Abstract
Efforts to understand human physiology through the study of champion athletes and record performances have been ongoing for about a century. For endurance sports three main factors – maximal oxygen consumption 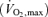 , the so-called ‘lactate threshold’ and efficiency (i.e. the oxygen cost to generate a give running speed or cycling power output) – appear to play key roles in endurance performance.
, the so-called ‘lactate threshold’ and efficiency (i.e. the oxygen cost to generate a give running speed or cycling power output) – appear to play key roles in endurance performance. 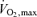 and lactate threshold interact to determine the ‘performance
and lactate threshold interact to determine the ‘performance 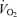 ‘ which is the oxygen consumption that can be sustained for a given period of time. Efficiency interacts with the performance
‘ which is the oxygen consumption that can be sustained for a given period of time. Efficiency interacts with the performance 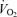 to establish the speed or power that can be generated at this oxygen consumption. This review focuses on what is currently known about how these factors interact, their utility as predictors of elite performance, and areas where there is relatively less information to guide current thinking. In this context, definitive ideas about the physiological determinants of running and cycling efficiency is relatively lacking in comparison with
to establish the speed or power that can be generated at this oxygen consumption. This review focuses on what is currently known about how these factors interact, their utility as predictors of elite performance, and areas where there is relatively less information to guide current thinking. In this context, definitive ideas about the physiological determinants of running and cycling efficiency is relatively lacking in comparison with 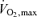 and the lactate threshold, and there is surprisingly limited and clear information about the genetic factors that might pre-dispose for elite performance. It should also be cautioned that complex motivational and sociological factors also play important roles in who does or does not become a champion and these factors go far beyond simple physiological explanations. Therefore, the performance of elite athletes is likely to defy the types of easy explanations sought by scientific reductionism and remain an important puzzle for those interested in physiological integration well into the future.
and the lactate threshold, and there is surprisingly limited and clear information about the genetic factors that might pre-dispose for elite performance. It should also be cautioned that complex motivational and sociological factors also play important roles in who does or does not become a champion and these factors go far beyond simple physiological explanations. Therefore, the performance of elite athletes is likely to defy the types of easy explanations sought by scientific reductionism and remain an important puzzle for those interested in physiological integration well into the future.
Introduction
Faster, Higher, Stronger: these simple descriptions have been of interest to humans since the beginning of recorded history. In this context, integrative physiology has long been served by so-called ‘experiments in nature.’ These include asking fundamental questions about the ability of various animal species to function in harsh environments and studies on unique human patients with clinical conditions that offer the opportunity to ask important questions about physiological regulation (for examples see Hagberg et al. 1982; Faraci et al. 1984; Schrage et al. 2005). Along similar lines, studies on both human and animal performance in athletic events can provide important insights and raise critical questions about oxygen transport, muscle performance and metabolism, cardiovascular control, and the operation of various components of the nervous system (Joyner, 1991).
Historical note
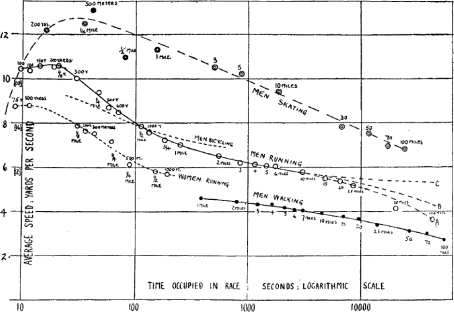
One of the first analyses of world records from A. V. Hill in 1925 (Hill, 1925) related the decline in running speed as race distance increased to the topic of muscle fatigue (Fig. 1). Even before then, the Italian physiologist Mosso, who was interested in fatigue associated with manual labour, noted ‘It is not will, not the nerves, but it is the muscle that finds itself worn out after the intense work of the brain.’ But Mosso hedged his bets and also commented that ‘fatigue of brain reduces the strength of the muscles’ (DiGiulio et al. 2006).
Figure 1. A. V. Hill's (Hill, 1925) original plot of world record performance time on the X-axis versus performance speed on the Y-axis.
The top tracing is for speed-skating, the middle tracing is for running by males, and the bottom tracing is for running by women. The shape of the curve led to Hill's original ideas about differing causes of muscle fatigue for exercise bouts of different durations.
In Hill's analysis he speculated that the factors limiting performance in events of less than a minute and more than an hour are probably not dependent solely on the energy supply to the contracting muscles and discussed the physiological determinants of performance in the context of ideas about energy stores, oxygen demand and oxygen debt. He also speculated that there were ‘three types of fatigue’ (a concept he found to be inexact) including: (1) one associated with short violent efforts; (2) the exhaustion ‘which overcomes the body when an effort of moderate intensity is continued for a long time’; and (3) fatigue associated with a more general ‘wear-and-tear’. The first two types of fatigue were thought to be primarily ‘muscular’.
Additionally, for the second and third types of fatigue, which occur during endurance exercise, Hill speculated that as distances increase beyond about 10 miles (∼16 km), ‘The continued fall in the curve, as the effort is prolonged, is probably due to the second and third types of fatigue which we discussed above, either to the exhaustion of the material of the muscle, or to the incidental disturbances which may make a man stop before his muscular system has reached its limit. A man of average size running in a race must expend about 300 g of glycogen per hour; perhaps a half of this may be replaced by its equivalent of fat. After a very few hours therefore the whole glycogen supply of his body will be exhausted. The body, however, does not readily use fat alone as a source of energy; disturbances may arise in the metabolism; it will be necessary to feed a man with carbohydrate as the effort continues. Such feeding will be followed by digestion; disturbances of digestion may occur – other reactions may ensue. For very long distances the case is far more complex than for the shorter ones, and although, no doubt, the physiological principles can be ascertained, we do not know enough about them yet to be able further to analyse the curves.’ These comments and the work of Scandinavian physiologists in the 1930s set the stage for the concept of carbohydrate loading and a number of dietary and feeding strategies that have been shown to delay fatigue (Christensen, 1939; Sherman & Costill, 1984; Murray, 1998).
Focus of this review
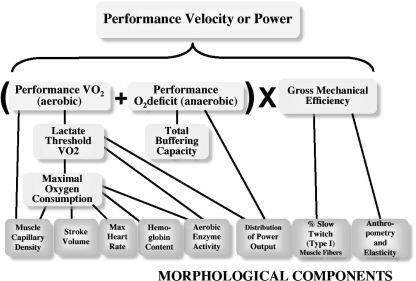
In this review for The Journal of Physiology's 2008 Olympic Issue, we will focus on current models of human performance, review the physiological ‘ideas’ that led to these models, and ask what these models explain and more importantly do not explain. Figure 2 presents our concepts using a model like Hill's that is focused on ‘performance velocity’ and how it is determined by maximum rates of aerobic energy production, anaerobic capacity and how efficiently the energy being used is converted to movement.
Figure 2. Overall schematic of the multiple physiological factors that interact as determinants of performance velocity or power output.
This figure serves as the conceptual framework for the ideas discussed in this review.
