Abstract
Low-volume ‘sprint’ interval training (SIT) stimulates rapid improvements in muscle oxidative capacity that are comparable to levels reached following traditional endurance training (ET) but no study has examined metabolic adaptations during exercise after these different training strategies. We hypothesized that SIT and ET would induce similar adaptations in markers of skeletal muscle carbohydrate (CHO) and lipid metabolism and metabolic control during exercise despite large differences in training volume and time commitment. Active but untrained subjects (23 ± 1 years) performed a constant-load cycling challenge (1 h at 65% of peak oxygen uptake 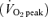 before and after 6 weeks of either SIT or ET (n = 5 men and 5 women per group). SIT consisted of four to six repeats of a 30 s ‘all out’ Wingate Test (mean power output ∼500 W) with 4.5 min recovery between repeats, 3 days per week. ET consisted of 40–60 min of continuous cycling at a workload that elicited ∼65%
before and after 6 weeks of either SIT or ET (n = 5 men and 5 women per group). SIT consisted of four to six repeats of a 30 s ‘all out’ Wingate Test (mean power output ∼500 W) with 4.5 min recovery between repeats, 3 days per week. ET consisted of 40–60 min of continuous cycling at a workload that elicited ∼65% 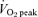 (mean power output ∼150 W) per day, 5 days per week. Weekly time commitment (∼1.5 versus∼4.5 h) and total training volume (∼225 versus∼2250 kJ week−1) were substantially lower in SIT versus ET. Despite these differences, both protocols induced similar increases (P < 0.05) in mitochondrial markers for skeletal muscle CHO (pyruvate dehydrogenase E1α protein content) and lipid oxidation (3-hydroxyacyl CoA dehydrogenase maximal activity) and protein content of peroxisome proliferator-activated receptor-γ coactivator-1α. Glycogen and phosphocreatine utilization during exercise were reduced after training, and calculated rates of whole-body CHO and lipid oxidation were decreased and increased, respectively, with no differences between groups (all main effects, P < 0.05). Given the markedly lower training volume in the SIT group, these data suggest that high-intensity interval training is a time-efficient strategy to increase skeletal muscle oxidative capacity and induce specific metabolic adaptations during exercise that are comparable to traditional ET.
(mean power output ∼150 W) per day, 5 days per week. Weekly time commitment (∼1.5 versus∼4.5 h) and total training volume (∼225 versus∼2250 kJ week−1) were substantially lower in SIT versus ET. Despite these differences, both protocols induced similar increases (P < 0.05) in mitochondrial markers for skeletal muscle CHO (pyruvate dehydrogenase E1α protein content) and lipid oxidation (3-hydroxyacyl CoA dehydrogenase maximal activity) and protein content of peroxisome proliferator-activated receptor-γ coactivator-1α. Glycogen and phosphocreatine utilization during exercise were reduced after training, and calculated rates of whole-body CHO and lipid oxidation were decreased and increased, respectively, with no differences between groups (all main effects, P < 0.05). Given the markedly lower training volume in the SIT group, these data suggest that high-intensity interval training is a time-efficient strategy to increase skeletal muscle oxidative capacity and induce specific metabolic adaptations during exercise that are comparable to traditional ET.
Prolonged sessions of moderate-intensity exercise (e.g. ≥ 1 h at ∼65% of peak oxygen uptake 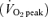 ), performed repeatedly for at least several weeks, increases skeletal muscle oxidative capacity and alters substrate utilization during matched-work exercise, resulting in improved endurance capacity (Gollnick et al. 1973). Although less-widely appreciated, numerous studies have shown that brief, repeated sessions of ‘all out’ high-intensity or sprint-type interval training (SIT) elicits changes in skeletal muscle energy metabolism that resemble traditional endurance training (ET) (Henriksson & Reitman, 1976; Saltin et al. 1976; Gibala et al. 2006a). The relatively few studies that have directly compared skeletal muscle metabolic adaptations to interval and continuous training have yielded equivocal results, and in all cases the total volume of work performed was similar between groups (Henriksson & Reitman, 1976; Saltin et al. 1976; Eddy et al. 1977; Fournier et al. 1982; Gorostiaga et al. 1991; Edge et al. 2006). Recently, we (Gibala et al. 2006a) examined molecular and cellular adaptations in resting human skeletal muscle after six sessions of SIT or ET performed over 2 weeks. By design, total training time commitment and exercise volume was markedly lower in the SIT group, yet we found similar improvements in the maximal activity of cytochrome c oxidase (COX) and the protein content of COX subunits II and IV after training in both groups. Although previously speculated by others (Coyle, 2005), to our knowledge this was the first study to demonstrate that SIT was indeed a very ‘time-efficient’ strategy to improve skeletal muscle oxidative capacity and exercise performance (Gibala et al. 2006a).
), performed repeatedly for at least several weeks, increases skeletal muscle oxidative capacity and alters substrate utilization during matched-work exercise, resulting in improved endurance capacity (Gollnick et al. 1973). Although less-widely appreciated, numerous studies have shown that brief, repeated sessions of ‘all out’ high-intensity or sprint-type interval training (SIT) elicits changes in skeletal muscle energy metabolism that resemble traditional endurance training (ET) (Henriksson & Reitman, 1976; Saltin et al. 1976; Gibala et al. 2006a). The relatively few studies that have directly compared skeletal muscle metabolic adaptations to interval and continuous training have yielded equivocal results, and in all cases the total volume of work performed was similar between groups (Henriksson & Reitman, 1976; Saltin et al. 1976; Eddy et al. 1977; Fournier et al. 1982; Gorostiaga et al. 1991; Edge et al. 2006). Recently, we (Gibala et al. 2006a) examined molecular and cellular adaptations in resting human skeletal muscle after six sessions of SIT or ET performed over 2 weeks. By design, total training time commitment and exercise volume was markedly lower in the SIT group, yet we found similar improvements in the maximal activity of cytochrome c oxidase (COX) and the protein content of COX subunits II and IV after training in both groups. Although previously speculated by others (Coyle, 2005), to our knowledge this was the first study to demonstrate that SIT was indeed a very ‘time-efficient’ strategy to improve skeletal muscle oxidative capacity and exercise performance (Gibala et al. 2006a).
