Abstract
The dorsal-side-up body posture of standing quadrupeds is maintained by coordinated activity of all limbs. Somatosensory input from the limbs evokes postural responses when the supporting surface is perturbed. The aim of this study was to reveal the contribution of sensory inputs from individual limbs to the posture-related modulation of pyramidal tract neurons (PTNs) arising in the primary motor cortex. We recorded the activity of PTNs from the limb representation of motor cortex in the cat maintaining balance on a platform periodically tilted in the frontal plane. Each PTN was recorded during standing on four limbs, and when two or three limbs were lifted from the platform and thus did not signal its displacement to motor cortex. By comparing PTN responses to tilts in different tests we found that the amplitude and the phase of the response in the majority of them were determined primarily by the sensory input from the corresponding contralateral limb. In a portion of PTNs, this input originated from afferents of the peripheral receptive field. Sensory input from the ipsilateral limb, as well as input from limbs of the other girdle made a much smaller contribution to the PTN modulation. These results show that, during postural activity, a key role of PTNs is the feedback control of the corresponding contralateral limb and, to a lesser extent, the coordination of posture within a girdle and between the two girdles.
When standing, quadrupeds maintain a specific, dorsal-side-up body posture due to the activity of the postural control system. This system is driven by sensory feedback signals and generates corrective motor responses when the body orientation deviates from the desired one (for review, see Horak & Macpherson, 1996; Macpherson et al. 1997a; Massion, 1998; Deliagina & Orlovsky, 2002; Deliagina et al. 2006b).
Lesion experiments have shown that the forebrain, and the motor cortex in particular, are not necessary for the occurrence of essential aspects of postural behaviour (Magnus, 1924; Chambers & Lin, 1957; Bard & Macht, 1958; Adkins et al. 1971; Dubrovsky et al. 1974; Beloozerova & Sirota, 1988, 1993a). On the other hand, recent experiments with recording the activity of cortical neurons in freely behaving animals have shown that this activity strongly correlates with postural corrections (Beloozerova et al. 2003b, 2005), suggesting that the motor cortex is involved in some aspects of the postural control. However, the role of the motor cortex in postural control is not clear (for discussion of this problem, see Jacobs & Horak, 2007). To understand the functional role of cortical activity in the control of body posture, one has to answer two questions: (1) What is the origin of posture-related cortical activity? (2) What are the motor effects of this activity?
In the present study, we addressed the first of these questions and assessed the origin of posture-related cortical activity. We used our previously developed experimental design. A cat stood on a platform and maintained balance when the platform was periodically tilted in the frontal plane. It was previously shown that in these experimental conditions the postural system equally well compensates for predictable and unpredictable perturbations, suggesting that it can well operate on the feedback principal (Beloozerova et al. 2005). We examined the tilt-related activity of the main cortical output – pyramidal tract neurons (PTNs) from the limb representation of the motor cortex. In the previous study it was found that almost all PTNs (both from the forelimb and from the hindlimb representations) were profoundly modulated in the rhythm of tilts (Beloozerova et al. 2005). What are the sources of this modulation?
The postural system operates on the basis of sensory information. It was shown that corrective postural responses, underlying trunk stabilization in quadrupeds (in the feedback mode of postural control), are driven primarily by the signals from limb mechanoreceptors rather than by vestibular and visual inputs (Inglis & Macpherson, 1995; Deliagina et al. 2000; Stapley et al. 2002; Beloozerova et al. 2003b). The contribution of sensory signals from different limbs to the generation of PTN postural responses was examined in the present study.
In standing quadrupeds, each of the four limbs participates in supporting the body weight. When the animal's posture is perturbed, each of the limbs contributes to the generation of a corrective motor response (Macpherson, 1988a,b; Macpherson et al. 1997a; Beloozerova et al. 2003b). To join the efforts of individual limbs, they have to be accurately coordinated. Thus, the postural control system performs two main functions – the intralimb coordination based on local reflexes in each of the limbs, and the interlimb coordination based on interlimb influences (Deliagina et al. 2006a; see also the functional scheme of the postural system in Fig. 9B. The goal of the present study was to assess the participation of the motor cortex in these two aspects of postural control. If the motor cortex is primarily involved in the intralimb coordination, the PTN activity would mainly depend on the afferent signals from the ‘own’ limb (to which the neuron projects). If the motor cortex participates in the interlimb coordination, the PTN activity would reflect the postural activity of other limbs.
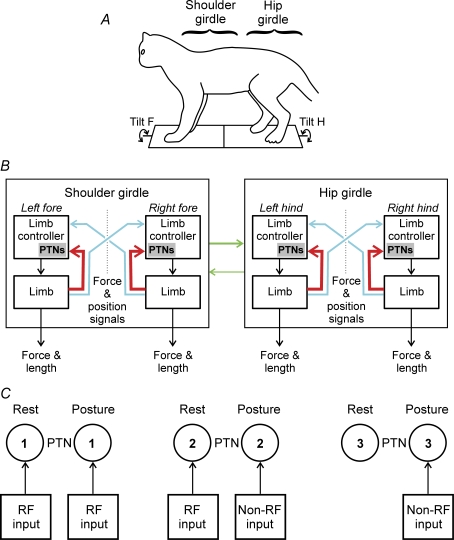
Figure 9. Role of PTNs in the postural system stabilizing the dorsal-side-up trunk orientation in the cat.
A and B, proposed scheme for sensorimotor processing in the postural system stabilizing the dorsal-side-up trunk orientation in the cat (adapted from Deliagina et al. 2006a). A, the system consists of two subsystems, one for the shoulder girdle and the other for the hip girdle. They compensate for tilts of the anterior and posterior parts of the body, respectively (Tilt F and Tilt H). B, each subsystem includes two controllers, one for the left limb and one for the right limb. Each limb controller contains a reflex mechanism driven by somatosensory input from its own limb. These local reflexes partly compensate for tilts. The limb controllers also receive somatosensory input (direct or subjected to processing) from the opposite limbs. The motor responses to these crossed influences are added to the local reflexes. The forelimb controllers exert influences on the hindlimb controllers promoting their coordination. Reversed influences are much smaller. The PTNs constitute a part of each limb controller; they are primarily involved in the feedback control of their own limb. The corresponding sensory influences are shown by thick red lines. The PTNs are less involved in the coordination of activity between the two limbs within a girdle, and between the shoulder and hip girdles. The corresponding influences are shown by thin blue and green lines. C, role of signals from the receptive field in modulation of PTNs. Three types of PTNs are shown: 1, PTN in which the receptive field input (RF input) controls the activity both at rest and in the postural task; 2, PTN in which the receptive field input controls the activity only at rest – the activity in the postural task is controlled by a different sensory input (Non-RF input); 3, PTN in which the receptive field input is absent – the activity in the postural task is controlled by a special sensory input (Non-RF input).
To assess a contribution of input from a given limb to the PTN activity, in the present study we used the recently developed method of varying the number of limbs supporting the body (Deliagina et al. 2006a). In the cat balancing on the tilting platform, we lifted one limb or a group of limbs from the platform, and compared the PTN responses to the platform tilts in control and in the limb-lifted condition. These experiments have shown that the tilt-related modulation of the activity in a PTN depended primarily on the sensory input from the corresponding contralateral limb. The input from the ipsilateral limb, as well as the inputs from the limbs of the other girdle made a much smaller contribution to the PTN modulation. These findings strongly suggest that, in the postural task, the PTNs are primarily involved in the feedback control of their own limb (intralimb coordination) and, to a lesser extent, in the coordination of activity between the two limbs within a girdle, and between the two girdles.
It is known that, in the resting animal, the PTNs controlling a given limb usually receive excitatory or inhibitory influences from a certain group of receptors of this particular limb (from the area termed ‘peripheral receptive field’), and respond to different manipulations with the limb such as touch, muscle palpation, flexion of joints, etc. (Asanuma, 1989). Do the sensory signals from the receptive field, observed in the resting animal, contribute to the generation of PTN responses in the postural task, or are these responses caused by other signals? To answer this question, for individual PTNs we compared the pattern of responses to tilt with that expected from the data on their receptive fields at rest. We have found that sensory input from the receptive field could be responsible for the tilt-related modulation in only a proportion of PTNs, whereas in other PTNs the modulation was caused by another sensory input.