In general, we focus on endurance exercise performance because it is our area of expertise, and there are relatively more data on the physiological adaptations that contribute to endurance performance, and (especially for running) there are accurate records extending for more than 100 years. There is also at least some physiological data on champion athletes over almost the same period of time.
Overview of current ideas about human performance
As noted above, most models of athletic performance focus on distance running and endurance cycling. First, there are excellent records and standard events. Second, there is comprehensive physiological data on a large number of elite athletes. Third, it is possible, using treadmills and cycle ergometers, to reasonably simulate in a laboratory what is happening during actual competition. We should also note that for the purposes of this review we assume that environmental conditions are ideal and do not add any additional challenges to physiological regulation (most notably the challenges associated with high altitude and/or high environmental temperatures).
Several well-accepted concepts (Joyner, 1991, 1993; Coyle, 1995; Bassett & Howley, 2000) have emerged related to endurance exercise performance velocity and the first component issue is the level of aerobic metabolism that can be maintained during a race (i.e. performance 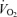 ; Fig. 2). The upper limit for this is ‘maximal’ oxygen uptake. This is usually achieved during relatively large muscle mass exercise and represents the integrative ability of the heart to generate a high cardiac output, total body haemoglobin, high muscle blood flow and muscle oxygen extraction, and in some cases the ability of the lungs to oxygenate the blood (Mitchell et al. 1958; Kanstrup & Ekblom, 1984; Rowell, 1986; Dempsey, 1986; Saltin & Strange, 1992; Bassett & Howley, 2000). By the 1930s very high values for
; Fig. 2). The upper limit for this is ‘maximal’ oxygen uptake. This is usually achieved during relatively large muscle mass exercise and represents the integrative ability of the heart to generate a high cardiac output, total body haemoglobin, high muscle blood flow and muscle oxygen extraction, and in some cases the ability of the lungs to oxygenate the blood (Mitchell et al. 1958; Kanstrup & Ekblom, 1984; Rowell, 1986; Dempsey, 1986; Saltin & Strange, 1992; Bassett & Howley, 2000). By the 1930s very high values for 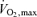 in athletes were observed and identified as a marker of elite performance (Robinson et al. 1937). Champion endurance athletes have
in athletes were observed and identified as a marker of elite performance (Robinson et al. 1937). Champion endurance athletes have 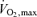 values of between 70 and 85 ml kg−1 min−1, with values in women typically averaging about 10% lower due to lower haemoglobin concentrations and higher levels of body fat (Saltin & Astrand, 1967; Pollock, 1977; Durstine et al. 1987; Pate et al. 1987).
values of between 70 and 85 ml kg−1 min−1, with values in women typically averaging about 10% lower due to lower haemoglobin concentrations and higher levels of body fat (Saltin & Astrand, 1967; Pollock, 1977; Durstine et al. 1987; Pate et al. 1987).
In summary, 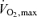 values 50–100% greater than those seen in normally active healthy young subjects are seen in champion endurance athletes and the most striking adaptations to training that contribute to these high
values 50–100% greater than those seen in normally active healthy young subjects are seen in champion endurance athletes and the most striking adaptations to training that contribute to these high 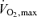 values include increased cardiac stroke volume, increased blood volume, increased capillary density and mitochondrial density in the trained muscles (Costill et al. 1976). Of these, the most dominant factor is a high stroke volume (Ekblom & Hermansen, 1968; Coyle et al. 1984; Martin et al. 1986).
values include increased cardiac stroke volume, increased blood volume, increased capillary density and mitochondrial density in the trained muscles (Costill et al. 1976). Of these, the most dominant factor is a high stroke volume (Ekblom & Hermansen, 1968; Coyle et al. 1984; Martin et al. 1986).
Once it became reasonably clear that elite runners had high values for 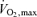 it also became clear that for events lasting beyond 10 or 15 min, most or all of the competition was performed at an average pace that did not evoke
it also became clear that for events lasting beyond 10 or 15 min, most or all of the competition was performed at an average pace that did not evoke 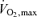 , with much of the 42 km marathon run at approximately 75–85%
, with much of the 42 km marathon run at approximately 75–85%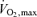 while 10 km is performed at 90–100%
while 10 km is performed at 90–100%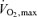 and 5 km at close to
and 5 km at close to 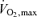 (Costill et al. 1973; Bassett & Howley, 2000). Along these lines, it has recently been shown that maximal aerobic metabolism can decline acutely during the course of a 5–8 min laboratory performance bout. This decline is caused by a fall in stroke volume and accelerated muscle fatigue due to reduced blood and oxygen delivery and increased anaerobic metabolism (Gonzalez-Alonso & Calbet, 2003; Mortensen et al. 2005). This does not invalidate the concept of
(Costill et al. 1973; Bassett & Howley, 2000). Along these lines, it has recently been shown that maximal aerobic metabolism can decline acutely during the course of a 5–8 min laboratory performance bout. This decline is caused by a fall in stroke volume and accelerated muscle fatigue due to reduced blood and oxygen delivery and increased anaerobic metabolism (Gonzalez-Alonso & Calbet, 2003; Mortensen et al. 2005). This does not invalidate the concept of 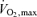 , but rather indicates that the maximal rate of aerobic ATP resynthesis during a race is dynamic and that truly accurate models of energy turnover during actual competition would require instantaneous measurements and calculation of fluxes through multiple metabolic pathways (e.g. total ATP turnover with contributions from both aerobic and anaerobic components as well as energy stores).
, but rather indicates that the maximal rate of aerobic ATP resynthesis during a race is dynamic and that truly accurate models of energy turnover during actual competition would require instantaneous measurements and calculation of fluxes through multiple metabolic pathways (e.g. total ATP turnover with contributions from both aerobic and anaerobic components as well as energy stores).
Lactate threshold
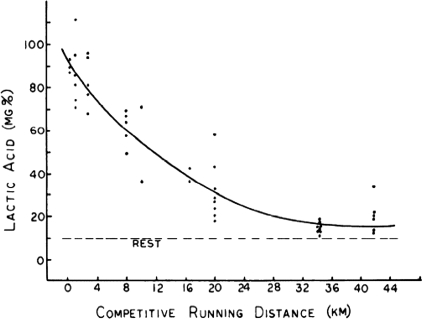
Based on the concepts above the question then became what fraction of 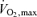 might be sustained for periods of time extending to several hours (i.e. the marathon) and what is the rate of glycolysis in the active muscles at this rate of mitochondrial oxidation. This question led to observations showing a curvilinear relationship between blood lactate values during exercise and the distance of the effort (Fig. 3; Costill, 1970) and led to the concept that the rate of aerobic metabolism maintained during a performance bout (i.e. performance
might be sustained for periods of time extending to several hours (i.e. the marathon) and what is the rate of glycolysis in the active muscles at this rate of mitochondrial oxidation. This question led to observations showing a curvilinear relationship between blood lactate values during exercise and the distance of the effort (Fig. 3; Costill, 1970) and led to the concept that the rate of aerobic metabolism maintained during a performance bout (i.e. performance 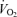 ; Fig. 2) can be better described by the degree of muscle glycolytic stress reflected in lactate production in addition to
; Fig. 2) can be better described by the degree of muscle glycolytic stress reflected in lactate production in addition to 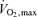 (Farrell et al. 1979; La Fontaine et al. 1981).
