Abstract
Agonist-activated Ca2+ signals in non-excitable cells are profoundly influenced by calcium entry via both store-operated and store-independent conductances. Recent studies have demonstrated that STIM1 plays a key role in the activation of store-operated conductances including the Ca2+-release-activated Ca2+ (CRAC) channels, and that Orai1 comprises the pore-forming component of these channels. We recently demonstrated that STIM1 also regulates the activity of the store-independent, arachidonic acid-regulated Ca2+ (ARC) channels, but does so in a manner entirely distinct from its regulation of the CRAC channels. This shared ability to be regulated by STIM1, together with their similar biophysical properties, suggested that these two distinct conductances may be molecularly related. Here, we report that whilst the levels of Orai1 alone determine the magnitude of the CRAC channel currents, both Orai1 and the closely related Orai3 are critical for the corresponding currents through ARC channels. Thus, in cells stably expressing STIM1, overexpression of Orai1 increases both CRAC and ARC channel currents. Whilst similar overexpression of Orai3 alone has no effect, ARC channel currents are specifically increased by expression of Orai3 in cells stably expressing Orai1. Moreover, expression of a dominant-negative mutant Orai3, either alone or in cells expressing wild-type Orai1, profoundly and specifically reduces currents through the ARC channels without affecting those through the CRAC channels, and siRNA-mediated knockdown of either Orai1 or Orai3 markedly inhibits ARC channel currents. Importantly, our data also show that the precise effects observed critically depend on which of the three proteins necessary for effective ARC channel activity (STIM1, Orai1 and Orai3) are rate limiting under the specific conditions employed.
In non-excitable cells, the agonist-activated entry of Ca2+ influences the intracellular Ca2+ signals generated at all levels of stimulation. Typically, although not exclusively, such entry occurs via highly Ca2+-selective conductances. At high agonist concentrations where sustained depletion of intracellular Ca2+ stores occurs, entry via capacitative or store-operated channels, such as the Ca2+-release-activated Ca2+ channel (CRAC channel) and its close relatives, acts to modulate the amplitude of the resulting elevated Ca2+ signals (Hoth & Penner, 1992, 1993; Zweifach & Lewis, 1993; Parekh & Penner, 1997; Parekh & Putney, 2005). In contrast, during the oscillatory Ca2+ signals seen at low agonist concentrations where emptying of the stores is only partial and transient (Park et al. 2000), store-independent pathways for Ca2+ entry are indicated (Shuttleworth & Thompson, 1996; Shuttleworth, 1999, 2004). Perhaps the most extensively characterized of these store-independent pathways is that mediated by the arachidonic acid-regulated Ca2+-selective channel (ARC channel) (Mignen & Shuttleworth, 2000; Shuttleworth et al. 2004). These channels have been shown to be present in a variety of different cell types including acutely dissociated acinar cells of the exocrine pancreas and parotid gland, as well as in several cell lines (Mignen et al. 2003, 2005). Importantly, evidence indicates that these channels appear to have a major role in providing the predominant mode of Ca2+ entry at the low agonist concentrations that induce oscillatory Ca2+ signals, where they act to modulate the frequency of these oscillations (Mignen et al. 2001; Mignen et al. 2005).
Although it is clear that the CRAC and ARC channels are entirely distinct entities, their basic biophysical properties display many similarities – features that largely reflect the fact that they are both highly Ca2+-selective conductances. Recently, these similarities have been shown to extend to the role of STIM1 in their regulation. STIM1 is a member of the stromal interacting molecule family of proteins and was demonstrated to play a critical role in the regulation of store-operated conductances such as CRAC channels. In this, STIM1 appears to act as the sensor for the depletion of intracellular stores via an N-terminal Ca2+-binding EF-hand that is located in the lumen of the endoplasmic reticulum (Roos et al. 2005; Liou et al. 2005; Zhang et al. 2005; Spassova et al. 2006). However, STIM1 also regulates the activity of the ARC channels, but does so via an entirely distinct mechanism (Mignen et al. 2007). Thus, the STIM1-dependent regulation of the ARC channels does not involve store depletion, or any subsequent translocation of STIM1 to sites close the plasma membrane, and is independent of the Ca2+-binding function of the N-terminal EF-hand in STIM1. Instead, the regulation of the ARC channels is specifically dependent on the pool of STIM1 that constitutively resides in the plasma membrane (Mignen et al. 2007). Despite these clear differences in the precise mechanism of their regulation by STIM1, the shared ability of these two conductances to be regulated by the same protein, along with their overall similarity in basic biophysical properties, suggested that they may be, in some way, molecularly related.
Recently, understanding of the molecular nature of the CRAC channels has undergone a major advance with the identification of members of the Orai proteins as a critical component of the channels themselves (Feske et al. 2006; Vig et al. 2006a,b; Zhang et al. 2006; Prakriya et al. 2006; Yeromin et al. 2006). The Orai proteins comprise a family of highly conserved proteins of which three discrete homologs are expressed in mammalian cells (Feske et al. 2006; Zhang et al. 2006; Gwack et al. 2007). Currently, the evidence suggests that only Orai1 (or Orai in Drosophila) is required for the formation of functional CRAC channels (Feske et al. 2006; Zhang et al. 2006; Peinelt et al. 2006; Soboloff et al. 2006; Mercer et al. 2006; Prakriya et al. 2006). However, the presence of additional Orai family members raises the possibility that these other proteins may comprise key components of the ARC channels. Given the importance of oscillatory [Ca2+]i signals for the appropriate regulation of a variety of cellular responses in a frequency-dependent manner, and the demonstrated specific role of the ARC channels in modulating this frequency, the possibility of identifying the key molecular components that make up the channel would represent a powerful tool in understanding the function and regulation of these important channels at a molecular level. Here, for the first time, we present evidence demonstrating that both Orai1 and the closely related protein Orai3 are essential components of the ARC channels.