In the present study, we sought to confirm and extend the findings of our previous work showing similar increases in muscle oxidative capacity after 2 weeks of SIT or ET (Gibala et al. 2006a). This previous study was limited in that the duration of training was relatively short and it could be argued that the very intense nature of SIT might stimulate rapid skeletal muscle remodelling (possibly due to altered fibre recruitment), whereas adaptations to lower-intensity ET accrue more slowly. In addition, our previous study involved a single marker of muscle oxidative capacity in resting biopsy samples, and thus provided limited insight regarding the potential for changes in substrate metabolism during exercise. In consideration of these issues, the unique purpose of the present study was to compare changes in markers of skeletal muscle carbohydrate (CHO) and lipid metabolism and metabolic control during matched-work exercise, before and after 6 weeks of low-volume SIT or high-volume ET. The SIT protocol was modelled on previous work in our laboratory and consisted of four to six 30 s ‘all out’ cycling tasks performed three times per week for 6 weeks (Burgomaster et al. 2006; Gibala et al. 2006a). By contrast, the ET protocol was modelled on public health guidelines (American College of Sports Medicine, 1998) and consisted of 40–60 min of continuous cycling at ∼65% 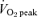 5 days per week for 6 weeks. Whereas previous studies have examined adaptations in resting skeletal muscle after matched-work interval and continuous training (Henriksson & Reitman, 1976; Saltin et al. 1976; Eddy et al. 1977; Fournier et al. 1982; Gorostiaga et al. 1991; Edge et al. 2006), the present study was unique in that we assessed changes during exercise and, by design, the total weekly exercise volume was ∼90% lower in the SIT group (i.e. 225 versus 2250 kJ week−1 in the SIT and ET groups, respectively). We hypothesized that 6 weeks of SIT and ET would induce similar adaptations in muscle oxidative capacity and selected measures of whole-body and skeletal muscle substrate metabolism during exercise despite large differences in total training time commitment and exercise volume.
5 days per week for 6 weeks. Whereas previous studies have examined adaptations in resting skeletal muscle after matched-work interval and continuous training (Henriksson & Reitman, 1976; Saltin et al. 1976; Eddy et al. 1977; Fournier et al. 1982; Gorostiaga et al. 1991; Edge et al. 2006), the present study was unique in that we assessed changes during exercise and, by design, the total weekly exercise volume was ∼90% lower in the SIT group (i.e. 225 versus 2250 kJ week−1 in the SIT and ET groups, respectively). We hypothesized that 6 weeks of SIT and ET would induce similar adaptations in muscle oxidative capacity and selected measures of whole-body and skeletal muscle substrate metabolism during exercise despite large differences in total training time commitment and exercise volume.
Methods
Subjects
Twenty young healthy men and women (n = 5 men and 5 women per group) volunteered for the study (Table 1). A preliminary screening process was employed to establish that subjects: (a) were free of risk factors associated with cardiovascular, pulmonary or metabolic disease; (b) were deemed safe to begin a physical activity programme; and (c) other than activities of daily living, were not engaged in a regular exercise training programme (i.e. ≤ 2 sessions per week and ≤ 30 min per session, for at least 1 year prior to the study). The experimental procedures and potential risks were fully explained to the subjects prior to the study, and all subjects provided written, informed consent. The experimental protocol was approved by the McMaster University and Hamilton Health Sciences Research Ethics Board.
Table 1.
Subject characteristics and peak oxygen uptake data before and after 3 and 6 weeks of exercise training
| SIT Group | ET Group | |||||
|---|---|---|---|---|---|---|
| Pretraining | 3 week | 6 week | Pretraining | 3 week | 6 week | |
| Age (years) | 24 ± 1 | — | — | 23 ± 1 | — | — |
| Height (cm) | 171 ± 2 | — | — | 175 ± 4 | — | — |
| Weight (kg) | 69 ± 3 | 69 ± 3 | 68 ± 3 | 75 ± 4 | 75 ± 4 | 75 ± 4 |
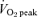 (ml kg−1 min−1)* (ml kg−1 min−1)*
|
41 ± 2 | 44 ± 2 | 44 ± 2 | 41 ± 2 | 42 ± 2 | 45 ± 2 |
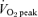 (l min−1)* (l min−1)*
|
2.8 ± 0.2 | 3.0 ± 0.1 | 3.0 ± 2 | 3.0 ± 0.3 | 3.2 ± 0.3 | 3.2 ± 0.3 |
Values are means ± s.e.m., n = 10 per group.
P < 0.05, main effect for training (3 week = 6 week > pretraining).
Pre-experimental procedures
Subjects initially performed a progressive exercise test (increasing 1 W every 2 s) on an electronically braked cycle ergometer (Lode BV, Excalibur Sport V2.0, the Netherlands) in order to determine their peak oxygen uptake 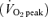 using an on-line gas collection system (Moxus Modular VO2 System, AEI Technologies Inc., Pittsburgh, PA, USA). The value used for
using an on-line gas collection system (Moxus Modular VO2 System, AEI Technologies Inc., Pittsburgh, PA, USA). The value used for 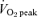 corresponded to the highest value achieved over a 30 s collection period. Subjects subsequently performed a familiarization ride in order to determine the workload that elicited ∼65% of their
corresponded to the highest value achieved over a 30 s collection period. Subjects subsequently performed a familiarization ride in order to determine the workload that elicited ∼65% of their 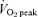 . All subjects also performed a 30 s test of all out effort (Wingate Test) on the same cycle ergometer against a resistance equivalent to 0.075 kg (kg body mass)−1. After the familiarization procedures, subjects were assigned to either a sprint training (SIT) group or an endurance training (ET) group in a matched fashion based on sex and
. All subjects also performed a 30 s test of all out effort (Wingate Test) on the same cycle ergometer against a resistance equivalent to 0.075 kg (kg body mass)−1. After the familiarization procedures, subjects were assigned to either a sprint training (SIT) group or an endurance training (ET) group in a matched fashion based on sex and 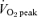 (Table 1).
(Table 1).