(Farrell et al. 1979; La Fontaine et al. 1981).
Figure 3. Plot or blood lactic acid concentration versus race distance (Costill, 1970).
This figure is an example of the diminishing contribution of so-called ‘anaerobic’ energy sources as race distance increases. This paper also set the stage for a number of later investigations related to the fraction of 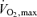 (e.g. performance
(e.g. performance 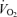 ) that could be sustained in competition.
) that could be sustained in competition.
In this context, as running speed or power output on a cycle ergometer increases in untrained subjects there is typically no sustained rise in blood lactate concentration until about 60% of 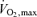 is reached. In trained subjects this value can be 75–90% of
is reached. In trained subjects this value can be 75–90% of 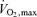 (Fig. 4). There is a long history of investigation about what causes this rise in blood lactate levels and also how lactate (and/or hydrogen ion) does or does not contribute to fatigue. For this review the important summary points include: (1) the initial appearance of blood lactate is not synonymous with hypoxia in the skeletal muscle, and (2) the lactate molecule per se does not ‘cause’ muscle fatigue (Holloszy et al. 1977; Holloszy & Coyle, 1984; Robergs et al. 2004).
(Fig. 4). There is a long history of investigation about what causes this rise in blood lactate levels and also how lactate (and/or hydrogen ion) does or does not contribute to fatigue. For this review the important summary points include: (1) the initial appearance of blood lactate is not synonymous with hypoxia in the skeletal muscle, and (2) the lactate molecule per se does not ‘cause’ muscle fatigue (Holloszy et al. 1977; Holloszy & Coyle, 1984; Robergs et al. 2004).
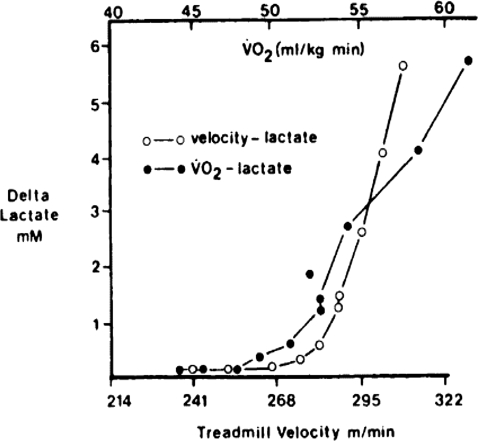
Figure 4. Individual record of treadmill velocity and 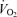 versus blood lactate concentration in subject capable of breaking 2:30 h for the marathon (Farrell et al. 1979).
versus blood lactate concentration in subject capable of breaking 2:30 h for the marathon (Farrell et al. 1979).
In untrained subjects the upturn in lactic acid concentrations is seen at about 60% of 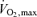 . Trained subjects can usually exercise at 75–85% of
. Trained subjects can usually exercise at 75–85% of 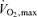 before there is a marked increase in blood lactate concentration. This figure also illustrates the concept of performance
before there is a marked increase in blood lactate concentration. This figure also illustrates the concept of performance 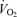 and performance velocity.
and performance velocity.
What appears to be occurring is that the maximum rate of fat oxidation is inadequate to meet the ATP demands of muscles contracting at moderate and high intensities. This causes intracellular signalling events to occur which stimulate glycogenolysis and glycolysis and ultimately the rate of pyruvate delivery to the mitochondria progressively exceeds the ability of the mitochondria to oxidize pyruvate and this leads to accelerated generation of lactic acid (Holloszy et al. 1977; Holloszy & Coyle, 1984; Robergs et al. 2004). The associated hydrogen ion is then a likely culprit in muscle fatigue and also activates group III and IV skeletal muscle afferents that evoke important cardiovascular and autonomic reflexes (Pryor et al. 1990).
While the physiological determinants of the lactate threshold are extremely complex, they are determined mainly by the oxidative capacity of the skeletal muscle (Holloszy et al. 1977; Davies et al. 1982; Holloszy & Coyle, 1984; Gregg et al. 1989a,b). This capacity is highly plastic and can essentially increase more than twofold in the trained skeletal muscle of humans or animals who engage in 20–120 min of training at a requisite intensity (Holloszy et al. 1977; Dudley et al. 1982; Holloszy & Coyle, 1984). This more than doubling of oxidative capacity is one of the factors that is linked to the high ‘lactate threshold’ values seen in elite endurance athletes (Fig. 2). As noted above, these elite athletes have 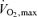 values that are 50–100% above those seen in normally active sedentary young people and their lactate threshold occurs at a higher percentage of their
values that are 50–100% above those seen in normally active sedentary young people and their lactate threshold occurs at a higher percentage of their 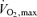 . This means that in elite athletes the absolute oxygen consumptions (power output and/or speed) that can be generated for long periods of time before reaching the lactate threshold is essentially doubled allowing sustained running speeds of 20 km h−1 or cycling power outputs of 400 W.
. This means that in elite athletes the absolute oxygen consumptions (power output and/or speed) that can be generated for long periods of time before reaching the lactate threshold is essentially doubled allowing sustained running speeds of 20 km h−1 or cycling power outputs of 400 W.
Other key factors that reduce muscle fatigability and lactate production during exercise at 85–90% 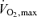 , when only a fraction of the total limb muscle mass is simultaneously recruited, is the quantity of muscle mass that the athlete can recruit to share in sustaining power production (Fig. 2). Elite cyclists appear capable of rotating power production through 20–25% more muscle mass throughout a 1 h bout of cycling, thus reducing the relative power production and stress on a given fibre (Coyle et al. 1988; Coyle, 1995). Additionally, this ‘power sharing’ among fibres would also reduce the glycolytic stress and lactate production per fibre due to more total mitochondrial sharing for a given rate of aerobic metabolism. These factors should operate in a complementary way that reduces the stress per mitochondria and muscle fibre.
, when only a fraction of the total limb muscle mass is simultaneously recruited, is the quantity of muscle mass that the athlete can recruit to share in sustaining power production (Fig. 2). Elite cyclists appear capable of rotating power production through 20–25% more muscle mass throughout a 1 h bout of cycling, thus reducing the relative power production and stress on a given fibre (Coyle et al. 1988; Coyle, 1995). Additionally, this ‘power sharing’ among fibres would also reduce the glycolytic stress and lactate production per fibre due to more total mitochondrial sharing for a given rate of aerobic metabolism. These factors should operate in a complementary way that reduces the stress per mitochondria and muscle fibre.
