Abstract
Increasing the mechanical load on skeletal muscle results in increased expression of insulin-like growth factor I (IGF-I), which is thought to be a critical step in the induction of muscle hypertrophy. To determine the role of the IGF-I receptor in load-induced skeletal muscle hypertrophy, we utilized a transgenic mouse model (MKR) that expresses a dominant negative IGF-I receptor specifically in skeletal muscle. Skeletal muscle hypertrophy was induced in the plantaris muscle using the functional overload (FO) model, a model which has previously been shown to induce significant elevations of IGF-I expression in skeletal muscle. Adult male wild-type (WT) and MKR mice were subjected to 0, 7 or 35 days of FO. In control or unchallenged animals, the plantaris mass was 11% greater in WT compared to the MKR mice (P < 0.05). After 7 days of FO, plantaris mass increased significantly by 26% and 62% in WT and MKR mice, respectively (P < 0.05). After 35 days of FO, WT and MKR mice demonstrated significant increases of 100% and 122%, respectively, in plantaris mass (P < 0.05). Further, at no time point was the degree of hypertrophy significantly different between the WT and MKR mice. Previous research suggests that IGF-I induces muscle growth through activation of the Akt–mTOR signalling pathway; therefore, we measured the phosphorylation status of Akt and p70s6k in the WT and MKR mice after 7 days of FO. Significant increases of ∼100% and ∼200% in Akt (Ser-473) and p70s6k (Thr-389) phosphorylation were measured in overloaded plantaris from both WT and MKR mice, respectively. Moreover, no differences were detected between the WT and MKR mice. These data suggest that increased mechanical load can induce muscle hypertrophy and activate the Akt and p70s6k independent of a functioning IGF-I receptor.
The mechanisms that regulate skeletal muscle mass are now receiving enormous scientific attention. Recent investigations have begun to unravel various transcription- and translation-mediated mechanisms that contribute to the regulation of skeletal muscle mass (Rennie et al. 2004). In particular, considerable attention has been focused on mechanisms that regulate the activation of selective protein kinases and phosphatases, especially those downstream of tyrosine kinase receptors such as insulin-like growth factor I. Recent evidence has documented quite convincingly that a mechanical load applied to skeletal muscle can induce significant activation of various signalling pathways leading to enhanced activation of ribosomal machinery. At this time, however, it is unclear how changes in load are translated into the activation of various signalling pathways that ultimately lead to an increase in muscle mass.
It is well established that increases in mechanical load result in an increase in the expression of insulin-like growth factor I (IGF-I) at both the mRNA and protein levels (Adams & Haddad, 1996). Further, the increase in IGF-I levels occur independently of any change in growth hormone levels and are directly produced by the ‘loaded’ muscle (Goldberg, 1967; DeVol et al. 1990). Based on these observations it is often suggested that IGF-1 is responsible and critical for the induction of skeletal muscle hypertrophy, especially in response to increases in mechanical loading such as occur during resistance exercise. (DeVol et al. 1990; Adams & Haddad, 1996).
The anabolic effects of IGF-I have been demonstrated in both muscle cell lines and in vivo animal models (Adams & McCue, 1998; Chakravarthy et al. 2000; Rommel et al. 2001; Vyas et al. 2002). For example, the addition of exogenous IGF-I to cultured myotubes results in an enlargement of myotube diameters and a higher protein content (Rommel et al. 2001; Vyas et al. 2002), while the delivery of exogenous IGF-I either through osmotic pumps or genetic over-expression results in enhanced muscular mass in rodents (Adams & McCue, 1998; Musaro et al. 2001). However, IGF-I alone does not seem to prevent muscle atrophy (Criswell et al. 1998). Thus, it is clear that the addition of exogenous IGF-I to muscle can effectively induce muscle growth. However, at this time no data have convincingly determined if the endogenous IGF-I system is the key factor necessary for initiation of the growth response that occurs in muscle following the application of chronic mechanical load.