Experimental protocol
Each subject served as their own control and performed two experimental trials, before and after a 6 week exercise training programme (see below). Upon arrival at the laboratory, the lateral portion of one thigh was prepared for the extraction of needle biopsy samples from the vastus lateralis muscle (Bergström, 1975). Two small incisions were made in the skin and overlying fascia after injection of a local anaesthetic (2% lidocaine). A biopsy was obtained at rest, and then subjects commenced cycling for 60 min on an electronically braked cycle ergometer (Lode BV) at a workload designed to elicit ∼65% of pretraining 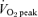 . Heart rate was determined using telemetry (Polar Electro, Woodbury, NY, USA) and expired gases were collected for the determination of
. Heart rate was determined using telemetry (Polar Electro, Woodbury, NY, USA) and expired gases were collected for the determination of 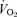 ,
, 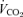 and respiratory exchange ratio (RER) using a metabolic cart (Moxus Modular VO2 System) and used to estimate rates of whole-body fat and carbohydrate oxidation (Peronnet & Massicotte, 1991). A second muscle biopsy was obtained immediately after exercise. The second experimental trial was performed 96 h after the final exercise training session and was identical in all respects to the first experimental trial, including power output which was set at the same absolute workload (i.e. 65% of pretraining
and respiratory exchange ratio (RER) using a metabolic cart (Moxus Modular VO2 System) and used to estimate rates of whole-body fat and carbohydrate oxidation (Peronnet & Massicotte, 1991). A second muscle biopsy was obtained immediately after exercise. The second experimental trial was performed 96 h after the final exercise training session and was identical in all respects to the first experimental trial, including power output which was set at the same absolute workload (i.e. 65% of pretraining 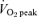 ).
).
Training protocol
The training protocols were initiated several days after the first experimental trial (Table 2). ET consisted of continuous cycling on an ergometer, 5 days per week (Monday–Friday) for 6 weeks, at a power output corresponding to ∼65% 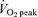 . Subjects performed 40 min of exercise per training session for the first 2 weeks. Exercise time was increased to 50 min per session during weeks 3 and 4, and subjects performed 60 min of exercise per session during the final 2 weeks.
. Subjects performed 40 min of exercise per training session for the first 2 weeks. Exercise time was increased to 50 min per session during weeks 3 and 4, and subjects performed 60 min of exercise per session during the final 2 weeks. 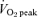 tests were re-administered after 3 weeks of training and training loads were adjusted in order to maintain a training intensity equivalent to ∼65%
tests were re-administered after 3 weeks of training and training loads were adjusted in order to maintain a training intensity equivalent to ∼65%  . SIT consisted of repeated Wingate Tests on an ergometer 3 days per week (Monday, Wednesday and Friday) for 6 weeks. The number of Wingate Tests performed during each training session increased from four during week 1–2, to five during week 3–4, and finally to six during week 5–6. For all training sessions, the recovery interval between Wingate Tests was fixed at 4.5 min, during which time subjects cycled at a low cadence (< 50 r.p.m.) against a light resistance (30 W) to reduce venous pooling in the lower extremities and minimize feelings of light-headedness or nausea. The endurance training programme was based on general guidelines recommended by leading public health agencies (American College of Sports Medicine, 1998) whereas the SIT programme was modelled on recent studies conducted in our laboratory that have examined metabolic and performance adaptations to low-volume, high-intensity interval training (Burgomaster et al. 2005, 2006, 2007; Gibala et al. 2006a). By design, the protocols differed substantially in terms of total exercise training volume and time commitment in order to evaluate adaptations in skeletal muscle metabolism to two diverse training programmes.
. SIT consisted of repeated Wingate Tests on an ergometer 3 days per week (Monday, Wednesday and Friday) for 6 weeks. The number of Wingate Tests performed during each training session increased from four during week 1–2, to five during week 3–4, and finally to six during week 5–6. For all training sessions, the recovery interval between Wingate Tests was fixed at 4.5 min, during which time subjects cycled at a low cadence (< 50 r.p.m.) against a light resistance (30 W) to reduce venous pooling in the lower extremities and minimize feelings of light-headedness or nausea. The endurance training programme was based on general guidelines recommended by leading public health agencies (American College of Sports Medicine, 1998) whereas the SIT programme was modelled on recent studies conducted in our laboratory that have examined metabolic and performance adaptations to low-volume, high-intensity interval training (Burgomaster et al. 2005, 2006, 2007; Gibala et al. 2006a). By design, the protocols differed substantially in terms of total exercise training volume and time commitment in order to evaluate adaptations in skeletal muscle metabolism to two diverse training programmes.
Table 2.
Summary of sprint interval training (SIT) and endurance training (ET) protocols
| Variable | SIT Group (n = 10) | ET Group (n = 10) |
|---|---|---|
| Protocol | 30 s × 4–6 repeats, 4.5 min rest | 40–60 min cycling |
| (3 × per week) | (5 × per week) | |
| Training intensity | ‘All out’ maximal effort | 65% of 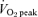
|
| (workload) | (∼500 W) | (∼150 W) |
| Weekly training | ∼10 min | ∼4.5 h |
| time commitment | (∼1.5 h including rest) | |
| Weekly training volume | ∼225 kJ | ∼2250 kJ |
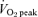 , peak oxygen uptake.
, peak oxygen uptake.
Dietary controls
Subjects were instructed to continue their normal dietary and physical activity practices throughout the experiment but to refrain from alcohol and exercise for 48 h before each trial. Exercise was performed 3 h after a standardized meal. Subjects recorded their dietary intake for 24 h prior to the pretrial so that their individual pattern of food intake could be replicated in the 24 h before the post-trial. Subsequent dietary analyses (Nutritionist Five, First Data Bank, San Bruno, CA, USA) revealed no differences in total energy intake or macronutrient composition prior to the two trials and no differences between groups (Table 3).
Table 3.
Nutritional data
| SIT Group | ET Group | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Energy intake (MJ) | 11 ± 1 | 10 ± 1 | 11 ± 1 | 10 ± 1 |
| Carbohydrate (%) | 60 ± 2 | 59 ± 2 | 56 ± 3 | 55 ± 4 |
| Fat (%) | 23 ± 2 | 24 ± 2 | 26 ± 2 | 26 ± 2 |
| Protein (%) | 17 ± 1 | 17 ± 1 | 18 ± 2 | 19 ± 2 |
| Protein (g (kg body wt)−1) | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.2 |
Data collected for 24 h prior to each experimental trial. Values are means ± s.e.m., n = 10 and n = 9 for SIT and ET groups, respectively. No difference between trials or groups.
Muscle analyses
Muscle samples were initially sectioned into several pieces under liquid nitrogen and one of the pieces was freeze-dried, powdered and dissected free of all non-muscle elements. The samples were stored at −80°C until required for analyses.
Enzyme activities
One piece of frozen wet muscle was chipped from the resting samples and homogenized to a 50 times dilution as described by Henriksson et al. (1986). The homogenate was subsequently analysed to determine the maximal activity of citrate synthase (CS) on a spectrophotometer (Ultrospec 3000 pro UV/Vis) using a method described by Carter et al. (2001). The remaining homogenate was further diluted to 150 times (Henriksson & Reitman, 1976) and analysed for the maximal activity of 3-hydroxyacyl CoA dehydrogenase (β-HAD) on a fluorometer (Hitachi F-2500, Hitachi Instruments, Tokyo, Japan) using a method described by Chi et al. (1983). Total protein content of all homogenates was determined by the method of Bradford (1976) and enzyme activities are expressed as moles per kilogram of protein per h.