Methods
Cell lines and constructs
Maintenance of the HEK293 cell line stably transfected with the human m3 muscarinic receptor (m3-HEK cells) used in this study was as previously described (Mignen & Shuttleworth, 2000), and the cell line stably expressing STIM1 (STIM1-stable) was generated using the Flp-In(tm)-293 system (Invitrogen) following the manufacturers' instructions. Briefly, this system involves the introduction of a Flp recombination target site into the genome of the cell, followed by the incorporation of an expression vector containing the gene of interest (STIM1) at this site via Flp recombinase-mediated DNA recombination, and subsequent selection with hygromycin B. The Orai1, Orai2 and Orai3 constructs each bearing a C-terminal FLAG tag were as described by Gwack et al. (2007). The Orai1 R91W SCID mutant was subcloned from the original MO7O vector into pBSII SK+ and mutated using QuikChange II (Stratagene). The construct was confirmed by complete sequencing and then returned to the MO7O vector for transfection. The STIM1-stable cell line stably expressing either Orai1 or Orai3 were generated by transfection with the appropriate construct, followed by selection using 5 μg ml−1 puromycin. In experiments involving transient expression, cells were transfected with 0.5 or 1 μg DNA of the selected construct, together with 0.25 μg of an EYFP construct using a Amaxa Nucleofector II following the manufacturers' guidelines.
Electrophysiology
Cells were patch-clamped 42–50 h after transfection using the EYFP fluorescence as a marker for transfected cells. Whole-cell recordings of currents through CRAC and ARC channels were made as previously described (Mignen et al. 2007), using alternating 250 ms voltage pulses to −80 mV and +60 mV delivered every 2 s from a holding potential of 0 mV. Current–voltage relationships were recorded using 150 ms voltage ramps from −100 to +60 mV. The standard extracellular (bath) solution contained (mm): NaCl 140, MgCl2 1.2, CaCl2 10, CsCl 5, d-glucose 30, Hepes 10 (pH 7.4). Standard pipette solutions for the recording of ARC channel currents contained (mm) Cs+ acetate 140, NaCl 10, MgCl2 3.72, EGTA 10, 3.5 Ca2+, Hepes 10 (pH 7.2). Calculated free [Ca2+] and [Mg2+] in this solution were 100 nm, and 3 mm, respectively. This concentration of free Mg2+ was designed to inhibit the activation of MIC/MagNum currents. As an additional sensitive check for the activation of these conductances, currents were monitoring at +60 mV, and experiments were terminated on occasions when significant changes in the currents at this voltage were observed. For recording CRAC channel currents, this solution was modified by removing all calcium, and adding 2 μm of the potent InsP3 receptor agonist adenophostin A. Currents were sampled at 20 kHz during the voltage steps and digitally filtered off-line at 1 kHz. Initial current–voltage relationships obtained before activation of either the CRAC channel or ARC channel currents, were averaged and used for leak subtraction of subsequent current recordings. In addition, where possible, La3+ (100 μm) was added at the end of the experiment to inhibit the currents through the activated CRAC or ARC channels, and the resulting values compared with those recorded initially on achieving the whole-cell condition. This was particularly critical for the CRAC channel recordings as a check that the adenophostin A had not begun to activate the CRAC channels immediately on going whole-cell. The experiments using the cells stably expressing Orai1 resulted in unusually large currents (≥ 150 pA) raising the possibility of saturating the intracellular buffers with the entering Ca2+. Because of this we monitored the developing current by pulsing to −40 mV rather than the usual −80 mV. Once the developing currents had fully stabilized, we obtained their magnitude at −80 mV to allow direct comparison with the other data. No obvious differences in the relationships between the different currents were observed at −40 mV versus−80 mV. All experiments were carried out at room temperature (20–22°C). All data are presented as mean ± s.e.m. Where appropriate, statistical significance was determined using Student's t test, with a value of P < 0.05 taken as significant.
Western blotting
After resolving on 7% SDS-PAGE gels, proteins were transferred onto nitrocellulose, and analysed by Western blotting using the appropriate primary antibody, and goat anti-mouse HRP secondary antibodies (1 : 2000 dilution). Labelled bands were visualized by chemiluminescence (Western Lightning, Pierce) and exposure to Biomax XAR film. GOK/STIM1 mouse primary antibodies targeted to an N-terminal domain (BD Bioscience) were used at 1 : 250 dilution. Loading controls used monoclonal β-actin antibodies (Sigma) at a 1 : 5000 dilution.
