Abstract
It is not known whether frontal cerebral rhythms of the two hemispheres are implicated in fine motor control and balance. To address this issue, electroencephalographic (EEG) and stabilometric recordings were simultaneously performed in 12 right-handed expert golfers. The subjects were asked to stand upright on a stabilometric force platform placed at a golf green simulator while playing about 100 golf putts. Balance during the putts was indexed by body sway area. Cortical activity was indexed by the power reduction in spatially enhanced alpha (8–12 Hz) and beta (13–30 Hz) rhythms during movement, referred to as the pre-movement period. It was found that the body sway area displayed similar values in the successful and unsuccessful putts. In contrast, the high-frequency alpha power (about 10–12 Hz) was smaller in amplitude in the successful than in the unsuccessful putts over the frontal midline and the arm and hand region of the right primary sensorimotor area; the stronger the reduction of the alpha power, the smaller the error of the unsuccessful putts (i.e. distance from the hole). These results indicate that high-frequency alpha rhythms over associative, premotor and non-dominant primary sensorimotor areas subserve motor control and are predictive of the golfer's performance.
A large body of evidence indicates that electroencephalographic (EEG) alpha (about 8–12 Hz) and beta (about 14–30 Hz) oscillations markedly decrease in power over sensorimotor cortical areas during the preparation and execution of voluntary self-paced movements; this reduction is known as event-related desynchronization (ERD; Gastaut, 1952; Babiloni et al. 1999; Pfurtscheller & Lopes da Silva, 1999; Pfurtscheller et al. 2000; Neuper & Pfurtscheller, 2001). These sensorimotor EEG oscillations are collectively called ‘mu rhythm’ and can be distinguished from other alpha or beta rhythms (e.g. parieto-occipital alpha) that show changes in amplitude during sensory information processing (Pfurtscheller & Lopes da Silva, 1999). Furthermore, alpha and beta ERD are generally related to fine cognitive-motor performance (Klimesch et al. 1997; Klimesch, 1999; Pfurtscheller & Lopes da Silva, 1999). These rhythms mainly reflect the mode of transfer and processing of sensorimotor information among cortical and thalamic structures (Pfurtscheller & Lopes da Silva, 1999).
Among EEG rhythms, alpha rhythms seem to be especially implicated in the sporting performance of athletes. Marked differences in alpha power have been observed between expert sportsmen and non-athletes (Hatfield et al. 1984; Collins et al. 1990; Salazar et al. 1990; Crews & Landers, 1993; Shaw, 1993, 1996; Loze et al. 2001; Gualberto Cremades, 2002; Del Percio et al. 2007a). Alpha power (8–12 Hz) over the occipital cortex has been found to increase before best shots in expert air pistol marksmen; this is thought to be a sign of cortical inhibition in the period of stillness that occurs at certain phases of a skilled motor act (Loze et al. 2001). Furthermore, alpha power has been found to be higher over the left than the right hemisphere of skilled marksmen during shot preparation (Hatfield et al. 1984), and before the best shots of élite archers (Salazar et al. 1990; Landers et al. 1994; Shaw, 1996). This hemispherical asymmetry of alpha rhythms has been challenged by other evidence showing that sporting performance is associated with bilateral or preponderant modulation of alpha rhythms over the right hemisphere (Collins et al. 1990; Crews & Landers, 1993; Del Percio et al. 2007a). A possible cause of these contrasting results is the use of standard EEG techniques with poor spatial resolution.
In this high-resolution EEG study, we evaluated whether frontal alpha and beta rhythms of the two hemispheres are implicated in fine motor control and balance, by simultaneously examining EEG and stabilometric data obtained in right-handed expert golfers during putts.
Methods
Subjects and ethical approval
Seven men and five women expert golfers were recruited. They had been practising golf for more than 8 years and at least five times a week, and regularly compete in national and international competitions. Their mean age was 20.8 ± 1 years (range: 16–25 years). All golfers were right-handed as measured by the Edinburgh Inventory (mean 56.3 ± 6.2%). All subjects gave their informed consent according to the Declaration of Helsinki, and were free to withdraw from the study at any time. The procedure was approved by the local Institutional Ethics Committee (I Medical School, University of Rome ‘Sapienza’).