IGF-I is thought to induce muscle growth through the activation and increased proliferation of satellite cells and the enhancement of protein translation resulting in an increase in the rate of protein synthesis (for review see Adams, 2002). At this time, most of the data demonstrating an effect of IGF-I on satellite cells are restricted to cell culture models, although Barton-Davis et al. (1999) did find that muscle hypertrophy induced by adeno-associated virus (AAV)-delivery of IGF-I was prevented by irradiating the muscle limb. Irradiation is thought to prevent satellite cell proliferation by preventing the cell from entering the cell cycle; however, irradiation experiments are complicated by the possibility that the irradiation may have non-specific effects on other cell types. Thus, at this time very few in vivo data exist that directly support the hypothesis that increases in IGF-I concentration enhances satellite cell activation.
The data, however, are quite convincing that IGF-I can rapidly increase protein synthesis of the muscle cell resulting in an increase in cellular protein content. In both cultured myotubes and animal models, IGF-I effectively increases protein synthesis through the activation of the PI3K–Akt–mTOR signalling pathway (Rommel et al. 2001; Li et al. 2002; Vyas et al. 2002). Activation of the Akt–mTOR signalling pathway is thought to increase protein synthesis by enhancing the initiation phase of protein translation (for review see Baar et al. 2006). Thus, IGF-I is a potent activator of muscle growth and appears to mediate this effect through enhanced protein translation and potentially through increased satellite cell activation.
Mechanical loading of skeletal muscle is an effective way to increase the activation of the Akt–mTOR signalling pathway (Baar & Esser, 1999; Bodine et al. 2001; Spangenburg & McBride, 2006), although the means by which this pathway is activated remain poorly defined. It is largely assumed that load-induced production of IGF-I by the skeletal muscle is responsible for the increased activation of the Akt–mTOR signalling process (Adams, 2002; Frost & Lang, 2007). However, no investigation has convincingly demonstrated that the IGF-I receptor is the primary upstream signal necessary for load-induced activation of the Akt–mTOR signalling pathway. Thus, the purpose of this investigation was to determine if an active IGF-I receptor is necessary for mechanical load-induced muscle growth and activation of the Akt–mTOR signalling pathway.
Methods
Animals
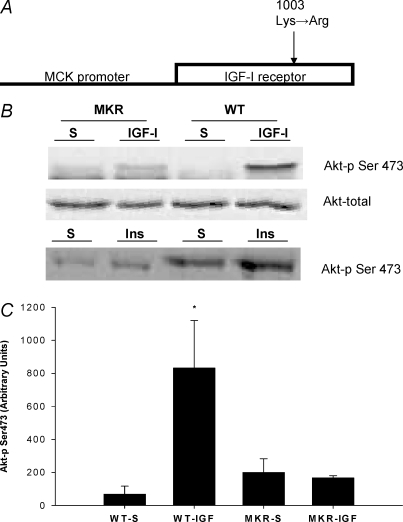
The generation of IGF receptor mutant (MKR) mice has been previously described (Fernandez et al. 2001, 2002). The muscle creatine kinase promoter was used to drive muscle-specific expression of a mutant human IGF-I receptor cDNA. The mutation Lys1003→Arg1003 abolishes ATP binding within the β subunit of the human IGF-I receptor and results in a kinase-inactive human IGF-I receptor that acts in a dominant negative fashion (see Fig. 1A) (Fernandez et al. 2001, 2002). The transgene for muscle creatine kinase promoter/human IGF-I receptor was injected into the eggs of female FVB/N mice. All experiments were performed in male homozygous transgenic mice or wild-type mice (8–10 weeks of age). The wild-type mice were strain-matched FVB mice. Mice were kept under a 12 h light–dark cycle and were fed standard diets. The study was conducted under the guidelines accepted by the American Physiological Society and received prior approval from the Institutional Animal Care and Use Committee at the University of California–Davis or the University of Maryland.
Figure 1. Description and evaluation of the transgenic mouse model.
A, the transgenic mouse (MKR) employed in this study contains a transgene driven by the muscle creatine kinase (MCK) promoter which provides the transgene with skeletal muscle specific gene expression. No detection of the transgene was apparent in cardiac muscle (Fernandez et al. 2001). The MCK promoter drives expression of a mutated version human IGF-I receptor. The mutation, a conversion of Lys to Arg (1003), results in abolished ATP binding within the β subunit of the human IGF-I receptor, resulting in a kinase-inactive human IGF-I receptor that acts in a dominant negative fashion (see previous descriptions, Fernandez et al. 2001, 2002). B, an example Akt immunoblot for muscle from WT and MKR mice that was injected with either IGF-I or saline. Direct injection of recombinant IGF-I or insulin (Ins) (50 μg ml−1; 50 μl total volume) in the tibalis anterior muscle failed to significantly increase Akt phosphorylation (Ser473) in the MKR, while the injection of IGF-I or insulin significantly increased Akt phosphorylation in the WT animals. The contralateral leg was injected with 50 μl of saline (S; vehicle used to dissolve the IGF-I or insulin). C, quantification of the phosphorylated (Ser473) form of Akt from the WT and MKR mice that underwent injections with either IGF-I or saline. *Statistically different from all of the groups of mice (P < 0.05). (WT-S n = 3; WT-IGF-I n = 3, MKR-S n = 4; MKR-IGF-I n = 4.)