RT-PCR
m3-HEK cells were transfected (Amaxa Nucleofector II) with either the STIM1 construct alone, or STIM1 plus the relevant Orai1, Orai2 or Orai3 construct. Total RNA was extracted 48 h later using RNAeasy (Qiagen). To ensure removal of any contaminating DNA, the total RNA was treated with DNA-free (Ambion) following the manufacturers' protocol. RT-PCR was performed using Superscript One-Step RT-PCR (Invitrogen) using a 1 μg template RNA for each sample. All samples were probed with the appropriate primers (0.2 μm) for STIM1, Orai 1, Orai2 and Orai3 as follows: STIM1-S (5′-GCGGGAGGGTACTGAG-3′), STIM1-AS (5′-TCCATGTCATCCACGTCGTCA-3′), Orai1-S (5′-CAACTCGGTCAAGGA GTCCC-3′), Orai1-AS (5′-GTGAGCGGTAGAAGTGGACGG-3′), Orai2-S (5′-CACCTCTAA CCACCACTCGG-3′), Orai2-AS (5′-GAACTTGATCCAGCAGAGCAG-3′), Orai3-S (5′-GAG TGACCACGAGTACCCACC-3′), and Orai3-AS (5′-GGGTACCATGATGGCTGTGG-3′). In addition, ‘no RT’ control reactions were performed omitting the reverse transcriptase to confirm the absence of genomic DNA. cDNA synthesis was performed at 50°C for 30 min, and denaturation temperature was 94°C for 3 min. For amplification, denaturation was 94°C for 30 s per cycle, annealing temperature was 55°C for 30 s per cycle and extension temperature 72°C for 1 min per cycle. The final extension temperature was 72°C for 10 min. The Orai1 primer samples were amplified for 22 cycles, the Orai2 for 27 cycles, the Orai 3 and Stim1 for 25 cycles, with each being compared to its own control (STIM1 alone). Quantitative RT-PCR was used to assess the effectiveness of siRNA knockdown. For this, m3-HEK cells were transfected as above, with 1 μm of either an Alexa Fluor 546 tagged negative control siRNA (Qiagen), or similarly tagged siRNA targeting Orai1, Orai2 or Orai 3 as appropriate. Constructs were identical to those used by Gwack et al. (2007). Total RNA was extracted 24 h later using RNAeasy (Qiagen). The integrity of the RNA was confirmed (Bioanalyser, Agilent), and reverse transcribed prior to real-time PCR analysis using an Applied Biosystems 7900HT Sequence Detection System, and predesigned (Applied Biosystems) primer pairs for Orai1, Orai2 and Orai3. Data were normalized to β-actin. Percentate changes in RNA levels relative to the controls were calculated using the 2−ΔΔCT method (Livak & Schmittgen, 2001).
Results
Effects of transient overexpression of STIM1 and Orai proteins ARC channel activities
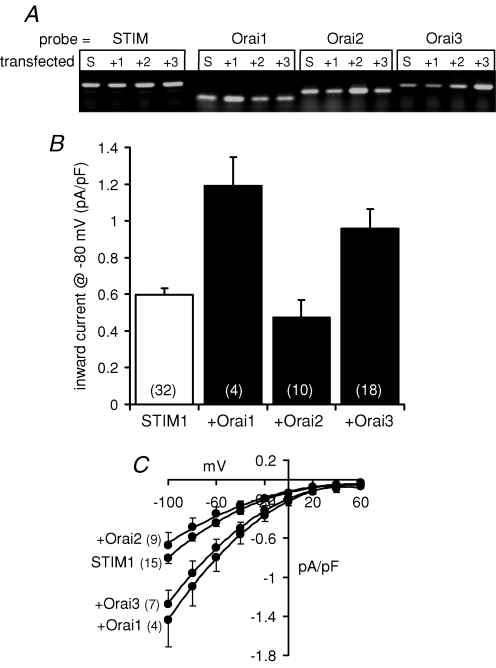
We began by examining the effects of overexpression of each of the three known mammalian members of the Orai family of proteins on ARC channel activities in the m3-HEK cell line. In previous studies, we have extensively characterized the biophysical properties, and regulation, of the coexisting CRAC conductances and ARC conductances in these cells (Mignen & Shuttleworth, 2000; Mignen & Shuttleworth, 2001; Mignen et al. 2001), and we recently demonstrated that the activities of both these channels were profoundly influenced by the levels of STIM1 expression (Mignen et al. 2007). The key importance of adequate STIM1 levels is emphasized by reports from several groups who have found that expression of Orai1 only induces an increase in store-operated Ca2+ entry and/or CRAC channel activity when coexpressed with STIM1, and that when expressed alone Orai1 can actually result in an inhibition of these parameters (Peinelt et al. 2006; Soboloff et al. 2006; Mercer et al. 2006). We therefore chose to examine the effects of each of the individual Orai proteins cotransfected with STIM1 on the ARC channel currents in these cells. Levels of each of the different Orai transcripts were examined by RT-PCR analysis of the translated mRNA levels (Fig. 1A), which indicated a specific, 2- to 3-fold increase in each of the relevant transcript level compared with their respective controls. The ARC channels were activated as previously described (Mignen et al. 2001) by exposure to exogenous arachidonic acid (8 μm) – a concentration that maximally activates these conductances, without causing any of the non-specific affects on membrane integrity that are routinely seen when using higher concentrations (≥ 20 μm). Previously, we have shown that the expression STIM1 alone in the m3-HEK cells results in an increase in inward currents through the ARC channels from a value of 0.33 ± 0.02 pA pF−1 (n = 13) at −80 mV, to 0.6 ± 0.03 pA pF−1 (n = 32) (Mignen et al. 2007). The simultaneous expression of Orai1 and STIM1 in these cells resulted in an approximate doubling of these currents to reach a value of 1.19 ± 0.16 pA pF−1 (n = 4) at −80 mV (Fig. 1A). Similarly, expression of Orai3 with STIM1 increased ARC channel currents by almost 50% (to a mean value of 0.88 ± 0.06 pA pF−1, n = 18, at −80 mV). In contrast, expression of Orai2 along with STIM1 resulted in an inward current at −80 mV of 0.47 ± 0.10 pA pF−1 (n = 10), a value that is not significantly different from that seen in the cells expressing STIM1 alone (P= 0.12). Together, these data suggest that both Orai1 and Orai3, but probably not Orai2, might be involved in the makeup of the ARC channels.
Figure 1. Effect of expression of STIM1, and the different Orai proteins, on ARC channel currents in the m3-HEK cells.
A, RT-PCR analysis of the overexpression of Orai1, 2 and 3 in m3-HEK cells. RT-PCR analyses were performed on cells transfected with a STIM1 construct alone (S) as an internal control, or along with constructs encoding Orai1 (+1), Orai2 (+2) or Orai3 (+3). Each of these was then probed with appropriate primers for STIM1, Orai1, Orai2 or Orai3, as indicated. B, cells were transfected with 0.6 μg DNA (STIM1) either alone, or with 0.2 μg of the relevant Orai DNA. Values are mean inward currents, +s.e.m. (n, numbers in parentheses), measured at −80 mV following activation by addition of 8 μm exogenous arachidonic acid. C, mean (± s.e.m.) current–voltage relationships of the maximally activated ARC channel currents in cells expressing STIM1 alone, or STIM1 plus each of the different Orai proteins, as indicated. n, numbers in parentheses.