Experimental procedure and recordings
All subjects were asked to stand upright upon a 60 × 60 cm stabilometric force platform (ARGO by RGM Genova, Italy) at a golf green simulator (1.5 m × 3 m; see online supplemental material, Supplemental Fig. 1). The surface of the green simulator was covered with a special green moquette (kindly provided by the Italian Federation of Golf for the purpose of the study). In the golf green simulator, the distance between the starting point of the ball and the hole was 2.1 m. Hole diameters were 108 mm (standard), 80 mm or 60 mm. For each subject, the hole diameter associated with more than 30% unsuccessful putts was used, as ascertained during a preliminary training phase in which subjects familiarized themselves with the golf green simulator. The training phase always started with the 108 mm-diameter hole (standard) and consisted of about 50 putts within about 15 min. Subjects then performed about 100 putts (interstroke interval of 15 s) in 10 separate recording blocks (interblocks interval of about 90 s). The golf putting performance was self-paced, in that the subjects were asked to relax between two consecutive putts and to start the golf putting performance when they felt ready. A device based on optic technologies was used to define the time of impact between the putter and ball.
The stabilometric force platform recorded the amplitude of the subject's body sway in the anteroposterior and mediolateral directions, with an acquisition rate of 100 Hz. While the subjects stood on the stabilogram, the EEG data were continuously recorded (bandpass: 0.01–100 Hz, sampling rate: 256 Hz; EB-Neuro Be-plus, Firenze, Italy) from 56 scalp electrodes (cap) positioned over the whole scalp according to an augmented 10–20 system (see Supplemental Fig. 2). Electrical reference was located between the Afz and Fz electrodes, and the ground electrode was located between the Pz and Oz electrodes. Electrode impedance was kept below 5 kOhm. In parallel, the recording of bipolar electro-oculographic data (EOG; bandpass: 0.1–100 Hz; sampling rate: 256 Hz) monitored blinking and eye movements. Furthermore, electromyographic data (EMG; bandpass: 0–100 Hz; sampling rate: 256 Hz) from the right anterior tibialis muscle, right gastrocnemius lateralis muscle and right external oblique muscle were collected to monitor muscle activity involved in upright standing.
Analysis of stabilometric data
The data recorded from the stabilometric force platform allowed the computation of ‘sway area’ index, which was expressed in mm2 s−1. The sway area measured the mean area spanned by the body centre of pressure (COP) during the period of interest for each golf putt (i.e. 10 s, from −5 s to +5 s, the zero time being the instant of the impact between the putter and ball). The sway area was calculated as the area swept by the line connecting the mean COP position with each point of the COP described path, and the recording time.
Spectral analysis and computation of alpha event-related desynchronization/event-related synchronization (ERD/ERS) percentages
Recorded EEG, EOG and EMG data were segmented in single trials of 10 s, each spanning −5 s to + 5 s. The EEG epochs with ocular, muscular and other types of artifact were preliminarily identified by a computerized automatic procedure (Moretti et al. 2003). The software package included procedures for (i) EOG artifact detection and correction; (ii) EMG analysis; (iii) EEG artifact analysis; and (iv) optimization of the ratio between artifact-free EEG channels and EEG single trials to be rejected. The EEG epochs contaminated by ocular artifacts were then corrected by an autoregressive method (Moretti et al. 2003). Finally, two expert electroencephalographists (C.D.P. and N.M.) manually confirmed this automatic selection and correction, with special attention to residual contaminations of the EEG epochs due to eye movements, blinking and head movements during the putts. Indeed, eye–head movements (e.g. gaze movements between the ball and the target hole) could be critical in the baseline period, before the golfer concentrates on the stroke. Therefore, only the EEG epochs totally free from artifact residuals were accepted for the subsequent analyses.