A brief account of a part of this study has been published in abstract form (Karayannidou et al. 2006).
Methods
Recordings were obtained from two adult cats, a male and a female. Some of the methods have been described (Beloozerova et al. 2005; Prilutsky et al. 2005; Deliagina et al. 2006a) and will be reported briefly here. Experiments were conducted in accordance with NIH guidelines and were approved by the Barrow Neurological Institute Animal Care and Use Committee.
Surgical procedures
Surgery was performed using aseptic procedures. Anaesthesia was induced using ketamine (8 mg kg−1), which was followed by 2–5% isofluorane mixed with oxygen (flow rate 0.8 l min−1) administered by inhalation for the length of the surgical procedure. The skin and fascia were removed from the dorsal surface of the skull. At 10 points around the circumference of the head, stainless steel screws were screwed into the skull and connected together with a wire; the screw heads and the wire were then inserted into a plastic cast to form a circular base. Later, while searching for neurons before behavioural tests, awake cats were rigidly held by this base. The base was also used for fixation of connectors, a miniature micro drive, preamplifiers, contacts for stimulating electrodes, and a protective and electrically shielding cap.
A portion of the skull and dura above the left motor cortex, over approximately 0.6 cm2, were removed. The area of the motor cortex was visually identified by the surface features and photographed. The aperture was then covered by a plastic plate 1 mm thick, in which approximately 100 holes (0.36 mm in diameter) had been drilled and filled by sterile wax. The plate was fastened to the surrounding bone by orthodontic resin (Densply Caulk).
Two 26-gauge hypodermic guide tubes were implanted vertically above the medullar pyramid at the Horsley and Clarke coordinates (P 10, L 0.5) and (P 10, L 1.5), at the depth of H 0 for insertion of stimulating electrodes into the pyramidal tract later in the awake state.
Following the surgery, the cat was placed in a warm padded cage and respiration and reflexes were monitored until it regained conscious. Analgesic enrofloxacin (Baytril, purchased from Bayer; 2.5–5.0 mg kg−1) was administered intramuscularly on the day of surgery and two times a day for five to seven subsequent days. Triple antibiotic ointment bacitracin–neomycin–polymyxin was applied daily to wounds margins around the head implant for the duration of experiments.
Identification of cortical motor area
After several days of recovery, experiments were initiated by placing the animal in the head-restraining device. The cat was positioned on a table equipped with a foam rubber pad, encouraged to take a ‘sphinx’ position, and allowed to rest for several minutes. Then the base attached to the skull during surgery was fastened to the restraining device so that the resting position of the cat's head was approximated. This procedure minimized stress on the neck while the head was temporarily immobilized and the body was put in a comfortable position. Over several days, a number of sessions of increasing duration were used to accustom the cat to the head restrainer. After several training sessions, all cats sat quietly with their head restrained. They did not seem to be disturbed by the restrainer because they frequently fell asleep. Then neuronal recordings were initiated.
A detailed description of the area of recording was given earlier (Beloozerova et al. 2005). In brief, the area immediately adjacent to and inside the lateral half of the cruciate sulcus in the cat is considered to be the motor cortex. This is based on a considerable body of data obtained by means of inactivation, stimulation and recording techniques (Nieoullon & Rispal-Padel, 1976; Phillips & Porter, 1977; Armstrong & Drew, 1983a, 1985a,b; Vicario et al. 1983; Martin & Ghez, 1985, 1993; Beloozerova & Sirota, 1993b; Drew, 1993), as well as on histological considerations (Hassler & Muhs-Clement, 1964; Myasnikov et al. 1994; Ghosh, 1997). The fine mapping of the body parts in the cortex varies in different subjects, however (e.g. Myasnikov et al. 1997). In order to delineate the fore and hindlimb representations of the left motor cortex in each subject, three approaches have been used: (1) somatic receptive fields mapping, (2) observation of neuronal activity during voluntary movements, and (3) intracortical microstimulation.
Cell recording and identification
Neuronal activity was recorded extracellularly from the left motor cortex using either platinum–tungsten quartz insulated microelectrodes (40 μm outer diameter) pulled to a fine tip and mechanically sharpened (Reitboeck, 1983), or commercially available tungsten varnish insulated electrodes (125 μm outer diameter, Frederick Haer & Co). The impedance of the electrodes was 2–4 MΩ. After the electrode reached the depth of the cortex where clear responses of many neurons to limb movements could be observed (presumably layer V), two 200 μm platinum–iridium wires were slowly inserted and lowered into the medullar pyramid through the guide tubes implanted during surgery. Pulses of graded intensity (0.2 ms duration, up to 0.5 mA) were delivered through this bipolar electrode. The wires were fixed at the position which was most effective in eliciting antidromic responses in neurons of the motor cortex, and served as the pyramidal tract-stimulating electrode during subsequent experiments. The criterion for identification of antidromic responses was the test for collision of spikes (Bishop et al. 1962; Fuller & Schlag, 1976).
Signals from the microelectrode preamplifier, as well as from the platform position and body position sensors were amplified (CyberAmp 380, Axon Instruments), digitized with a sampling frequency of 30 kHz (microelectrode), and 400 Hz (sensors), displayed on the screen, and recorded to the disc of a computer by means of data acquisition software (Power-1401/Spike-2).
Before, during and after testing in each postural task, all encountered neurons were tested for antidromic activation. In addition, the waveform analysis was employed to discriminate and identify the spikes of a single neuron using the Power-1401/Spike-2 system waveform-matching algorithm. Only the neurons with a stable response latency and spike shape, which consistently satisfied the collision test, were used for the analysis.
The somatic receptive fields were examined in resting animals under conditions of head restraint. Stimulation was produced by palpation of muscle bellies, tendons, etc., and by passive movements of joints. The size of receptive fields was determined by listening to the audio monitor, and measuring the entire area, from which action potentials could be elicited. A directional selectivity was assessed by comparing the number of spikes elicited by stimulation in the optimal direction and the direction opposite from optimal.
Postural tests
The basic experimental arrangement for postural tests was the same as in our previous study (Beloozerova et al. 2005). The unrestrained cats were trained to quietly stand on a platform, which consisted of two parts – the F-platform under the forelimbs and the H-platform under the hindlimbs (Fig. 1A). They were rewarded by a paste food continuously ejected from the feeder. The feeder (a plastic tube of 18 mm outer diameter and 6 mm inner diameter) was positioned in front of the cat at a height of 21–23 cm (Fig. 1A). The platforms under the cat were periodically tilted in the frontal (roll) plane of the animal. A sine-like tilt trajectory was used, with a period of 1 s and amplitude of ± 15 deg. The cats were easily engaged in this postural task and maintained equilibrium during tilts. They tended to compensate for the platform tilts by performing lateral displacements of the body in relation to the supporting platform (postural corrections), which allowed them to hold the mouth against the feeder and keep licking food despite the platform tilts. Postural tests differed in the composition of the group of limbs, which supported the body (Deliagina et al. 2006a). This composition is reflected in the name of each test.
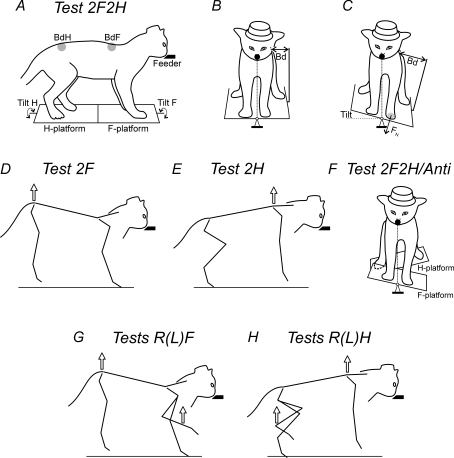
Figure 1. Postural tests.
The cat was standing on two platforms, one under the forelimbs (F-platform) and one under the hindlimbs (H-platform). The platforms were tilted in the frontal (roll) plane. The cat was continuously licking food from a feeder (feeder position is indicated by the filled bars in the side views and by the filled circles in the front views). A–C, in-phase tilts of the two platforms. A, the body outline (view from the right side) is shown for the horizontal position of the platforms. B and C, the body outline (view from the front) is shown for two positions of the platforms, horizontal and 15 degrees L, respectively. Postural corrections were characterized by measuring the lateral displacement of the upper point of the fore and hind parts of the trunk in relation to the corresponding platform (body displacements, BdF and BdH, in A–C). The normal component of the contact force produced by each limb was measured by means of a force plate (shown for only the left forelimb, FN in C). D, lifting the hindquarters. E, lifting the forequarters. F, antiphase tilt of the two platforms. G, lifting the hindquarters and one forelimb. H, lifting the forequarters and one hindlimb.