CRAC and ARC channel currents in cells stably expressing STIM1
If both Orai1 and Orai3 are important for ARC channel function, then further experiments would probably require the transient overexpression of up to three different proteins (STIM1, Orai1 and Orai3), the levels of each of which appeared to have the potential to markedly influence the magnitude of the recorded currents. We therefore chose to develop a HEK293 cell line in which at least one of these proteins, namely STIM1, was being stably expressed at a constant level (STIM1-stable cells). In the particular system utilized (Flp-In™-293), the STIM1 construct is actually integrated into a single specific location in the genome and is therefore expressed at a predicted constant level in all cells, i.e. the STIM1-stable cells are isogenic.
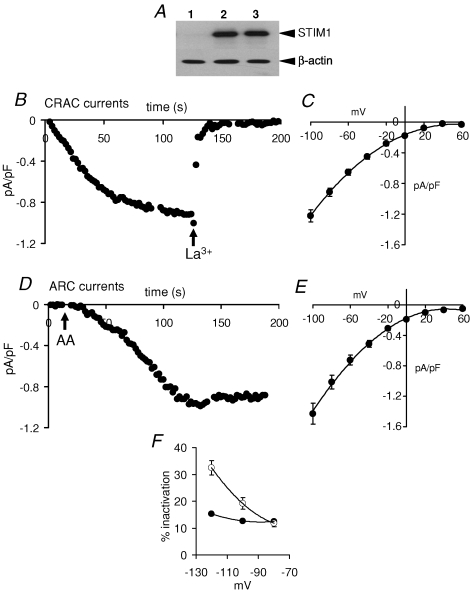
Overexpression of STIM1 in this STIM1-stable cell line was confirmed by Western blot (Fig. 2A). Maximal depletion of intracellular Ca2+ stores using a Ca2+-free pipette solution containing the potent InsP3 receptor agonist adenophostin A (2 μm) (Mignen et al. 2001), resulted in the development of a current displaying marked inward rectification, and a reversal potential of > 60 mV, and which was completely inhibited by La3+ (100 μm) (Fig. 2B and C). These features are consistent with this current reflecting the activity of the endogenous store-operated CRAC channels (Mignen et al. 2001, 2007). Mean values of the inward CRAC channel currents measured in this way were 0.87 ± 0.06 pA pF−1 at −80 mV (n = 29). In separate experiments, the arachidonic acid-regulated conductance in these cells was activated, as before, by exposure to exogenous arachidonic acid (8 μm). Importantly, as previously demonstrated for the m3-HEK cells (Mignen et al. 2001), preliminary studies confirmed that this concentration fails to induce any detectible release of intracellular Ca2+ in the STIM1-stable cells. Addition of arachidonic acid induced the appearance of currents consistent with the activation of the endogenous Ca2+-selective ARC channels (Fig. 2D and E), again displaying marked inward rectification, a reversal potential of > 60 mV, and inhibition by La3+ (100 μm). Inward currents through these ARC channels measured at −80 mV were 0.95 ± 0.06 pA pF−1 (n = 26). In both cases, the values recorded represent an approximate doubling of the magnitude of the respective currents recorded in the parental Flp-In™-293 cells without stable expression of STIM1 (inward currents at −80 mV equal to 0.42 ± 0.04 pA pF−1, n = 5, and 0.41 ± 0.03 pA pF−1, n = 6, respectively). In addition to their entirely distinct modes of activation, these two currents could be distinguished by examination of their kinetics during brief pulses to negative voltages (Mignen & Shuttleworth, 2000). Thus, the store-operated conductance displayed a characteristic fast inactivation during such brief hyperpolarizing pulses, the magnitude of which was clearly voltage dependent, reaching values greater than 30% at −120 mV (Fig. 2F). In contrast, rapid time-dependent changes in the magnitude of the arachidonate-activated conductance during such pulses were smaller (< 15%), and showed no voltage dependence. These features conform to those reported for the endogenous CRAC and ARC channels in HEK293 cells and, together with their specific modes of activation, confirm that the currents observed in these STIM1-stable cells represent the activities of these two distinct, yet coexisting, conductances.
Figure 2. Activation of CRAC and ARC currents in cells stably expressing STIM1.
A, representative Western blot showing STIM1 protein levels in control cells (lane 1) and in cells stably expressing STIM1 (STIM1-stable cells, lanes 2 and 3). Gels were stripped and reprobed with β-actin as a loading control. B, representative trace showing the activation of CRAC channel currents in a STIM1-stable cell. CRAC channels were maximally activated by use of a Ca2+-free pipette solution containing adenophostin (2 μm), and traces show the leak-subtracted inward currents measured at −80 mV. La3+ (100 μm) was added as indicated (arrow). C, mean current–voltage relationship (± s.e.m., n = 17) of the maximally activated CRAC channel currents. D, representative trace showing the activation of ARC channel currents in a STIM1-stable cell. ARC channels were maximally activated by addition where indicated (arrow) of exogenous arachidonic acid (8 μm). Traces show the leak-subtracted inward currents measured at −80 mV. E, mean current–voltage relationship (± s.e.m., n = 16) of the maximally activated ARC channel currents. F, mean (± s.e.m.) per cent fast-inactivation shown by CRAC channel current (○), and ARC channel currents (•) in STIM1-stable cells during brief pulses to the voltages indicated.
