are larger in rat than in mouse FDB muscle.
A, SOD1, SOD2, and total SOD activity in mouse (open bars; n = 4) and rat (filled bars; n = 5) FDB muscles. B, changes in myoplasmic ROS concentration measured with CM-H2DCF in mouse (○; n = 20) and rat (•; n = 15) FDB fibres during repeated tetanic stimulation. Data expressed relative to the fluorescence signal before the stimulation period, which was set to 100% in each cell. Values are mean (± s.e.m.). *P < 0.05 and **P < 0.01 mouse versus rat muscle.
Changes in myoplasmic ROS levels induced by repeated tetanic stimulation were assessed with the fluorescent indicator CM-H2DCF in isolated WT mouse and rat FDB fibres. The CM-H2DCF fluorescence increased during fatiguing stimulation in rat but not in mouse fibres and hence it was significantly larger in rat fibres from the 50th tetanus until the end of stimulation (Fig. 2B). Fibres were exposed to 1 mm hydrogen peroxide 5 min after the end of the stimulation period. This resulted in a prompt increase in the fluorescence signal with an amplitude that was similar in mouse and rat fibres: after 2 min it was 309 ± 43% and 382 ± 54% of the original in mouse and rat fibres, respectively (P > 0.05), and after 4 min the fluorescence signal was saturated (≥ ∼800%).
Basal properties of SOD2 overexpressing muscle fibre
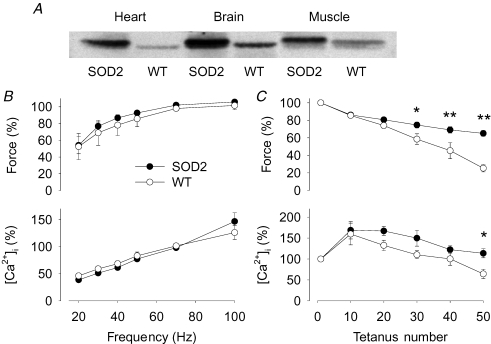
The strain of SOD2 overexpressing mice used in the present study (PAC817) showed a major increase in the expression of SOD2 in heart, brain and skeletal muscle (Fig. 3A). Under control conditions, there was no significant difference between WT and SOD2 overexpressing FDB fibres regarding the force (287 ± 22 versus 311 ± 55 kPa) and [Ca2+]i (1.04 ± 0.32 versus 1.14 ± 0.17 μm) in 70 Hz tetani (n = 4 in each group). The force–frequency and [Ca2+]i–frequency relationships were also similar in WT and SOD2 overexpressing fibres (Fig. 3B). Consequently, Ca50 was not significantly different between the two groups: 0.43 ± 0.12 μm in WT and 0.54 ± 0.09 μm in SOD2 overexpressors.
Figure 3. FDB fibres overexpressing SOD2 are more fatigue resistant than WT fibres.
A, Western blot analysis of SOD2 levels in heart, brain and skeletal muscle of SOD2 overexpressing and WT mice. B, the force–frequency (top) and [Ca2+]i–frequency (bottom) relationships in SOD2 overexpressing (•) and WT (○) FDB fibres. C, tetanic force (top) and [Ca2+]i (bottom) during fatigue induced by repeated tetanic stimulation. Data are means ± s.e.m. (n = 4) and values obtained in 70 Hz tetani produced under control conditions were set to 100% in each cell. *P < 0.05 and **P < 0.01 SOD2 overexpressing versus WT fibres.
Fatigue properties of SOD2 overexpressing fibres
Force was significantly lower in WT than in SOD2 overexpressing FDB fibres towards the end of a series of 50 fatiguing tetani (Fig. 3C). Decreased tetanic [Ca2+]i is a hallmark in fatigue induced by repeated tetani (Allen et al. 1989; Westerblad & Allen, 1991) and tetanic [Ca2+]i in fatigued WT fibres was significantly lower (64 ± 11% of the original) than in SOD2 overexpressing fibres (114 ± 11%) (Fig. 3C).