A contribution of afferentation from individual girdles (shoulder or hip) to the periodical modulation of PTNs was examined in the following tests.
Test 2F2H
The cat was standing on the platform with two forelimbs and two hindlimbs, and compensated for the platform tilts producing corrective movements by all four limbs (Fig. 1A–C).
Test 2F
The hindquarters of the cat were suspended in a hammock and slightly lifted, so that the hindlimbs were hanging freely and did not touch the platform, and thus were largely deprived of the ability to signal displacements of the platform to the motor cortex. In this test, postural function, i.e. compensation for the platform tilts, was performed by two forelimbs standing on the platform (Fig. 1D).
Test 2H
The forequarters of the cat were suspended in the hammock and slightly lifted, so that the forelimbs were hanging freely and did not touch the platform. They were thus deprived of the ability to signal displacements of the platform to the motor cortex. In this test, postural function was performed by two hindlimbs standing on the platform (Fig. 1E).
Test 2F2H/Anti
In this test, the two platforms were uncoupled, and the cat stood with its forelimbs on the F-platform and with its hindlimbs on the H-platform, while the platforms were tilted in antiphase (Fig. 1F).
A contribution of afferentation from individual limbs (right (R) or left (L)) of the same girdle to the tilt-related modulation of PTNs was examined in the following tests.
Tests RF and LF
The hindquarters of the cat were suspended in a hammock. In addition, one of the forelimbs was lifted. For this purpose, an experimenter took the limb by hand (in the elbow or the ankle region), lifted it for a few centimetres from the platform, and kept in this position during the test (about 10 s). Thus, in this test both of the hindlimbs and one of the forelimbs were deprived of the ability to signal displacements of the platform to the motor cortex. The postural corrective movements in these tests were performed by the right (test RF) or left (test LF) forelimb (Fig. 1G).
Tests RH and LH
The forequarters of the cat were suspended in the hammock. In addition, one of the hindlimbs was lifted. Thus, both of the forelimbs and one of the hindlimbs were not signalling displacements of the platform to the motor cortex. The corrective movements were performed by the right (test RH) or the left (test LH) hindlimb (Fig. 1H).
The following parameters were recorded during the postural tests: (i) the tilt angle of the platform (Tilt in Fig. 1A); (ii) postural corrections, that is the lateral displacements of the body in relation to the platform, separately in the fore and hind parts of the trunk (BdF and BdH in Fig. 1A); (iii) the normal component of contact forces under each foot (FN in Fig. 1C).
A representative example of the data recording (tests 2F2H and 2F) is shown in Fig. 2A. For each PTN, responses to 30–60 tilt cycles were collected in each test. Each of the cycles was divided into 20 equal bins, the peak of the right tilt was taken as the cycle onset. For each cycle, a phase histogram of a PTN spike activity was generated, then the activity was averaged over all cycles of the test (Fig. 2B). The Rayleigh test for directionality (P < 0.05) was used to determine whether the activity of the PTN was modulated in relation to tilts (Batshelet, 1981; Fisher, 1993). Only PTNs whose activity in test 2F2H was modulated were selected for this study.
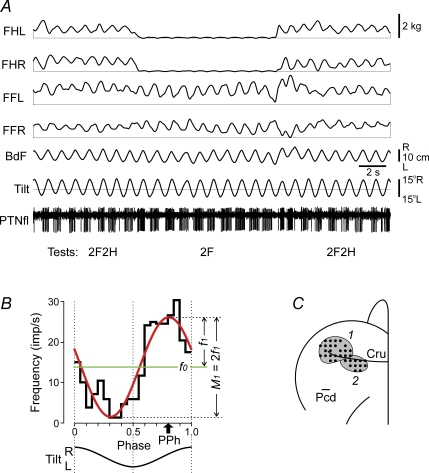
Figure 2. Representative example of postural and PTN responses in test 2F2H (standing on all four limbs) and test 2F (standing on two forelimbs), along with areas of recording in the motor cortex.
A, the following traces are shown: PTNfl – activity of a PTN from the forelimb representation of the motor cortex; Tilt – tilt of the platform; BdF – lateral position of the body; FHL, FHR – contact forces under hindlimbs, left and right; FFL, FFR – contact forces under forelimbs, left and right. B, characteristics of neuronal activity: the histogram of spike activity in the tilt cycle (black thick line); 1st harmonic of Fourier image (red line) with its amplitude (f1) and the value of modulation (M1) indicated; mean frequency in the tilt cycle (f0, green line); preferred phase of the discharge (PPh, arrow). C, areas of recording within representations of the fore- and hindlimb in the left motor cortex (1 and 2, respectively; Cru, cruciate sulcus; Pcd, postcruciate dimple). Microelectrode entry points into the cortex (cortical plate openings through which penetrations were made) are combined from two cats and shown by black circles.
For each modulated PTN, the following characteristics of the activity were assessed using Fourier image of the spike sequence (Fig. 2B): f(ϕ) =f0+f1cos(ϕ−ϕ1) +r(ϕ), where ϕ is the phase of the tilt cycle. The constant component f0 of the image provides the average frequency during the test. The first harmonic f1cos(ϕ−ϕ1) is a sine approximation of the one-peak modulation. (We have previously found that 94% of PTNs have one dominating peak in their response to tilts (Beloozerova et al. 2005).) The phase of the peak of the first harmonic indicates the preferred phase of neuronal discharge, ϕ1. Finally, r(ϕ) is the remainder after the first two terms of the series (a sum of higher harmonics). As a measure of periodical, tilt-related PTN modulation, we used the peak-to-peak value of the first harmonic (M1= 2f1).
All quantitative data are presented as the mean ± s.e.m. Statistical comparisons were made using Student's ttest, with the significance level set at P= 0.05.
Histological procedures
At the termination of experiments, cats were deeply anaesthetized with pentobarbital sodium. Several reference lesions were made in the region of the motor cortex from which neurons were sampled. Cats were then perfused with isotonic saline followed by a 10% formalin solution. Frozen brain sections of 50 μm thickness were cut in the regions of recording and stimulating electrodes. The tissue was stained for Nissl substance with cresyl violet. Position of stimulation electrodes in the medullar pyramids was verified by observation of electrode track gliosis. Positions of recording tracks in the motor cortex were estimated in relation to the reference lesions.
Results
Postural motor responses
Postural motor responses in different tests were similar in the two cats and did not differ from those described in our previous paper (Deliagina et al. 2006a). When all limbs were standing (test 2F2H), tilts of the platform evoked postural corrections, i.e. lateral displacements of the trunk in the direction opposite to tilt, with a peak-to-peak value of 6–8 cm (Fig. 2A). These corrective movements were caused by extension of the limbs on the side moving down, and flexion of the limbs on the opposite side. Due to postural corrections, cats maintained the dorsal side-up orientation, and stabilized the head position against the feeder.
When the two parts of the platform were tilted in antiphase (test 2F2H/Anti), cats stabilized the dorsal side-up orientation of both the forequarters and hindquarters. Corrective movements in this test were in antiphase relative to the corresponding platform, with the values similar to that in test 2F2H.
When only two forelimbs (test 2F) or only two hindlimbs (test 2H) were standing on the platform, tilts of the platform evoked postural corrections – lateral displacements of the forequarters or the hindquarters, respectively, in the direction opposite to tilt. These corrective movements were caused by extension of the limb on the side moving down, and flexion of the limb on the opposite side. The values of corrective movements in these tests were similar to that in test 2F2H. In test 2F, the cat effectively stabilized the head position against the feeder. In test 2H, the cat effectively stabilized the dorsal-side-up orientation of the hindquaters, with postural corrections of 5–6 cm peak-to-peak. In tests 2F and 2H, the active pair of limbs was loaded by half of the body weight.