IGF-I and insulin injections
To ensure the efficacy of the transgenic mouse model, the mice underwent intramuscular injections of recombinant IGF-I or insulin into the tibialis anterior (TA) muscle. Briefly, the mice were anaesthetized with 2–3% isoflurane. Under sterile conditions, a small incision was made through the skin exposing the TA muscle. The TA muscle of the mice was injected with 50 μl of recombinant IGF-I (50 μg ml−1, Sigma-Aldrich, St Louis, MO, USA) and the contralateral TA muscle was injected with 50 μl of saline (vehicle for IGF-I). For the insulin injections, the TA muscle was injected with 50 μl of recombinant insulin (50 μg ml−1, Sigma-Aldrich), while the contralateral TA muscle was injected with 50 μl of saline (vehicle for insulin). Both muscles were removed 15 min after the injections and immediately frozen in liquid nitrogen. The animals were subsequently killed by exsanguination while under anaesthesia.
Functional overload surgeries and muscle removal
To produce mechanical overload of the plantaris muscle, animals were subjected to bilateral functional overload according to previously described methodology (Spangenburg & Booth, 2006). Briefly, the mice were anaesthetized with 2–3% isoflurane. Under aseptic conditions, a small incision was made to the posterior lower limb, exposing the ankle extensor muscles and Achilles tendon. The entire soleus and approximately one-half of the medial/lateral gastrocnemius muscles were carefully removed, with care taken to ensure that no damage was done to the plantaris neural-vascular supply. The wound was irrigated with sterile saline and the skin incision closed with subcuticular sutures. The identical procedure was performed on both hindlimbs, producing a bilateral overload of the plantaris muscle. At 7 and 35 days post-surgery, the animals were anaesthetized with 2–3% isoflurane and the plantaris muscles were carefully dissected and weighed. The muscle was then immediately frozen in liquid nitrogen and stored at −80°C for later use. The animals were subsequently killed by exsanguination while under anaesthesia.
Plantaris and TA muscle homogenization
The plantaris or TA muscle was homogenized on ice in buffer containing 50 mm Hepes (pH 7.4), 0.1% Triton X-100, 4 mm EGTA, 10 mm EDTA, 15 mm Na4P2O7H2O, 100 mmβ-glycerophosphate, 25 mm NaF, 50 μg ml−1 leupeptin, 50 μg ml−1 pepstatin, 40 μg ml−1 aprotinin, 5 mm Na3VO4, and 1 mm PMSF as previously described (Sitnick et al. 2006; Spangenburg et al. 2006). After homogenization, the samples were stored at −80°C. The protein concentration of the samples was determined in triplicate via the Bradford procedure (Bio-Rad protein assay, Hercules, CA, USA).
SDS-PAGE, Western blotting, and immunodetection
Homogenates of the muscle were solubilized in loading buffer (2.5 mm Tris-HCl, pH 6.8, 20% glycerol, 2% SDS, 5%β-mercaptoethanol, and 0.025% bromophenol blue) and boiled at 98°C for 5 min as previously described (Morris et al. 2004). Seventy-five micrograms of total protein was then loaded (μg per sample per lane) onto 10% SDS-PAGE gels. All gels were run at 150 V for 1 h to separate proteins. The gels were then transferred onto PVDF membranes (Millipore, Billerica, MA, USA) at 50 V for 1 h at 4°C in transfer buffer (25 mm Tris base, 192 mm glycine, and 20% methanol). To confirm successful transfer of protein and equal loading of lanes, the membranes were stained with Ponceau S (data not shown). After successful transfer, the membrane was placed in blocking buffer (5% non-fat dry milk in TBS-T (0.1% Tween-20) for 1 h at room temperature, serially washed (3 × 5 min) and incubated with primary antibody in dilution buffer (5% BSA in TBS-T) overnight at 4°C. After another serial wash with TBS-T (3 × 5 min), the membranes were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h followed by another serial wash with TBS-T (3 × 5 min). Enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA) was used to detect the HRP activity by exposure to Kodak-XAR5 autoradiographic film for the appropriate durations to keep the integrated optical densities (IOD) within a linear and non-saturated range for all bands of each membrane. The IODs were quantified using ImageQuant densitometry software (Molecular Dynamics, Sunnyvale, CA, USA).