Immunoblotting
An aliquot of freeze-dried muscle was added to 250 μl homogenizing buffer containing 50 mm Tris (pH 7.5), 1 mm EDTA, 1 mm EGTA, 10% glycerol, 1% Triton X-100, 50 mm NaF, 5 mm sodium pyrophosphatase, 1 mm DTT and 2 μl protease inhibitor cocktail. The sample was homogenized on ice and subsequently centrifuged at 1000 g for 5 min. The supernatant was collected and the protein content was determined (Pierce BCA Protein Assay Reagent). The sample was subsequently diluted with homogenizing buffer to 3 mg (ml protein)−1. A 200 μl aliquot of sample was mixed with 50 μl loading buffer and heated at 100°C for 5 min. For each blot, a standard and a positive control was loaded along with 40 μl each sample onto a 5% polyacrylamide stacking gel and separated using a 10% polyacrylamide separating gel of 1.5 mm thickness at 180 V with a running time of ∼45 min in Tris-glycine electrophoresis buffer. The gels were electroblotted onto nitrocellulose membranes in transfer buffer (37 mm Tris base, 140 mm glycine and 20% methanol) for 90 min at 90 V in a cold room. Membranes were then incubated in Tris-buffered saline Tween (TBST; 10 mm Tris base, 150 mm NaCl and 0.05% Tween 20) with 5% skimmed milk for 1 h, and washed twice with TBST for 3 min. The membranes were subsequently incubated overnight at 4°C in PBS containing 1% bovine serum albumin and primary antibodies against the E1α subunit of pyruvatp dehydrogenase (PDH) (Molecular Probes, Eugene, OR, USA) or peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC-1α) (Chemicon, Temecula, CA, USA). Following incubation with the primary antibody, the membranes were rewashed with TBST and exposed to appropriate anti-species horseradish peroxidase-conjugated secondary antibodies (Cell Signalling Technology, Danvers, MA, USA) at a 1 : 10 000 dilution in blocking buffer for 60 min at room temperature (20°C). Membranes were then washed again with TBST before being exposed to a chemiluminescent liquid (Immuno-Star HRP Substrate Kit, Bio-Rad) for 2 min. Membranes were exposed using a Biorad Chemi-Doc System for 5 min and the density of the bands was determined using associated image-analysis software.
Metabolites
An aliquot of freeze-dried muscle was extracted on ice using 0.5 m perchloric acid (PCA) containing 1 mm EDTA, neutralized with 2.2 m KHCO3, and the resulting supernatant was analysed for creatine, phosphocreatine (PCr), ATP and lactate using enzymatic assays adopted for fluorometry (Hitachi). Another aliquot of freeze-dried muscle was incubated in 2.0 n HCl and heated for 2 h at 100°C to hydrolyse the glycogen to glucosyl units. The solution was neutralized with an equal volume of 2.0 n NaOH and analysed for glucose using an enzymatic assay adapted for fluorometry (Passoneau & Lowry, 1993).
Statistical analyses
Exercise capacity data, whole-body substrate oxidation and cardiorespiratory data and enzyme activity data were analysed by using a two-factor mixed ANOVA, with the between factor ‘group’ (SIT versus ET) and repeated factor ‘condition’ (pretraining versus post-training). Muscle metabolite data were analysed by using a three-factor mixed ANOVA, with the factors ‘group’ (SIT versus ET), ‘condition’ (pretraining versus post-training) and ‘time’ (0 versus 60 min). The level of significance for analyses was set at P < 0.05 and significant interactions or main effects were determined using Tukey's honestly significant difference post hoc test. All data are presented as means ± s.e.m. based on 10 subjects per group.
Results
Exercise capacity and cardiorespiratory data
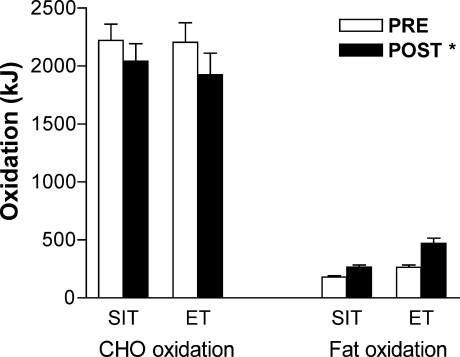
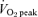 increased after training, with no difference between groups (main effect for condition, P < 0.05; Table 1). Peak power output elicited during the Wingate Test increased by 17% and 7% in the SIT and ET groups, respectively, with no difference between groups (main effect for condition, P < 0.05), whereas, mean power output was increased by 7% only in the SIT group (P < 0.05). Oxygen uptake during the constant-load cycling challenge was similar before and after training; however, mean heart rate and ventilation were reduced (main effects, P < 0.05; Table 4). Mean respiratory exchange ratio during exercise was reduced after training (main effect, P < 0.05; Table 4) and calculated rates of whole-body fat and carbohydrate oxidation were increased and decreased, respectively, with no difference between groups (main effects, P < 0.05; Fig. 1).
increased after training, with no difference between groups (main effect for condition, P < 0.05; Table 1). Peak power output elicited during the Wingate Test increased by 17% and 7% in the SIT and ET groups, respectively, with no difference between groups (main effect for condition, P < 0.05), whereas, mean power output was increased by 7% only in the SIT group (P < 0.05). Oxygen uptake during the constant-load cycling challenge was similar before and after training; however, mean heart rate and ventilation were reduced (main effects, P < 0.05; Table 4). Mean respiratory exchange ratio during exercise was reduced after training (main effect, P < 0.05; Table 4) and calculated rates of whole-body fat and carbohydrate oxidation were increased and decreased, respectively, with no difference between groups (main effects, P < 0.05; Fig. 1).
Table 4.
Cardiorespiratory data during cycling exercise at 65% 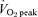 before and after 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET)
before and after 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET)
| SIT Group | ET Group | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Heart rate (beats min−1)* | 160 ± 5 | 151 ± 6 | 157 ± 5 | 144 ± 5 |
| RER* | 0.977 ± 0.01 | 0.965 ± 0.01 | 0.967 ± 0.01 | 0.941 ± 0.01 |
| Ventilation (l min−1)* | 48 ± 3 | 42 ± 3 | 47 ± 5 | 42 ± 4 |
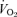 (ml kg−1 min−1) (ml kg−1 min−1) |
27.7 ± 1.0 | 26.6 ± 1.0 | 26.1 ± 1.1 | 25.3 ± 1.8 |
Values are means ± s.e.m. (n = 10 per group). RER, respiratory exchange ratio; 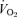 , oxygen uptake.