When only one limb was standing on the platform (tests RF, LF, RH, LH), this limb was loaded by a half of the body weight. Tilts of the platform evoked corrective motor responses in the standing limb – extension when the platform under the limb was moving downward, and flexion when it was moving upward. In tests RF and LF, the position of the forequarters was effectively stabilized; in tests RH and LH the position of the hindquarters was also stabilized but less effectively.
Responses of individual PTNs
The activity of 149 PTNs (74 from the forelimb representation of the left motor cortex and 75 from the hindlimb representation) whose activity was modulated in relation to tilts and to postural responses evoked by the tilts were analysed (68 neurons from cat no. 1, and 81 neurons from cat no. 2). Figure 2C shows the microelectrode entry points into the cortex (cortical plate openings through which penetrations were made); they were combined from two cats. Matching between individual maps was accomplished by normalizing the length of the cruciate sulcus (Cru) and the distance from it to the postcruciate dimple (Pcd).
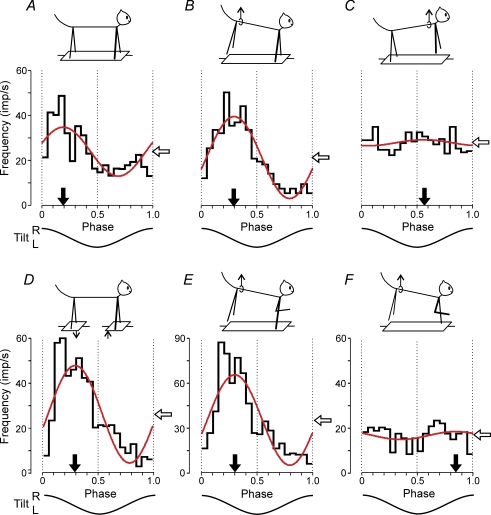
Figure 3 shows a representative example of PTN responses to tilts (PTN no. 3566). This neuron was recorded from the forelimb representation of the left motor cortex. In control (test 2F2H, Fig. 3A) the neuron exhibited a pronounced modulation of its discharge frequency, with the modulation value M1= 21.8 imp s−1, and the phase of the peak (preferred phase) ϕ1= 0.19. When the hindquarters were lifted and only the forelimbs were standing on the platform (test 2F, Fig. 3B), the response increased (M1= 36.2 imp s−1), and the preferred phase (ϕ1= 0.29) differed by only 0.1 from that in control. By contrast, when the forequarters were lifted and the hindlimbs were standing on the platform (test 2H, Fig. 3C), the response decreased significantly (M1= 2.6 imp s−1) and its temporal pattern changed – the neuron was slightly more active not in the first but in the second half of the cycle (ϕ1= 0.57). During antiphase tilts (test 2F2H/Anti, Fig. 3D) the response increased (M1= 44 imp s−1), and the preferred phase (in the cycle of P1 platform) was similar to that in control (ϕ= 0.29).
Figure 3. Activity of a PTN from the forelimb representation during different postural tests.
A, control (test 2F2H). B, standing on two forelimbs (test 2F). C, standing on two hindlimbs (test 2H). D, antiphase tilts of the F and H platforms (test 2F2H/Anti). E, standing on the right forelimb (test RF). F, standing on the left forelimb (test LF). For each test there are shown: (1) the phase histogram of spike activity in the tilt cycle, (2) the 1st harmonic of Fourier image (red line), (3) the mean frequency of discharge (white arrow), and (4) the preferred phase (black arrow).
When only the right forelimb was standing on the platform (test RF, Fig. 3E) the amplitude of modulation increased considerably (M1= 54 imp s−1), and the preferred phase was similar to that in control (ϕ1= 0.3). However, when only the left forelimb was standing on the platform (test LF, Fig. 3F) the response was small (M1= 3.6 imp s−1) and the neuron was slightly more active in the second half of the cycle (ϕ1= 0.85). From Fig. 3 one can also see that the mean frequency of the neuron (white arrow) in most of the tests was similar.
The results illustrated in Fig. 3 demonstrated that this particular PTN received its main tilt-related input from the contralateral (right) forelimb, and additional very small inputs from the ipsilateral forelimb and from the hindlimbs. The phases of additional inputs differed considerably from that of the main input. One should note, however, that lifting of the limb strongly reduced tilt-related movements of this limb but some small movements could still remain due to mechanical influences of the supporting limb. These residual limb movements could produce small rhythmical influences on PTNs of the lifted limb.
Results shown for PTN no. 3566 in Fig. 3 were typical for both fore- and hindlimb PTNs: in the majority of them, the tilt-related modulation was determined mainly by input from the contralateral fore- or hindlimb, respectively. These common features were reflected in the population characteristics of PTNs.
Population characteristics of PTNs
Three characteristics were used to describe postural responses in the forelimb and in the hindlimb PTN populations: (i) The value of modulation, M1; (ii) the mean frequency in the tilt cycle, f0– these values were averaged over the whole (forelimb or hindlimb) population of PTNs; and (iii) the distribution of preferred phases of response (ϕ1) of forelimb or hindlimb PTNs over the tilt cycle. To evaluate the contribution of different limbs to the generation of PTN responses to tilts, we compared these three values across different tests.
Influences from shoulder and hip girdles
Forelimb PTNs
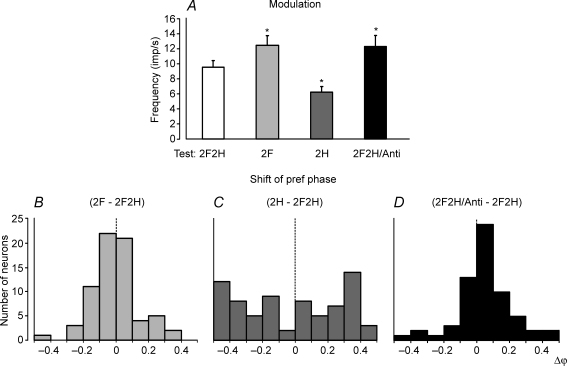
A contribution of postural mechanisms of an individual (shoulder or hip) girdle to the periodical modulation of the forelimb PTNs was examined by lifting the hind- or forequarters, as well as by tilting them in antiphase. Figure 4A shows that, when the cat stood on the forelimbs only (test 2F), the response slightly increased as compared to control (test 2F2H). By contrast, standing on only the hindlimbs (test 2H) led to a considerable decrease of the response. The values of responses in different tests (mean ± s.e.m.) are given in Table 1, together with the mean value of frequency in the tilt cycle.
Figure 4. Population characteristics of forelimb PTN responses in tests revealing influences from shoulder and hip girdles.
A, mean value of modulation. B–D, algebraic differences between preferred phases of individual PTNs in tests 2F and 2F2H (B), in tests 2H and 2F2H (C), and in tests 2F2H/Anti and 2F2H (D).
Table 1.
Characteristics of inputs to PTNs from different girdles
| Forelimb PTNs | Hindlimb PTNs | |||
|---|---|---|---|---|
| Modulation (imp s−1) | Frequency (imp s−1) | Modulation (imp s−1) | Frequency (imp s−1) | |
| 2F2H | 9.5 ± 0.8 | 11.9 ± 1.1 | 17.7 ± 1.5 | 18.2 ± 1.4 |
| 2F | 12.4 ± 1.3* | 13.4 ± 1.2 | 7.6 ± 0.8* | 17.3 ± 1.7 |
| 2H | 6.2 ± 0.7* | 15.6 ± 1.5* | 13.5 ± 1.4* | 19.1 ± 1.5 |
| 2F2H/Anti | 12.1 ± 1.5* | 13.2 ± 1.1* | 14.4 ± 1.5 | 16.6 ± 1.4 |
Lifting of the forequarters and lifting of the hindquarters produced also very different effects on the phases of PTN responses. A histogram in Fig. 4B shows phase shift in test 2F as compared to control. In the majority of neurons (58/69, or 84%), the phase shift was less than 0.2. By contrast, in test 2H, phase shift in the majority of neurons (49/73, or 67%) was more than 0.2 (Fig. 4C). Thus, lifting of the hindquarters produced a weak effect on the phases of forelimb PTNs, whereas lifting of the forequarters produced a strong effect.