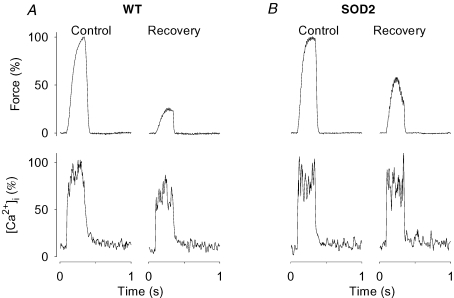
Prolonged low-frequency force depression in SOD2 overexpressing fibres
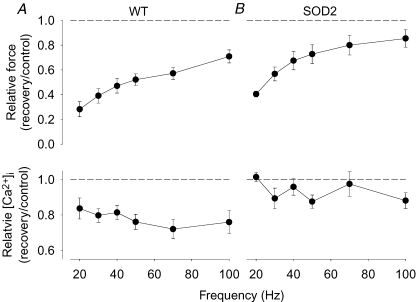
The force and [Ca2+]i relationships were assessed 30 min after fatiguing stimulation and both WT and SOD2 overexpressing fibres then showed a marked reduction in force at low stimulation frequencies (Fig. 4, upper part). Figure 5 shows original force and [Ca2+]i records from 30 Hz tetani produced either in a WT (A) or a SOD2 overexpressing (B) fibre. The decreased force after 30 min recovery was accompanied by decreased [Ca2+]i in the WT fibre, while [Ca2+]i showed little change in the SOD2 overexpressing fibre. The relatively larger decrease in force than in [Ca2+]i in the WT fibre can be explained by the fact that 30 Hz tetani lie on the steep part of the force–[Ca2+]i relationship (Allen et al. 2007). Mean [Ca2+]i data show significant (P < 0.01) decreases at all stimulation frequencies in WT fibres (Fig. 4, lower part). On the other hand, Ca50 was significantly increased in SOD2 overexpressing fibres by 0.30 ± 0.07 μm (P < 0.05), while there was only a tendency for an increase in WT fibres (increased by 0.11 ± 0.04 μm; P= 0.08). Thus, the decrease in force at low stimulation frequencies was mainly due to decreased SR Ca2+ release in WT fibres, whereas it was caused by decreased myofibrillar Ca2+ sensitivity in SOD2 overexpressing fibres.
Figure 4. Both WT and SOD2 overexpressing fibres display marked PLFFD but the underlying mechanism differs.
Mean data (± s.e.m.) of the relative change in tetanic force (top) and [Ca2+]i (bottom) obtained in mouse WT (A) and SOD2 overexpressing (B) fibres (n = 4). Relative changes were calculated as ratio 30 min after (recovery) to before (control) fatiguing stimulation; dashed lines indicate no change.
Figure 5. PLFFD is associated with decreased tetanic [Ca2+]i in WT but not in SOD2 overexpressing fibres.
Typical original records of force (top) and [Ca2+]i (bottom) obtained in 30 Hz contractions produced in a WT (A) and a SOD2 overexpressing (B) fibre. Contractions were produced before (Control) and 30 min after (Recovery) fatiguing stimulation. Tetanic force and [Ca2+]i in control are set to 100%.
The effect of dithiothreitol and N-acetylcysteine on the prolonged low-frequency force depression
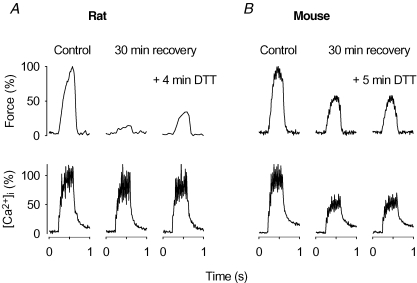
Rat fibres displaying PLFFD were exposed to the reducing agent DTT (0.5 mm) while following changes in force and [Ca2+]i in 30 Hz tetani. Application of DTT resulted in a prompt increase in force production in all fibres (n = 5), which reached a maximum during the first 5 min of exposure. Original records from one of these experiments are shown in Fig. 6A and mean data showed that DTT exposure caused a significant (P < 0.05) increase in the force at 30 Hz from 37 ± 9% to 46 ± 9% of the 30 Hz force before fatiguing stimulation. On the other hand, DTT had no effect on [Ca2+]i during the 30 Hz contractions, which was 115 ± 8% and 110 ± 7% of the control before and after DTT application.
Figure 6. PLFFD can be partially reversed by the reductant DTT in rat but not in mouse FDB fibres.
Representative original records of force (top) and [Ca2+]i (bottom) obtained in 30 Hz contractions produced in a rat (A) and a mouse (B) fibre. Contractions were produced before (Control) and after the end of fatiguing stimulation at the indicated times in the absence and presence of 0.5 mm DTT. Tetanic force and [Ca2+]i in control are set to 100%.