As exercise extends beyond about 2 h the problem becomes one of fuel availability as (Hill predicted) the glycogen content in skeletal muscle becomes depleted and the modest ability of active muscle to take up glucose from blood (via either the liver or from feeding) can limit the rate of oxidative ATP generation and thus the pace that can be sustained. In some (but not all) subjects the associated reductions in blood glucose evoke frank symptoms of hypoglycaemia that limit the ability of the individual to continue exercising (Christensen, 1939; Coyle et al. 1983, 1986). Other highly trained subjects show remarkable resistance to hypoglycaemia and for these athletes muscle glycogen depletion is probably more important. In response to these events, a number of pre-competition dietary strategies and during-exercise energy replacement regimens and products have been developed (Murray, 1998). When these are used in an optimal manner muscle glycogen stores can be augmented by 40% before exercise, and hypoglycaemia can be avoided with the net effect being that the duration of exercise at about the lactate threshold can be extended by about one-third (from 2 to 3 h to 4 h) (Coyle et al. 1983, 1986; Sherman & Costill, 1984).
Performance 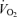 and anaerobic metabolism
and anaerobic metabolism
Without practical direct calorimetric methods to measure instantaneous rates of heat and work production during endurance exercise (Webb et al. 1988; Scott, 2000), the best practical estimation of the rates of actual metabolic energy production and ATP turnover is obtained from measures of oxygen consumption (i.e. indirect calorimetry) during an endurance performance bout. During marathon running the relative amount of anaerobic metabolism is small yet in events lasting 13–30 min (i.e. 5 and 10 km running), it will be significant, contributing perhaps 10–20% of total ATP turnover. This anaerobic contribution to ATP turnover during endurance performance bouts is noted in Fig. 2 and has classically been estimated from measures of post-exercise oxygen consumption and may equal the energy provided by 50–80 ml kg−1 of oxygen uptake (Fig. 2) (Bangsbo et al. 1993). However, the rate at which this energy might be generated and consumed is difficult to estimate in a definitive way.
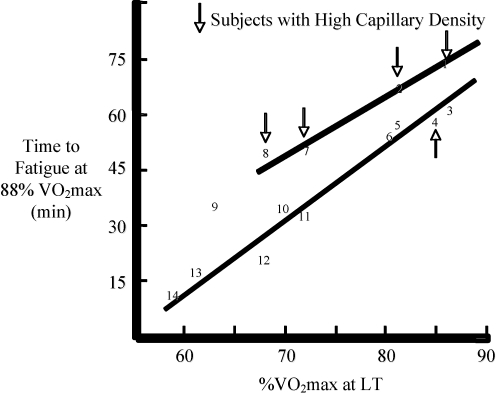
Figure 2 also makes the point that the rate of total ATP turnover during endurance performance reflects the interplay of aerobic and anaerobic metabolism with lactate generation serving to maintain the NAD+ needed for continued glycolysis and generation of pyruvate. An example of this interplay appears to be the influence of high skeletal muscle capillary density, serving to remove or recycle within muscle fatiguing metabolites (e.g. hydrogen ions). As shown in Fig. 5, exercise time to fatigue at 88% 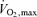 in a population of cyclists (n = 14, individually numbered) possessing the same
in a population of cyclists (n = 14, individually numbered) possessing the same 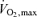 (i.e. 4.9 l min−1), as expected, was related to the percentage of
(i.e. 4.9 l min−1), as expected, was related to the percentage of 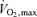 at the blood lactate threshold. However, some subjects (see upper line in Fig. 5) were able to exercise longer than normal (see lower line in Fig. 5) even when accounting for their lactate threshold (i.e. subjects 1, 2, 7 and 8 in Fig. 4). For the most part, these individuals (i.e. 1, 2, 7 and 8) possessed an unusually high muscle capillary density which may have allowed their exercising muscles to better tolerate anaerobic metabolism and lactic acid production. For this reason, Fig. 2 indicates that ‘Performance
at the blood lactate threshold. However, some subjects (see upper line in Fig. 5) were able to exercise longer than normal (see lower line in Fig. 5) even when accounting for their lactate threshold (i.e. subjects 1, 2, 7 and 8 in Fig. 4). For the most part, these individuals (i.e. 1, 2, 7 and 8) possessed an unusually high muscle capillary density which may have allowed their exercising muscles to better tolerate anaerobic metabolism and lactic acid production. For this reason, Fig. 2 indicates that ‘Performance 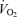 , might be directly influenced by muscle capillary density, independent of its important role in delivering oxygen and reducing diffusion gradients, but also by removing waste products and limiting acidosis in the contracting muscles.
, might be directly influenced by muscle capillary density, independent of its important role in delivering oxygen and reducing diffusion gradients, but also by removing waste products and limiting acidosis in the contracting muscles.
Figure 5. Time to fatigue during exercise at 88% of 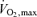 plotted against lactate threshold (LT) in 14 highly trained cyclists and triathletes (data plotted from Coyle et al. 1988; Coyle, 1995).
plotted against lactate threshold (LT) in 14 highly trained cyclists and triathletes (data plotted from Coyle et al. 1988; Coyle, 1995).
These athletes all had similar 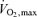 values and uniformly high muscle oxidative enzymes. A subgroup of 4 athletes (subjects 1, 2, 7 and 8) with exceptionally high capillary density seemed to ‘overachieve’ in comparison with their lactate threshold values compared with other members of the group.
values and uniformly high muscle oxidative enzymes. A subgroup of 4 athletes (subjects 1, 2, 7 and 8) with exceptionally high capillary density seemed to ‘overachieve’ in comparison with their lactate threshold values compared with other members of the group.
An additional point from Fig. 5 is that much remains to be learned about subtle factors that delay or accelerate fatigue during events performed at intensities above 80–90% of 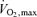 . Small increases in total energy expenditure or reductions in oxygen delivery will have disproportionate effects and accelerate fatigue (Mortensen et al. 2005) during very intense exercise. At this time it remains unclear if laboratory tests can detect the subtle adaptations in the very best performers who seem to be able manage their metabolism in a way that permits maximum efficient energy use.
. Small increases in total energy expenditure or reductions in oxygen delivery will have disproportionate effects and accelerate fatigue (Mortensen et al. 2005) during very intense exercise. At this time it remains unclear if laboratory tests can detect the subtle adaptations in the very best performers who seem to be able manage their metabolism in a way that permits maximum efficient energy use.
Efficiency
The next factor that makes an important contribution in endurance exercise performance velocity has been termed ‘economy’ or ‘efficiency.’ In the above sections we outlined how 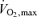 and the lactate threshold operate to determine ‘Performance
and the lactate threshold operate to determine ‘Performance 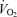 , (Fig. 2). The next question then is how much speed or power can be generated for that level of oxygen consumption? The oxygen cost of endurance running (ml kg min−1) at a given speed can vary about 30–40% among individuals (Farrell et al. 1979; Conley & Krahenbuhl, 1980; Joyner, 1991), as shown in Fig. 6. When cycling at a given power output, the oxygen cost and thus gross mechanical efficiency also varies from one person to another, but by a somewhat lesser amount compared with running (i.e. 20–30%) (Coyle, 1995).
, (Fig. 2). The next question then is how much speed or power can be generated for that level of oxygen consumption? The oxygen cost of endurance running (ml kg min−1) at a given speed can vary about 30–40% among individuals (Farrell et al. 1979; Conley & Krahenbuhl, 1980; Joyner, 1991), as shown in Fig. 6. When cycling at a given power output, the oxygen cost and thus gross mechanical efficiency also varies from one person to another, but by a somewhat lesser amount compared with running (i.e. 20–30%) (Coyle, 1995).