, oxygen uptake.
Significant difference versus post-training (main effect for condition, such that pre-training ≠ post-training, no difference between groups), P < 0.05.
Figure 1. Whole-body carbohydrate and fat oxidation during exercise that consisted of 60 min at 65% 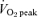 before (PRE) and after (POST) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
before (PRE) and after (POST) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
Values are means ± s.e.m. (n = 10 per group). *Main effect for condition (P < 0.05), such that carbohydrate and fat oxidation PRE and POST are different.
Skeletal muscle mitochondrial enzymes and PGC-1α
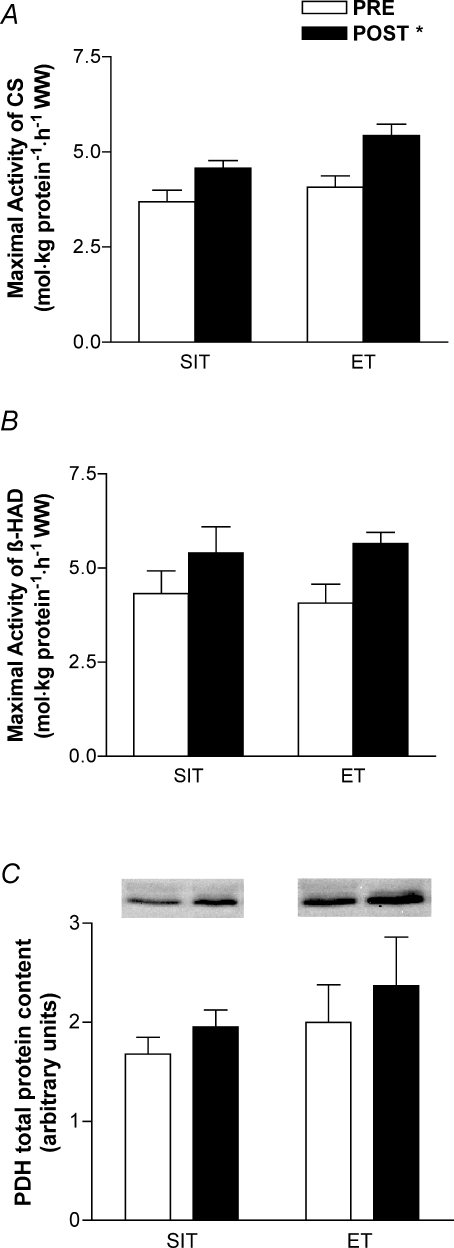
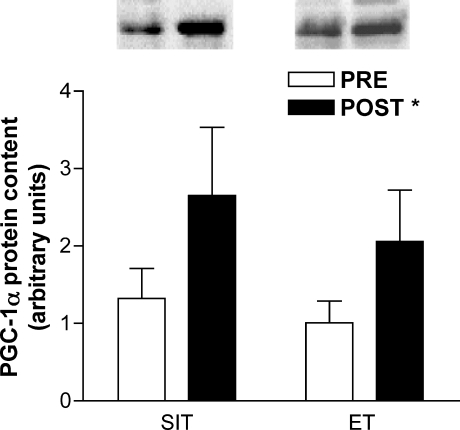
The maximal activities of CS and β-HAD and the total protein content of PDH were increased after training, but there were no differences between groups (main effect for condition, P < 0.05; Fig. 2). Similarly, PGC-1α protein content increased after training but there was no difference between groups (main effect for condition, P < 0.05; Fig. 3).
Figure 2. Maximal activity or total protein content of mitochondrial enzymes citrate synthase (CS; A), 3-hydroxyacyl CoA dehydrogenase (β-HAD; B) and pyruvate dehydrogenase (PDH; C) measured in biopsy samples obtained before (PRE) and after (POST) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
Values are means ± s.e.m. (n = 10 per group); WW, wet weight. *Main effect for condition (P < 0.05), such that post-training (POST) > pretraining (PRE).
Figure 3. Total protein content of PGC-1α measured in biopsy samples obtained before (PRE) and after (POST) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
Values are means ± s.e.m. (n = 10 per group); WW, wet weight. *Main effect for condition (P < 0.05), such that post-training (POST) > pretraining (PRE).
Skeletal muscle metabolites
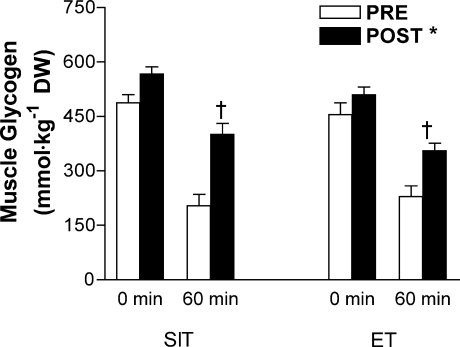
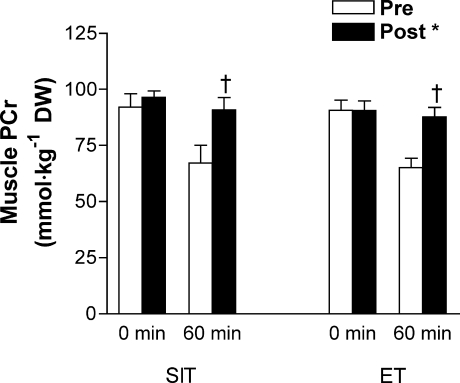
Muscle glycogen content was higher at 60 min of exercise post-training compared to pretraining, with no difference between groups (condition × time interaction, P < 0.05; Fig. 4). Net muscle glycogenolysis during exercise was also reduced after training in both SIT (post-training, 166 ± 20; pretraining, 283 ± 28 mmol (kg dry wt)−1; P < 0.05) and ET (post-training, 154 ± 25; pretraining, 226 ± 15 mmol (kg dry wt)−1; P < 0.05), with no difference between groups (main effect for condition, P < 0.05). Muscle PCr content was higher at 60 min of exercise post-training compared to pretraining with no difference between groups (condition × time interaction, P < 0.05; Fig. 5), whereas creatine showed a reciprocal change and was lower after 60 min of exercise post-training compared to pretraining (condition × time interaction, P < 0.05; Table 5). Muscle ATP was unchanged by acute exercise; however, ATP was reduced after 6 weeks of SIT compared to ET (group–condition interaction, P < 0.05; Table 5). Lactate accumulation was reduced after training; however, the relative exercise-induced changes were modest and overall there were no significant effects (Table 5).