The artifact-free EEG trials were spatially enhanced by surface Laplacian estimation (regularized 3-D spline function; Babiloni et al. 1996, 1998). The single-trial analysis was carefully repeated on the Laplacian-transformed EEG data, to discard computational artifacts. These artifact-free Laplacian-transformed EEG trials were subdivided into two groups: the Successes group refers to EEG trials associated with the successful putts and the Failures group refers to EEG trials associated with the unsuccessful putts. Error in the unsuccessful golf putt was defined as the final distance (cm) between the golf ball and hole. The mean number of EEG trials was 42.9 ± 4.6 (s.e.m.) for the Successes group, while it was 26.6 ± 2.6 (s.e.m.) for the Failures group. Of note, the number of the EEG trials for each condition was used as a covariate in the statistical analysis.
Laplacian-transformed EEG trials were used as an input for power spectrum analysis, which was performed with a standard fast Fourier transform (FFT) algorithm using the Welch technique and Hanning windowing function (Matlab; MathWorks, Natick, Massachusetts USA). Negative values of event-related percentage changes in alpha band power represented the ERD (Pfurtscheller & Lopes da Silva, 1999; Pfurtscheller et al. 1997). Conversely, positive values indicated the event-related synchronization (ERS; Pfurtscheller & Lopes da Silva, 1999). Specifically, the alpha and beta ERD/ERS was calculated using the well-known standard formula (Pfurtscheller & Aranibar, 1979; Pfurtscheller & Neuper, 1994; Pfurtscheller et al. 1997; Pfurtscheller & Lopes da Silva, 1999):
where E indicates the alpha or beta power density during the event period and R indicates the alpha/beta power density during the baseline period. In the present study, the ‘event’ period was the movement period (from 1 s before to the impact between putter and ball as a zero time), whereas the baseline period was defined as the time interval between −5 s and −4 s. We separately computed the ERD/ERS for the Successes and Failures conditions in the range 6–30 Hz. For the determination of the alpha and beta sub-bands, we preliminarily defined the alpha and beta reactive frequencies (Pfurtscheller et al. 2001, 2006). In each subject, the alpha (or beta) reactive frequency indicated the frequency bin showing the highest ERD within 8–12 (or 14–30) Hz. Based on the alpha and beta reactive frequencies, two alpha and beta sub-bands of interest were considered: the low-frequency alpha and beta bands ranged from the reactive frequency minus 2 Hz to the reactive frequency, and the high-frequency alpha and beta bands ranged from the reactive frequency to the reactive frequency plus 2 Hz. For an individual alpha (or beta) reactive frequency of 10 (or 20) Hz, the low-frequency alpha (or beta) band is 8–10 (or 18–20) Hz, and the high-frequency alpha (or beta) band is 10–12 (or 20–22) Hz.
Topographic mapping of the alpha and beta ERD/ERS percentage
Topographic maps (256 hues) of the ERD/ERS at the two alpha and beta sub-bands were calculated on a 3-D cortical model using a spline interpolating function (Babiloni et al. 1996). This model is based on the magnetic resonance data of 152 subjects digitized at the Brain Imaging Center of the Montreal Neurological Institute (SPM96, http://www.mni.mcgill.ca), and is commonly considered an acceptable template for the rendering of group neuroimaging data.
Statistical analysis
Statistical comparisons for the EEG and stabilometric data were performed by analysis of variance (ANOVA). With the ANOVA, Mauchley's test evaluated the sphericity assumption when necessary. Correction of the degrees of freedom was made with the Greenhouse–Geisser procedure, and Duncan's test was used for post hoc comparisons (P < 0.05). Four ANOVAs (one for each alpha and beta sub-band) tested the hypothesis that, compared to the Failures condition, the Successes condition shows stronger alpha and beta ERD, indicating a stronger cortical sensorimotor activity associated with the successful putts. The ANOVAs had ERD/ERS amplitude for a certain sub-band as a dependent variable, and the factors were condition (Successes, Failures) and electrode (Fz, FCz, C3, Cz, C4). The number of Laplacian-transformed EEG trials was used as a covariate. We selected Fz, FCz, C3, Cz and C4 electrodes for this analysis, as these electrodes roughly overlie primary sensorimotor areas (C3 and C4 electrodes), the supplementary motor area and motor cingulate (Cz and FCz), and the medial prefrontal and anterior cingulate areas (Fz), which are thought to be related to the control of fine motor sequences (Ashe et al. 2006).