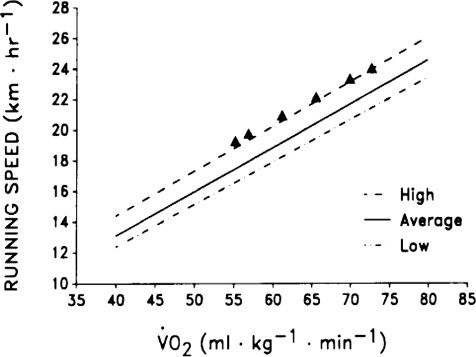
Figure 6. Regression lines for high, average and low running economy (efficiency) in elite endurance athletes based on values gleaned from a number of sources (Joyner, 1991).
Since there has been little systematic data collected above ∼18 km h−1 the filled triangles in the figure are individual data from a limited number of champions with exceptional running economy. This figure emphasizes the importance of efficiency among groups of elite performers with relatively uniform 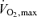 and lactate threshold values. It is also of note that the physiological determinants of efficiency (especially for running) are poorly understood.
and lactate threshold values. It is also of note that the physiological determinants of efficiency (especially for running) are poorly understood.
Gross mechanical efficiency when endurance-trained cyclists generate 300 W can vary from 18.5 to 23.5% and it appears that more than one-half of this variability is related to the percentage of type I (slow twitch) muscle fibres of the vastus lateralis muscle (Coyle et al. 1992). The efficiency with which the chemical energy of ATP hydrolysis is converted to physical work depends greatly on the velocity of sarcomere and muscle fibre shortening. Type I (slow twitch) fibres display greater mechanical efficiency when cycling at cadences of 60–120 r.p.m. Therefore, it is not surprising that elite endurance cyclists typically possess a higher percentage of type I muscle fibres, given that they are more efficient. Although type I muscle fibres in untrained humans possess higher mitochondrial density compared with type II fibres (fast twitch), it is important to note that with intense interval training, mitochondrial activity can be increased to equally high levels in both fibre types (Chi et al. 1983). Thus, with intense endurance training over years, the main functional advantage of type I fibres appears to be efficiency when cycling rather than total oxidative ability, although type I fibre seem to retain a greater ability to oxidize fat.
It is also of note that many champion cyclists chose pedal cadences of around 90 r.p.m. This is a cadence that may actually increase whole body oxygen consumption slightly for a given total body power output from the 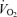 minimum which usually occurs at 50–60 r.p.m. In a comprehensive engineering/physiology analysis of this problem Hansen et al. (2002) noted that subjects with higher levels of myosin heavy chain I (MHC I, the predominant myosin in type I fibres) self-selected higher pedal rates and these rates closely matched the rate of peak mechanical efficiency. In this context, they speculated that motor control patterns in these subjects might favour a faster cadence so that relatively low total muscle forces (probably from fatigue-resistant motor units) per pedal stroke could generate the needed power so that the higher force (and more fatigable) motor units could be conserved. On a speculative note, with lower force per contraction there might be less compression of the microcirculation in the active muscle and better distribution of blood flow in a way that is consistent with the concepts presented in Fig. 5.
minimum which usually occurs at 50–60 r.p.m. In a comprehensive engineering/physiology analysis of this problem Hansen et al. (2002) noted that subjects with higher levels of myosin heavy chain I (MHC I, the predominant myosin in type I fibres) self-selected higher pedal rates and these rates closely matched the rate of peak mechanical efficiency. In this context, they speculated that motor control patterns in these subjects might favour a faster cadence so that relatively low total muscle forces (probably from fatigue-resistant motor units) per pedal stroke could generate the needed power so that the higher force (and more fatigable) motor units could be conserved. On a speculative note, with lower force per contraction there might be less compression of the microcirculation in the active muscle and better distribution of blood flow in a way that is consistent with the concepts presented in Fig. 5.
Running is a more complicated movement than cycling in that it elicits more stretch on the muscle prior to contraction and there is more potential to capture mechanical energy in the elastic elements of tissue. However, although there has been long-standing interest in identifying the biomechanical and anatomical factors that allow one person compared with another to expend 30–40% less energy per kilogram of body to move at a given velocity, the aetiology of differences in running economy generally remain a mystery, and biomechanical descriptions of running are not good predictors of running economy (Kyröläinen et al. 2001; Williams, 2007).
Elite endurance runners typically possess a predominance of type I muscle fibres and it would seem logical that they are more mechanically efficient at the velocities of distance running (Costill et al. 1976; Fink et al. 1977; Bosco et al. 1987). However, running and walking economy has not often been highly correlated with a person's percentage of type I muscle fibres (Morgan & Craib, 1992; Hunter et al. 2005). This agrees with the idea that running economy reflects the interaction of numerous factors including muscle morphology, elastic elements and joint mechanics in the efficient transfer of ATP to running speed.
The extent to which cycling efficiency or running economy can be improved with training has also been of long-standing interest. Until recently, it was generally believed that cycling efficiency and running economy did not improve much with training (Moseley et al. 2004). At best, running economy might sometimes increase slightly over the course of 2 months when explosive-type weight training is added to an endurance training program (Paavolainen et al. 1999; Millet et al. 2002). However, the conclusion that efficiency does not change with training was based on cross-sectional comparisons of relatively small numbers of endurance athletes (Moseley et al. 2004).
In this context, there are no comprehensive longitudinal data on groups of endurance athletes followed over several years to determine the trainability of cycling efficiency or running economy. However, there are at least two cases reporting that running economy can be improved over years of training in elite athletes (Conley et al. 1984; Jones, 2006). In fact, the current world record holder for the women's marathon displayed a remarkable 14% improvement in running economy over the course of 5 years of training (Jones, 2006). Furthermore, cycling efficiency was observed to increase 8% over the course of 7 years in an elite endurance cyclist (Coyle, 2005). In general, these case reports suggest that muscular efficiency and running economy might indeed improve with continued endurance training at a rate of approximately 1–3% per year. One possible contributing factor is that at least some of the fast myosin in endurance-trained muscle shifts to a different and perhaps more efficient isoform (Green et al. 1984). Additionally, in some models of extreme muscle use there can be a complete conversion of fast twitch to slow twitch muscle fibres, whether this occurs in elite athletes who train for two or more hours per day for many years is not known and it is further not known if such a shift would explain any improvements in efficiency that might occur with years of training (Pette, 2001).
Integrating current ideas about physiological limiting factors
The concepts above and in Fig. 2 suggest that 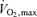 and lactate threshold interact to determine how long a given rate of aerobic and anaerobic metabolism can be sustained (i.e. performance
and lactate threshold interact to determine how long a given rate of aerobic and anaerobic metabolism can be sustained (i.e. performance 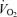 ) and efficiency then determines how much speed or power (i.e. performance velocity) can be achieved at a give amount of energy consumption. These relationships were hinted at by Hill in his 1925 paper (Hill, 1925) and were clearly defined in the period between 1970 and the early 1990s (Costill, 1970; Costill et al. 1973; Joyner, 1991; Joyner, 1993; Coyle, 1995; Bassett & Howley, 2000).