Expression of Orai1 and Orai3 in the STIM1-stable cells
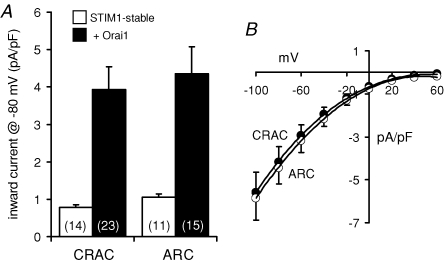
Transient overexpression of an Orai1 construct in these STIM1-stable cells resulted in marked increases in the recorded currents through both the CRAC channels and the ARC channels. Thus, depletion of internal Ca2+ stores in these cells resulted in the development of large inward currents at −80 mV that measured 3.93 ± 0.61 pA pF−1 (n = 23). In a similar way, addition of exogenous arachidonic acid (8 μm) also induced the activation of large inward currents measuring 4.35 ± 0.73 pA pF−1 at −80 mV (n = 15). Both these values represent an approximate 4.5-fold increase over the corresponding currents recorded in the untransfected STIM1-stable cells (Fig. 3A). The demonstration that overexpression of Orai1 induces a clear increase in the measured currents through the CRAC channels is consistent with several previous reports (Zhang et al. 2006; Peinelt et al. 2006; Soboloff et al. 2006; Mercer et al. 2006; Prakriya et al. 2006; Gross et al. 2007). However, our data show that Orai1 is also an essential component for the functional integrity of the store-independent ARC channels.
Figure 3. The effect of overexpression of Orai1 on CRAC and ARC channel currents in the STIM1-stable cells.
A, mean inward currents at −80 mV (+s.e.m.) were recorded in STIM1-stable cells (open bars) and following transfection with an Orai1 construct (1 μg DNA – filled bars). n, numbers in parentheses. B, mean (± s.e.m.) current–voltage relationship of the currents through the CRAC channels (•, n = 16), and the ARC channels (○, n = 17) in STIM1-stable cells overexpressing Orai1.
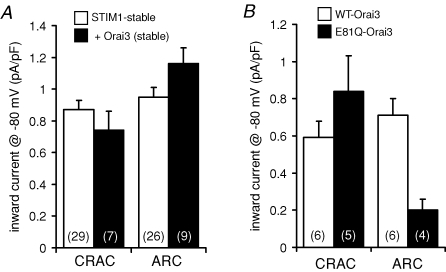
We next examined the possible requirement for Orai3 for the formation of functional ARC channels, as suggested by the data obtained in the m3-HEK cells. Transient overexpression of Orai3 in the STIM1-stable cells resulted in mean inward currents through the CRAC channels of 0.59 ± 0.09 pA pF−1 (n = 6) at −80 mV. The corresponding measured inward currents through the ARC channels were 0.71 ± 0.09 pA pF−1 (n = 6). These data indicate that overexpression of Orai3 actually induced small, but significant, decreases (P= 0.02 and 0.03, respectively) in the currents through both the CRAC and ARC channels, compared with the corresponding currents in the untransfected STIM1-stable cells. The decline in the CRAC channel currents could be due to the transfected Orai3 interacting with endogenous Orai1 to effect a reduction in the available Orai1 for the formation of homomeric CRAC channels. However, the decrease in ARC channel currents is more difficult to explain, especially given the clear increase induced by a similar transfection of Orai3 (along with STIM1) in the m3-HEK cells (Fig. 1). Increasing the concentration of DNA transfected (from 0.5 μg to 1.0 μg) failed to induce any significant change in the recorded currents through either the CRAC or ARC channels (data not shown). As this same construct has previously been shown to express effectively in the plasma membrane of HEK293 cells (Gwack et al. 2007), it is unlikely that this could account for the failure of Orai3 to affect these currents. Finally, to eliminate the possibility that the failure of the expression of Orai3 to induce any increase in ARC channel currents might result from non-specific negative effects of the transient expression of the protein, we chose to develop a cell line that stably expressed both STIM1 and Orai3 together. In these cells, measured inward currents at −80 mV were 0.74 ± 0.12 pA pF−1 (n = 7) for the CRAC channels and 1.16 ± 0.10 pA pF−1 (n = 9) for the ARC channels (Fig. 4A). Neither of these values were significantly different from the corresponding currents recorded in the parental STIM1-stable cells (P= 0.18 and 0.05, respectively).
Figure 4. The effect of Orai3 on CRAC and ARC channel currents in the STIM1-stable cells.
A, effect of stable expression of Orai3 on currents through the CRAC and ARC channels in the STIM1-stable cells. Mean inward currents at −80 mV (+s.e.m.) were recorded in STIM1-stable cells (open bars) and in same cells stably expressing Orai3 (filled bars). n, numbers in parentheses. B, the effect of transient overexpression (0.5 μg DNA) of wild-type Orai3 (open bars) and the E81Q mutant Orai3 (filled bars) in STIM1-stable cells on currents through CRAC channels and ARC channels. Values represent mean inward currents at −80 mV (+s.e.m.). n, numbers in parentheses.
Clearly, these data from the overexpression of Orai3 in the STIM1-stable cells are inconsistent with those observed in the m3-HEK cells, where overexpression of Orai3 and STIM1 induced an approximate 50% increase in the ARC channel currents (Fig. 1). To try and resolve this discrepancy, we considered the alternative approach of examining the effect of expressing a dominant-negative mutant of Orai3 in the cells stably expressing STIM1. Previous studies have shown that changing the glutamate at position 106 to a glutamine in Orai1 (or the equivalent mutation at position 178 in the Drosophila Orai) acts as a dominant negative and profoundly inhibits both CRAC channel activity and store-operated Ca2+ entry (Prakriya et al. 2006; Vig et al. 2006a; Yeromin et al. 2006; Gwack et al. 2007). The corresponding residue in Orai3 is the glutamate at position 81 (Prakriya et al. 2006; Gwack et al. 2007). Expression of the E81Q-mutant of Orai3 in the STIM1-stable cells resulted in inward currents through the CRAC channels of 0.84 ± 0.19 pA pF−1 (n = 5) at −80 mV (Fig. 4B), a value that was not significantly different from that seen following expression of the wild-type Orai3 (see above, P= 0.14). In contrast, expression of the same E81Q-mutant Orai3 markedly reduced ARC channel currents in the STIM1-stable cells, with inward currents through these channels equal to 0.20 ± 0.06 pA pF−1 (n = 4) at −80 mV (Fig. 4B), representing a 72% reduction compared with the values seen on expression of the wild-type Orai3 (see above, P < 0.001). These data indicate that, despite the inability of overexpression of Orai3 to induce an increase in the currents through the ARC channels, the presence of functional Orai3 protein is clearly essential for the activity of these channels.