An interaction of influences from the two girdles upon the forelimb PTNs was examined in test 2F2H/Anti, with antiphase tilts of the fore- and hindquarters. As shown in Fig. 4A and in Table 1, the response of PTNs to tilts and their mean frequency slightly increased in test 2F2H/Anti as compared to control (P < 0.05). We compared the phases of responses of individual PTNs in test 2F2H/Anti and test 2F2H. (In test 2F2H/Anti, phase measurements were performed in relation to the tilt cycle of the F-platform.) It was found that the phase shift in test 2F2H/Anti in relation to control was small (less than 0.2) in the majority of neurons (50/63, or 79%; Fig. 4D).
To summarize, these results suggest that the tilt-related modulation of the forelimb PTNs is primarily based on the sensory information coming from the forelimb afferents.
Hindlimb PTNs
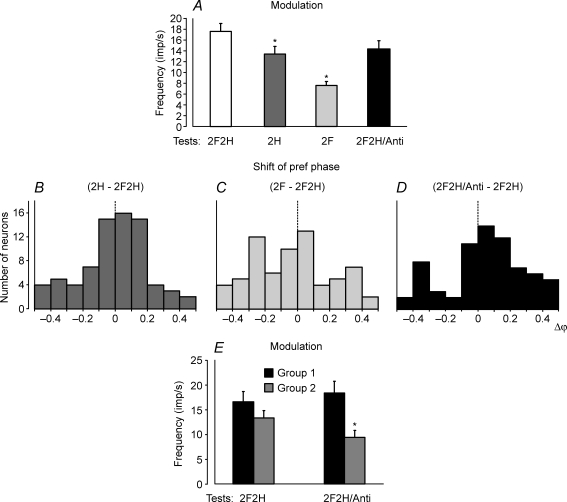
A contribution of postural mechanisms of individual girdles to the periodical modulation of the hindlimb PTNs was examined with the same methods as the forelimb PTNs, that is, by lifting the fore- or hindquarters, as well as by tilting them in antiphase. When the cat stood on the hindlimbs only (test 2H) the response decreased slightly as compared to control (test 2F2H). By contrast, standing on only the two forelimbs (test 2F) led to a considerable decrease of the response (Fig. 5A and Table 1). The mean frequencies in these tests did not differ significantly (Table 1).
Figure 5. Population characteristics of hindlimb PTN responses in tests revealing influences from shoulder and hip girdles.
A, mean value of modulation. B–D, algebraic differences between preferred phases of individual PTNs in tests 2H and 2F2H (B), tests 2F and 2F2H (C), and in tests 2F2H/Anti and 2F2H (D). E, mean value of modulation in group 1 (phase shift in D is less than 0.2) and group 2 neurons (phase shift in D is more than 0.2).
Lifting of the hindquarters and lifting of the forequarters produced different effects on the phases of PTN responses. A histogram in Fig. 5B shows the phase shift in test 2H in relation to control. In the majority of neurons (53/75, or 70%) it was less than 0.2. By contrast, in test 2F phase shift in the majority of neurons (35/68, or 51%) was more than 0.2 (Fig. 5C).
An interaction of influences from the two girdles upon the hindlimb PTNs was examined in test 2F2H/Anti. As shown in Fig. 5A and in Table 1, the response of PTNs to tilts in test 2F2H/Anti was similar to that in control. The mean frequency in tests 2F2H and 2F2H/Anti did not differ significantly. We also compared the phases of responses of individual PTNs in these tests. (In test 2F2H/Anti, phase measurements were performed in relation to the tilt cycle of the H-platform.) It was found that the population of hindlimb PTNs was not homogeneous – the phase shift in test 2F2H/Anti in relation to control was small (less than 0.2) in about a half of neurons (39/70, or 56%), and was larger (more than 0.2) in the other half (31/70, or 44%; Fig. 5D). We will designate these neurons as groups 1 and 2, respectively. The groups 1 and 2 also differed in the value of response in test 2F2H/Anti.
In Fig. 5E, we compare the responses of groups 1 and 2 PTNs in tests 2F2H and 2F2H/Anti. For group 1, the responses in tests 2F2H and 2F2H/Anti were similar (16.6 ± 2.0 against 18.4 ± 2.4 imp s−1; P > 0.05). For group 2, the response decreased with antiphase tilts (from 13.3 ± 1.5 imp s−1 in test 2F2H to 9.5 ± 1.4 imp s−1 in test 2F2H/Anti; P < 0.05).
To summarize, these results suggest that, for a portion of hindlimb PTNs (group 1), the tilt-related modulation is mainly caused by sensory influences from the hindlimbs. In another portion (group 2), sensory influences from the forelimbs also contribute noticeably to the modulation.
Influences from ipsilateral and contralateral limb
Forelimb PTNs
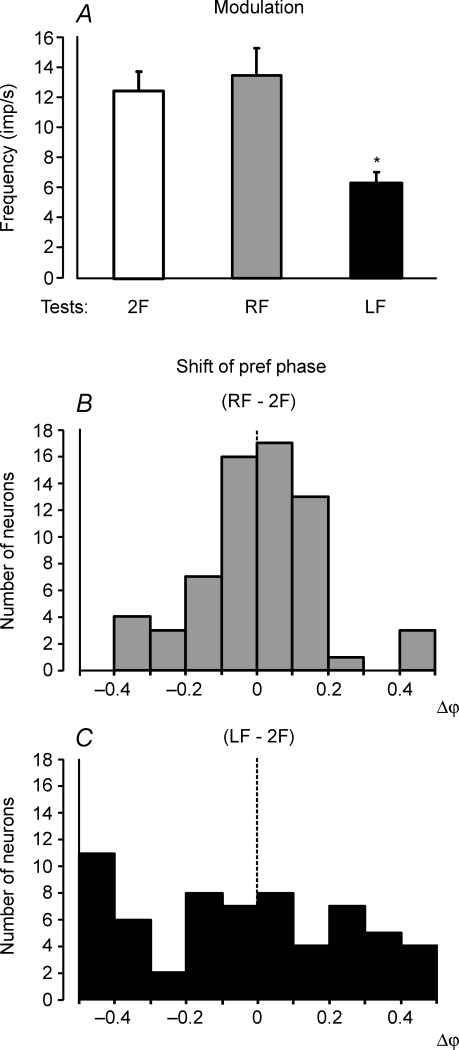
The contribution of postural mechanisms of a single forelimb to the periodical modulation of forelimb PTNs was examined by lifting one of the forelimbs in addition to lifting the hindlimbs.
Figure 6A shows that standing on the right forelimb only (test RF) caused a slight increase of the response compared to test 2F (standing on both forelimbs). By contrast, standing only on the left forelimb (test LF) caused a considerable decrease of the response. The values of responses in these tests (mean ± s.e.m.) as well as the mean values of the mean frequency in the cycle are given in Table 2. The mean frequencies in tests 2F, LF and RF did not differ significantly.
Figure 6. Population characteristics of forelimb PTN responses in tests revealing influences from individual limbs of the same girdle.
A, mean value of modulation. B and C, algebraic differences between preferred phases of individual PTNs in tests RF and 2F, and in tests LF and 2F, respectively.
Table 2.
Characteristics of inputs to PTNs from different limbs of the same girdle
Lifting of the right forelimb and lifting of the left forelimb produced different effects on the phases of PTN responses. A histogram in Fig. 6B shows shift of the preferred phase in test RF in relation to test 2F. In the majority of neurons (53/64 or 83%) the shift of preferred phase was less than 0.2. By contrast, in test LF the shift of the preferred phase was more than 0.2 in the majority of neurons (35/62 or 56%; Fig. 6C).
To summarize, these results suggest that the sensory input from the contralateral limb of the shoulder girdle contributes much more strongly to the tilt-related modulation of the forelimb PTNs than the input from the ipsilateral limb.