We also followed the effect of DTT on 30 Hz force and [Ca2+]i in mouse fibres showing PLFFD and records from one representative experiment are shown in Fig. 6B. Mean data (n = 4) showed no significant effect of DTT on either the force (47 ± 15 versus 50 ± 16%) or [Ca2+]i (69 ± 9 versus 67 ± 9%). Thus, DTT partially reversed the fatigue-induced prolonged decrease in myofibrillar Ca2+ sensitivity in rat fibres, but had no significant effect on the decreased SR Ca2+ release in mouse fibres.
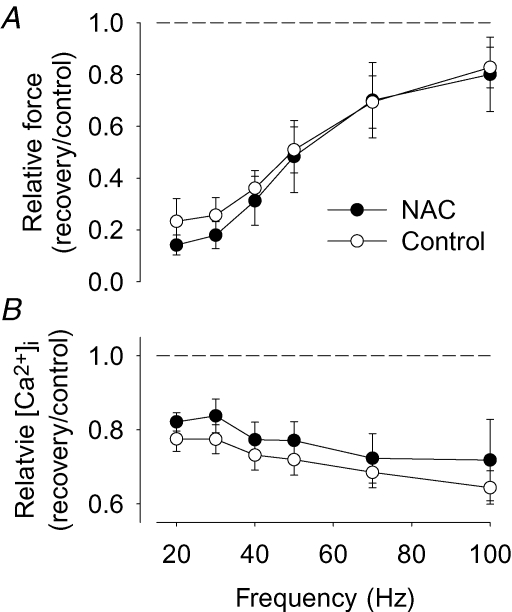
In a final set of experiments, we studied whether exposure to an antioxidant during fatiguing stimulation might prevent the prolonged depression of SR Ca2+ release in WT mouse fibres. The non-specific antioxidant NAC (0.5 mm) was applied 2 min before the start of fatiguing stimulation and removed immediately after the end of stimulation. The presence of NAC had no significant effect on the number of tetani required to decrease force to 40% (62 ± 9 tetani in control (n = 7) and 71 ± 11 tetani in the presence of NAC (n = 4)). A marked PLFFD accompanied by a significant reduction of tetanic [Ca2+]i at all stimulation frequencies was present 30 min after fatiguing stimulation both in the presence and absence of NAC (Fig. 7).
Figure 7. The antioxidant NAC had no effect on PLFFD in mouse FDB fibres.
Mean data (± s.e.m.) of the relative change in tetanic force (A) and [Ca2+]i (B) obtained in mouse FDB fibres fatigued in the presence of 0.5 mm NAC (n = 4; •) and in control (n = 7; ○). Relative changes were calculated as ratio 30 min after (recovery) to before (control) fatiguing stimulation; dashed lines indicate no change.
While it is clear that tetanic [Ca2+]i is decreased during PLFFD in WT mouse fibres, the controls for the SOD2 overexpressing fibres showed a tendency of decreased myofibrillar Ca2+ sensitivity (a mean increase of Ca50 of ∼0.1 μm). We therefore also measured changes in Ca50 in the WT mouse fibres used in the DTT experiments (before adding DTT) and in control fibres used in the NAC experiments. Ca50 was then found to be increased after fatigue by 0.14 ± 0.03 μm (P < 0.01; n = 11). Thus, WT mouse fibres showed both a decrease in tetanic [Ca2+]i and a slight decrease in myofibrillar Ca2+ sensitivity.
Discussion
We studied the involvement of ROS in the long-lasting, fatigue-induced force depression at low stimulation frequencies (PLFFD). The major novel finding is that (i) PLFFD in WT rat fibres and SOD2 overexpressing mouse fibres was due to reduced myofibrillar Ca2+ sensitivity rather than decreased tetanic [Ca2+]i, which was the predominant cause in WT mouse fibres, and (ii) these different mechanisms of PLFFD appear to be due to differences in SOD activity.