) and efficiency then determines how much speed or power (i.e. performance velocity) can be achieved at a give amount of energy consumption. These relationships were hinted at by Hill in his 1925 paper (Hill, 1925) and were clearly defined in the period between 1970 and the early 1990s (Costill, 1970; Costill et al. 1973; Joyner, 1991; Joyner, 1993; Coyle, 1995; Bassett & Howley, 2000).
The Marathon
In 1991, these concepts were used (Joyner, 1991) to predict that a much faster world record for the marathon was ‘physiologically’ possible based on the idea that marathon running speed was essentially predicted by the equation:
When reasonable estimates of the ‘best’ values ever recorded for these three parameters were used in this equation a predicted optimal marathon time of around 1:45 h emerged. Even when assumptions about wind resistance were added, times well under 2 h seemed possible. In retrospect, one overlooked possibility was that the 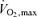 values used in the estimates came from laboratory studies typically conducted while the subjects ran up a grade of 5–10% and these values may be ∼10% higher than those seen during level running (Morgan et al. 1989). However, even if the highest
values used in the estimates came from laboratory studies typically conducted while the subjects ran up a grade of 5–10% and these values may be ∼10% higher than those seen during level running (Morgan et al. 1989). However, even if the highest 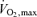 values seen during graded running protocols are not attainable during level running in many people, there are high enough
values seen during graded running protocols are not attainable during level running in many people, there are high enough 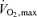 and lactate threshold values that might result in sustainable oxygen uptakes, which in combination with outstanding running economy, would generate a marked improvement in current world record time. These comments reinforce the conclusions of this earlier modelling effort that either there are unknown factors that operate at high speeds that make such time ‘not’ achievable or that for some reason ‘best in class’ values for every factor are unlikely to occur in the same person (Lucia et al. 2002).
and lactate threshold values that might result in sustainable oxygen uptakes, which in combination with outstanding running economy, would generate a marked improvement in current world record time. These comments reinforce the conclusions of this earlier modelling effort that either there are unknown factors that operate at high speeds that make such time ‘not’ achievable or that for some reason ‘best in class’ values for every factor are unlikely to occur in the same person (Lucia et al. 2002).
Some unanswered questions
In the context of the ideas above there are a number of fundamental unanswered questions. We have already highlighted questions about the determinants of efficiency especially for running, and for both running and cycling a key question is how ‘trainable’ this factor is. Additionally, we have discussed several factors beyond mitochondrial content and oxidative enzymes that may permit some athletes to operate for prolonged periods at especially high fractions of their 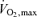 . These factors may also be important in events like long distance cycling and cross country skiing that occur over varied terrain and are not conducted at an even physiological pace. In these competitions there are frequent bursts of more intense near-maximal activity lasting from a few seconds to a few minutes that are followed by periods of relative recovery.
. These factors may also be important in events like long distance cycling and cross country skiing that occur over varied terrain and are not conducted at an even physiological pace. In these competitions there are frequent bursts of more intense near-maximal activity lasting from a few seconds to a few minutes that are followed by periods of relative recovery.
A fundamental question is the role genetics plays in the attainment of world class status and truly elite athletic performance. There are a number of studies showing that key elements of the response to training in sedentary persons is widely variable and has a genetic component (Rankinen et al. 2006). There have also been reports suggesting that common single nucleotide polymorphisms might be over represented in either groups of elite endurance athletes or in sedentary subjects that respond most to training. The most notable example is the idea that I (for insertion) variant of the angiotensin converting enzyme (ACE) gene is over represented among elite endurance athletes. However, in the largest cohort of elite athletes who have been both rigorously phenotyped and genotyped this association has not been confirmed and to date there are no genetic markers identified in humans that have been clearly shown to be more frequent in elite endurance athletes (Rankinen et al. 2000).
Another interesting example relates to the gene encoding for the skeletal muscle isoform of AMP deaminase. There is a common mutation of this gene that may be associated with lower exercise capacity and ‘trainability’ in untrained subjects (Rico-Sanz et al. 2003). While the frequency of the gene may be lower in elite endurance athletes there are still a number of elite performers who carry it so it does not appear to preclude the attainment of elite status and there is at least one example of an elite performer with essentially no AMP deaminase activity (Lucia et al. 2007; Rubio et al. 2005).
In this context, finding genetic markers that are strongly predictive of either success in endurance athletic performance or somehow preclude it is likely to be a daunting task because of the many cultural and environmental factors that contribute to success in sport, the many physiological factors that interact as determinants of performance, and the heroic nature of the training required. Ideas about culture are highlighted by the observation that while East African runners currently dominate international competition previously athletes from Australia and New Zealand, preceded by Eastern Europeans and even earlier the Finns showed remarkable levels of success. This geographical diversity argues against a simple genetically based set of answers to the problem of elite performance in endurance competition. In a parallel way, there is a low signal to noise ratio for many proposed genetic factors that might contribute to multifactorial medical conditions like heart disease, diabetes and hypertension and clear causal associations between genotype and phenotype are slow to emerge (Morgan et al. 2007).
Concluding remarks
Our concepts about factors that regulate and potentially limit endurance performance are not a radical departure from the intuitive logic introduced by Mosso and Hill. Elite athletic performance involves integration of muscular, cardiovascular and neurological factors that function cooperatively to efficiently transfer the energy from aerobic and anaerobic ATP turnover into velocity and power. The past four decades of research have described in great detail the cardiovascular and muscular factors that govern oxygen delivery to active muscles, oxidative ATP rephosphorylation and markers of metabolic stress. However, little advancement seems to have been made in identifying neurological factors that might alter motor unit recruitment during prolonged exercise in ways that limit fatigue. Although it has become increasingly apparent that muscular efficiency and economy are hugely important, the physiological determinants of running economy remain a mystery while myosin type appears important to cycling efficiency at cadences chosen in competition.
The outcome of all Olympic endurance events is decided at intensities above 85% 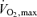 and most require athletes to be relatively fatigue resistant at intensities that stimulate significant anaerobic metabolism. At this time, the literature contains insufficient data that specifically describe the actual total energy demands of competition, the amount of muscle that is active during competition, and the complex neural patterns by which power and velocity are maintained as fatigue and failure develop in the nervous, cardiovascular and muscular systems. Such data are needed both in absolute and temporal terms. In this context, more work is needed on highly trained athletes performing very intense exercise in real or simulated competitions.
and most require athletes to be relatively fatigue resistant at intensities that stimulate significant anaerobic metabolism. At this time, the literature contains insufficient data that specifically describe the actual total energy demands of competition, the amount of muscle that is active during competition, and the complex neural patterns by which power and velocity are maintained as fatigue and failure develop in the nervous, cardiovascular and muscular systems. Such data are needed both in absolute and temporal terms. In this context, more work is needed on highly trained athletes performing very intense exercise in real or simulated competitions.