Antibodies
The primary antibodies phospho Ser473-AKT (1 : 1000 dilution), AKT (1 : 1000), phospho Thr389-p70S6 kinase (p70S6K) (1 : 500), and p70S6K (1 : 500) were purchased from Cell Signalling Technologies, Inc. (Beverly, MA, USA). Anti-rabbit and anti-mouse secondary antibodies (1 : 2000) were purchased from Cell Signalling Technologies, Inc. Antibody specificity was verified by molecular weight, positive controls (where possible) and lack of secondary antibody signal in the absence of the primary antibody.
Muscle contractile experiments
These experiments were performed according to previously describe methodology (Spangenburg et al. 1998). In brief, single EDL muscles were surgically excised with ligatures at each tendon (5–0 silk suture) and mounted in a bath between a fixed post and force transducer (Aurora 300B-LR) operated in isometric mode. The muscle was maintained in physiological saline solution (PSS; pH 7.6) containing (mm) 119 NaCl, 5 KCl, 1 MgSO4, 5 NaHCO3, 1.25 CaCl2, 1 KH2PO4, 10 Hepes, 10 dextrose, and maintained at 30°C under aeration with 95% O2–5% CO2 throughout the experiment. Resting tension, muscle length and stimulation current were iteratively adjusted for each muscle to obtain optimal twitch force. During a 5 min equilibration, single twitches were elicited at every 30 s with electrical pulses (0.5 ms) via platinum electrodes running parallel to the muscle. Optimal resting tension was determined and isometric tension was evaluated by 250 ms trains of pulses delivered at 1, 10, 20, 40, 60, 80, 100, 150 and 300 Hz. Following a 15 min rest period, the muscle was stimulated to fatigue by delivering tetanic trains (100 Hz for 100 ms) every 2 s for 5 min. After the experimental protocol, the muscle rested for 5 min at which time muscle length was determined with a digital micrometer; muscle was trimmed proximal to the suture connections, blotted and weighed. The cross-sectional area for each muscle was determined by dividing the mass of the muscle (g) by the product of its length (Lo, mm) and the density of muscle (1.06 g cm−3; Mendez & Keys, 1960) and was expressed as square millimetres. Muscle output was then expressed as isometric tension (N cm−2) determined by dividing the tension (N) by the muscle cross-sectional area.
Statistics
All data are expressed as means ± s.e.m., except where designated. Statistical significance was determined using a one-way analysis of variance for multiple comparisons followed by a Tukey's post hoc test. A P-value of < 0.05 was considered significant.
Results
To determine if the IGF-I receptor is critical for the induction of hypertrophy following chronic mechanical loading we employed a previously described transgenic mouse that over-expressed a mutated IGF-I receptor (MKR) selectively in skeletal muscle (Fig. 1A) (Fernandez et al. 2001, 2002). To confirm that the IGF-I receptor was in fact dysfunctional in the skeletal muscle, recombinant IGF-I (50 μg ml−1) was directly injected into the tibalis anterior muscle. While the injected IGF-I induced an 11-fold increase in Ser473 phosphorylation of Akt in the wild-type mice (WT), there was no significant increase in Akt phosphorylation in the MKR mice (Fig. 1B and C). In addition, the MKR mice did not have the ability to respond to insulin injections when compared to the WT mice (Fig. 1B). The lack of response in the MKR mice was not due to a difference in the amount of Akt protein between WT and MKR muscles since the TA muscle from WT and MKR mice had similar amounts of total Akt (Fig. 1B). Skeletal muscles from MKR mice were significantly smaller compared to age-matched WT animals (Figs 2 and 4A), confirming previously published findings with this transgenic line (Fernandez et al. 2001, 2002), and further supporting the effectiveness of the mutated IGF-I receptor in these experiments. It should be pointed out that although the mutated IGF1-R affected the postnatal growth of the skeletal muscles, it did not affect the ability of the muscles to produce tension.
