Abstract
Striatum, the main input nucleus of basal ganglia, is involved in the learning of cognitive and motor sequences in response to environmental stimuli. Striatal output neurons (medium spiny neurons, MSNs) integrate cortical activity and the two main classes of interneurons (GABAergic and cholinergic interneurons) tightly regulate the corticostriatal information transfer. We have explored the transmission between cortex and striatal interneurons and their capability to develop activity-dependent long-term plasticity based on the quasi-coincident cortical and striatal activities (spike-timing-dependent plasticity, STDP). We have observed glutamatergic monosynaptic connections between cortical cells and both striatal interneurons. Excitatory postsynaptic current latencies and rise times revealed that a cortical stimulation activates GABAergic interneurons before cholinergic, and both interneurons before MSNs. In addition, we have observed that striatal interneurons are able to develop bidirectional long-term plasticity and that there is a cell-specificity of STDP among striatal interneurons. Indeed, in GABAergic interneurons, long-term depression (LTD) and long-term potentiation (LTP) are induced by post-pre and pre-post STDP protocols, respectively. Cholinergic interneurons displayed a partially reversed STDP when compared to GABAergic interneurons: post-pre protocols induced LTP as well as LTD (the induction of either LTP or LTD is correlated with rheobase) and pre-post protocols induced LTD. The cell-specificity of STDP also concerned the receptors activated for the induction of LTP and LTD in GABAergic and cholinergic interneurons: in GABAergic interneurons LTP and LTD required NMDA receptor-activation whereas, in cholinergic interneurons, LTP was underlain by NMDA receptor-activation and LTD by metabotropic glutamate receptors.
Basal ganglia are involved in the learning of cognitive and motor sequences related to environmental stimuli (Graybiel et al. 1994; Kimura, 1995; Hollerman et al. 2000; Schultz et al. 2003; Graybiel, 2005; Yin & Knowlton, 2006). Striatum, the main input nucleus of basal ganglia, is considered to play a major role in this process. Indeed, the striatum receives glutamatergic inputs from the whole cortex. In turn, it relays the integrated cortical information toward the basal ganglia output nuclei through which it operates a selected activation of behavioural effectors. As learning and memory are believed to be underlain by long-term synaptic efficacy changes (Bliss & Collingridge, 1993; Martin et al. 2000; Martin & Morris, 2002; Lynch, 2004; Malenka & Bear, 2004), the corticostriatal long-term plasticity provides a basic mechanism for the function of basal ganglia in procedural learning and memory.
The striatum comprises two main neuronal populations: the GABAergic output neurons (medium spiny neurons, MSNs) and the interneurons, composed mainly of GABAergic and cholinergic cells. Regarding striatal GABAergic interneurons, three classes have been described: (1) the parvalbumin-positive cells (also named fast-spiking interneurons according to electrophysiological criteria; Kawaguchi, 1993), (2) the calretinin-positive cells (Figueredo-Cardenas et al. 1996; from which electrophysiological features remain to be determined, Tepper & Bolam, 2004), and (3) the NO-synthase-positive cells (also named persistent and low-threshold spiking cells, PLTS, according to electrophysiological criteria; Kawaguchi, 1993).
MSNs are thought to act as coincidence detectors of cortical activity; they require a strong and synchronized cortical activity to discharge (Calabresi et al. 1987; Nisenbaum & Wilson, 1995; Wilson, 1995). MSN excitability is further regulated by the striatal interneurons, which consequently exert a critical role in cortico-basal ganglia information processing. Fast-spiking GABAergic interneurons exert a powerful inhibitory weight on MSNs (Kita, 1996; Plenz & Kitai, 1998; Koos & Tepper, 1999). Because they also receive cortical inputs (Bennett & Bolam, 1994; Ramanathan et al. 2002; Mallet et al. 2005), they are considered to provide a feed-forward mechanism increasing the selectivity of MSN responsiveness to cortical inputs. Cholinergic interneurons also receive cortical inputs (Thomas et al. 2000; Reynolds & Wickens, 2004) and regulate the excitability of MSNs. These cells, which fire tonically in vivo provide a synchronized signal throughout the striatal network in response to the occurrence of sensory cues predictive of reward (Aosaki et al. 1994; Kimura et al. 2003; Morris et al. 2004; Apicella, 2007).
A growing body of evidence indicates that striatal interneurons control striatal microcircuits as well as corticostriatal information processing. Nevertheless, most of the studies on corticostriatal synaptic transmission have focused on MSNs. Consequently, the integration of cortical information by interneurons and the subsequent long-term activity-dependent synaptic plasticity they could develop remain poorly documented.
The temporal relationship between activity in presynaptic and postsynaptic elements is now recognized as a critical factor that can rule the induction of activity-dependent long-term synaptic plasticity, a process named spike-timing-dependent plasticity (STDP). In various mammalian brain structures, long-term potentiation (LTP) was induced when postsynaptic activation followed presynaptic activation, and long-term depression (LTD) when postsynaptic activation preceded presynaptic activation (Abbott & Nelson, 2000; Bi & Poo, 2001; Sjöström & Nelson, 2002; Dan & Poo, 2004, 2006). Interestingly, we showed that corticostriatal synapses at the level of MSNs constitute an exception to this rule in mammals since these cells displayed STDP with a reversed orientation (Fino et al. 2005).
In the present study, we investigated the transmission and the activity-dependent long-term plasticity in striatal fast-spiking GABAergic and cholinergic interneurons. We have observed glutamatergic monosynaptic connections between cortical cells and both striatal interneurons. We determined a sequence of activation of striatal neurons following a cortical activation: both striatal interneurons were activated before MSNs. We report here a cell-specificity of the striatal STDP. Indeed, GABAergic interneurons displayed a strictly orientated STDP: post-pre protocols induced LTD and pre-post protocols led to LTP and both forms of plasticity required NMDA receptor-activation. Cholinergic interneurons displayed a partially reversed STDP: post-pre protocols induced LTP and LTD (depending on the excitability level of the cells) and pre-post protocols induced LTD. LTP depended on the activation of NMDA receptors whereas LTD was underlain by metabotropic glutamate receptor-activation.
Methods
Electrophysiological recordings
Whole-cell recordings were performed on horizontal brain slices (330 μm) from Sprague–Dawley rats (postnatal days 15–18). Such slices preserved the corticostriatal projections from the pyramidal cells of the somatosensory cortical area to their terminal sites in the adjacent dorsal striatum (Fino et al. 2005). Animals were killed by decapitation and brains were immediately removed. All experiments were performed in accordance with local animal welfare committee (Institute of Biology, College de France) and EU guidelines (directive 86/609/EEC). Patch-clamp recordings were made as previously described (Venance et al. 2004; Fino et al. 2005). Briefly, borosilicate glass pipettes (11–15 MΩ) contained (mm): 105 potassium gluconate, 30 KCl, 10 Hepes, 0.3 EGTA (adjusted to pH 7.35 with KOH). The composition of the extracellular solution was (mm): 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, and 10 μm pyruvic acid bubbled with 95% O2 and 5% CO2. All recordings were performed at 32°C using a temperature control system (Bioptechs ΔTC3, Butler, PA, USA) and slices were continuously superfused at 2–3 ml min−1 with the extracellular solution. Individual neurons were identified using infrared-differential interference contrast microscopy with CCD camera (Hamamatsu C2400-07; Hamamatsu, Japan). Signals were amplified using an EPC9-2 amplifier (HEKA Elektronik, Lambrecht, Germany). The range of access resistance was 80–120 MΩ. The liquid junction potential was calculated (−13.6 mV) and corrected. Current-clamp recordings were filtered at 2.5 kHz and sampled at 5 kHz and voltage-clamp recordings were filtered at 5 kHz and sampled at 10 kHz using the program Pulse-8.53 (HEKA Elektronik). Off-line analysis was performed using Igor software (Wavemetrics, Lake Oswego, OR, USA). All control electrophysiological recordings were realized in perforated patch configuration at 32°C and without any pharmacological treatments or ionic modifications to preserve the local striatal microcircuits involved in corticostriatal transmission. For pharmacological experiments, all chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, Illkirch, France) except 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), dl-2-amino-5-phosphonopentanoic acid (AP5) and LY341495 (Tocris, Ellisville, MO, USA).