Effects of Orai3 in cells stably expressing Orai1
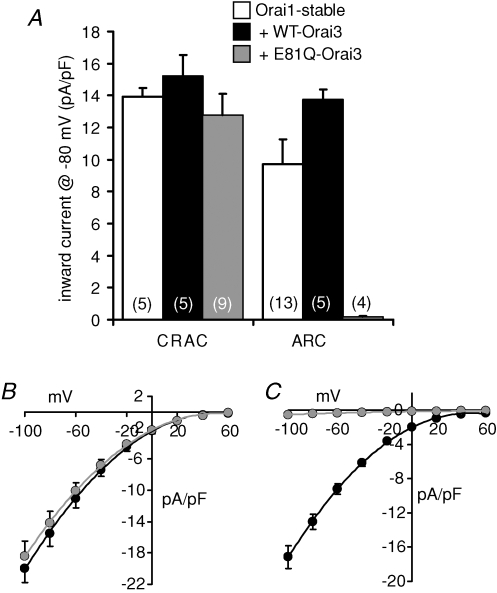
Given that the above data indicate that Oria3 is an essential component for functional ARC channel activity, the fact that profound increases in currents through the ARC channels are seen on overexpression of Orai1 alone (see above) suggests that endogenous levels of Orai1 in the STIM1-stable cells must be limiting for ARC channel activity. This limitation would be absent, or at least largely mitigated, in the presence of overexpressed Orai1. We therefore considered that the large ARC channel currents seen in the cells overexpressing Orai1 might be used to reveal more clearly the effect of Orai3 on these currents. To examine this, we developed a cell line that stably expressed both STIM1 and Orai1 together. As seen in the cells transiently expressing Orai1, activation of store-operated CRAC channels by depletion of internal Ca2+ stores in these Orai1-stable cells resulted in the development of large inward currents measuring 13.91 ± 0.57 pA pF−1 (n = 5) at −80 mV (Fig. 5A). Similarly, activation of the ARC channels by addition of exogenous arachidonic acid (8 μm) also induced the development of large inward currents measuring 9.71 ± 1.57 pA pF−1 (n = 13) at −80 mV (Fig. 5A).
Figure 5. The effect of Orai3 on CRAC and ARC channel currents in STIM1-stable cells stably expressing Orai1.
A, mean inward (+s.e.m.) currents through the CRAC channels and the ARC channels recorded at −80 mV in the STIM1-stable cells stably expressing Orai1 (open bars), and in the same cells following transfection (1 μg DNA) with either wild-type Orai3 (black bars), or the E81Q mutant Orai3 (grey bars). n, numbers in parentheses. B, mean (± s.e.m.) current–voltage relationships of CRAC channels following transfection with wild-type Orai3 (black circles, n = 5) or the E81Q mutant Orai3 (grey circles, n = 9). C, mean (± s.e.m.) current–voltage relationships of ARC channels following transfection with wild-type Orai3 (black circles, n = 5) or the E81Q mutant Orai3 (grey circles, n = 4).
Transient expression of Orai3 in these Orai1-stable cells resulted in the appearance of inward currents through the CRAC channels of 15.19 ± 1.35 pA pF−1 (n = 5) at −80 mV, a value that was not significantly different from that recorded in the untransfected Orai1-stable cells (P= 0.07) (Fig. 5A). Similar overexpression of Orai3 in these cells resulted in inward currents through the ARC channels of 13.74 ± 0.66 pA pF−1 at −80 mV (n = 5), representing a significant increase of more than 40% over the same currents measured in the untransfected cells stably expressing Orai1 (P= 0.02) (Fig. 5A). We next compared these effects of expressing the wild-type Orai3 in the Orai1-stable cells with those seen on transfection of the dominant-negative E81Q-mutant of Orai3. Transient overexpression of the E81Q-mutant Orai3 resulted in inward currents through the CRAC channels of 12.79 ± 1.30 pA pF−1 (n = 9) at −80 mV, a value that was not significantly different from that recorded in the same cells expressing the wild-type Orai3 (see above, P= 0.11) (Fig. 5A and B). In marked contrast, expression of the E81Q-mutant Orai3 in the Orai1-stable cells essentially eliminated the large inward currents at −80 mV through the ARC channels, reducing them to a value of only 0.19 ± 0.06 pA pF−1 (n = 4), a reduction of almost 99% compared with the same currents measured in the Orai1-stable cells expressing the wild-type Orai3 (Fig. 5A and C).
Clearly then, the large increases in ARC channel currents induced by expression of Orai1 are critically dependent on the presence of functional Orai3, whilst this has no effect on the corresponding increase in CRAC channel currents induced by expression of Orai1. This supports our contention that endogenous levels of Orai1 are a limiting factor for ARC channel activity in the STIM1-stable cells and, importantly, confirms the essential and specific requirement of Orai3 for ARC channel activity.