Figure 2. Absolute gastrocnemius muscle mass in WT (n = 8) and MKR (n = 8) animals at 8–10 weeks of age.
*Statistically different WT mice (P < 0.05).
Figure 4.
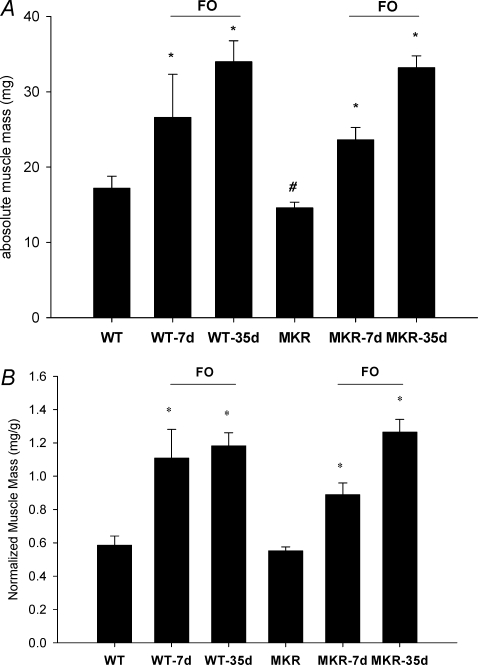
A–B, effects of functional overload (FO) of the plantaris muscle in the WT and MKR mice. Changes in absolute (A) and normalized (B) plantaris muscle mass after varying time points (7 or 35 days) of FO of the plantaris muscle in WT and MKR mice. *Statistically different from the control for WT and MKR mice; #statistically different from the WT control mice (P < 0.05). (WT n = 5; WT-7d FO n = 7; WT-35d FO n = 8; MKR n = 5; MKR-7d FO n = 10; MKR-35d FO n = 9.)
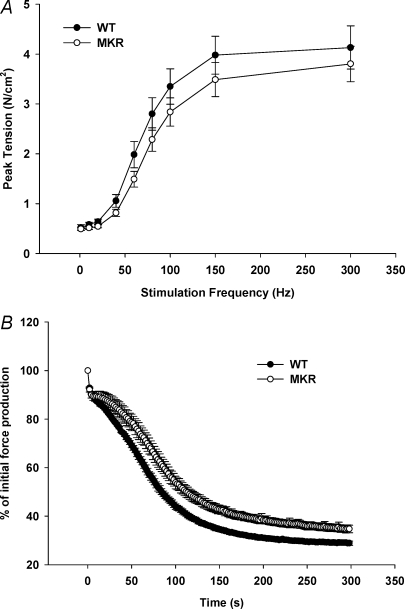
Contractile property measurements were made to address the possibility that mutation of the IGF-I receptor resulted in alterations in force production capabilities of the MKR mice. Previous reports have suggested that IGF-I can affect force production of skeletal muscle (Gonzalez et al. 2003; Anderson et al. 2006) Contractile testing of the extensor digitorum longus (EDL) muscle demonstrated that WT and MKR mice produced similar tensions at a range of frequencies (Fig. 3A). Further, the fatigue response of the EDL in MKR muscles was not impaired, but was in fact slightly better than the fatigue response seen in WT mice (Fig. 3B).
Figure 3. EDL contractile function measurements in 8- to 10-week-old WT and MKR mice.
A, in vitro force production measured at varying frequency stimulations (1–300 Hz) in WT (•) and MKR mice (○) (WT n = 5; MKR n = 5). B, in vitro fatigue development measured in WT (•) and MKR mice (○). (WT n = 5; MKR n = 5.)