Figure 4. Muscle glycogen concentration measured at rest and during cycling exercise that consisted of 60 min at 65% 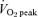 before (PRE) and after (POST) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
before (PRE) and after (POST) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
Values are means ± s.e.m. (n = 10 per group); DW, dry weight. *Main effect for condition (P < 0.05), such that post-training (POST) > pretraining (PRE). †Condition (PRE and POST) × time (0 and 60 min) interaction (P < 0.05), such that POST 60 min > PRE 60 min in both groups.
Figure 5. Muscle phosphocreatine (PCr) concentration measured at rest and during cycling exercise that consisted of 60 min at 65% 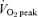 before (Pre) and after (Post) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
before (Pre) and after (Post) 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET).
Values are means ± s.e.m. (n = 10 per group); DW, dry weight. *Main effect for condition (P < 0.05), such that post-training (Post) > pretraining (Pre). †Condition (Pre and Post) × time (0 and 60 min) interaction (P < 0.05), such that Post 60 min > Pre 60 min in both groups.
Table 5.
Muscle metabolite and enzyme data at rest and following 60 min of exercise at 65% 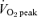 before and after 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET)
before and after 6 weeks of sprint interval training (SIT) or 6 weeks of endurance training (ET)
| SIT Group | ET Group | ||||
|---|---|---|---|---|---|
| Rest | 60 min | Rest | 60 min | ||
| Cr | PRE | 54 ± 6 | 79 ± 8 | 51 ± 4 | 77 ± 4 |
| Post | 49 ± 5 | 55 ± 5† | 64 ± 6 | 54 ± 4† | |
| ATP | pre | 22 ± 1 | 24 ± 1 | 22 ± 1 | 22 ± 1 |
| Post | 19 ± 1# | 20 ± 1# | 23 ± 1 | 25 ± 1 | |
| Lactate | PRE | 13 ± 2 | 36 ± 9 | 20 ± 2 | 37 ± 4 |
| Post | 15 ± 2 | 30 ± 5 | 20 ± 2 | 27 ± 3 | |
Values are means ± s.e.m. (n = 10 per group). Metabolite data expressed in mmol (kg dry wt)−1. Cr, creatine.
Significant difference condition × time interaction (P < 0.05) for creatine, such that post-training (post) 60 min > pretraining (pre) 60 min in both groups
significant difference group–condition interaction (P < 0.05) for ATP, such that post < pre in SIT versus ET.
Discussion
The major novel finding from the present study was that 6 weeks of SIT elicited adaptations in selected markers of skeletal muscle CHO and lipid metabolism and metabolic control during exercise that were comparable to those elicited by ET, despite a much lower training volume and time commitment. By design, weekly training volume was ∼90% lower in the SIT group (∼225 versus∼2250 kJ week−1 for ET) and necessitated a training time commitment that was only ∼one-third of that of the ET group (∼1.5 versus 4.5 h). Most of the training time in the SIT group was spent in recovery between short, intense bursts of all out cycling and actual weekly exercise time was only ∼10 min, as compared to ∼4.5 h of continuous moderate-intensity cycling in the ET group. The present results confirm and extend our recent findings (Gibala et al. 2006a) of similar increases in muscle oxidative capacity and volitional exercise performance after six sessions of SIT or ET over 2 weeks. To our knowledge, the present study is the first to directly compare SIT versus ET in a standardized manner with respect to changes in skeletal muscle metabolism during matched-work exercise.
Skeletal muscle oxidative capacity
As in our previous investigation to assess changes after 2 weeks of training (Gibala et al. 2006a), in the present study we found similar increases in muscle oxidative capacity after 6 weeks of SIT or ET despite large differences in training volume. It is interesting that the relative increase in maximal activity of CS after 6 weeks in the present study (∼25% in both groups) was similar to that observed for COX after 2 weeks in our previous study (Gibala et al. 2006a). Collectively, these findings suggest that much of the increase in mitochondrial content occurs relatively ‘early’ in the training process, which is supported by another recent study that showed increased COX protein content after 1 week (three sessions) of SIT, yet no further increase after 6 weeks of training (Burgomaster et al. 2007). Data from animal investigations also illustrate the potential for brief bouts of intense interval exercise to elicit rapid changes in muscle oxidative capacity. For example, Terada et al. (2001) showed that 8 days of high-intensity, intermittent swimming training (lasting < 5 min day−1) increased maximal activity of citrate synthase in rat skeletal muscle to a level similar to that induced by 6 h of daily low-intensity training.
In terms of comparing SIT versus ET, a novel aspect of the present study was the measurement of other mitochondrial enzymes that are commonly used to reflect the maximal capacity for skeletal muscle CHO (PDH) and lipid oxidation (β-HAD). Although previously speculated by others (Harmer et al. 2000), this is the first study to show that SIT increases the total protein content of PDH (E1α subunit) in human skeletal muscle, the magnitude of which was similar to that previously reported for ET (LeBlanc et al. 2004). The training-induced increase in maximal activity of β-HAD in the present study is in contrast to our previous findings (Burgomaster et al. 2006) of no change after 2 weeks of SIT. These data suggest that a minimum volume of intense, interval-based exercise training may be necessary in order to elicit changes in the maximal capacity for lipid oxidation in skeletal muscle. Consistent with this interpretation, Talanian et al. (2007) recently reported that seven sessions of high-intensity interval training over 2 weeks increased the maximal activity of β-HAD and protein content of plasma membrane associated fatty acid binding protein (FABPpm) in human skeletal muscle. Each training session in that study (Talanian et al. 2007) consisted of 10 bouts of cycling for 4 min at 90% 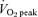 with 2 min of rest between intervals. The total training time commitment (∼5 h) and exercise volume (∼3000 kJ) over the 2 week training period was thus substantially higher than in our recent short-term studies employing Wingate Test-based exercise training (Burgomaster et al. 2005, 2006, 2007).
with 2 min of rest between intervals. The total training time commitment (∼5 h) and exercise volume (∼3000 kJ) over the 2 week training period was thus substantially higher than in our recent short-term studies employing Wingate Test-based exercise training (Burgomaster et al. 2005, 2006, 2007).