Perforated patch-clamp experiments
Amphotericin B (Sigma-Aldrich) was used to perform perforated patch-clamp experiments. The concentration of amphotericin B in the patch-clamp pipette solution was 200 μg ml−1. The perforated patch prevents dialysis of intracellular content and therefore avoids the loss of intracellular molecules that could be involved in long-term plasticity occurrence and maintenance.
Stimulation protocols
Electrical stimulations of the cerebral cortex were performed with a bipolar electrode (Phymep, Paris, France) placed in layer V of the somatosensory cortex (mean distance from corpus callosum 586 ± 10 μm, n = 110) (Fig. 1A). The recording sites in the dorsal striatum were at a distance of around 1.5 mm away from the cortical stimulation site. Electrical stimulations were monophasic and at constant current (Stimulator WPI, Stevenage, UK), without detectable polarization of electrodes with time. Stimulation intensity was 329.4 ± 13.4 μA on average. There was no significant difference in the current intensity of cortical stimulations between each stimulation protocol group (post-pre and pre-post STDP protocols) and between GABAergic and cholinergic interneurons. This indicates that the orientation of induced synaptic plasticities (LTP versus LTD) was not related to the stimulation intensity. Currents were adjusted in order to evoke striatal excitatory postsynaptic currents (EPSCs) ranging from 50 to 200 pA amplitudes. Repetitive stimuli were applied at a frequency of 0.1 Hz, a frequency for which no short- or long-term changes in EPSC amplitudes were induced (Fino et al. 2005).
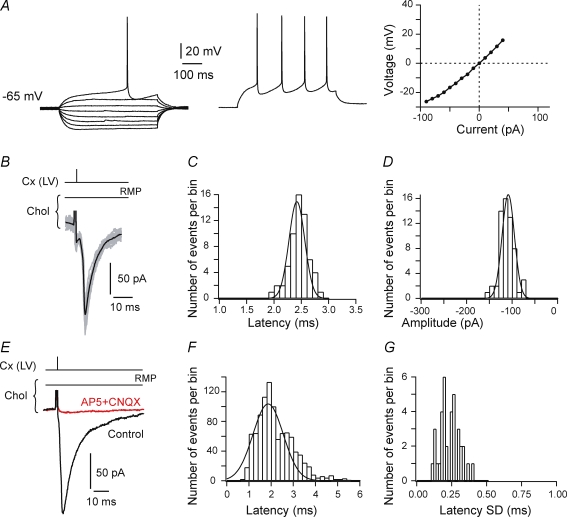
Figure 1. Corticostriatal transmission in striatal fast-spiking GABAergic interneurons.
A, schematic representation of the horizontal corticostriatal brain slice preparation. The stimulating electrode was placed in layer V of the somatosensory cortex (mean distance from the cortical border of corpus callosum 586 ± 10 μm, n = 110) and the recording electrode in the adjacent striatum at a mean distance from the striatal border of corpus callosum of 543 ± 34 μm. B, characteristic membrane properties and spiking pattern of a fast-spiking GABAergic interneuron. Raw traces show individual voltage responses to a series of 500 ms current pulses from −90 pA to +130 pA with 20 pA increasing current steps (left side) and to +30 pA above AP threshold (middle panel, spike frequency = 40 Hz). Note the linear I–V relationship (right panel). C, fast-spiking GABAergic interneuron EPSCs evoked by cortical layer V (Cx (LV)) stimulations. Black line represents the average of 20 consecutive EPSC raw traces (grey lines). A low variability of cortically evoked EPSC amplitudes should be noted. D, latency distribution of 60 EPSCs recorded in a single GABAergic interneuron (well fitted by a Gaussian curve). Note the short latency centred on 1.7 ms. E, amplitude distribution of 60 EPSCs recorded in the same GABAergic interneuron shown in D (distribution well fitted by a Gaussian curve centred around −121 pA). F, cortically evoked EPSCs on fast-spiking GABAergic interneurons were glutamatergic since they were totally inhibited by AP5 (50 μm) and CNQX (20 μm) treatments. This is illustrated by a representative experiment showing averages of 15 traces recorded in control conditions (‘Control’) and after AP5 and CNQX treatment (‘AP5+CNQX’). G, latency distribution of EPSCs was fitted with one Gaussian function centred on 1.67 ± 0.03 ms (n = 784 EPSCs from 28 GABAergic interneurons). H, SD distribution of averaged EPSC latencies recorded in 28 GABAergic interneurons indicates a monosynaptic transmission between cortex and fast-spiking GABAergic interneurons (mean value of latency SD was 0.23 ± 0.02 ms).
STDP experiments consisted of time shifting the presynaptic stimulation with a postsynaptic action potential (AP) evoked by the direct application of a depolarizing current step. Cortical stimulations and striatal evoked APs were delivered 100 times at 1 Hz. The time interval between pre- and postsynaptic stimulations (Δt) was measured from the stimulation artifact (produced by cortical stimulation and recorded at the level of the striatum) to the peak of the striatally evoked AP. Neurons were recorded for 10 min in control and for at least 1 h after the cellular conditioning protocol. Input resistances and injected currents were monitored throughout the experiments and variations of these parameters in excess of 20% led to the rejection of the experiment. To investigate STDP pharmacology, we first recorded 10 min of control baseline, then drugs were applied in the bath 10 min before STDP protocol and remained present continuously until the end of the recording (at least 1 h after the cellular conditioning protocol).
Data analysis
All results were expressed as mean ± s.e.m. and statistical significance was assessed using Student's t test, the non-parametric Mann–Whitney test when appropriate, or the Pearson test for correlations at the significance level (P) indicated. Neuronal input resistance was calculated from the voltage responses obtained after injecting a hyperpolarizing current (−10 pA, 100 ms duration). The amplitude of APs was estimated as the potential difference between their voltage threshold (the abrupt increase in slope depolarization) and the peak of the spike waveform. Instantaneous firing frequency was calculated as the reciprocal of the interspike interval. The spike frequency adaptation (SFA) (i.e. a gradual reduction of the firing frequency) was calculated using the formula: adaptation ratio =Ffinal/Finitial, where Finitial is the initial spike frequency (1/first interspike) and Ffinal is the average frequency calculated from the last two interspike intervals. To determine the long-term synaptic efficacy changes, EPSC mean amplitudes, measured 60 min after the induction protocol, were the average of 25 evoked EPSCs (each of the 25 EPSCs was normalized to the mean of the EPSC amplitudes recorded before the induction protocol). Synaptic efficacy changes were classified as either LTP or LTD when the mean of normalized EPSC amplitudes was significantly different from the control baseline (see online Supplemental material, Fig. 1). Histograms of the long-term synaptic efficacy changes induced in striatal interneurons by STDP protocols represent the distributions of normalized EPSC amplitude changes and 25 normalized EPSCs were plotted for each cell.
Plasticity loci were determined by the mean variance analysis method (Clements & Silver, 2000). Briefly, EPSC coefficients of variation (CVs) were calculated by the ratio of the standard deviation and the mean EPSC amplitude. The plasticity loci were deduced from the relationship between the normalized CV−2 ((CV−2 after induction of plasticity)/(CV−2 control)) and the normalized EPSC amplitudes ((EPSC mean amplitude after induction of plasticity)/(control EPSC mean amplitude)). EPSC amplitude is proportional to npq with n being the number of releasing sites, p the probability of release and q the quantum size. It is admitted that n and p describe presynaptic events and q is a postsynaptic indicator. If normalized CV−2 > normalized EPSC amplitude, both n and p can vary, and if normalized CV−2= normalized EPSC amplitude, only n can vary. In both cases, changes in EPSC amplitude reflect mainly presynaptic modifications. If normalized CV−2 < normalized EPSC amplitude, changes in EPSC amplitude are related to variations of n, p and q, indicating a mixed (pre- and postsynaptic) origin of the modifications. Finally, a variation of normalized EPSC amplitude without any variation of normalized CV−2 indicates postsynaptic modifications since only q can vary.
Results
Electrophysiological properties of striatal interneurons
Striatum, besides MSNs, is composed of two main types of interneurons, GABAergic and cholinergic. These different types of striatal neurons were easily distinguished based on their electrophysiological characteristics (Kawaguchi, 1993; Kawaguchi et al. 1995; Fino et al. 2007) (Table 1; Figs 1B and 2A). Numerous passive and active membrane properties allowed us to identify GABAergic interneurons, cholinergic interneurons or MSNs (for details see Table 1). In particular, resting membrane potential (RMP), AP duration, after-hyperpolarization (AHP) duration and frequency discharge constitute parameters which are significantly different among these three striatal neuronal populations (Table 1).