An additional ANOVA tested the hypothesis that, compared to the Failures condition, the Successes condition shows a reduced sway area of the stabilometric data, indicating a better upright balance associated with the successful rather than unsuccessful putts. Specifically, the ANOVA used the sway areas as a dependent variable, with Successes or Failures as the factor.
Results
Behavioural and stabilometric data
Measurements of upright balance and golf performance were analysed in parallel. The mean number of successful putts was 62.5 ± 3.1%. For the unsuccessful putts, mean error value was 13.1 ± 1.8 cm. The mean (± s.e.m.) values of subjects' body sway area during the putts was 180 ± 45 mm2 s−1 for the Successes condition, and 165 ± 43 mm2 s−1 for the Failures condition. Despite the difference in golf performance, the ANOVA of the sway area showed no difference between successful and unsuccessful putts (F1,11= 0.36; P= 0.55), thus indicating that subjects' upright balance did not explain the result of that performance.
EEG recordings
The analysis of the EEG data determined the ‘reactive frequencies’ at which alpha (8–12 Hz) and beta (14–30 Hz) rhythms presented the highest values of ERD over the primary sensorimotor cortex of both hemispheres during the successful and unsuccessful putts. Figure 1 shows the mean ERD in the range of 6–30 Hz for the Successes and Failures conditions at the electrodes overlying the arm and hand region of the left (C3 electrode) and of the right (C4 electrode) primary sensorimotor cortex. It is noted the predominant alpha ERD values over the right primary sensorimotor cortex (C4 electrode) during the successful putts. The mean ± s.e.m. values of alpha reactive frequency was 9.5 ± 0.5 Hz for both the Successes and the Failures conditions, while mean values of reactive beta frequency was 19.3 ± 0.9 Hz for the Successes condition and 20.7 ± 1.1 Hz for the Failures condition. There was no significant interconditions difference in the alpha and beta reactive frequencies as evaluated by an ANOVA for each band (P > 0.3). These results indicated the ‘reactive frequencies’ for the subsequent topographical analysis of the ERD at low- and high-frequency alpha and beta rhythms.
Figure 1. Mean event-related desynchronization (ERD) in the range 6–30 Hz for the Successes and Failures conditions at the electrodes overlying the arm and hand region of the left (C3 electrode) and of the right (C4 electrode) primary sensorimotor cortex.
Figure 2 maps topographical details of low- and high-frequency alpha and beta ERD/ERS percentages for the Successes and Failures conditions. The corresponding difference maps (Successes minus Failures) are also reported. For both Successes and Failures conditions, there was an evident low- and high-frequency alpha and beta ERD over the whole scalp; it was more represented in frontal and central areas. Compared to the Failures, the Successes condition was characterized by a stronger low- and high-frequency alpha ERD in frontal and central areas (see the difference maps). The low- and high-frequency beta ERD showed smaller differences between the Successes and Failures conditions. These findings indicated the prominent sensorimotor alpha ERD during the successful putts.
Figure 2. Topographical distribution of low- and high-frequency alpha and beta ERD/ERS percentages for the Successes and Failures conditions.
The corresponding difference maps (Successes versus Failures) are also reported. Colour scale: maximum ERD and ERS are coded in white and violet, respectively. The maximal (%) value of the ERD/ERS percentages is reported under the maps. The maximum ERD difference in the Successes compared to the Failures condition is coded in white. In contrast, the maximum ERD difference in the Failures compared to the Successes condition is coded in violet.
The ANOVA for the high-frequency alpha ERD/ERS percentages pointed to a statistically significant interaction (F4,44= 4.33; P < 0.005) between the condition (Successes, Failures) and the electrode (Fz, FCz, C3, Cz, C4). Duncan post hoc testing indicated that the high-frequency alpha ERD was higher in amplitude in the Successes than in the Failures condition at the Fz (P= 0.01), Cz (P= 0.05) and C4 (P= 0.004) electrodes, giving statistical support to the prominent sensorimotor alpha ERD during the successful putts (see Fig. 3).