Hindlimb PTNs
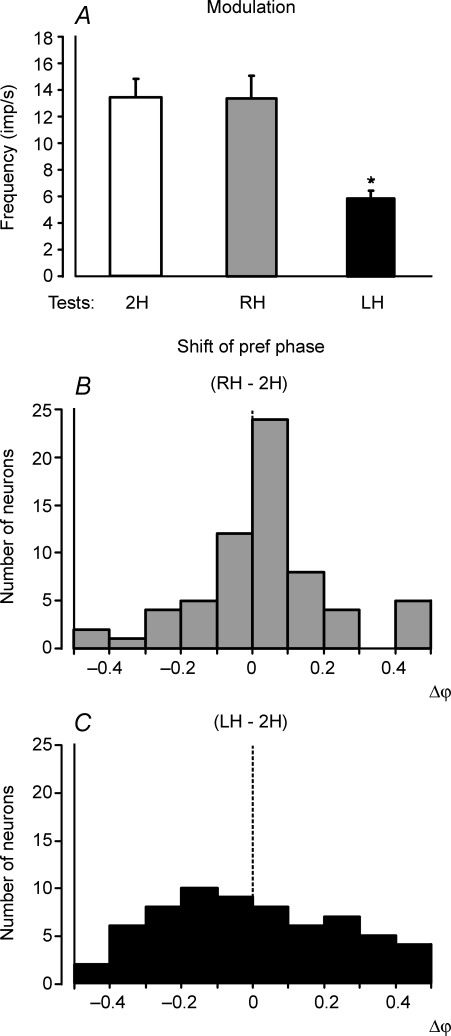
The contribution of postural mechanisms of a single hindlimb to the periodical modulation of hindlimb PTNs was examined in the same way as used for the forelimb PTNs. Figure 7A shows that standing on the right hindlimb (test RH) caused only a slight decrease of the response as compared to test 2H (standing on both hindlimbs). By contrast, standing on the left hindlimb (test LH) caused a significant decrease of the response. The values of the responses in these tests (mean ± s.e.m.), as well as the mean values of the mean frequency, are shown in Table 2. The mean frequencies in these tests did not differ significantly.
Figure 7. Population characteristics of hindlimb PTN responses in tests revealing influences from individual limbs of the same girdle.
A, mean value of modulation. B and C, algebraic differences between preferred phases of individual PTNs in tests RH and 2H, and in tests LH and 2H, respectively.
Lifting of the left or right hindlimb produced also different effects on the phases of PTN responses. A histogram in Fig. 7B shows the phase shift in test RH in relation to test 2H. In the majority of neurons (49/65, or 75%) the phase shift was less than 0.2. By contrast, in test LH the phase shift was more than 0.2 in nearly half of the neurons (32/65, or 49%) (Fig. 7C).
To summarize, these results suggest that the sensory input from the contralateral hindlimb contributes much more strongly to the tilt-related modulation of hindlimb PTNs than the input from the ipsilateral limb. One should note that, though lifting of the limb strongly reduced tilt-related movements of this limb, some small movements could still remain due to mechanical influences of the supporting limb. These residual limb movements could produce small rhythmical influences on PTNs of the lifted limb.
Role of input from receptive field in modulation of PTNs
Somatosensory receptive fields were tested in 133 PTNs (70 PTNs from the forelimb and 63 PTNs from the hindlimb representation). We found that 118 (89%) PTNs had excitatory receptive fields on the contralateral fore- or hindlimb, respectively. Only one PTN, which was recorded from the hindlimb area, had a receptive field stretching on both forelimb and hindlimb. Fourteen PTNs (10%) did not have any receptive field, and one cell was inhibited by passive manipulation of the hindlimb. Most of the receptive fields were ‘deep’, i.e. the cells responded to movements of joints and/or palpation of muscles. A summary of the positions of receptive fields of PTNs on different segments of the limbs is given in Table 3.
Table 3.
Receptive fields of forelimb (n = 70) and hindlimb (n = 63) PTNs
| Receptive field position | Forelimb PTNs (%) | Hindlimb PTNs (%) |
|---|---|---|
| Digits | 17 (1, 16) | 18 (7, 11) |
| Palm/Sole | 7 (1, 6) | 6 (3, 3) |
| Wrist/Ankle | 16 (10, 6) | 16 (6, 10) |
| Elbow/Knee | 16 (10, 6) | 17 (10, 7) |
| Shoulder/Hip | 21 (10, 11) | 20 (7, 13) |
| Whole Limb | 11 (1, 10) | 11 (0, 11) |
| Both Limbs | 0 | 2 |
| None | 11 | 10 |
Percentage of PTNs is indicated with a given receptive field defined by a segment and a joint. Numbers in brackets show portion of PTNs with similar and different (separated by a comma) responses to passive and active limb movements.
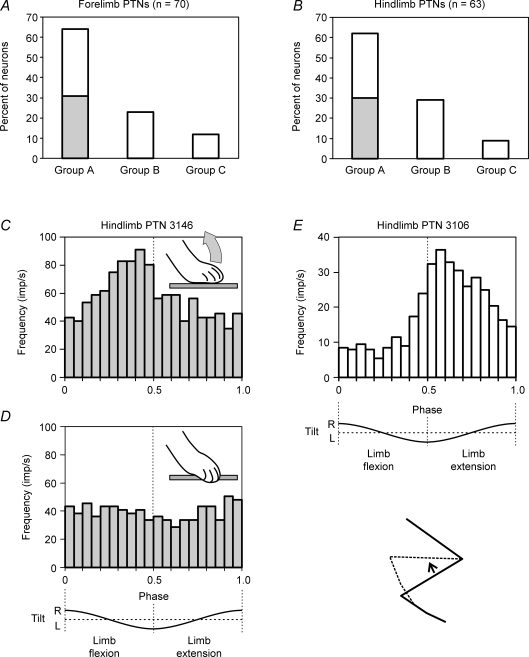
We separated the forelimb population and the hindlimb population into three groups each (Fig. 8A and B). Group A (64% of the forelimb PTNs and 62% of the hindlimb PTNs) included the cells with a directional preference in their response to receptive field stimulation. Group B (24 and 29%, respectively) included the cells with no such preference. Group C PTNs (12 and 9%, respectively) had no receptive fields.
Figure 8. Role of sensory input from the receptive field in modulation of PTNs.
A and B, relative number of PTNs in the forelimb population (A) and hindlimb population (B) which had a receptive field with a directional preference (group A), no such preference (group B), or did not have a receptive field (group C). A proportion of group A PTNs with similar responses to passive and active limb movements is shown in grey. C and D, an example of the hindlimb PTN (no. 3146) driven during postural task by afferents of its receptive field. At rest, the PTN was activated with the dorsal flexion of toes (inset in C). During postural tests, the PTN was also activated during dorsal flexion of toes in the first half of the cycle (C). When the paw was positioned on the edge of the platform so that during balancing toes did not flex dorsally (inset in D) and the afferents of the receptive field were not activated, the PTN was not modulated (D). E, an example of a hindlimb PTN (no. 3106) not driven by afferents of its receptive field during postural task. At rest, the PTN was activated by knee flexion (inset). In postural tests, the PTN was activated during knee extension.
For individual group A PTNs, we have compared the preferred direction of their response during passive flexion–extension movements of the limb with the direction of maximal response to active flexion–extension movements during postural corrections. In a half of PTNs these directions were the same. Those were PTNs from the forelimb representation (in 31% of the forelimb population, Fig. 8A) and also from the hindlimb area (in 30% of the hindlimb population, Fig. 8B). In another half of PTNs the preferred directions of responses in passive and active conditions were different.
An example of PTNs with similar responses in passive and active conditions is shown in Fig. 8C and D. This hindlimb PTN had a receptive field on the distal part of the limb. It was activated by passive dorsal flexion of the toes (inset in Fig. 8C). In the postural task, when standing on the tilting platform with the toes directed outward, the dorsal flexion of toes occurred in the first half of the cycle, when the right side of the platform moves upwards and the leg is shortening. In the postural task, the neuron was active during the first half of the cycle (Fig. 8C). Such similarity between the phases of activity in the passive and active conditions suggests that receptive field input might contribute to the tilt-related modulation of the PTN. We have directly demonstrated this by positioning the paw near the edge of the platform, so that the toes were flexed ventrally around its edge, and tilt of the platform did not result in their dorsal flexion and thus did not activate the receptive field afferents (inset in D). Under these conditions, the PTN was no longer modulated in response to tilts (Fig. 8D).
Figure 8E shows a hindlimb PTN with different responses in passive and active conditions. At rest, this cell was activated during flexion of the knee (inset in E). However, in the postural tests the neuron was active in the second half of the tilt cycle, i.e. during extension of the knee. It seems unlikely that, in the postural task, the response in this PTN could be generated on the basis of its receptive field input.