References
- Bangsbo J, Michalsik L, Petersen A. Accumulated O2 deficit during intense exercise and muscle characteristics of elite athletes. Int J Sports Med. 1993;14:207–213. doi: 10.1055/s-2007-1021165. [DOI] [PubMed] [Google Scholar]
- Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Bosco C, Montanari G, Ribacchi R, Giovenali P, Latteri F, Iachelli G, Faina M, Colli R, Dal Monte A, La Rosa M. Relationship between the efficiency of muscular work during jumping and the energetics of running. Eur J Appl Physiol Occup Physiol. 1987;56:138–143. doi: 10.1007/BF00640636. [DOI] [PubMed] [Google Scholar]
- Chi M, Hintz CS, Coyle EF, Martin WH, 3rd, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol. 1983;244:C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- Christensen EH. Untersuchungen uber die Verbrennungsvorgange bei langdauernder, scwherer Muskelarbeit. Skand Arch Physiol. 1939;81:152–161. [Google Scholar]
- Conley DK, Burkett LN, Millar AL. Following Steve Scott: physiological changes accompanying training. Physician Sports Med. 1984;12:103–106. [Google Scholar]
- Conley DL, Krahenbuhl GS. Running economy and distance running performance of highly trained athletes. Med Sci Sports Exercise. 1980;12:357–360. [PubMed] [Google Scholar]
- Costill DL. Metabolic responses during distance running. J Appl Physiol. 1970;28:251–255. doi: 10.1152/jappl.1970.28.3.251. [DOI] [PubMed] [Google Scholar]
- Costill D, Fink WJ, Pollock ML. Muscle fiber composition and enzyme activities of elite distance runners. Med Sci Sports. 1976;8:96–100. [PubMed] [Google Scholar]
- Costill DL, Thomason H, Roberts E. Fractional utilization of the aerobic capacity during distance running. Med Sci Sports. 1973;5:248–252. [PubMed] [Google Scholar]
- Coyle EF. Integration of the physiological factors determining endurance performance ability. Exerc Sport Sci Rev. 1995;23:25–63. [PubMed] [Google Scholar]
- Coyle EF. Improved muscular efficiency displayed as Tour de France champion matures. J Appl Physiol. 2005;98:2191–2196. doi: 10.1152/japplphysiol.00216.2005. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–172. doi: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol. 1988;64:2622–2630. doi: 10.1152/jappl.1988.64.6.2622. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Hagberg JM, Hurley BF, Martin WH, Ehsani AA, Holloszy JO. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol. 1983;55:230–235. doi: 10.1152/jappl.1983.55.1.230. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Martin WH, 3rd, Sinacore DR, Joyner MJ, Holloszy JO. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol. 1984;57:1857–1864. doi: 10.1152/jappl.1984.57.6.1857. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Davies KJA, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol Endocrinol Metab. 1982;242:E418–E427. doi: 10.1152/ajpendo.1982.242.6.E418. [DOI] [PubMed] [Google Scholar]
- Dempsey JA. Is the lung built for exercise? Med Sci Sports Exerc. 1986;18:143–155. [PubMed] [Google Scholar]
- DiGiulio C, Daniele F, Tipton CM. Angelo Mosso and muscular fatigue: 116 years after the first Congress of Physiologists: IUPS commemoration. Adv Physiol Educ. 2006;30:51–57. doi: 10.1152/advan.00041.2005. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- Durstine JL, Pate RR, Sparling PB, Wilson GE, Senn MD, Bartoli WP. Lipid, lipoprotein, and iron status of elite women distance runners. Int J Sports Med. 1987;8:119–123. doi: 10.1055/s-2008-1025716. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol. 1968;25:619–625. doi: 10.1152/jappl.1968.25.5.619. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Kilgore DL, Jr, Fedde MR. Oxygen delivery to the heart and brain during hypoxia: Pekin duck vs bar-headed goose. Am J Physiol Regul Integr Comp Physiol. 1984;247:R69–R75. doi: 10.1152/ajpregu.1984.247.1.R69. [DOI] [PubMed] [Google Scholar]
- Farrell P, Wilmore JH, Coyle EF, Billing JE, Costill DL. Plasma lactate accumulation and distance running performance. Med Sci Sports. 1979;25:1091–1097. doi: 10.1249/00005768-199310000-00002. [DOI] [PubMed] [Google Scholar]
- Fink WJ, Costill DL, Pollack ML. Submaximal and maximal working capacity of elite distance runners. Part II. Muscle fiber composition and enzyme activities. Ann NY Acad Sci. 1977;30:323–327. doi: 10.1111/j.1749-6632.1977.tb38210.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- Green HJ, Klug GA, Reichmann H, Wiehrer W, Pette D. Exercise-induced fibre type transitions with regard to myosin, parfalbumin, and sarcoplasmic reticulum in muscles of the rat. Pflugers Arch. 1984;400:432–438. doi: 10.1007/BF00587545. [DOI] [PubMed] [Google Scholar]
- Gregg SG, Mazzeo RS, Budinger TF, Brooks GA. Acute anemia increases lactate production and decreases clearance during exercise. J Appl Physiol. 1989a;67:756–764. doi: 10.1152/jappl.1989.67.2.756. [DOI] [PubMed] [Google Scholar]
- Gregg SG, Willis WT, Brooks GA. Interactive effects of anemia and muscle oxidative capacity on exercise endurance. J Appl Physiol. 1989b;67:765–770. doi: 10.1152/jappl.1989.67.2.765. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Coyle EF, Carroll JE, Miller JM, Martin WH, Brooke MH. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol. 1982;52:991–994. doi: 10.1152/jappl.1982.52.4.991. [DOI] [PubMed] [Google Scholar]
- Hansen EA, Andersen JL, Nielsen JS, Sjogaard G. Muscle fibre type, efficiency, and mechanical optima affect freely chosen pedal rate during cycling. Acta Physiol Scand. 2002;176:185–194. doi: 10.1046/j.1365-201X.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Hill AV. Athletic records. Lancet. 1925;5:481–486. [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Rennie MJ, Hickson RC, Conlee RK, Hagberg JM. Physiological consequences of the biochemical adaptations to endurance exercise. Ann NY Acad Sci. 1977;301:441–450. doi: 10.1111/j.1749-6632.1977.tb38220.x. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Bamman MM, Larson-Meyer DE, Joanisse DR, McCarthy JP, Blaudeau TE, Newcomer BR. Inverse relationship between exercise economy and oxidative capacity in muscle. Eur J Appl Physiol. 2005;94:558–568. doi: 10.1007/s00421-005-1370-z. [DOI] [PubMed] [Google Scholar]
- Jones AM. The physiology of the world record holder for the women's marathon. Int J Sports Sci Coaching. 2006;1:101–116. [Google Scholar]
- Joyner MJ. Modeling: optimal marathon performance on the basis of physiological factors. J Appl Physiol. 1991;70:683–687. doi: 10.1152/jappl.1991.70.2.683. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Physiologic limiting factors and distance running: Influence of gender and age on record performances. Exerc Sport Sci Rev. 1993;21:103–133. [PubMed] [Google Scholar]