Table 1.
Membrane properties of striatal fast-spiking GABAergic interneurons, cholinergic interneurons and medium spiny neurons (MSNs)
| Fast-spiking GABAergic interneurons (n = 45), a | Cholinergic interneurons (n = 65), b | MSNs (n = 50), c | a vs. b | a vs. c | b vs. c | |
|---|---|---|---|---|---|---|
| Passive membrane properties | ||||||
| RMP (mV) | −72.8 ± 0.7 | −61.9 ± 0.4 | −75.3 ± 0.9 | < 0.001 | < 0.05 | < 0.001 |
| Ri (MΩ) | 209.7 ± 7.4 | 307.3 ± 16.9 | 225.7 ± 12.9 | < 0.001 | n.s. | < 0.001 |
| Action potential properties | ||||||
| AP threshold (mV) | −30.2 ± 1.0 | −41.6 ± 0.9 | −37.4 ± 0.5 | < 0.001 | < 0.001 | < 0.001 |
| Rheobase (pA) | 161.9 ± 9.0 | 40.1 ± 3.1 | 163.6 ± 8.8 | < 0.001 | n.s. | < 0.001 |
| Delay to first spike (ms) | 248.5 ± 16.0 | 281.0 ± 13.2 | 414.6 ± 4.3 | n.s. | < 0.001 | < 0.001 |
| AP amplitude (mV) | 46.3 ± 1.0 | 61.3 ± 1.0 | 68.5 ± 1.3 | < 0.001 | < 0.001 | < 0.001 |
| AP duration (ms) | 1.4 ± 0.1 | 5.2 ± 0.2 | 3.3 ± 0.2 | < 0.001 | < 0.001 | < 0.001 |
| fAHP peak amplitude (mV) | 12.8 ± 0.4 | 12.0 ± 0.3 | 8.6 ± 0.3 | n.s. | < 0.001 | < 0.001 |
| fAHP time to peak (ms) | 1.84 ± 0.15 | 25.7 ± 1.5 | 16.1 ± 0.6 | < 0.001 | < 0.001 | < 0.001 |
| sAHP duration (ms) | 17.9 ± 0.8 | 64.6 ± 2.9 | 38.3 ± 1.3 | < 0.001 | < 0.001 | < 0.001 |
| AP rise/AP decay | 1.43 ± 0.13 | 4.22 ± 0.31 | 2.63 ± 0.13 | < 0.001 | < 0.001 | < 0.001 |
| Action potential trains | ||||||
| Initial firing rate (Hz) | 34.4 ± 2.0 | 9.4 ± 0.5 | 12.5 ± 0.4 | < 0.001 | < 0.001 | < 0.001 |
| Final firing rate (Hz) | 22.2 ± 1.7 | 8.0 ± 0.4 | 13.0 ± 0.4 | < 0.001 | < 0.001 | < 0.001 |
| SFA ratio | 2.0 ± 0.2 | 1.2 ± 0.1 | 0.96 ± 0.02 | < 0.001 | < 0.001 | < 0.001 |
RMP, resting membrane potential; Ri, input resistance; AP, action potential; AHP, afterhyperpolarization (f, fast; s, slow); SFA, spike frequency adaptation. The AP characteristics were measured when injecting a current of 30 pA above AP threshold. AP rise/AP decay is the ratio of AP rise (dV/dt; mV ms−1) and AP decay (dV/dt; mV ms−1). P < 0.001, extremely significant; P < 0.01, very significant; P < 0.05, significant; n.s., not significant.
Figure 2. Corticostriatal transmission in striatal cholinergic interneurons.
A, characteristic membrane properties and spiking pattern of a cholinergic interneuron. Raw traces show individual voltage responses to a series of 500 ms current pulses from −90 pA to +50 pA with 20 pA increasing current steps (left side) and to +30 pA above AP threshold (middle panel, spike frequency = 8 Hz). Note the linear I–V relationship (right panel). B, EPSCs evoked by cortical layer V (Cx (LV)) stimulations in cholinergic interneurons. Black line represents the average of 20 consecutive EPSC raw traces (grey lines). A low variability of cortically evoked EPSC amplitudes should be noted. C, latency distribution of 60 consecutive EPSCs recorded in a single cholinergic interneuron (well fitted by a Gaussian curve). Note the short latency centred on 2.4 ms. D, amplitude distribution of 60 EPSCs recorded in the same cholinergic interneuron shown in C (distribution well fitted by a Gaussian curve centred around −108 pA). E, cortically evoked EPSCs on cholinergic interneurons were glutamatergic since they were totally inhibited by AP5 (50 μm) and CNQX (20 μm) treatments. This is illustrated by a representative experiment showing averages of 15 traces recorded in control conditions (‘Control’) and after AP5 and CNQX treatment (‘AP5+CNQX’). F, latency distribution of EPSCs was fitted with one Gaussian function centred on 1.86 ± 0.03 ms (n = 980 EPSCs from 35 cholinergic interneurons). G, SD distribution of averaged EPSC latencies recorded in 35 cholinergic interneurons indicates a monosynaptic transmission between cortex and cholinergic interneurons (mean value of latency SD was 0.23 ± 0.01 ms).
GABAergic interneurons (fast-spiking cells) (n = 45) were characterized by a hyperpolarized RMP (−72.8 ± 0.7 mV), a short spike duration (1.36 ± 0.07 ms), a short AHP (fast-AHP and slow-AHP durations were 1.8 ± 0.2 ms and 17.9 ± 0.8 ms, respectively) and a high frequency discharge in response to depolarizing current pulses (34.4 ± 2.0 Hz, for +30 pA injected current above AP threshold) (Fig. 1B). It should be noted that in the present study, among striatal GABAergic interneurons, we have focused on fast-spiking GABAergic interneurons, which were easily distinguished electrophysiologically from another class of GABAergic interneurons, the PLTS cells (Kawaguchi, 1993; Kawaguchi et al. 1995; Fino et al. 2007). Briefly, PLTS cells (n = 11) were characterized by longer spike duration (2.6 ± 0.2 ms) and a longer AHP duration (fast-AHP and slow-AHP durations were 12.9 ± 0.9 ms and 27.6 ± 2.7 ms, respectively). In addition, they displayed a characteristic spiking pattern consisting of fast initial AP doublets at 66.2 ± 11.3 Hz. Cholinergic interneurons (long-lasting afterhyperpolarization cells) (n = 65) were characterized by a depolarized RMP (−61.9 ± 0.4 mV), a long AP duration (5.2 ± 0.2 ms), a long-lasting AHP (fast-AHP and slow-AHP durations were 25.7 ± 1.5 ms and 64.6 ± 2.9 ms, respectively) and a low firing rate in response to depolarizing current pulses (9.4 ± 0.5 Hz, for +30 pA injected current above AP threshold) (Fig. 2A). The electrophysiological properties of striatal interneurons were clearly distinct from those of MSNs (see details in Table 1) (Kawaguchi, 1993; Kawaguchi et al. 1995; Fino et al. 2007). MSNs were identified by a very hyperpolarized RMP (−75.3 ± 0.9 mV, n = 50), a medium range (when compared to striatal interneurons) of AP duration (3.3 ± 0.2 ms), AHP duration (fast-AHP and slow-AHP durations were 16.1 ± 0.6 ms and 38.3 ± 1.3 ms, respectively), and firing rate (12.5 ± 0.4 Hz). In addition, they are characterized by a long delay to first spike (414.6 ± 4.3 ms), a fast inward rectification, and a long depolarizing ramp to spike threshold underlain by potassium currents (Calabresi et al. 1987; Nisenbaum & Wilson, 1995).