A previous study of PLFFD in FDB fibres from WT mice showed a reduced SR Ca2+ release, leading to decreased tetanic [Ca2+]i, that could explain the decrease in force (Westerblad et al. 1993), which agrees with the present finding in WT mouse fibres. Rat FDB fibres also showed a marked PLFFD but this was mainly caused by reduced myofibrillar Ca2+ sensitivity rather than decreased tetanic [Ca2+]i. We hypothesized that this difference between rat and mouse fibres was due to a higher SOD activity, and hence more effective conversion of superoxide to hydrogen peroxide, in rat fibres. This hypothesis was based on the finding that myofibrillar Ca2+ sensitivity is affected by exposure to very low concentrations of hydrogen peroxide (Andrade et al. 2001). In accordance with the hypothesis, we observed a markedly higher SOD activity in rat than in WT mouse fibres. Moreover, only rat fibres showed an increased CM-H2DCF fluorescence during fatiguing stimulation (see Fig. 2B). This is consistent with a higher SOD activity in rat than in WT mouse fibres because this ROS indicator is distributed in the cytosol and is insensitive to superoxide (Zhu et al. 1994; Vanden Hoek et al. 1997; Murrant & Reid, 2001). We also studied muscle fibres from SOD2 overexpressing mice and the PLFFD observed in these was caused by decreased myofibrillar Ca2+ sensitivity, which again is in accordance with our hypothesis.
The present results show an association between a fatigue-induced prolonged decrease in SR Ca2+ release and a lower SOD activity and hence the reduced SR Ca2+ release may be related to increased superoxide levels. The precise mechanism(s) involved remains to be established but the long lasting nature of the phenomenon indicates some protein modification. It should be noted that action potential activated SR Ca2+ release is a delicate process involving a complex of many proteins that can be differentially affected by ROS, calpains, nitric oxide and its derivatives, changes in energy metabolites, etc.
Prolonged elevation of [Ca2+]i has been suggested as a causative factor underlying the fatigue-induced prolonged decrease in SR Ca2+ release (Chin & Allen, 1996; Chin et al. 1997; Westerblad et al. 2000), possibly via activation of Ca2+-dependent proteases known as calpains (Murphy et al. 2006). However, when cross-bridge force production in mouse FDB fibres was inhibited by BTS, the number of fatiguing tetani required to induce fatigue was increased more than two-fold and this increased Ca2+ exposure had no effect on SR Ca2+ release during recovery (Bruton et al. 2006). Moreover, the present results show that compared to WT fibres, SOD2 overexpressing fibres were more fatigue resistant and hence exposed to higher [Ca2+]i during fatiguing stimulation and still they showed no or little decrease in tetanic [Ca2+]i after 30 min of recovery. Thus, it appears that increased [Ca2+]i on its own is not responsible for the fatigue-induced prolonged inhibition of SR Ca2+ release.
Mitochondria can produce superoxide and the increased mitochondrial respiration during repetitive contractions of skeletal muscle may be accompanied by increased ROS production (Smith & Reid, 2006). Interestingly, mitochondria are mainly located close to the Z-disc in mouse FDB fibres (Lännergren et al. 1999), i.e. close to the sites of SR Ca2+ release but at some distance from the myosin filaments. Furthermore, it has recently been shown that superoxide can be produced by NADPH oxidase in a Ca2+-dependent manner. Activation of this enzyme, which is preferentially localized to the transverse tubular membrane, affects the SR Ca2+ release channels (Hidalgo et al. 2006; Espinosa et al. 2006).
The superoxide anion as such is unlikely to have widespread effects in muscle cells since it cannot easily diffuse through membranes, although it may pass through voltage-dependent anion channels in the mitochondrial membrane (Han et al. 2003). Superoxide is rapidly converted to hydrogen peroxide by SOD and non-enzymatic processes. It can also be converted to hydroxyl radicals or interact with nitric oxide to form peroxynitrite; both these highly reactive radicals undergo electron transfer reactions with almost any molecule at a diffusion limited rate (Murrant & Reid, 2001). Thus, superoxide is produced close to the SR Ca2+ release sites in a contraction-dependent manner and a prolonged exposure to increased superoxide might directly or indirectly (e.g. via hydroxyl radicals or peroxynitrite) impair SR Ca2+ release. These effects would then be limited in SOD2 overexpressing mouse fibres and rat fibres, which display a higher SOD activity than WT mouse fibres and therefore the superoxide accumulation would be limited by an effective conversion to hydrogen peroxide. It should be noted that SOD2 overexpressing fibres were more fatigue resistant than their WT control fibres (see Fig. 3C). Therefore, we cannot exclude the possibility that SOD2 overexpressing fibres would also show the prolonged inhibition of SR Ca2+ release if they were more extensively fatigued in order to obtain the same force decrease as that induced in their WT control fibres.