Next, we examined whether a functional IGF-1 receptor was critical for producing muscle hypertrophy in response to an increase in mechanical load by subjecting the plantaris muscle from WT and MKR mice to functional overload (FO) for either 7 or 35 days. In control animals, the plantaris wet weight was significantly greater (11%) in WT compared to MKR mice (Fig. 4A). In WT mice, the response of the plantaris to loading was a significant increase in wet weight of 26% and 100% after 7 and 35 days, respectively (Fig. 4A). In comparison, plantaris mass significantly increased by 62% and 127% in the MKR mice following 7 and 35 days of FO, respectively (Fig. 4A). Given that MKR mice are on average 6% smaller than WT mice (WT = 28.65 ± 1.65 (s.d.) and MKR = 27.02 ± 2.07 (s.d.)), muscle wet weight was normalized to the body weight. The results for normalized muscle weights (Fig. 4B) show the same trends as the results for absolute muscle weights, with the exception that when normalized to body weight the plantaris weight of control mice is similar between WT and MKR mice.
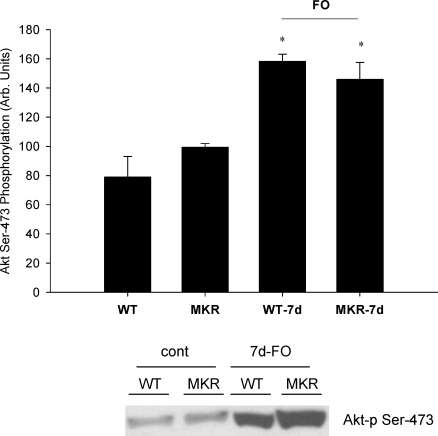
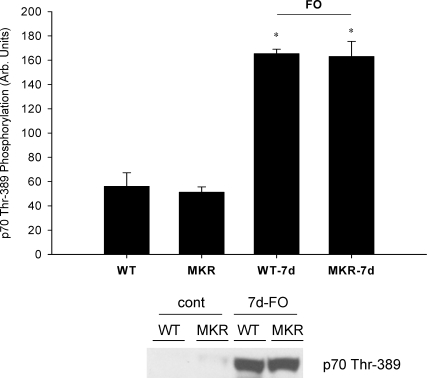
Previous studies have suggested that the Akt–mTOR signalling pathway is a critical regulator of muscle growth in response to mechanical loading (Bodine et al. 2001). Moreover, it is often suggested that the upstream activator of Akt–mTOR is IGF-I acting through the IGF-I receptor (Adams, 2002). Consequently, we measured the phosphorylation status of Akt and p70s6k after seven days of functional overload. Significant increases in the phosphorylation status of Akt (Ser473) and p70s6k (Thr389) were detected in the plantaris of both WT and MKR mice after 7 days of functional overload (Figs 5 and 6). No differences were measured in phosphorylation status or total protein amounts for either Akt or p70s6k at any time point (data not shown).
Figure 5. Effects of 7 days of functional overload (FO) and the absence of a functional IGF-I receptor on changes in Akt phosphorylation (Ser473).
WT and MKR refer to muscle masses measured from control animals, while WT-7d FO and MKR-7d FO refer to mice subjected to functional overload surgery for 7 days. No differences were detected in total Akt from any group (data not shown). *Statistically different from the control for WT and MKR mice (P < 0.05). (WT n = 3; WT-7d FO n = 3; MKR n = 3; MKR-7d FO n = 3.)
Figure 6. Effects of 7 days of functional overload (FO) and the absence of a functional IGF-I receptor on changes in p70s6k phosphorylation (Thr389).
WT and MKR refer to muscle masses measured from control animals, while WT-7d FO and MKR-7d FO refer to mice subjected to functional overload surgery for 7 days. No differences were detected in total p70s6k from any group (data not shown). *Statistically different from the control for WT and MKR mice (P < 0.05). (WT n = 3; WT-7d FO n = 3; MKR n = 3; MKR-7d FO n = 3.)
Discussion
These data represent the first demonstration that the IGF-I receptor is not necessary for the induction of skeletal muscle growth in response to mechanical loading. The transgenic mice employed in these studies express a mutated IGF-I receptor specifically in skeletal muscle that acts in a dominant negative fashion to prevent activation of the IGF-1R in response to binding of endogenous or exogenous IGF-I. Thus, if up-regulation of IGF-I and receptor activation are critical factors for load-induced skeletal muscle hypertrophy, then mice deficient in IGF-IR mediated signalling should not respond to functional overload with an increase in muscle mass. However, these data demonstrate quite clearly that signalling through the IGF-IR is not necessary for load-induced muscle hypertrophy since the MKR mice retain the capacity to induce muscle growth in response to functional overload. Furthermore, we demonstrate that components of the Akt–mTOR signalling pathway, thought to be critical for the induction muscle growth, were activated equally by the overload stimulus in both the WT and MKR mice. Thus, these data suggest that although exogenous IGF-I can induce muscle hypertrophy, the production of endogenous IGF-I by skeletal muscle and activation of the IGF-IR are not necessary for the activation of Akt-mediated signalling or the induction of muscle growth in response to mechanical load.