Reliable monosynaptic corticostriatal transmission in striatal fast-spiking GABAergic and cholinergic interneurons
Cortical stimulation evoked postsynaptic currents in striatal interneurons with a success rate of 96% for fast-spiking GABAergic (n = 45) and 90% for cholinergic (n = 65) interneurons (Figs 1C and 2B). Once corticostriatal transmission occurred, no failure was observed in fast-spiking GABAergic as well as in cholinergic interneurons indicating a very reliable and efficient transmission. Latency and amplitudes of the cortically evoked EPSCs for GABAergic and cholinergic interneurons displayed very narrow Gaussian distributions (see examples in Figs 1D and E, and 2C and D). Cortically evoked EPSCs in GABAergic and cholinergic interneurons were glutamatergic since they were totally blocked by CNQX (20 μm) and AP5 (50 μm) treatments (n = 4 GABAergic interneurons and n = 6 cholinergic interneurons) (Figs 1F and 2E). NMDA currents represent on average 12.0 ± 6.2% (n = 7) and 16.5 ± 7.7% (n = 4) of the glutamatergic-evoked responses in fast-spiking GABAergic interneurons and in cholinergic interneurons, respectively. EPSC latencies displayed very narrow distributions centred on 1.67 and 1.86 ms for GABAergic (n = 28) and cholinergic (n = 35) interneurons, respectively (Figs 1G and 2F). EPSC latency SDs were less than 1 ms (0.23 ± 0.02 ms and 0.23 ± 0.01 ms for GABAergic and cholinergic interneurons, respectively) denoting a monosynaptic corticostriatal transmission for both interneuron subtypes (Figs 1H and 2G).
Temporal sequence of activation of striatal neurons in response to a cortical stimulation
In response to a cortical stimulation, the precise temporal sequence of activation of the different striatal neuronal populations could be determined (Supplemental Fig. 2). Based on their shortest latency (1.93 ± 0.03 ms) and rise time (2.70 ± 0.05 ms) fast-spiking GABAergic interneurons (n = 20) were activated first, followed by cholinergic interneurons (n = 35) (latency: 2.07 ± 0.02 ms, P < 0.001, and rise time: 3.97 ± 0.04 ms, P < 0.001). Cortical stimulation activated MSNs after striatal interneurons. Indeed, latency and rise time of fast-spiking GABAergic interneurons were significantly (P < 0.001) shorter than those of MSNs (n = 31). Although the latency of cortically evoked EPSCs in cholinergic interneurons was not significantly different to that of MSNs, the rise time was significantly (P < 0.001) shorter (Supplemental Fig. 2).
Cell-specific spike-timing-dependent plasticity
The temporal relationship between activity in presynaptic and postsynaptic elements can lead to the induction of long-term synaptic efficacy changes known as STDP (Abbott & Nelson, 2000; Bi & Poo, 2001; Sjöström & Nelson, 2002; Dan & Poo, 2004, 2006). Using STDP protocols, we examined the influence of the temporal relationship between the discharges of striatal interneurons and the activation of their cortical afferents on the induction of long-term synaptic plasticity. STDP protocols consisted of evoking an AP in a single striatal interneuron a few milliseconds before (post-pre protocol) or after (pre-post protocol) the stimulation of cortical afferents. This pairing protocol was repeated 100 times at 1 Hz frequency (Figs 3A and 5A).
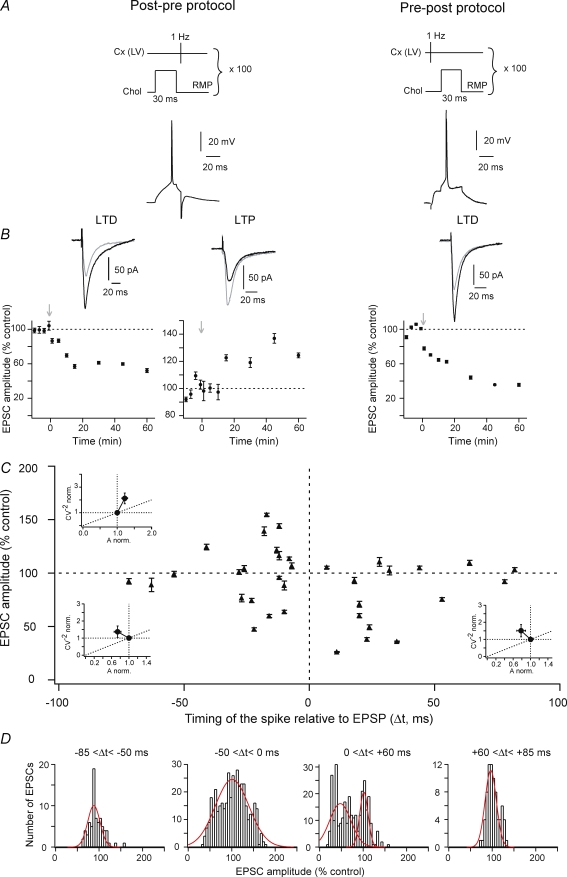
Figure 3. Fast-spiking GABAergic interneuron spike-timing-dependent plasticity.
A, schematic representation of STDP protocols and the corresponding raw traces of the postsynaptic GABAergic interneuron recording. An AP was evoked in the fast-spiking GABAergic interneuron a few milliseconds before (post-pre protocol) or after (pre-post protocol) a cortical stimulation (100 paired stimulations at 1 Hz). B, representative experiments of long-term plasticity induced by post-pre and pre-post protocols and EPSCs evoked in control (black) and 60 min after induction protocol (grey). Long-term synaptic efficacy changes were −63.7 ± 7.4% for LTD induced by post-pre protocol and +74.6 ± 11.7% for LTP induced by pre-post protocol (arrows indicate the induction protocols). C, STDP protocols induced bidirectional long-term synaptic plasticity (black triangles represent the mean ± s.e.m. of EPSC amplitude changes measured 60 min after induction protocol in 28 GABAergic interneurons). Post-pre protocols induced LTD for −40 < Δt < 0 ms (n = 10). Pre-post protocols induced LTP for 0 < Δt < +60 ms (n = 10). No long-term plasticity occurred for Δt < −40 ms and Δt > +60 ms (n = 8). Insets: normalized CV−2 were plotted as a function of the normalized EPSC amplitude to determine the loci of the STDP. Mean variance analysis suggests that LTD has a mainly postsynaptic origin and LTP is mainly underlain by presynaptic mechanisms. Δt and induced plasticities were significantly correlated (r= 0.588, P < 0.01). This indicates a time-dependency of the STDP for GABAergic interneurons. D, histograms of EPSC amplitude changes. Histogram for post-pre protocols for −40 < Δt < 0 ms displayed a bimodal distribution fitted with a sum of two Gaussian functions, plotted individually, centred on 43.1 ± 1.2% and 97.3 ± 1.8% (n = 250 EPSCs, 10 GABAergic interneurons). This indicates the induction of either LTD (n = 9) or absence of plasticity (n = 1). Histogram for pre-post protocols for 0 < Δt < +60 ms displayed a unimodal distribution fitted with one Gaussian function centred on 141.1 ± 3.3% (n = 250 EPSCs, 10 GABAergic interneurons). This indicates that pre-post protocols induced LTP (n = 10). Histograms for post-pre and pre-post protocols for Δt < −40 ms and Δt > +60 ms displayed unimodal distributions fitted with one Gaussian function centred on 94.5 ± 2.8% for Δt < −40 ms (n = 100 EPSCs, 4 GABAergic interneurons) and 89.9 ± 2.4% for Δt > +60 ms (n = 100 EPSCs, 4 GABAergic interneurons), indicating an absence of long-term plasticity for both Δt.
Figure 5. Cholinergic interneuron spike-timing-dependent plasticity.