- Kanstrup I-L, Ekblom B. Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc. 1984;16:256–262. [PubMed] [Google Scholar]
- Kyröläinen H, Belli A, Komi PV. Biomechanical factors affecting running economy. Med Sci Sports Exerc. 2001;33:1330–1337. doi: 10.1097/00005768-200108000-00014. [DOI] [PubMed] [Google Scholar]
- La Fontaine TP, Londeree BR, Spath WK. The maximal steady state versus selected running events. Med Sci Sports Exercise. 1981;13:190–193. [PubMed] [Google Scholar]
- Lucia A, Hoyos J, Pérez M, Santalla A, Chicharro JL. Inverse relationship between VO2max and economy/efficiency in world-class cyclists. Med Sci Sports Exerc. 2002;34:2079–2084. doi: 10.1249/01.MSS.0000039306.92778.DF. [DOI] [PubMed] [Google Scholar]
- Lucia A, Martin MA, Esteve-Lanao J, San Juan AF, Rubio JC, Oliván J, Arenas J. C34T mutation of the AMPD1 gene in an elite white runner. Br J Sports Med. 2007;40:e7. doi: 10.1136/bjsm.2005.019208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, 3rd, Coyle EF, Bloomfield SA, Ehsani AA. Effects of physical deconditioning after intense endurance training on left ventricular dimensions and stroke volume. J Am Coll Cardiol. 1986;7:982–989. doi: 10.1016/s0735-1097(86)80215-7. [DOI] [PubMed] [Google Scholar]
- Millet G, Jaouen B, Borrani F, Candau R. Effects of concurrent endurance and strength training on running economy and. VO2 kinetics. Med Sci Sports Exerc. 2002;34:1351–1359. doi: 10.1097/00005768-200208000-00018. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest. 1958;37:538–547. doi: 10.1172/JCI103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DW, Baldini FD, Martin PE, Kohrt WM. Ten kilometer performance and predicted velocity at VO2max among well-trained male runners. Med Sci Sports Exerc. 1989;21:78–83. doi: 10.1249/00005768-198902000-00014. [DOI] [PubMed] [Google Scholar]
- Morgan DW, Craib M. Physiological aspects of running economy. Med Sci Sports Exerc. 1992;24:456–461. [PubMed] [Google Scholar]
- Morgan TM, Krumholz HM, Lifton RP, Spertus JA. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. JAMA. 2007;297:1551–1561. doi: 10.1001/jama.297.14.1551. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, Gonález-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley L, Achten J, Martin JC, Jeukendrup AE. No differences in cycling efficiency between world-class and recreational cyclists. Int J Sports Med. 2004;25:374–379. doi: 10.1055/s-2004-815848. [DOI] [PubMed] [Google Scholar]
- Murray R. Rehydration strategies – balancing substrate, fluid, and electrolyte provision. Int J Sports Med. 1998;19:S133–S135. doi: 10.1055/s-2007-971978. [DOI] [PubMed] [Google Scholar]
- Paavolainen L, Häkkinen K, Hämäläinen I, Nummela A, Rusko H. Explosive-strength training improves 5-km running time by improving running economy and muscle power. J Appl Physiol. 1999;86:1527–1533. doi: 10.1152/jappl.1999.86.5.1527. [DOI] [PubMed] [Google Scholar]
- Pate RR, Sparling PB, Wilson GE, Cureton KJ, Miller BJ. Cardiorespiratory and metabolic responses to submaximal and maximal exercise in elite women distance runners. Int J Sports Med. 1987;8:91–95. doi: 10.1055/s-2008-1025712. [DOI] [PubMed] [Google Scholar]
- Pette D. Historical perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part 1. Cardiorespiratory aspects. Ann NY Acad Sci. 1977;301:310–322. doi: 10.1111/j.1749-6632.1977.tb38209.x. [DOI] [PubMed] [Google Scholar]
- Pryor SL, Lewis SF, Haller RG, Bertocci LA, Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's disease) J Clin Invest. 1990;85:1444–1449. doi: 10.1172/JCI114589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen T, Bray MS, Hagberg JM, Pérusse L, Roth SM, Wolfarth B, Bouchard C. The human gene map for performance and health-related fitness phenotypes: the 2005 update. Med Sci Sports Exerc. 2006;38:1863–1888. doi: 10.1249/01.mss.0000233789.01164.4f. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Wolfarth B, Simoneau JA, Maier-Lenz D, Rauramaa R, Rivera MA, Boulay MR, Chagnon YC, Pérusse L, Keul J, Bouchard C. No association between the angiotensin-converting enzyme ID polymorphism and elite endurance athlete status. J Appl Physiol. 2000;88:1571–1575. doi: 10.1152/jappl.2000.88.5.1571. [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J, Rankinen T, Joanisse DR, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Associations between cardiorespiratory responses to exercise and the C34T AMPD1 gene polymorphism in the HERITAGE Family Study. Physiol Genomics. 2003;14:161–166. doi: 10.1152/physiolgenomics.00165.2002. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R502–R516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- Robinson S, Edward HT, Dill DB. New records in human power. Science. 1937;85:409–410. doi: 10.1126/science.85.2208.409. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation Regulation During Physical Stress. New York: Oxford University Press; 1986. pp. 1–416. [Google Scholar]
- Rubio JC, Martin MA, Rabadán M, Gómez-Gallego F, San Juan AF, Chicharro JL, Pérez M, Arenas J, Lucia A. Frequency of the C34T mutation of the AMPD1 gene in world-class endurance athletes: does this mutation impair performance? J Appl Physiol. 2005;8:2108–2112. doi: 10.1152/japplphysiol.01371.2004. [DOI] [PubMed] [Google Scholar]
- Saltin B, Astrand P-O. Maximal oxygen uptake in athletes. J Appl Physiol. 1967;23:353–358. doi: 10.1152/jappl.1967.23.3.353. [DOI] [PubMed] [Google Scholar]
- Saltin B, Strange S. Maximal oxygen uptake: ‘old’ and ‘new’ arguments for a cardiovascular limitation. Med Sci Sports Exerc. 1992;24:30–37. [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol. 2005;99:1866–1871. doi: 10.1152/japplphysiol.00616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CB. Energy expenditure of heavy to severe exercise and recovery. J Theor Biol. 2000;207:293–297. doi: 10.1006/jtbi.2000.2174. [DOI] [PubMed] [Google Scholar]
- Sherman WM, Costill DL. The marathon: dietary manipulation to optimize performance. Am J Sports Med. 1984;12:44–51. doi: 10.1177/036354658401200107. [DOI] [PubMed] [Google Scholar]
- Webb PW, Saris WH, Schoffelen PF, Van Ingen Schenau GJ, Ten Hoor F. The work of walking: a calorimetric study. Med Sci Sports Exerc. 1988;20:331–337. doi: 10.1249/00005768-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Williams KR. Biomechanical factors contributing to marathon race success. Sports Med. 2007;37:420–423. doi: 10.2165/00007256-200737040-00038. [DOI] [PubMed] [Google Scholar]