In contrast to superoxide, hydrogen peroxide can readily pass through membranes and hence exert widespread effects in cells. Experiments on intact mouse FDB fibres showed that exogenous hydrogen peroxide has time- and concentration-dependent effects preferentially on myofibrillar Ca2+ sensitivity (Andrade et al. 1998, 2001). Recent studies on mouse FDB fibres performed at high temperature (37°C) showed a contraction-induced decrease in myofibrillar Ca2+ sensitivity that could be prevented by antioxidants and reversed by reducing agents (Moopanar & Allen, 2005, 2006). Moreover, hydrogen peroxide exposure of skinned rat muscle fibres had little effect on action potential-mediated SR Ca2+ release, whereas the myofibrillar Ca2+ sensitivity was substantially affected in a glutathione-dependent way (Posterino et al. 2003; Lamb & Posterino, 2003). Thus, the force depression in SOD2 overexpressing mouse fibres and rat fibres, which was mainly due to decreased myofibrillar Ca2+ sensitivity, can be explained by a greater capacity of these fibres to convert superoxide to hydrogen peroxide during fatiguing stimulation. In accordance, myoplasmic ROS levels, as detected by CM-H2DCF, increased during fatigue in rat but not in WT mouse fibres.
ROS effects on fatigue-induced changes in muscle function are generally larger at high temperatures (Diaz et al. 1994; Moopanar & Allen, 2005). The present study was performed at ∼24°C, which is lower than the ambient temperature of mouse FDB muscles (∼30°C) (Bruton et al. 1998). The reason for using this temperature is that force and [Ca2+]i are followed over a long time when recovery processes are studied. Therefore the function of the muscle preparation has to be stable over time and the force production of single FDB fibres show a marked decrease over time even without fatiguing stimulation at high temperature (Lännergren & Westerblad, 1987; Moopanar & Allen, 2005). Using the lower temperature, we show clear differences in the recovery process that are associated with differences in ROS metabolism. Furthermore, we have previously shown ROS-dependent effects on the contraction-mediated glucose uptake in mouse skeletal muscles studied at 25°C (Sandström et al. 2006). Thus, ROS affects muscle function even at temperatures below the in vivo temperature, but larger or additional effects at higher temperatures cannot be excluded.
Decreased force production and deleterious ROS effects on muscle function have been associated with numerous muscle disorders (Supinski et al. 1999; Polkey & Moxham, 2001; Supinski & Callahan, 2005; Keeton & Binder-Macleod, 2006; Smith & Reid, 2006). In this context, the present findings may have clinical implications. ROS-mediated impairments in myofibrillar function can be prevented or limited by antioxidants and reversed by reducing agents, whereas changes in SR Ca2+ handling are more robust (Andrade et al. 1998, 2001; Mishima et al. 2005; Moopanar & Allen, 2005, 2006). In line with this, Fig. 6 shows that application of the reductant DTT partially reversed the decreased myofibrillar Ca2+ sensitivity and hence increased force production in rat fibres, whereas it had no effect on the decreased SR Ca2+ release in mouse fibres. Moreover, the addition of the non-specific antioxidant NAC did not prevent the prolonged fatigue-induced impairment of SR Ca2+ release in mouse fibres (see Fig. 7), despite the fact that NAC has been shown to decrease the myoplasmic ROS concentration and the oxidation of glutathione during 10 min of intense repeated tetanic stimulation in mouse muscle (Sandström et al. 2006). Thus, in the clinical setting a prolonged force depression caused by decreased myofibrillar Ca2+ sensitivity would be sensitive to antioxidant treatment, while SR impairments would be more therapy resistant.
In conclusion, we show that fatigue-induced prolonged low-frequency force depression can be caused by two ROS-dependent mechanisms: decreased SR Ca2+ release associated with low SOD activity and reduced myofibrillar Ca2+ sensitivity associated with high SOD activity.
Acknowledgments
This study was supported by the Muscle Dystrophy Association, the United Mitochondrial Disease Foundation, the Swedish Research Council, the Swedish Foundation for Strategic Research (Functional Genomics and INGVAR), the Swedish National Center for Sports Research, and Funds of Karolinska Institutet. N.P. is supported by the Swedish Institute.