A, schematic representation of STDP protocols and the corresponding raw traces of the postsynaptic cholinergic interneuron recording. An AP was evoked in the cholinergic interneuron a few milliseconds before (post-pre protocol) or after (pre-post protocol) cortical stimulation (100 paired stimulations at 1 Hz). B, representative experiments of long-term plasticity induced by post-pre and pre-post protocols and EPSCs evoked in control (black) and 60 min after induction protocol (grey). Long-term synaptic efficacy changes were −47.8 ± 2.3% for LTD and +24.4 ± 1.8% for LTP induced by post-pre protocols, and −64.4 ± 6% for LTD induced by pre-post protocols (arrows indicate the induction protocols). C, STDP protocols induced bidirectional long-term synaptic plasticity (black triangles represent the mean ± s.e.m. of EPSC amplitude changes measured 60 min after induction protocol in 35 cholinergic interneurons). Post-pre protocols induced LTD as well as LTP for −50 < Δt < 0 ms (n = 17). Pre-post protocols induced LTD for 0 < Δt < +60 ms (n = 12). No more long-term plasticity occurred for Δt < −50 ms and Δt > +60 ms (n = 6). Insets: normalized CV−2 were plotted as a function of the normalized EPSC amplitude to determine the loci of the STDP. Mean variance analysis suggests that post-pre-induced LTP is mainly underlain by presynaptic mechanisms and LTD has a mainly postsynaptic origin. Pre-post-induced LTD appears to be mainly postsynaptic. Δt and induced plasticities were not significantly correlated for cholinergic interneurons (due to the non-oriented post-pre plasticities). D, histograms of EPSC amplitude changes. Histograms for post-pre for −50 < Δt < 0 ms displayed a unimodal distribution fitted with one Gaussian function centred on 100.9 ± 2.7% (n = 425 EPSCs, 17 cholinergic interneurons). This wide distribution indicates that post-pre protocols led to either LTP or LTD, or an absence of plasticity. Histograms for pre-post protocols for 0 < Δt < +60 ms displayed bimodal distributions fitted with a sum of two Gaussian functions centred on 47.3 ± 2.0% and 101.6 ± 0.8% (n = 300 EPSCs, 12 cholinergic interneurons). This indicates that pre-post protocols led to either LTD or absence of plasticity. Histograms for post-pre and pre-post protocols for Δt < −50 ms and Δt > +60 ms displayed unimodal distributions fitted with one Gaussian function centred on 88.0 ± 2.3% for Δt < −50 ms (n = 75 EPSCs, 3 cholinergic interneurons) and 96.5 ± 2.1% for Δt > +60 ms (n = 75 EPSCs, 3 cholinergic interneurons), indicating an absence of long-term plasticity for both Δt.
Striatal fast-spiking GABAergic interneuron STDP
STDP protocols induced efficient bidirectional long-term synaptic efficacy changes in fast-spiking GABAergic interneurons with a success rate of 95% (n = 20) for time intervals between pre- and postsynaptic activation (Δt) comprised between −40 to +60 ms (minus sign indicates that the postsynaptic AP precedes the presynaptic cortical stimulation) (Fig. 3).
Post-pre protocols (Fig. 3A) induced significant LTD with a success rate of 90% (n = 10) for −40 < Δt < 0 ms (Fig. 3C). An example of post-pre LTD is illustrated by a representative experiment showing a depression of −63.7 ± 7.4% (Fig. 3B). It should be noted that among 10 GABAergic interneurons tested, only one did not develop significant long-term plasticity (Fig. 3C). The bimodal distribution of EPSC amplitude changes observed for −40 < Δt < 0 ms (the two peaks were centred on 43.1 ± 1.2% and 97.3 ± 1.8%, n = 250 EPSCs measured from 10 GABAergic interneurons) illustrated the occurrence of LTD (n = 9) or the absence of plasticity (n = 1) (Fig. 3D). The mean value of the EPSC amplitude depression (estimated 1 h after the STDP protocol induction) was −39.5 ± 8.3% (n = 10). This EPSC amplitude depression was significantly different from the control baseline (P < 0.01) (see the averaged baseline shown in Supplemental Fig. 1A, n = 14). No more long-term synaptic efficacy change was observed for Δt beyond −40 ms (n = 4) (Fig. 3C), as also illustrated by the unimodal distribution of EPSC amplitudes changes centred on 94.5 ± 2.8% (n = 100 EPSCs measured from 4 GABAergic interneurons) (Fig. 3D, left panel). Mean variance analysis of the cortically evoked synaptic events indicated that the spike-timing-dependent LTD arose mainly from the postsynaptic element (Fig. 3C).
Pre-post STDP protocols (Fig. 3A) induced exclusively LTP, since significant EPSC amplitude potentiation occurred with a success rate of 100% (n = 10) for 0 < Δt < +60 ms (n = 10) (Fig. 3C). A pre-post LTP is illustrated by a representative experiment showing a potentiation of +74.6 ± 11.7% (Fig. 3B). The exclusive occurrence of LTP is also illustrated by the unimodal distribution of EPSC amplitude changes centred on 141.1 ± 3.3% (n = 250 EPSCs measured from 10 GABAergic interneurons) (Fig. 3D). The mean value of EPSC amplitude potentiation was +43.8 ± 10.4%. This EPSC amplitude potentiation was significantly different from the baseline (P < 0.001) (see the averaged baseline for pre-post protocols shown in Supplemental Fig. 1B, n = 14). No more long-term plasticity was observed for Δt > +60 ms (n = 4) (Fig. 3C), as also illustrated by the unimodal distribution of EPSC amplitude changes centred on 89.9 ± 2.4% (n = 100 EPSCs measured from 4 GABAergic interneurons) (Fig. 3D, right panel). Mean variance analysis of the cortically evoked synaptic events indicated that the spike-timing dependent LTP had mainly a presynaptic origin (Fig. 3C).
Receptors involved in striatal fast-spiking GABAergic interneuron STDP
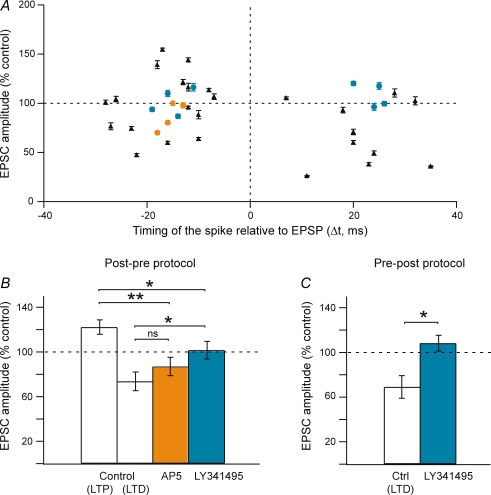
We have explored which receptors have to be activated to allow the induction of STDP in fast-spiking GABAergic interneurons. Pharmacology was investigated for −40 < Δt < +60 ms since this Δt allows a reliable induction of either LTD (post-pre protocol for −40 < Δt < 0 ms) or LTP (pre-post protocol for 0 < Δt < +60 ms) (Fig. 3C). For post-pre protocols (−40 < Δt < 0 ms), when AP5 (NMDA receptor antagonist, 50 μm) was applied, LTD was no longer observed (Fig. 4A and B). The mean EPSC amplitude value (92.5 ± 6.5%, n = 4) did not significantly differ from the baseline, indicating the lack of STDP induced synaptic plasticity. Similarly, for pre-post protocols (0 < Δt < +60 ms), AP5 prevented the induction of LTP and we observed an absence of plasticity (Fig. 4A and C). The mean EPSC amplitude value, 96.3 ± 11.9% (n = 6) was not significantly different from the baseline. In conclusion, LTD as well as LTP were both underlain by NMDA receptor-activation in striatal fast-spiking GABAergic interneurons.
Figure 4. Fast-spiking GABAergic interneuron LTP and LTD are NMDA receptor-activation dependent.
A, synaptic efficacy changes induced by STDP protocols within −40 < Δt < +60 ms were plotted in control (n = 20) and AP5 (50 μm) treatment (n = 10). B, long-term synaptic efficacy changes induced by post-pre protocols for −40 < Δt < 0 ms, in control (n = 10) (open bar) and in AP5 treatment (50 μm) (n = 6) (filled bar). In control, post-pre protocols induced significant LTD (−39.5 ± 8.3%, n = 10) and in AP5 treatment no more LTD was induced and an absence of long-term plasticity was observed (92.5 ± 6.5%, n = 6). C, long-term synaptic efficacy changes induced by pre-post protocols for 0 < Δt < +40 ms in control (n = 10) (open bar) and in AP5 treatment (n = 4) (filled bar). In control, pre-post protocols induced significant LTP (+42.2 ± 11.6%, n = 9) and in AP5 treatment no more LTP was observed but an absence of long-term plasticity (96.3 ± 11.9%, n = 4). AP5 abolished both LTD and LTP induced, respectively, by post-pre and pre-post protocols in control. This indicates that in GABAergic interneuron LTD and LTP are NMDA receptor-activation dependent.
Striatal cholinergic interneuron STDP
STDP protocols were able to induce reliable and robust bidirectional long-term synaptic plasticity in cholinergic interneurons with a success rate of 86% (n = 29) for −50 < Δt < +60 ms (Fig. 5).