Figure 3. Mean (± s.e.m.) values of high-frequency alpha ERD at the Fz, FCz, C3, Cz, C4 electrodes for the Successes and Failures conditions.
Asterisks indicate the probability levels of the post hoc Duncan testing (*P < 0.05, **P < 0.005).
The ANOVAs for the low-frequency alpha and for the low- and high-frequency beta ERD/ERS percentages showed no statistically significant differences (P > 0.1), and were not further considered.
Control analyses
As illustrated before, the high-frequency alpha ERD was higher in amplitude in the Successes than in the Failures condition at the Fz, Cz and C4 electrodes (P < 0.05–0.004). A first control analysis was performed to evaluate whether the present statistical results were associated with a difference in the baseline high-frequency alpha absolute power. To this end, the baseline high-frequency alpha absolute power was used as a dependent variable for an ANOVA design which used condition (Successes, Failures) and electrode (Fz, FCz, C3, Cz, C4) as factors, and the number of the Laplacian-transformed EEG trials as a covariate. The results showed no statistically significant effect (P > 0.15). It can be concluded that the above alpha ERD results were not significantly affected by the baseline alpha power.
A second control analysis was performed to compare the latency of the high-frequency alpha ERD peak in the Successes and Failures conditions. To this aim, Laplacian EEG time series were bandpassed (Bartlett function), squared, averaged across 120 ms periods (to 8 samples s−1), and averaged across all EEG single trials (Pfurtscheller & Lopes da Silva, 1999). Figure 4 shows the group grand average waveforms of the high-frequency alpha ERD/ERS percentages for the Successes and Failures conditions at Fz, FCz, Cz, C3 and C4 electrode sites. The latencies of the alpha ERD peaks were similar in the two conditions, whereas the peak amplitude was higher in the Successes than the Failures condition. The latency of the alpha ERD peak in the single subjects was used as a dependent variable for an ANOVA design which used condition (Successes, Failures) as a factor. The ANOVA results showed no statistically significant effect (P > 0.8). It was concluded that the peak latency of the high-frequency alpha ERD was similar during the successful and unsuccessful putts.
Figure 4. The grand average waveforms of the high-frequency alpha ERD for the Successes and Failures conditions at Fz, FCz, Cz, C3, and C4 electrode sites.
If the alpha ERD is related to a good performance, then it should be higher in amplitude in the unsuccessful putts in which the ball is close to the hole. To evaluate this control hypothesis, the high-frequency alpha ERD in the Failures condition was correlated with the error (cm) from the hole (Pearson test, P < 0.05). Results showed that the high-frequency alpha ERD at Fz was positively correlated with the error (r= 0.89, P= 0.0001, n = 12, Fig. 5 top). The same was true for the high-frequency alpha ERD/ERS percentages at the C4 and Cz electrodes (Cz: r= 0.62, P= 0.03, n = 12; C4: r= 0.62, P= 0.03, n = 12; Fig. 5 central and bottom). These findings indicate that, in the unsuccessful putts, the stronger the alpha ERD, the lower the error from the hole.
Figure 5. Scatterplots showing the linear correlation (Pearson test, P < 0.05) between the high-frequency alpha ERD at Fz, Cz and C4 electrodes and the error from the hole (cm) during the unsuccessful putts.
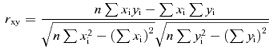 |
Discussion
In the present study, we investigated how the sensorimotor alpha and beta rhythms were related to upright balance and fine arm and hand motor control during the putts of expert golfers. We report that the body sway area is similar in the successful and unsuccessful putts, indicating that visual–vestibular integration on the basis of eyes-open upright balance is not essential for successful performance in the current experimental conditions, characterized by quite fine bimanual motor sequences. We also report that, notably, the 10–12 Hz frequency alpha ERD was greater in amplitude during the successful than the unsuccessful putts over the frontal midline (Fz and Cz electrodes) and arm and hand region of the right primary sensorimotor area (C4 electrode). The stronger the alpha ERD, the lower the error in the unsuccessful putts. These results taken together indicate that high-frequency alpha rhythms over frontal areas are specifically implicated in the fine motor control behind the successful putts. Therefore, they are in agreement with the notion that high- but not low-frequency alpha ERD displays a motorotopic representation over the primary sensorimotor cortex and is associated with active task-specific processes (Arroyo et al. 1993; Toro et al. 1994; Pfurtscheller et al. 1997, 2000; Pfurtscheller & Lopes da Silva, 1999). In line with its meaning (Klimesch, 1999), the lack of difference of low-frequency alpha ERD between successful and unsuccessful putts suggests that general arousal, attention and effort did not characterize winning performance.