The neurons with similar responses to passive and active limb movements were found among cells whose receptive fields were positioned on different segments of the limbs; they constituted about a half of these PTNs (Table 3, numbers in brackets). However, among the forelimb PTNs with distal receptive fields on the palm and digits, only 2 out of 24 (9%) neurons exhibited similar responses.
Discussion
Possible functions of PTNs in the control of posture
When standing, the cat maintains a specific, dorsal-side-up body orientation in the frontal plane due to the activity of the postural control system. This system is driven by sensory feedback signals and generates corrective motor responses when the body orientation in the frontal plane deviates from the desired one. General organization of the postural system in the cat has been characterized in the previous study (Deliagina et al. 2006a). It was concluded that the system consists of two subsystems, one for the shoulder girdle and the other for the hip girdle (Fig. 9A). They compensate for tilts of the anterior and posterior parts of the body, respectively. Each subsystem includes two controllers, one for the left limb and one for the right limb (Fig. 9B). Each limb controller contains a reflex mechanism driven by somatosensory input from its own limb. These local reflexes partly compensate for tilts. The limb controllers also receive somatosensory input from the contralateral limbs. The motor responses to these crossed influences are added to the local reflexes. The forelimb controllers exert influences on the hindlimb controllers promoting coordination of the fore- and hindlimbs. Reversed influences are much weaker.
Recent experiments, with recording the activity of pyramidal tract neurons (PTNs) in the cat maintaining balance on the tilting platform have shown that their activity strongly correlates with tilts of the platform and with postural corrections elicited by the tilts (Beloozerova et al. 2005). This finding suggests that the motor cortex is involved in the control of body posture. In the present study, we assessed a function of the motor cortex in this motor behaviour. In accordance with the general structure of the trunk stabilizing system (Fig. 9B), cortical functions could be as follows. First, the motor cortex could participate in the control of an individual limb, by sending corrective motor commands based on local somatosensory inputs. In this case, PTNs should be driven by somatosensory input from their own limb (to which they project). Second, the motor cortex could participate in the coordination of limbs within a girdle (shoulder or hip). In this case, the PTNs projecting to a given limb should be driven by afferents of the contralateral limb. Third, the motor cortex could participate in the coordination of the two girdles. In this case, PTNs of a given girdle should be driven by afferents of the other girdle. We addressed this question by investigating the origin of posture-related activity of PTNs. To assess a contribution of input from a given limb to the PTN activity, we used the method of varying the number of limbs supporting the body (Deliagina et al. 2006a).
The main result of this study is that the tilt-related modulation of the activity in a given PTN depends primarily on the sensory input from its own (target) limb. This conclusion was primarily based on the finding that standing on the target limb alone did not reduce the value of tilt-related PTN modulation and did not change the phase of this modulation, despite sensory inputs from three other limbs being severely attenuated (Figs 6 and 7). Another finding supporting this conclusion was a disappearance of modulation of a PTN when we managed to inactivate its receptive field (Fig. 8D). These results strongly suggest that, in the postural task, the PTNs constitute a part of the limb controller, and they are primarily involved in the feedback control of their own limb, that is, in the intralimb coordination. The corresponding sensory influences are shown by large red arrows in the scheme for sensorimotor processing in the postural system (Fig. 9B).
The input from the opposite limb, as well as the input from the limbs of the other girdle make a much smaller contribution to the PTN modulation. This suggestion was based on the finding that lifting of the target limb strongly reduced the value of tilt-related PTN modulation (as compared to standing limb) and changed the phase of this modulation (Figs 6 and 7). However, it is necessary to note that a decrease of modulation in the lifted limb could be caused not only by a reduced sensory input from this limb, but also by a reconfiguration of the control system (the brain does not engage the muscles that cannot do the task). These results suggest that, in the postural task, the PTNs are much less involved in the coordination of activity between the two limbs within a girdle, and between the two girdles. The corresponding sensory inputs are shown by small blue arrows in Fig. 9B. The whole population of PTNs, however, was not homogeneous in respect to the relative role of the three inputs. First, the input from the foreign girdle was usually much weaker in the forelimb PTNs than in the hindlimb PTNs. Second, for a portion of hindlimb PTNs, the tilt-related modulation was mainly caused by sensory influences from the hindlimbs, while in another portion sensory influences from the forelimbs noticeably contributed to the modulation (Fig. 5E).
A striking similarity was found between the mean frequencies of PTNs in different postural tests – they ranged from 11.9 to 16.9 imp s−1 in forelimb PTNs and from 16.6 to 20.0 imp s−1 in hindlimb PTNs. This was in contrast with the tilt-related modulation of PTNs, whose value strongly differed in different tests (Tables 1 and 2). We suggest that this finding reflects different sources of two components of PTN activity – somatosensory input for the phasic responses and central origin for the background activity.
Sensory origin of PTN responses
A distinctive feature of the feedback mode of postural control is the reflex origin of corrective motor responses (Horak & Macpherson, 1996; Deliagina et al. 2006b). The present study has shown that afferent input from the ‘own’ limb is the primary source of PTN responses to tilts of the animal. This input determines, to a large extent, the duration of responses, their phase and amplitude. Which afferents of the limb are responsible for generating the signals driving PTNs in the postural task? How is the afferent activity processed before it reaches the PTNs? Some data relating to these problems were obtained in the present study.
It is known that the limb areas of the motor cortex have specific afferent projections from the corresponding limbs (‘peripheral receptive fields’), which can be revealed in the quiescent state of the animal (Asanuma, 1989; Armstrong & Drew, 1983a). This input comes from different groups of muscle, joint, and cutaneous afferents (Oscarsson & Rosen, 1966a,b; Landgren & Silfvenius, 1971; for discussion see Deliagina et al. 2000; Duysens et al. 2000). It was suggested that this afferent input provides precise information essential for standing and walking reflexes (Welt et al. 1967). It was found that peripheral receptive fields of neurons of the cat motor cortex strongly correlate with their motor effects determined by microstimulation (Asanuma et al. 1968). By contrast, rather weak correlation was found between the locomotor-related neuronal discharges in the cat motor cortex and peripheral receptive fields (Armstrong & Drew, 1983b,c).
In the present study, we compared (i) the PTN responses to tilts and (ii) the afferent signals that the PTN presumably receives from its receptive field during tilts. In a portion of PTNs (34%), the response pattern well corresponded to the pattern which one could expect provided the PTN was driven by its receptive field input. One can suggest that these PTNs were controlled (at least partly) by the receptive field input (this category of PTNs is shown schematically in Fig. 9C, 1). For a few PTNs, we demonstrated that inactivation of the receptive field input leads to a complete attenuation of the PTN responses to tilts (Fig. 8C and D), strongly suggesting that this input completely determines the PTN responses. Another, although a less likely explanation as we believe, would be a reconfiguration of the control system (induced by the limb reconfiguration), which leads to inactivation of the PTN.
In the majority of PTNs (66%), however, the input from the receptive field could not be responsible (even partly) for the generation of PTN reactions to tilts. Similar results were obtained in our earlier experiments on rabbits (Beloozerova et al. 2003a) and cats (Beloozerova et al. 2005). One can suggest that, in this category of PTNs (2 in Fig. 9C), the somatosensory input from the receptive field (RF input) is replaced by another input (Non-RF input) when an active behaviour is taking place. In PTNs with no receptive field (3 in Fig. 9C), the response is due to different afferent input. This hypothesis could be further supported by the view that the somatosensory signals received from limb mechanoreceptors are processed in the spinal and brainstem networks before they reach the motor cortex (see, e.g. Landgren & Silfvenius, 1971; Asanuma, 1989). We also found that the proportion of PTNs not driven by receptive field was maximal in the distal parts of the forelimbs (Table 3), suggesting that their receptive fields deliver sensory information not for the postural control but for the control of other movements, e.g. voluntary movements in the distal joints.
To conclude, in the present study we analysed the role of the motor cortex in the control of body posture. An important feature of the postural activity is that postural motor output is generated mainly on the basis of sensory feedback signals (Horak & Macpherson, 1996), This contrasts to many other movements like stepping, the basic pattern of which is generated centrally, and sensory input only modulates this pattern (Orlovsky et al. 1999). In the present study we found that cortical output in the postural task, mediated by PTNs, is generated on the basis of somatosensory information coming mainly from the corresponding contralateral limb. Thus, during postural activity, a key role of the motor cortex is the feedback control of this limb.