For the post-pre protocol (Fig. 5A), bidirectional long-term plasticities (LTP and LTD) occurred with a success rate of 82% (n = 17) for −50 < Δt < 0 ms: significant LTP and LTD occurred with success rates of 40% (n = 8) and 30% (n = 6), respectively (Fig. 5C). Post-pre LTP and LTD are illustrated by representative experiments showing an EPSC amplitude depression of −47.8 ± 2.3% and a potentiation of +24.4 ± 1.8% (Fig. 5B). Among the sample of 17 cholinergic interneurons tested, only three did not develop long-term plasticity. The large unimodal distribution of EPSC amplitude changes for −50 < Δt < 0 ms centred on 100.9 ± 2.7% (n = 425 EPSCs measured from 17 cholinergic interneurons) reflected the fact that post-pre protocols led to LTP (n = 8), LTD (n = 6) and to an absence of plasticity (n = 3) (Fig. 5D). It should be noted, however, that LTP was induced at a higher rate than LTD by post-pre protocol since the LTP/LTD ratio was 1.3. The mean values of potentiation of EPSC amplitude were +27.5 ± 6.3% (n = 8) and the mean values of depression of EPSC amplitude were −30.4 ± 7.4% (n = 6). These values were significantly different from the baseline recorded in control (P < 0.01) (see averaged baseline data of the post-pre STDP experiments in Supplemental Fig. 1C, n = 20). No more synaptic efficacy changes were observed for Δt beyond −50 ms (Fig. 5C), as also illustrated by the unimodal distribution of EPSC amplitude changes centred on 88.0 ± 2.3% (n = 75 EPSCs measured from 3 cholinergic interneurons) (Fig. 5D, left panel). Mean variance analysis of the cortically evoked synaptic events indicated that post-pre LTP arose mainly from the presynaptic element and the post-pre LTD had mainly a postsynaptic origin (Fig. 5C).
We investigated if occurrences of LTP or LTD induced by post-pre protocol could be predicted by parameters related to synaptic transmission (EPSC rise time) or neuronal membrane properties (RMP, input resistance and rheobase) of cholinergic interneurons. No significant correlation was observed between RMP or input resistance and the occurrence of either LTP or LTD (Supplemental Fig. 3B and C). EPSC rise time is related to electrotonic distance, which has been proposed to be a key component for the orientation (LTP versus LTD) of cortical STDP (Sjöström & Hausser, 2006; Letzkus et al. 2006). We did not observe any significant correlation between EPSC rise times and synaptic efficacy changes induced by post-pre protocols in cholinergic interneurons (Supplemental Fig. 3A). Strikingly, the rheobase parameter was found to be a key component for the orientation of plasticities induced by post-pre STDP protocols. Indeed, a significant (P < 0.05) correlation was observed between rheobase and long-term synaptic efficacy changes (Fig. 6). Cholinergic interneurons displaying low rheobase values preferentially developed LTD whereas those with highest rheobases were correlated with induction of LTP. Accordingly, excitability of cholinergic interneurons appears to be a determinant in the orientation of post-pre STDP.
Figure 6. Level of excitability of cholinergic interneurons is determinant in the orientation of the plasticity induced by post-pre protocols.
A, rheobase and long-term synaptic plasticity induced by post-pre protocols (n = 16) in cholinergic interneurons were significantly correlated (r= 0.550, P < 0.05). This indicated that the level of excitability of cholinergic interneurons was a key component in the induction of either LTP or LTD by post-pre protocols. B, when post-pre LTD (n = 9) (upper panel, r= 0.602) and post-pre LTP (n = 10) (lower panel, r= 0.290) were considered separately, no significant correlation was found between rheobase and the plasticity induced. It should be noted that the absence of plasticity (n = 3) was considered in post-pre LTD and post-pre LTP analysis. These correlations included plasticities induced for −40 < Δt < 0 ms since this Δt corresponds to the maxima of induction rate and magnitude of both LTP and LTD.
Conversely, a pre-post STDP protocol (Fig. 5A) induced a unidirectional plasticity, orientated towards LTD (n = 8) or an absence of plasticity (n = 4) (Fig. 5C). Pre-post LTD is illustrated by a representative experiment showing a depression of −64.4 ± 6% (Fig. 5B). Significant LTD occurred with a success rate of 67% (n = 12) for 0 < Δt < +60 ms. The occurrence of LTD or the absence of plasticity is also evidenced by the bimodal distribution of EPSC amplitude changes: the two peaks were centred on 47.3 ± 2.0% and 101.6 ± 0.8% (n = 300 EPSCs measured from 12 cholinergic interneurons). The mean value of EPSC amplitude depression estimated 1 h after the pre-post protocol was −27.4 ± 8.3% (n = 12). This value was significantly different from the baseline recorded in control (P < 0.01) (see averaged baseline data of the pre-post experiments in Supplemental Fig. 1D, n = 15). No more synaptic efficacy changes were observed for Δt beyond +60 ms (Fig. 5C), as illustrated by the unimodal distribution of EPSC amplitude changes centred on 96.5 ± 2.1% (n = 75 EPSCs measured from 3 cholinergic interneurons) (Fig. 5D, right panel). Mean variance analysis indicated that the pre-post LTD had a mainly postsynaptic origin (Fig. 5C).
Receptors involved in striatal cholinergic interneuron STDP
We next explored the receptors involved in the induction of cholinergic interneuron STDP. Pharmacological experiments were performed for −40 < Δt < +40 ms. Indeed, such Δt corresponds to the maxima of induction rate and magnitude of both LTP and LTD (Fig. 5C). For post-pre protocol (−40 < Δt < 0 ms), when AP5 (50 μm) was applied (n = 4), LTP was no longer induced and either LTD or an absence of plasticity was observed (Fig. 7A and B). The mean value of EPSC amplitude changes in AP5 was 87.0 ± 8.3%, which was not significantly different from the mean value of LTD observed in control conditions, but still significantly different from the baseline. Conversely, after LY341495 (metabotropic glutamate receptor antagonist, 100 μm) treatment, no more significant long-term synaptic plasticity was observed (101.6 ± 7.9%, n = 4) (Fig. 7A and B). For pre-post protocols (0 < Δt < +40 ms), LY341495 prevented the induction of LTD and we observed an absence of long-term synaptic efficacy changes (108.3 ± 7.1%, n = 4) (Fig. 7A and C). In conclusion, LTD induced by post-pre or pre-post STDP protocols was underlain by metabotropic glutamate receptor-activation whereas LTP induced by post-pre STDP protocols was NMDA receptor-activation dependent.
Figure 7. Cholinergic interneuron LTP and LTD are NMDA and metabotropic glutamate receptor-activation dependent, respectively.
A, synaptic efficacy changes induced by STDP protocols within −40 < Δt < +40 ms were plotted in control (n = 26), AP5 (50 μm) (n = 4) and LY341495 (100 μm) treatments (n = 8). B, long-term synaptic efficacy changes induced by post-pre protocols for −40 < Δt < 0 ms in control (n = 16) (LTP and LTD), in AP5 (n = 4) or LY341495 (n = 4) treatments. In control, post-pre protocols induced significant LTP (+22.3 ± 6.4%, n = 9) and LTD (−26.3 ± 8.4%, n = 7). In AP5 treatment, LTP was no longer observed while LTD was still induced (87.0 ± 8.3%, n = 4). In LY341495 treatment, no more long-term plasticity was induced (101.6 ± 7.9%, n = 4). For post-pre protocols, LTP was NMDA receptor-activation dependent and LTD was metabotropic glutamate receptor-activation dependent. C, long-term synaptic efficacy changes induced by pre-post protocols for 0 < Δt < +40 ms in control (n = 8) and in LY341495 treatment (n = 4). In control, pre-post protocols induced LTD (−30.9 ± 10.1%, n = 10) and in LY341495 treatment, no more LTD was observed but an absence of long-term plasticity (108.3 ± 7.1%, n = 4). For pre-post protocols, LTD was metabotropic glutamate receptor-activation dependent.