The high-frequency alpha ERD over the right primary sensorimotor cortex was maximum during the successful putts, suggesting that a fine cortical control of the left arm and hand movements is crucial for that winning performance. Of note, these results contribute to the debate on the functional significance and hemispheric distribution of alpha rhythms recorded during sporting performance. An inhibitory increase in alpha power (8–12 Hz) in the left hemisphere before the performance of skilled marksmen (Hatfield et al. 1984) and archers (Salazar et al. 1990; Landers et al. 1994; Shaw, 1996) has already been shown; this would favour visual–spatial processes in the right hemisphere. Furthermore, a correlation has been reported between the reduction in power of right parietal alpha power (ERD) and skilled karate performance (Del Percio et al. 2007b). In contrast, other studies have pointed to a bilateral increase in alpha power during skilled karate performance (Collins et al. 1990), and an inhibitory increase in alpha power over the right hemisphere during skilled golf performance (Crews & Landers, 1993). Bearing in mind these data, the present study shows that baseline alpha power is not crucial either for the hemispherical alpha ERD topography or for successful putts. This finding indicates that relationships between the topography of baseline alpha power and subsequent cognitive–motor processes are quite complex, possibly depending on the specific tasks to be performed. Furthermore, it agrees with previous evidence showing that high baseline power of bilateral alpha rhythms predicts good cognitive performance in both visual stimulus encoding and memory formation (Neubauer & Freudenthaler, 1995; Klimesch, 1999; Babiloni et al. 2006), but not in successful performance of fine motor acts. It is still a matter of debate whether an enhanced alpha power represents a general pattern of cortical neural inhibition common to all skilled and target sports (Shaw, 1996; Palva & Palva, 2007). If this is the case, hemispheric differences in preparatory alpha power among sporting gestures might reflect specific inhibitory versus excitatory processes associated with exogenous versus endogenous direction of attention and with verbal versus spatial processes (Wertheim, 1981; Shaw, 1996; Klimesch, 1999). In this framework, golf putting performance would be characterized by excitatory desynchronization of frontal alpha rhythms during action execution, in line with the traditional model of alpha power modulation (Pfurtscheller & Lopes da Silva, 1999; Palva & Palva, 2007). On the whole, the present results indicate that high-resolution EEG techniques and analysis of high-frequency alpha rhythms (about 10–12 Hz) represent a powerful approach to mapping the topography of cortical task-specific processes accompanying sporting gestures.
We also report that the successful putts were additionally characterized by high-frequency alpha ERD over the medial prefrontal, cingulate and/or supplementary motor areas. These cortical areas play a pivotal role in the planning, selection and regulation of learned complex sequences performed with both arms and both hands, thanks to their bilateral anatomical connectivity (Rosenbaum et al. 1992, 2001; Wiesendanger & Wise, 1992; Ashe et al. 2006). The medial prefrontal cortex is bilaterally interconnected to the cingulate and supplementary motor areas (Rouiller et al. 1994), which have strong bilateral connections to motor effectors (Penfield & Welch, 1949; Brinkman, 1984; Lim et al. 1994; Kazennikov et al. 1998) and to basal ganglia nuclei (Wiesendanger et al. 1996). The putative specific role of these areas in the control of the successful putts can be derived from the following recent neurophysiological evidence. Medial prefrontal and anterior cingulate areas represent explicit processes (subject's intention, awareness) associated with bilateral motor sequences, having an inhibitory effect on automatic or implicit processes (Destrebecqz & Cleeremans, 2001; Destrebecqz et al. 2005), whereas cingulate and supplementary motor areas may represent the automatic or implicit processes, subserving temporal and ordinal representation of the motor sequence (Deiber et al. 1991; Ashe et al. 2006).