Acknowledgments
We thank Peter Wettenstein for exceptional engineering assistance. This work was supported by grants from NIH R01 NS-049884, the Swedish Research Council (no. 11554), Gösta Fraenckels Foundation, Erik and Edith Fernströms Foundation to T.G.D., and NIH R01 NS-39340, Barrow Neurological Foundation to I.N.B.
References
- Adkins RJ, Cegnar MR, Rafuse DD. Differential effects of lesions of the anterior and posterior sygmoid gyri in cats. Brain Res. 1971;30:411–414. doi: 10.1016/0006-8993(71)90092-8. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Topographical localization in the motor cortex of the cat for somatic afferent responses and evoked movements. J Physiol. 1983a;350:33–54. doi: 10.1113/jphysiol.1984.sp015187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1983b;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Discharges of pyramidal tract and other motor cortical neurons during locomotion in the cat. J Physiol. 1983c;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Electromyographic responses evoked in muscles of the forelimb by intracortical stimulation in the cat. J Physiol. 1985a;367:309–326. doi: 10.1113/jphysiol.1985.sp015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Forelimb electromyographic responses to motor cortex stimulation during locomotion in the cat. J Physiol. 1985b;367:327–351. doi: 10.1113/jphysiol.1985.sp015827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H. The Motor Cortex. New York: Raven Press; 1989. [Google Scholar]
- Asanuma H, Stoney SD, Jr, Abzug C. Relationship between afferent input and motor outflow in cat motosensory cortex. J Neurophysiol. 1968;31:670–681. doi: 10.1152/jn.1968.31.5.670. [DOI] [PubMed] [Google Scholar]
- Bard P, Macht MB. The behavior of chronically decerebrated cat. In: Wolstenholme GEW, O'Connor CM, editors. Neurological Basis of Behaviour. London: Churchill; 1958. pp. 55–71. [Google Scholar]
- Batshelet E. Circular Statistics in Biology. New York: Academic Press; 1981. [Google Scholar]
- Beloozerova IN, Sirota MG. Role of motor cortex in control of locomotion. In: Gurfinkel VS, Ioffe ME, Massion J, Roll JP, editors. Stance and Motion. Facts and Concepts. New York, London: Plenum Press; 1988. pp. 163–176. [Google Scholar]
- Beloozerova IN, Sirota MG. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J Physiol. 1993a;461:1–25. doi: 10.1113/jphysiol.1993.sp019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG. The role of the motor cortex in the control of vigour of locomotor movements in the cat. J Physiol. 1993b;461:27–46. doi: 10.1113/jphysiol.1993.sp019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J Neurophysiol. 2005;93:1831–1844. doi: 10.1152/jn.00577.2004. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA, Orlovsky GN, Popova LB, Deliagina TG. Activity of different classes of neurons of the motor cortex during postural corrections. J Neurosci. 2003a;23:7844–7853. doi: 10.1523/JNEUROSCI.23-21-07844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003b;90:3783–3793. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- Bishop PO, Burke W, Davis R. The identification of single units in central visual pathways. J Physiol. 1962;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WW, Lin CN. Corticospinal tract of the cat. An attempt to correlate the pattern of degeneration with deficits in reflex activity following neocortical lesions. J Comp Neurol. 1957;108:23–56. doi: 10.1002/cne.901080103. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Popova LB, Sirota MG, Swadlow H, Grant G, Orlovsky GN. Role of different sensory inputs for maintenance of body posture in sitting rat and rabbit. Motor Control. 2000;4:439–452. doi: 10.1123/mcj.4.4.439. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN. Comparative neurobiology of postural control. Curr Opin Neurobiol. 2002;12:652–657. doi: 10.1016/s0959-4388(02)00376-8. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology. 2006b;21:216–225. doi: 10.1152/physiol.00001.2006. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J Physiol. 2006a;573:211–224. doi: 10.1113/jphysiol.2006.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol. 1993;70:179–199. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B, Garcia-Rill E, Surkes MA. Effects of discrete precruciate cortex lesions on motor behavior. Brain Res. 1974;82:328–333. doi: 10.1016/0006-8993(74)90614-3. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical Analysis of Circular Data. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Fuller JH, Schlag J. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976;122:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Ghosh S. Identification of motor areas of the cat cerebral cortex based on studies of cortical stimulation and corticospinal connections. J Comp Neurol. 1997;380:191–214. doi: 10.1002/(sici)1096-9861(19970407)380:2<191::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hassler R, Muhs-Clement K. Architectonic construction of the sensomotor and parietal cortex in the cat. J Hirnforsch. 1964;20:377–420. [PubMed] [Google Scholar]
- Horak F, Macpherson J. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol. 1995;73:1181–1191. doi: 10.1152/jn.1995.73.3.1181. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayannidou A, Tamarova ZA, Sirota MG, Zelenin PV, Orlovsky GN, Deliagina TG, Beloozerova IN. 2006 Abstract Viewer/Itinerary Planner. DC, Washington: Society for Neuroscience; 2006. Integration of sensory inputs from different limbs in postural responses of pyramidal tract neurons. Program No. 32, 657.11. [Google Scholar]
- Landgren S, Silfvenius H. Nucleus Z, the medullary relay in the projection path to the cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol. 1971;218:551–571. doi: 10.1113/jphysiol.1971.sp009633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol. 1988a;60:204–217. doi: 10.1152/jn.1988.60.1.204. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol. 1988b;60:218–231. doi: 10.1152/jn.1988.60.1.218. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J, Lacobs R. Postural orientation, equilibrium, and the spinal cord. Adv Neurol. 1997a;72:227–232. [PubMed] [Google Scholar]
- Magnus R. Körperstellung. Berlin: Springer; 1924. [Google Scholar]
- Martin JH, Ghez C. Task-related coding of stimulus and response in cat motor cortex. Exp Brain Res. 1985;57:427–442. doi: 10.1007/BF00237829. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Differential impairments in reaching and grasping produced by local inactivation within the forelimb representation of the motor cortex in the cat. Exp Brain Res. 1993;94:429–443. doi: 10.1007/BF00230201. [DOI] [PubMed] [Google Scholar]
- Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;22:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- Myasnikov AA, Dykes RW, Avendano C. Cytoarchitecture and responsiveness of the medial ansate region of the cat primary somatosensory cortex. J Comp Neurol. 1994;49:401–427. doi: 10.1002/cne.903490307. [DOI] [PubMed] [Google Scholar]
- Myasnikov AA, Dykes RW, Leclerc SS. Correlating cytoarchitecture and function in cat primary somatosensory cortex: the challenge of individual differences. Brain Res. 1997;750:95–108. doi: 10.1016/s0006-8993(96)01337-6. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Rispal-Padel L. Somatotopic localization in cat motor cortex. Brain Res. 1976;105:405–422. doi: 10.1016/0006-8993(76)90590-4. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. From Mollusc to Man. Oxford: Oxford University Press; 1999. [Google Scholar]
- Oscarsson O, Rosen I. Organization of neurons in the cat cerebral cortex that are influenced from group I muscle afferents. J Physiol. 1966a;183:189–210. doi: 10.1113/jphysiol.1966.sp007860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O, Rosen I. Short-latency projections to the cat's cerebral cortex from skin and muscle afferents in the contralateral forelimb. J Physiol. 1966b;182:164–184. doi: 10.1113/jphysiol.1966.sp007816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG, Porter R. Corticospinal neurones. Their role in movement. Monogr Physiol Soc. 1977;34(v–xii):1–450. [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Reitboeck HJ. Fiber microelectrodes for electrophysiological recordings. J Neurosci Meth. 1983;8:249–262. doi: 10.1016/0165-0270(83)90038-9. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci. 2002;22:5803–5807. doi: 10.1523/JNEUROSCI.22-14-05803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario DS, Martin JH, Ghez C. Specialized subregiones in the cat motor cortex: a single unit analysis in the behaving animal. Exp Brain Res. 1983;51:351–367. [Google Scholar]
- Welt C, Aschoff J, Kameda K, Brooks VB. Intracortical organization of cat's sensory motor neurons. In: Purpura DP, Yahr MD, editors. Neurophysiological Basis of Normal and Abnormal Motor Activities. Hewlett, NY: Raven; 1967. pp. 255–293. [Google Scholar]