Discussion
The implication of striatum in procedural learning and motor habits formation has stimulated much interest in the forms of activity-dependent plasticity at corticostriatal synapses. So far, attention has been merely focused on corticostriatal connections with the MSNs, the main population of striatal neurons whose discharge generates a disinhibitory signal to cognitive and premotor neural networks supporting behavioural expression. Besides MSNs, the striatum comprises GABAergic and cholinergic interneurons, which regulate MSN excitability and consequently, the corticostriatal information processing. Although anatomical (Bennett & Bolam, 1994; Thomas et al. 2000; Ramanathan et al. 2002) and in vivo electrophysiological (Wilson et al. 1990; Reynolds & Wickens, 2004; Mallet et al. 2005) evidence has revealed the presence of excitatory cortical glutamatergic afferents targeting striatal interneurons, knowledge concerning the mode of corticostriatal transmission and plasticity at their level remains fragmentary. Using rat brain slices preserving cortical afferents (Fino et al. 2005), we observed a monosynaptic transmission that was highly reliable and efficient between pyramidal cells of the somatosensory cortex and fast-spiking GABAergic cells as well as cholinergic striatal interneurons. It should be noted that in vivo cortico-cholinergic interneuron transmission has been reported to be sparse and weak when the motor cortex is considered (Wilson et al. 1990; Reynolds & Wickens, 2004). The dissimilarity between such reports and our observation could come from the difference between the stimulated cortices, motor versus somatosensory.
We determined here a precise temporal sequence of activation for the striatal neuronal subtypes in the same experimental conditions. In response to a cortical stimulation, fast-spiking GABAergic interneurons were activated before cholinergic cells and both striatal interneurons were activated before MSNs. The earliest activation of striatal interneurons in response to a cortical stimulation is functionally important. Indeed, striatal interneurons are in a position to modulate the MSN excitability before they discharge in response to cortical inputs and relay other cortical information that MSNs do not receive directly. Therefore, interneurons can affect the corticostriatal function transfer. The feed-forward inhibitory function of GABAergic interneurons of striatum has been particularly well documented by in vivo recordings in the striatum of GAERS, the genetic model of absence epilepsy rats from Strasbourg (Slaght et al. 2004). During the crisis, the early activation of GABAergic interneurons triggered by the synchronized discharges of corticostriatal neurons exerts a shunting effect on the cortical excitatory synaptic inputs of MSNs, preventing these neurons from reaching the AP threshold. Interestingly, the innervation ratio provided by a single corticostriatal axon appears to be much higher onto GABAergic interneurons than onto MSNs, supporting the fact that corticostriatal integration by interneurons obeys specific rules (Ramanathan et al. 2002). To date, the operational mode of regulation of corticostriatal function transfer by the interneuronal feed-forward circuits still remains to be determined.
Evidence for a role of striatum in procedural learning has stimulated investigations into the long-term synaptic plasticity of corticostriatal transmission. However, despite the role of striatal interneurons in corticostriatal information transfer, long-term synaptic plasticity at their level remains poorly documented. To our knowledge, the capability of striatal GABAergic interneurons to develop activity-dependent long-term synaptic efficacy changes has never been reported in vivo or in vitro. Concerning striatal cholinergic interneurons, the only available data are from in vitro experiments reporting LTP in response to high frequency stimulation within the corpus callosum (Suzuki et al. 2001; Bonsi et al. 2004).
Here, by coupling stimulations applied in layer V of the somatosensory cortex with direct suprathreshold depolarization of the recorded cells, a strong spike-timing activity-dependent long-term plasticity was observed in striatal interneurons. Interestingly STDP of interneurons showed remarkable cell specificity. Yet STDP developed with a very high occurrence in GABAergic (95%) and cholinergic interneurons (86%), these values being similar to the percentage of MSNs expressing STDP with the same stimulation protocol (90%, Fino et al. 2005). The temporal window in which STDP is induced displays a cell-specificity. Indeed, it is narrower for MSNs (−30 < Δt < +30 ms) than for GABAergic (−40 < Δt < +60 ms) and cholinergic (−50 < Δt < +60 ms) interneurons.
In fast-spiking GABAergic interneurons, STDP was strictly orientated such that post-pre protocols induced LTD and pre-post protocols LTP. The orientation of this bidirectional plasticity was opposite to the STDP observed in MSNs (Fino et al. 2005) but conformed to the classical STDP rules described so far in other mammalian brain structures (Abbott & Nelson, 2000; Bi & Poo, 2001; Sjöström & Nelson, 2002; Dan & Poo, 2004, 2006). It should be noted that a morphological heterogeneity among fast-spiking GABAergic interneurons has been reported (Koos & Tepper, 1999): two-thirds display short dendritic trees (200–300 μm) whereas one-third display longer dendritic arborization (500–600 μm). Nevertheless, since we did not observe heterogeneity in the orientation or pharmacology of the STDP among fast-spiking GABAergic interneurons, it appears that the different morphologies of the dendritic trees are not determinants in STDP phenomenon.
Conversely, cholinergic interneurons displayed a partially reversed STDP: post-pre protocol induced LTP as well as LTD while pre-post protocol induced LTD. This finding, which provides the first observation of a STDP non-orientated ‘on one side’ of the spike timing conditioning protocol (for post-pre protocol), could be put in relation with a STDP observed in glycinergic interneurons of the cochlear nucleus (Tzounopoulos et al. 2004). In these cells, whereas a pre-post protocol induced LTD, a lack of plasticity was observed in the case of post-pre protocol. In striatal cholinergic interneurons, we determined that rheobase was a key parameter in the orientation of plasticity induced by post-pre protocols. Therefore, the membrane excitability of cholinergic interneurons appears to be a determinant in the induction of either LTP or LTD. Differences concerning the level of excitability could be due to synaptic inputs (glutamatergic, serotoninergic, dopaminergic, GABAergic or cholinergic) and/or to intrinsic properties even though no different subpopulations have been described, up to now, among striatal cholinergic interneurons (Kawaguchi, 1992, 1993). We have recently reported that an acute depletion of striatal dopamine modulates intrinsic properties of cholinergic interneurons (Fino et al. 2007). Indeed, dopamine depletion efficiently decreases the rheobase of cholinergic interneurons and consequently increases their excitability. In addition, the LTP and LTD evoked in cholinergic interneurons by post-pre protocols could also be explained by a difference in the distribution of glutamatergic receptors expressed at the level of the corticostriatal synapses activated. Indeed, whereas LTP induction in cholinergic interneurons depended on NMDA receptor-activation, LTD required metabotropic glutamate receptor-activation. Mean–variance analysis suggests that LTP has a mainly presynaptic origin and LTD arose mainly from the postsynaptic element. Therefore, a reasonable hypothesis could be that NMDA receptors involved in LTP are localized presynaptically as shown in cortex and cochlear nucleus (Sjöström et al. 2003, 2007; Duquid & Sjöström, 2006; Tzounopoulos et al. 2007) and metabotropic glutamate receptors underlying LTD are postsynaptic. Interestingly, such a pharmacological distinction in the STDP induction of LTD and LTP was not observed in striatal fast-spiking GABAergic interneurons, both forms of plasticity requiring activation of NMDA receptors. Nevertheless, mean–variance analysis suggests that LTP is mainly presynaptic whereas LTD is mainly postsynaptically localized. The pharmacological specificity of GABAergic and cholinergic interneurons offers an additional illustration of the cell-specific properties of STDPs among the striatal interneuronal populations.
The specific temporal sequence of activation and orientation of STDPs induced in striatal interneurons in response to cortical stimulation has important functional consequences for the integration of cortical information in striatum. Due to the strong inhibitory synaptic weight of GABAergic interneurons on MSNs (Koos & Tepper, 1999) and their numerous corticostriatal synapses (Ramanathan et al. 2002), their influence is expected to be major in cortico-basal ganglia information processing. Remarkably, the GABAergic interneurons and MSNs displayed a strictly opposite STDP orientation. The same cellular conditioning protocols inducing LTP in MSNs induced LTD in GABAergic interneurons and vice versa. Although opposite, these changes in synaptic weight may act synergistically in corticostriatal information transfer. Indeed, when a cellular conditioning induces LTP in MSNs, a LTD is induced in GABAergic interneurons leading to an increased potentiation of corticostriatal transmission. In contrast, a protocol inducing LTD in MSNs leads to a LTP in GABAergic interneurons, which could reinforce the depression of the corticostriatal transmission by MSNs.