Effects of reducing Orai levels on ARC channel activities
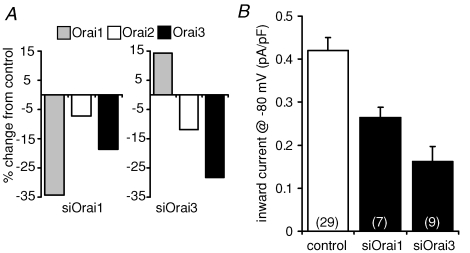
The above experiments have clearly indicated that both Orai1 and Orai3 are required for the development of functional ARC channel activity. However, a possible concern is that all these studies have relied on the overexpression of the relevant constructs (either in their wild-type form, or as dominant-negative mutants). An alternative approach is to reduce endogenous levels of these proteins by transfection of appropriate siRNA duplexes. We therefore examined the effect of transfection of siRNA duplexes targeting either Orai1 or Orai3 on ARC channel currents in the m3-HEK cells. Quantitative RT-PCR was used to examine the effectiveness of the corresponding siRNA duplexes to reduce the relevant Orai transcripts, and indicated an approximate 30–35% reduction in each case (Fig. 6A). Despite this relatively modest reduction in transcript levels, inward currents from the endogenous ARC channels were reduced from a value of 0.42 ± 0.03 pA pF−1 (n = 8) at −80 mV in control cells transfected with a scrambled siRNA, to 0.26 ± 0.02 pA pF−1 (n = 19) in cells transfected with the Orai1 siRNA construct – a reduction of almost 40% (Fig. 6B). Similar transfection with an siRNA duplex targeting Orai3 reduced the corresponding currents by more than 60% to a value of only 0.16 ± 0.01 pA pF−1 (n = 14) (Fig. 6B). Both of these reductions were highly significant (P= 0.0004 and 1.04 × 10−5, respectively). Together, these data are entirely consistent with our demonstrated requirement of both Orai1 and Orai3 for functional ARC channel activity. However, it should be noted that the quantitative PCR data indicate a rather complex response to these duplexes. Thus, the apparent reduction of approximately 15–20% in Orai3 in the cells transfected with the Orai1 siRNA (Fig. 6A) means that the observed reduction in ARC channel currents cannot be unequivocally assigned to an effect of Orai1 levels alone, as the observed changes in both Orai1 and Orai3 would be predicted to result in an inhibition of these currents. In contrast, transfection with the siRNA targeting Orai3 resulted in an unexpected small increase in Orai1 transcript levels. Interestingly a similar effect, although of much greater magnitude, was also seen in the data of Gwack et al. (2007). Based on our previous data, such an increase would be predicted to increase ARC channel currents, which means that the observed decrease in the currents recorded in these cells only serves to emphasize the essential requirement for Orai3 for the activity of these channels.
Figure 6. The effect of siRNA against Orai1 and Orai3 on ARC channel currents in m3-HEK cells.
A, effect of transfection of siRNA constructs against Orai1 (left panel) and Orai3 (right panel) on Orai protein levels. Values are means of duplicate assays and are expressed as the percentage change in RNA levels of Orai1 (grey), Orai2 (white), and Orai3 (black), as assessed by quantitative RT-PCR. B, mean (+s.e.m.) inward ARC channel currents measured at −80 mV in cells transfected with a control siRNA, or with siRNAs against Orai1, or Orai3 as indicated. n, numbers in parentheses.
Discussion
Recent studies have shown that the protein Orai1 is an essential component for the functional activity of the store-operated CRAC channels (Feske et al. 2006; Vig et al. 2006b; Zhang et al. 2006). Moreover, the effects of mutations of specific acidic residues in the putative transmembrane domains 1 and 3 in this protein indicated that Orai1 is an essential pore subunit of these channels (Prakriya et al. 2006; Vig et al. 2006a; Yeromin et al. 2006). The overall basic biophysical similarities shown by CRAC channels, and the store-independent, arachidonic acid-activated ARC channels, together with the demonstration that they are both critically regulated by the protein STIM1, albeit in an entirely distinct way, suggested that these two channels may be molecularly related. Based on this, we have examined the possibility that members of the Orai family of proteins may also be involved in forming functional ARC channels.
Here, we have demonstrated that overexpression of wild-type Orai1 along with STIM1 not only induced large increases in the recorded CRAC channel currents, as previously reported (Peinelt et al. 2006; Soboloff et al. 2006; Mercer et al. 2006; Gross et al. 2007; DeHaven et al. 2007), but also produced similar increases in currents through the ARC channels. Similar overexpression of Orai3 with STIM1 significantly increased ARC channel currents in the m3-HEK cells, but failed to do so in the STIM1-stable cells. However, further examination revealed that expression of a dominant-negative mutant Orai3 in the STIM1-stable cells profoundly, and specifically, reduced ARC channel currents without affecting the currents through the coexisting CRAC channels. More dramatically, expression of the same dominant-negative Orai3 mutant in cells stably expressing Orai1 essentially eliminated the large ARC channel currents recorded in these cells, whilst having no significant effect on currents through the CRAC channels. Finally, consistent with the above findings, we showed that siRNA knockdown or either Orai1 or Orai3 resulted in the significant inhibition of endogenous ARC channel currents in the m3-HEK cells. Based on these data, we conclude that Orai3, along with Orai1, does indeed play a critical, and specific, role in determining the activity of the ARC channels.
Why, then, did the overexpression of Orai3 fail to affect the ARC channels currents in the STIM1-stable cells, whilst this same procedure induced a clear increase in the m3-HEK cells? The evidence suggests that this results from the fact that constitutive levels of Orai1 in the STIM1-stable cells act as a limiting factor for functional ARC channel activity. Consistent with this, we showed that simple overexpression of Orai1 alone in these cells was sufficient to induce large increases in ARC channel currents. Moreover, in the presence of stably overexpressed Orai1, expression of Orai3 did indeed result in a significant increase in the ARC channel currents. Importantly however, the effects of overexpression of Orai1 on these currents are essentially obliterated by expression of a dominant-negative Orai3 mutant. We conclude therefore that both Orai1 and Orai3 are required essential components for the expression of functional ARC channels. Such an interacting combination of Orai1 and Orai3 is certainly feasible, as it has been shown that Orai1, in addition to forming homomultimers, can also form heteromultimers with Orai3 (Gwack et al. 2007), and both Orai1 and Orai3 are known to be expressed together in a wide variety of different tissues (Gwack et al. 2007).