Skeletal muscle metabolism during exercise
Training-induced shifts in substrate utilization during exercise have been well documented after several weeks of ET. In contrast to traditional ET, very few studies have examined changes in substrate utilization in working human skeletal muscle after SIT (Nevill et al. 1989; Harmer et al. 2000; Barnett et al. 2004; Burgomaster et al. 2006). Furthermore, the few studies that have examined sprint training-induced changes in skeletal muscle metabolism during exercise have incorporated brief ‘supramaximal’ exercise challenges in which the power output differs markedly between the pre- and post-training trials (Nevill et al. 1989; Parra et al. 2000; Barnett et al. 2004). We therefore compared changes in skeletal muscle metabolism during a prolonged bout of fixed-load exercise (1 h cycling at 65% of pretraining 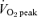 ) before and after 6 weeks of either SIT or ET. Consistent with our hypothesis, we observed similar reductions in net muscle glycogenolysis and phosphocreatine degradation during matched-work exercise after both forms of training, despite a marked decrease in training volume in the SIT group. Although reductions in glycogen utilization after several weeks of ET have been well established, the effect of interval training on muscle glycogenolysis is equivocal with most (Harmer et al. 2000; Parra et al. 2000; Burgomaster et al. 2006), but not all studies (Nevill et al. 1989; Barnett et al. 2004) showing a decrease in glycogen utilization during exercise. Similarly, reductions in phosphocreatine utilization during exercise have been reported after some (Parra et al. 2000), but not all (Nevill et al. 1989; Stathis et al. 1994; Harmer et al. 2000; Rodas et al. 2000; Barnett et al. 2004) sprint training studies.
) before and after 6 weeks of either SIT or ET. Consistent with our hypothesis, we observed similar reductions in net muscle glycogenolysis and phosphocreatine degradation during matched-work exercise after both forms of training, despite a marked decrease in training volume in the SIT group. Although reductions in glycogen utilization after several weeks of ET have been well established, the effect of interval training on muscle glycogenolysis is equivocal with most (Harmer et al. 2000; Parra et al. 2000; Burgomaster et al. 2006), but not all studies (Nevill et al. 1989; Barnett et al. 2004) showing a decrease in glycogen utilization during exercise. Similarly, reductions in phosphocreatine utilization during exercise have been reported after some (Parra et al. 2000), but not all (Nevill et al. 1989; Stathis et al. 1994; Harmer et al. 2000; Rodas et al. 2000; Barnett et al. 2004) sprint training studies.
A final observation with respect to skeletal muscle metabolic changes is that a reduction in ATP content at rest and during exercise following SIT but not ET. This finding is consistent with a previous study from our laboratory investigating metabolic adaptations after 2 weeks of SIT (Burgomaster et al. 2006) and work by Stathis et al. (1994) who reported a reduction in resting muscle ATP content after 7 weeks of SIT. During strenuous exercise, AMP produced from ATP hydrolysis can be deaminated by AMP deaminase, resulting in the formation of inosine monophosphate (IMP) and ammonia, and the subsequent breakdown of IMP to inosine and hypoxanthine resulting in a loss of adenine nucleotides from the muscle (Hellsten et al. 1998). Replacement of purine nucleotides lost from the muscle is a relatively slow, energy-consuming process and appears to continue for several days after intense exercise (Hellsten et al. 1998). Thus the lower ATP content measured after training in the present study may have been due to the stress of chronic training or the acute residual effects of the final training bout, which was performed approximately 72 h before tissue extraction.
Potential mechanisms involved in skeletal muscle remodelling after SIT
While the present study demonstrates the potency of SIT to elicit changes in muscle oxidative capacity and selected metabolic adjustments during exercise that resemble ET, the underlying mechanisms are unclear. From a cell signalling perspective, exercise is typically classified as either ‘strength’ or ‘endurance’, with short-duration, high-intensity work usually associated with increased skeletal muscle mass, and prolonged, low- to moderate-intensity work associated with increased mitochondrial mass and oxidative enzyme activity (Baar, 2006). Given the oxidative phenotype that is rapidly up-regulated by SIT, it is possible that metabolic adaptations to this type of exercise could be mediated in part through signalling pathways normally associated with traditional ET. A key regulator of oxidative enzyme expression in a number of cell types, including skeletal muscle, is PGC-1α (Koulmann & Bigard, 2006). PGC-1α coordinates mitochondrial biogenesis by interacting with various nuclear genes encoding for mitochondrial proteins. Acute exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle (Pilegaard et al. 2003) but to our knowledge only one previous study in humans has examined the effect of prolonged exercise on PGC-1α protein content. Russell et al. (2003) reported that endurance training for 6 weeks (3 days per week) increased PGC-1α protein content in type I, IIa and IIx fibres, which is consistent with data obtained in rats (Taylor et al. 2005). The present study is the first to show that low-volume SIT increases PGC-1α protein content in a similar manner to high-volume ET in human skeletal muscle. The potency of interval-based training in this regard is supported by the work of Terada et al. (2005), who showed increased skeletal muscle PGC-1α protein content after a single bout of high-intensity intermittent swimming exercise in rats. While the precise molecular events are unclear, increases in the AMP-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK) and calcium-signalling mechanisms appear to be important in the regulation of PGC-1α expression and activity (Koulmann & Bigard, 2006). Consistent with this interpretation, in a preliminary report we (Gibala et al. 2006b) showed activation of AMPK and p38 MAPK in response to an acute session of Wingate-based exercise training.
In summary, the results of the present study demonstrate that low-volume SIT is a time-efficient strategy to induce changes in selected markers of whole-body and skeletal muscle CHO and lipid metabolism during exercise that are comparable to changes induced by traditional high-volume ET. Skeletal muscle remodelling after SIT may be mediated in part through signalling pathways normally associated with traditional ET, but additional research is warranted to clarify the molecular mechanisms responsible for metabolic adaptations induced by these different acute exercise ‘impulses’. It is also important to stress that the relatively limited array of metabolic measurements performed in the present study may not be representative of other physiological adaptations normally associated with ET. For example, SIT may differ from ET with respect to changes induced in the cardiovascular and respiratory systems, metabolic control in other organs (e.g. liver or adipose tissue) and protection from various factors associated with chronic inactivity (e.g. insulin resistance or lipid dysregulation).