Another picture is expected concerning the impact of cholinergic interneuron STDP on corticostriatal information transfer. Indeed, striatal signals are conveyed to the output nuclei of the basal ganglia through direct (striato-nigral) and indirect (striato-pallido-subthalamonigral) pathways originating from partly segregated subpopulations of MSNs (Gerfen, 1992). MSNs of the direct and indirect pathways bear distinct patterns of muscarinic receptor expression. Indeed, MSNs of the direct pathway express both M1 (excitatory) and M4 (inhibitory) receptors and MSNs of the indirect pathway express M1 receptors (Acquas & Di Chiara, 2002). It is thus expected that a same change in the cortical synaptic weight of cholinergic interneurons will result in opposite effects in the two subpopulations of MSNs. Nevertheless, acetylcholine or muscarine has a mainly excitatory effect on the MSNs. Indeed, it induces an increase of the MSN activity (Perez-Rosello et al. 2005) or EPSCs in MSNs (Lin et al. 2004; Pakhotin & Bracci, 2007) due to M1 receptor-activation. In addition, acetylcholine has been shown to favour the induction of LTP in MSNs (Calabresi et al. 1999). Accordingly, the effect of acetylcholine being mainly excitatory, the STDP of cholinergic interneurons and MSNs should act together to either increase or depress the output of the striatum.
In conclusion, current conceptual models of the corticostriatal circuits rely essentially on the direct cortical connections with MSNs. This is particularly exemplified in the Parkinson's disease model of direct/indirect pathways in which the functional consequence of the loss of dopaminergic striatal transmission is predicted based on the sole expected changes in MSN excitability of the direct and indirect circuits. Striatal interneurons, however, are an integral component of the striatum, contributing to the processing of corticostriatal information. As stressed here, each interneuronal population reacts to cortical inputs with precise timing and expresses strong and specific STDP. Because the striatal functions in learning and memory may require the cooperation of the various striatal neuronal populations, it is of importance to consider the forms of plasticity in corticostriatal transmission in integrated striatal circuits. The understanding of the impact of interneuronal microcircuits on MSN physiology requires the mapping of the local interactions (chemical and electrical synapses) between all striatal neuronal subtypes. Functionally, the weight of the long-term synaptic plasticities developed by GABAergic and cholinergic interneurons in the cortical input integration by MSNs remains to be determined. Considering all the striatal neuronal populations, the cell-specificity of the STDP, reported here, is therefore determinant for the efficiency of the striatal output. Indeed, all the STDP developed by each neuronal population may act synergistically to either potentiate or depress the striatal output.
Acknowledgments
This work was supported by INSERM, ANR Mecarec and Collège de France.
Supplemental material
Online supplemental material for this paper can be accessed at:
Averages of normalized EPSC amplitudes recorded in control before STDP protocols for fast-spiking GABAergic interneurons for postpre protocols (A) (n=10) and pre-post protocols (B) (n=10), and for cholinergic interneurons for post-pre protocols (C) (n=17) and pre-post protocols (D) (n=12). Control EPSC amplitudes were recorded for 10 minutes (minus sign indicates time before the cellular conditioning protocol) and displayed no significant variation.
Temporal sequence of activation of striatal neurons in response to a cortical stimulation. A cortical stimulation induced the following sequence of activation: first fast-spiking GABAergic interneurons, second cholinergic interneurons and finally MSNs. This is illustrated by (A) latencies and (B) rise times of striatal neurons in response to a cortical stimulation. MSN data are taken from Fino et al. (2005) study.
The magnitudes of long-term synaptic efficacy changes induced by post-pre protocol on cholinergic interneurons were plotted against EPSC rise time in control (A), input resistance (B) and RMP (C). No significant correlation was found between these parameters and the magnitude of LTP or LTD induced by post-pre protocols (r values are indicated in each graph).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
http://jp.physoc.org/cgi/content/full/jphysiol.2007.144501/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.144501
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl.):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Acquas E, Di Chiara G. Handbook of Experimental Pharmacology. Vol. 154. Germany: Springer-Verlag; 2002. Dopamine–acetylcholine interactions; pp. 85–115. [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bonsi P, De Persis C, Calabresi P, Bernadi G, Pisani A. Coordinate high-frequency pattern of stimulation and calcium levels control the induction of LTP in striatal cholinergic interneurons. Learn Mem. 2004;11:755–760. doi: 10.1101/lm.82104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Bernardi G. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology. 1999;38:323–326. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Misgeld U, Dodt HU. Intrinsic membrane properties of neostriatal neurons can account for their low level of spontaneous activity. Neuroscience. 1987;20:293–303. doi: 10.1016/0306-4522(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Clements JD, Silver RA. Unveiling synaptic plasticity: a new graphical and analytical approach. Trends Neurosci. 2000;23:105–113. doi: 10.1016/s0166-2236(99)01520-9. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Duquid I, Sjöström PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Figueredo-Cardenas G, Medina L, Reiner A. Calretinin is largely localized to a unique population of striatal interneurons in rats. Brain Res. 1996;709:145–150. doi: 10.1016/0006-8993(95)01392-x. [DOI] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci Res. 2007;58:305–316. doi: 10.1016/j.neures.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptative motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kimura M. Role of basal ganglia in behavioural learning. Neurosci Res. 1995;22:353–358. doi: 10.1016/0168-0102(95)00914-f. [DOI] [PubMed] [Google Scholar]
- Kimura M, Yamada H, Matsumoto N. Tonically active neurons in the striatum encode motivational contexts of action. Brain Dev. 2003;25:S20–S23. doi: 10.1016/s0387-7604(03)90003-9. [DOI] [PubMed] [Google Scholar]
- Kita H. Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience. 1996;70:925–940. doi: 10.1016/0306-4522(95)00410-6. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Chung KK, de Castro D, Funk GD, Lipski J. Effects of muscarinic acetylcholine receptor-activation on membrane currents and intracellular messengers in medium spiny neurones of the rat striatum. Eur J Neurosci. 2004;20:1219–1230. doi: 10.1111/j.1460-9568.2004.03576.x. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J Neurosci. 1998;18:266–283. doi: 10.1523/JNEUROSCI.18-01-00266.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22:8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. The corticostriatal input to giant aspiny interneurons in the rat: a candidate pathway for synchronising the response to reward-related cues. Brain Res. 2004;1011:115–128. doi: 10.1016/j.brainres.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol. 2002;12:305–314. doi: 10.1016/s0959-4388(02)00325-2. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Multiple forms of long-term plasticity at unitary neocortical layer 5 synapses. Neuropharmacology. 2007;52:176–184. doi: 10.1016/j.neuropharm.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Slaght SJ, Paz T, Chavez M, Deniau JM, Mahon S, Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci. 2004;24:6816–6825. doi: 10.1523/JNEUROSCI.1449-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Miura M, Nishimura K, Aosaki T. Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J Neurosci. 2001;21:6492–6501. doi: 10.1523/JNEUROSCI.21-17-06492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Thomas TM, Smith Y, Levey AI, Hersch SM. Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse. 2000;37:252–261. doi: 10.1002/1098-2396(20000915)37:4<252::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Glowinski J, Giaume C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol. 2004;559:215–230. doi: 10.1113/jphysiol.2004.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The contribution of cortical neurons to the firing pattern of striatal spiny neurons. In: Houk JC, Davis JL, Beiser DG, editors. Models of Information Processing in the Basal Ganglia. Cambridge: MIT Press; 1995. pp. 29–50. [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Averages of normalized EPSC amplitudes recorded in control before STDP protocols for fast-spiking GABAergic interneurons for postpre protocols (A) (n=10) and pre-post protocols (B) (n=10), and for cholinergic interneurons for post-pre protocols (C) (n=17) and pre-post protocols (D) (n=12). Control EPSC amplitudes were recorded for 10 minutes (minus sign indicates time before the cellular conditioning protocol) and displayed no significant variation.
Temporal sequence of activation of striatal neurons in response to a cortical stimulation. A cortical stimulation induced the following sequence of activation: first fast-spiking GABAergic interneurons, second cholinergic interneurons and finally MSNs. This is illustrated by (A) latencies and (B) rise times of striatal neurons in response to a cortical stimulation. MSN data are taken from Fino et al. (2005) study.
The magnitudes of long-term synaptic efficacy changes induced by post-pre protocol on cholinergic interneurons were plotted against EPSC rise time in control (A), input resistance (B) and RMP (C). No significant correlation was found between these parameters and the magnitude of LTP or LTD induced by post-pre protocols (r values are indicated in each graph).