Numerous publications have demonstrated that the addition of exogenous IGF-I can induce muscle hypertrophy (Adams & McCue, 1998) and in some cases rescue muscle mass (Barton-Davis et al. 1998; Chakravarthy et al. 2000; Musaro et al. 2001). In response to an increase in mechanical load, skeletal muscles increase IGF-I mRNA and protein expression (DeVol et al. 1990; Adams & Haddad, 1996), which has led investigators to conclude that IGF-I is a critical factor involved in skeletal muscle hypertrophy. Unfortunately, this hypothesis has been difficult to test. For example, IGF-I knockout mice (IGF-I KO) exhibit a 30% reduction in body mass and low viability after birth (Liu et al. 1993). In addition, homozygote IGF-I KO mice are infertile and exhibit altered bone development making the study of these mice difficult (Baker et al. 1993). Interestingly, the IGF-I receptor knockout mice exhibit a more severe growth deficiency than the IGF-I KO mice and die of respiratory failure shortly after birth (Liu et al. 1993). Clearly, the MKR mouse model utilized in these studies does not demonstrate a phenotype that is as severe as the IGF-IR KO mice. This is likely to be due to the fact that expression of the mutated IGF-IR is being driven by the muscle creatine kinase promoter that has a postnatal expression pattern. Consequently, embryonic development of skeletal muscle in these MKR mice is probably not compromised. However, the MKR mice do exhibit a slight reduction in body mass and muscle mass (Fig. 2), indicating that the IGF-I receptor does contribute to postnatal muscle growth.
Our data do not support the current thinking with regard to how increased mechanical loading induces skeletal muscle hypertrophy, which proposes that mechanical loading (i.e. resistance exercise) results in enhanced IGF-I mRNA and protein production by the skeletal muscle resulting in an induction of muscle growth through the activation/proliferation of satellite cells and/or increase of protein synthesis through downstream activation of PI3K–AKt–mTOR and Raf–MEK–ERK signalling pathways (for review see Adams, 2002). Clearly, the MKR mice had no limitation in their ability to induce muscle growth, and in fact the signalling pathway by which IGF-I is thought to mediate the increase in protein synthesis, i.e. Akt–mTOR, was fully activated with the increase in load (Figs 4 and 5). Thus, IGF-I does not appear to be a limiting factor in the induction of muscle hypertrophy with increased mechanical loading, and other possible upstream signals should be considered. In fact, a few recent studies have suggested that the effects of mechanical loading may be mediated through other factors such as phospholipase-D to activate mTOR and its downstream pathways (Hornberger et al. 2006) and hepatocyte growth factor for the induction of satellite cell proliferation (Tatsumi et al. 2006).
One possible consideration is that IGF-I could be acting through the insulin receptor since previous findings show that IGF-I can bind to and activate the insulin receptor albeit with a lower affinity. However, in the initial description and characterization of these MKR mice, Fernandez et al. (2001) clearly demonstrated that the insulin receptor in skeletal muscle was also dysfunctional due to the formation of heterodimers with the mutated IGF-IR. In addition, we confirmed that direct muscular injections of recombinant insulin (50 ng ml−1 in 50 μl volume) failed to increase Akt phosphorylation (Ser473) in the MKR mice (Fig. 1B). Thus, these MKR mice do not have a functional IGF-I or insulin receptor. Previous reports have shown that these mice have a diabetic phenotype, which might lead one to predict that muscle growth in response to increased loading would be attenuated (Fluckey et al. 1996). Clearly, we did not observe that finding in these studies.
Another important consideration would be the role of IGF-II in these MKR mice. IGF-II is typically thought to contribute to the induction of myogenesis and is critical for myoblast differentiation (Tollefsen et al. 1989). IGF-II mRNA expression does increase with mechanical loading of the plantaris muscle (DeVol et al. 1990), although unlike IGF-I, IGF-II has not been shown to stimulate myotube hypertrophy (Vandenburgh et al. 1991). Thus, it is unlikely that IGF-II is acting in some sort of compensatory mechanism, unless the mechanistic properties of IGF-II have changed in these MKR mice resulting in the ability of IGF-II to have anabolic effects on skeletal muscle.