Acknowledgments
We thank our subjects for their time and effort, and John Moroz and Todd Prior for technical and analytical assistance. We are also thankful to Alicia Jury, Sophie Tanguay and Lindsay Gurr for help with testing and training. This work was supported by operating grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to M.J.G. and M.J.M., and an Experimental and Applied Science Research Grant on Nutrition and Human Performance from the American College of Sports Medicine Foundation (to K.A.B.). K.A.B. was also supported by an NSERC Canada Graduate Scholarship, K.R.H. held an Ontario Graduate Scholarship, and M.J.R. was supported by a Canadian Institutes of Health Research Canada Graduate Scholarship. S.L.M. is a National Health and Medical Research Council Peter Doherty Fellow (400446).
References
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- Baar K. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc. 2006;38:1939–1944. doi: 10.1249/01.mss.0000233799.62153.19. [DOI] [PubMed] [Google Scholar]
- Barnett C, Carey M, Proietto J, Cerin E, Febbraio MA, Jenkins D. Muscle metabolism during sprint exercise in man: influence of sprint training. J Sci Med Sport. 2004;7:314–322. doi: 10.1016/s1440-2440(04)80026-4. [DOI] [PubMed] [Google Scholar]
- Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A, Gibala MJ. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1970–R1976. doi: 10.1152/ajpregu.00503.2006. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Heigenhauser GJF, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time trial performance. J Appl Physiol. 2006;100:2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Hughes SC, Heigenhauser GJF, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98:1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky M. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001;79:386–392. [PubMed] [Google Scholar]
- Chi MM, Hintz CS, Coyle EF, Martin WH, III, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibres. Am J Physiol Cell Physiol. 1983;244:C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- Coyle EF. Very intense exercise-training is extremely potent and time efficient: a reminder. J Appl Physiol. 2005;98:1983–1984. doi: 10.1152/japplphysiol.00215.2005. [DOI] [PubMed] [Google Scholar]
- Eddy DO, Sparks KL, Adelizi DA. The effects of continuous and interval training in women and men. Eur J Appl Physiol Occup Physiol. 1977;37:83–92. doi: 10.1007/BF00421694. [DOI] [PubMed] [Google Scholar]
- Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96:97–105. doi: 10.1007/s00421-005-0068-6. [DOI] [PubMed] [Google Scholar]
- Fournier M, Ricci J, Taylor AW, Ferguson RJ, Montpetit RR, Chaitman BR. Skeletal muscle adaptation in adolescent boys: sprint and endurance training and detraining. Med Sci Sports Exerc. 1982;14:453–456. doi: 10.1249/00005768-198206000-00008. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Little PJ, van Essan M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006a;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham A, Hargreaves M. Effect of high-intensity interval exercise on signaling proteins involved in skeletal muscle remodeling in humans. Appl Physiol Nutr Metab. 2006b;31:S37. [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW, IV, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fibre composition of human skeletal muscle. J Appl Physiol. 1973;34:107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gorostiaga EM, Walter CB, Foster C, Hickson RC. Uniqueness of interval and continuous training at the same maintained exercise intensity. Eur J Appl Physiol Occup Physiol. 1991;63:101–107. doi: 10.1007/BF00235177. [DOI] [PubMed] [Google Scholar]
- Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Enger DM. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol. 2000;89:1793–1803. doi: 10.1152/jappl.2000.89.5.1793. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Sjodin B, Richter EA, Bangsbo J. Urate uptake and lowered ATP levels in human muscle after high-intensity intermittent exercise. Am J Physiol Endocrinol Metab. 1998;274:E600–E606. doi: 10.1152/ajpendo.1998.274.4.E600. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Chi MM, Hintz CS, Young DA, Kaiser KK, Salmons S, Lowry OH. Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am J Physiol Cell Physiol. 1986;251:C614–C632. doi: 10.1152/ajpcell.1986.251.4.C614. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976;97:392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Koulmann N, Bigard AX. Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflugers Arch. 2006;452:125–139. doi: 10.1007/s00424-005-0030-9. [DOI] [PubMed] [Google Scholar]
- LeBlanc PJ, Peters SJ, Tunstall RJ, Cameron-Smith D, Heigenhauser GJF. Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J Physiol. 2004;557:559–570. doi: 10.1113/jphysiol.2003.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill ME, Boobis LH, Brooks S, Williams C. Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol. 1989;67:2376–2382. doi: 10.1152/jappl.1989.67.6.2376. [DOI] [PubMed] [Google Scholar]
- Parra J, Cadefau JA, Rodas G, Amigo N, Cusso R. The distribution of rest periods affects performance and adaptations of energy metabolism induced by high-intensity training in human muscle. Acta Physiol Scand. 2000;169:157–165. doi: 10.1046/j.1365-201x.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- Passoneau JV, Lowry OH. Enzymatic Analysis: a Practical Guide. Totowa, NJ: Humana Press; 1993. [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: and update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodas G, Ventura JL, Cadefau JA, Cusso R, Parra J. A short training programme for the rapid improvement of both aerobic and anaerobic metabolism. Eur J Appl Physiol. 2000;82:480–486. doi: 10.1007/s004210000223. [DOI] [PubMed] [Google Scholar]
- Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-γ coactivator-1 and peroxisome proliferator-activated receptor-α in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Stathis CG, Febbraio MA, Carey MF, Snow RJ. Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J Appl Physiol. 1994;76:1802–1809. doi: 10.1152/jappl.1994.76.4.1802. [DOI] [PubMed] [Google Scholar]
- Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 2007;102:1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Lamb JD, Hurst RW, Chesser DG, Ellingson WJ, Greenwood LJ, Porter BB, Herway ST, Winder WW. Endurance training increases skeletal muscle LKB1 and PGC-1α protein abundance: effects of time and intensity. Am J Physiol Endocrinol Metab. 2005;289:E960–E968. doi: 10.1152/ajpendo.00237.2005. [DOI] [PubMed] [Google Scholar]
- Terada S, Kawanaka K, Goto M, Shimokawa T, Tabata I. Effects of high-intensity intermittent swimming on PGC-1α protein expression in rat skeletal muscle. Acta Physiol Scand. 2005;184:59–65. doi: 10.1111/j.1365-201X.2005.01423.x. [DOI] [PubMed] [Google Scholar]
- Terada S, Yokozeki T, Kawanaka K, Ogawa K, Higuchi M, Ezaki O, Tabata I. Effects of high-intensity swimming training on GLUT-4 and glucose transport activity in rat skeletal muscle. J Appl Physiol. 2001;90:2019–2024. doi: 10.1152/jappl.2001.90.6.2019. [DOI] [PubMed] [Google Scholar]