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue : cellular mechanisms. Physiol Rev. 2007 doi: 10.1152/physrev.00015.2007. in press. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol. 1998;85:478–483. doi: 10.1152/jappl.1998.85.2.478. [DOI] [PubMed] [Google Scholar]
- Bruton J, Pinniger GJ, Lännergren J, Westerblad H. The effects of the myosin-II inhibitor N-benzyl-p-toluene sulphonamide on fatigue in mouse single intact toe muscle fibres. Acta Physiol (Oxf) 2006;186:59–66. doi: 10.1111/j.1748-1716.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol. 1996;491:813–824. doi: 10.1113/jphysiol.1996.sp021259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Balnave CD, Allen DG. Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. Am J Physiol Cell Physiol. 1997;272:C550–C559. doi: 10.1152/ajpcell.1997.272.2.C550. [DOI] [PubMed] [Google Scholar]
- Diaz PT, Brownstein E, Clanton TL. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol. 1994;77:2434–2439. doi: 10.1152/jappl.1994.77.5.2434. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- Espinosa A, Leiva A, Pena M, Muller M, Debandi A, Hidalgo C, Carrasco MA, Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- Favero TG. Sarcoplasmic reticulum Ca2+ release and muscle fatigue. J Appl Physiol. 1999;87:471–483. doi: 10.1152/jappl.1999.87.2.471. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J Biol Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- Jones DA. High- and low-frequency fatigue revisited. Acta Physiol Scand. 1996;156:265–270. doi: 10.1046/j.1365-201X.1996.192000.x. [DOI] [PubMed] [Google Scholar]
- Keeton RB, Binder-Macleod SA. Low-frequency fatigue. Phys Ther. 2006;86:1146–1150. [PubMed] [Google Scholar]
- Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued single muscle fibres from frog and mouse. J Muscle Res Cell Motil. 1999;20:19–32. doi: 10.1023/a:1005412216794. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kranias EG, Schneider MF. Regulation of Ca2+ handling by phosphorylation status in mouse fast- and slow-twitch skeletal muscle fibers. Am J Physiol Cell Physiol. 1997;273:C1915–C1924. doi: 10.1152/ajpcell.1997.273.6.C1915. [DOI] [PubMed] [Google Scholar]
- Lunde PK, Dahlstedt AJ, Bruton JD, Lännergren J, Thorén P, Sejersted OM, Westerblad H. Contraction and intracellular Ca2+ handling in isolated skeletal muscle of rats with congestive heart failure. Circ Res. 2001;88:1299–1305. doi: 10.1161/hh1201.092041. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. Isolated single motor units in human muscle and their rate of discharge during maximal voluntary effort. J Physiol. 1971;217:12P–13P. [PubMed] [Google Scholar]
- Mishima T, Yamada T, Matsunaga S, Wada M. N-acetylcysteine fails to modulate the in vitro function of sarcoplasmic reticulum of diaphragm in the final phase of fatigue. Acta Physiol Scand. 2005;184:195–202. doi: 10.1111/j.1365-201X.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of mu-calpain in rat skeletal muscle. J Physiol. 2006;576:595–612. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrant CL, Reid MB. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech. 2001;55:236–248. doi: 10.1002/jemt.1173. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Moxham J. Clinical aspects of respiratory muscle dysfunction in the critically ill. Chest. 2001;119:926–939. doi: 10.1378/chest.119.3.926. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Cellini MA, Lamb GD. Effects of oxidation and cytosolic redox conditions on excitation–contraction coupling in rat skeletal muscle. J Physiol. 2003;547:807–823. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006;575:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Shabalina IG, Dufour E, Petrovic N, Backlund EC, Hultenby K, Wibom R, Nedergaard J, Cannon B, Larsson NG. SOD2 overexpression: enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J. 2005;24:4061–4070. doi: 10.1038/sj.emboj.7600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Diaphragmatic free radical generation increases in an animal model of heart failure. J Appl Physiol. 2005;99:1078–1084. doi: 10.1152/japplphysiol.01145.2004. [DOI] [PubMed] [Google Scholar]
- Supinski G, Stofan D, Callahan LA, Nethery D, Nosek TM, DiMarco A. Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol. 1999;87:783–791. doi: 10.1152/jappl.1999.87.2.783. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Allen DG, Lännergren J. Functional significance of Ca2+ in long-lasting fatigue of skeletal muscle. Eur J Appl Physiol. 2000;83:166–174. doi: 10.1007/s004210000275. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Zhu H, Bannenberg GL, Moldeus P, Shertzer HG. Oxidation pathways for the intracellular probe 2′,7′-dichlorofluorescein. Arch Toxicol. 1994;68:582–587. doi: 10.1007/s002040050118. [DOI] [PubMed] [Google Scholar]