It should be pointed out that these data do not question the ability of IGF-I to induce growth or to be used in a therapeutic sense, but only demonstrate that IGF-I is not necessary for the activation of Akt–mTOR-mediated pathways or muscle hypertrophy during mechanical loading and is not critical to induce growth. Exogenous IGF-I, whether delivered systemically or through genetic means, clearly has the ability to induce muscle hypertrophy (Adams & McCue, 1998; Barton-Davis et al. 1998; Schertzer & Lynch, 2006), and has potential value in treating muscle degenerative diseases and sarcopenia.
In conclusion, utilizing a transgenic mouse model in which skeletal muscles cannot respond to IGF-I or insulin due to the over-expression of a mutated IGF1R, we demonstrate that IGF-I receptor-mediated signalling is not necessary for the induction of skeletal muscle hypertrophy in adult mice following a chronic increase in mechanical loading. Thus, unless IGF-I can work through an unknown alternative receptor, it does not appear that IGF-I receptor is part of the upstream mechanism that activates Akt/mTOR signalling pathways during increased mechanical load. However, one must consider the possibility that mutation in the IGF-I receptor in the MKR mice results in an unexpected compensation that is allowing these mice to still respond to mechanical loading. Clearly, these experiments demonstrate the need for additional work to identify the upstream mechanisms responsible for activation of Akt-mediated signalling in response to loading and other growth stimuli.
Acknowledgments
The authors wish to thank Emily Pettycrew and Tamara Mahmood for their excellent technical assistance. This work was supported by National Institutes of Health Grant AR051396 (EES) and NIH NIDDK (DLR)
References
- Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996;81:2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47:2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Booth FW, DeMayo F, Schwartz RJ, Gordon SE, Fiorotto ML. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol Endocrinol Metab. 1998;275:E373–E379. doi: 10.1152/ajpendo.1998.275.3.e373. [DOI] [PubMed] [Google Scholar]
- DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol Endocrinol Metab. 1990;259:E89–E95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest. 2002;109:347–355. doi: 10.1172/JCI13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15:1926–1934. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckey JD, Vary TC, Jefferson LS, Farrell PA. Augmented insulin action on rates of protein synthesis after resistance exercise in rats. Am J Physiol Endocrinol Metab. 1996;270:E313–E319. doi: 10.1152/ajpendo.1996.270.2.E313. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967;213:1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li C, Parkhouse WS. Differential effects of des IGF-1 on Erks, AKT-1 and P70 S6K activation in mouse skeletal and cardiac muscle. Mol Cell Biochem. 2002;236:115–122. doi: 10.1023/a:1016164601887. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J Appl Physiol. 2004;96:398–404. doi: 10.1152/japplphysiol.00454.2003. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI3K/Akt/mTOR and PI3K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther. 2006;13:1657–1664. doi: 10.1038/sj.gt.3302817. [DOI] [PubMed] [Google Scholar]
- Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol. 2006;100:286–293. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF (–/–) mouse. Cytokine. 2006;34:125–130. doi: 10.1016/j.cyto.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Brown DA, Johnson MS, Moore RL. Exercise increases SOCS-3 expression in rat skeletal muscle: potential relationship to IL-6 expression. J Physiol. 2006;572:839–848. doi: 10.1113/jphysiol.2005.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenburg EE, McBride TA. Inhibition of stretch-activated channels during eccentric muscle contraction attenuates p70S6K activation. J Appl Physiol. 2006;100:129–135. doi: 10.1152/japplphysiol.00619.2005. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Ward CW, Williams JH. Effects of lactate on force production by mouse EDL muscle: implications for the development of fatigue. Can J Physiol Pharmacol. 1998;76:642–648. doi: 10.1139/cjpp-76-6-642. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- Tollefsen SE, Sadow JL, Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci U S A. 1989;86:1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol Cell Physiol. 1991;260:C475–C484. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- Vyas DR, Spangenburg EE, Abraha TW, Childs TE, Booth FW. GSK-3β negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2002;283:C545–C551. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]