Consistent with the majority of studies published to date, we could find no requirement for Orai3 in influencing the activity of the store-operated CRAC channels, which appear to depend on Orai1 alone (Mercer et al. 2006; Takahashi et al. 2007; Dehaven et al. 2007). Indeed, where studied, the other Orai family members have generally been reported as being variably much less effective, non-effective or even inhibitory to the activation of store-operated Ca2+ entry and CRAC channel activity (Mercer et al. 2006; Lis et al. 2007; Gross et al. 2007; Gwack et al. 2007). However, it has been reported that that coexpression of STIM1 and Orai3 at high levels was able to rescue store-operated Ca2+ entry in HEK293 cells pretreated with siRNA to Orai1 (Mercer et al. 2006), and that the combined overexpression of Orai3 and STIM1 was able, at least partially, to reconstitute Ca2+ influx in fibroblasts obtained from homozygous members of the SCID pedigree (Gwack et al. 2007). It seems, however, that these effects of Orai3 are, in some way, unique to the specific ‘rescue’ protocols used in these studies as the same reports note that overexpression of Orai3, either alone or with STIM1, failed to affect store-operated Ca2+ entry or CRAC-like currents in normal HEK293 cells (Feske et al. 2006), and knockdown of Orai3 had no effect on store-operated Ca2+ entry in cells not expressing the SCID mutation (Gwack et al. 2007). In addition, Lis et al. (2007) have recently reported that overexpression of Orai3 in STIM1-expressing HEK293 cells resulted in a 15- to 20-fold increase in store-operated channel currents. However, these authors make clear that the effect they describe was entirely dependent on the use of a specific vector and, using the same vector, the corresponding currents seen on overexpression of Orai1 were some 60-fold larger than those recorded in control STIM1-expressing cells (see also Peinelt et al. 2006). Moreover, as these authors note, many of the biophysical and pharmacological properties of the store-operated currents obtained under these conditions do not correspond with those of the endogenous CRAC channel currents (Lis et al. 2007). The potential problems of interpretation of data obtained from experiments involving excessive overexpression of proteins are well-known, and can be further exacerbated when the system under study involves essential interactions between multiple components, as is the case in the regulation of both store-operated and store-independent modes of Ca2+ entry. For this reason, in the studies reported here, we deliberately chose to use a system where the maximum currents recorded in any overexpression experiment were no more than 10- to 15-fold larger than the currents seen in untransfected cells, similar to the approach previously used by Zhang et al. (2006).
Our finding that ARC channel activity is dependent, not only on STIM1, but also on both Orai1 and Orai3 has certain important implications. First, as was previously shown for STIM1 (Mignen et al. 2007), the requirement of Orai1 for ARC channel activity means that simply demonstrating that a Ca2+ entry pathway displays a dependence on the presence of this protein does not, by definition, imply that such a pathway involves the CRAC channels or, indeed, store-operated pathways in general. Such conclusions would obviously require a precise characterization of the specific conductance involved. Secondly, as noted above, the requirement for three distinct proteins for normal functional activity of these channels can lead to significant complications in the interpretation of experiments involving simple overexpression and/or knockdown of any one of these components. Such complications were evident in the effects we observed with expression of Orai3 in the STIM1-stable cells versus those in the m3-HEK cells, where the nature of the resulting effects appeared to depend on the corresponding levels of Orai1 in each case.
In conclusion, we have shown that members of the Orai family of proteins are essential components of the store-independent ARC channels as well as the store-operated CRAC channels, although they differ in their precise composition. This, together with their shared ability to be regulated by STIM1 albeit via entirely distinct mechanisms (Mignen et al. 2007), indicates that ARC and CRAC channels are evolutionarily related. However, as demonstrated previously (Mignen et al. 2001; Shuttleworth et al. 2004), functional studies show that by adopting unique modes of activation they evolved to operate under different specific conditions of stimulation and to serve distinct roles in the overall regulation of agonist-activated Ca2+ signals.
Acknowledgments
We thank Drs Anjana Rao and Patrick Hogan (CBR Institute, Harvard) for generously providing essential constructs, and Pauline Leakey for excellent technical assistance. This work was supported by National Institutes of Health Grants GM040457 to T.J.S. In addition, O.M. was supported in part by funds from the Alfred and Eleanor Wedd Endowment.
References
- DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium Inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Gross SA, Wissenbach U, Philipp SE, Freichel M, Cavalie A, Flockerzi V. Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2007;282:1935–19384. doi: 10.1074/jbc.M701962200. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-Hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. IARC, a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the non-capacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J Biol Chem. 2001;276:21365–21374. doi: 10.1074/jbc.M102311200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J Biol Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Yule DI, Shuttleworth TJ. Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells. J Physiol. 2005;564:791–801. doi: 10.1113/jphysiol.2005.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ. What drives calcium entry during [Ca2+]i oscillations?– challenging the capacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ. Receptor-activated calcium entry channels – who does what, and when? Sci STKE. 2004:e40. doi: 10.1126/stke.2432004pe40. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Evidence for a non-capacitative Ca2+ entry during [Ca2+] oscillations. Biochem J. 1996;316:819–824. doi: 10.1042/bj3160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Murakami M, Watanabe H, Hasegawa H, Ohba T, Munehisa Y, Nobori K, Ono K, Iijima T, Ito H. Essential role of the N-terminus of murine Orai1 in store-operated Ca2+ entry. Biochem Biophys Res Commun. 2007;356:45–52. doi: 10.1016/j.bbrc.2007.02.107. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]