In conclusion, the findings of the present study indicate that the modulation of high-frequency alpha rhythms over associative, premotor and non-dominant primary sensorimotor areas may represent the basic physiological mechanism underlying the fine motor control which is the basis of the putts, and appears to be predictive of successful performance. In future research, these frontal alpha rhythms may be trained to produce a strong ERD during the execution of putts. Such training could be performed by alpha ERD neurofeedback (Hanslmayr et al. 2005) during computer simulation of putting performance (i.e. videogame, virtual reality), followed by testing EEG measurements carried out in the present experimental conditions.
Acknowledgments
We thank Professor Franco Chimenti, President of the Italian Federation of Golf, and Professor Luigi Frati, Dean of the Medical Faculty, for encouragement and advice. We thank also Dr Ivo Bruni (EB-Neuro) for technological partnership. The research was funded by The Institute of Sport Medicine and Science (CONI Servizi spa) and by the Italian Federation of Golf.
Supplemental material
Online supplemental material for this paper can be accessed at:
The golf green simulator used for the present study.
Electroencephalographic (EEG) electrode montage.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
http://jp.physoc.org/cgi/content/full/jphysiol.2007.141630/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.141630
References
- Arroyo S, Lesser RP, Gordon B, Uematso S, Jackson D, Webber R. Functional significance of the mu rhythm of human cortex: an electrophysiologic study with subdural electrodes. Electroencephalogr Clin Neurophysiol. 1993;87:76–87. doi: 10.1016/0013-4694(93)90114-b. [DOI] [PubMed] [Google Scholar]
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol. 2006;16:213–221. doi: 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Babiloni C, Carducci F, Fattorini L, Onorati P, Urbano A. Spline Laplacian estimate of EEG potentials over a realistic magnetic resonance-constructed scalp surface model. Electroencephalogr Clin Neurophysiol. 1996;98:363–373. doi: 10.1016/0013-4694(96)00284-2. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Carducci F, Babiloni C, Urbano A. Improved realistic Laplacian estimate of highly-sampled EEG potentials by regularization techniques. Electroencephalogr Clin Neurophysiol. 1998;106:336–343. doi: 10.1016/s0013-4694(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, Babiloni F. Human movement-related potentials vs. desynchronizationof EEG alpha rhythm: a high-resolution EEG study. Neuroimage. 1999;10:658–665. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Cappa S, Pasqualetti P, Rossi S, Miniussi C, Rossini PM. Functional frontoparietal connectivity during encoding and retrieval processes follows HERA model. A high-resolution study. Brain Res Bull. 2006;68:203–212. doi: 10.1016/j.brainresbull.2005.04.019. January. 15. [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey's cerebral cortex. short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Powell G, Davies I. An electroencephalographic study of hemispheric processing patterns during karate performance. J Sport Exerc Psychol. 1990;12:223–234. [Google Scholar]
- Crews DJ, Landers DM. Electroencephalographic measures of attentional patterns prior to the golf putt. Med Sci Sports Exerc. 1993;25:116–126. doi: 10.1249/00005768-199301000-00016. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Brancucci A, Bergami F, Marzano N, Fiore A, Di Ciolo E, Aschieri P, Andrea Lino A, Vecchio F, Iacoboni M, Gallamini M, Babiloni C, Eusebi F. Cortical alpha rhythms are correlated with body sway during quiet open-eyes standing in athletes: a high-resolution EEG study. Neuroimage. 2007a;36:822–829. doi: 10.1016/j.neuroimage.2007.02.054. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Marzano N, Tilgher S, Fiore A, Di Ciolo E, Aschieri P, Lino A, Toràn G, Babiloni C, Eusebi F. Pre-stimulus alpha rhythms are correlated with post-stimulus sensorimotor performance in athletes and non athletes: a high-resolution. EEG Study Clin Neurophysiol. 2007b;118:1711–1720. doi: 10.1016/j.clinph.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Destrebecqz A, Cleeremans A. Can sequence learning be implicit? New evidence with the process dissociation procedure. Psychon Bull Rev. 2001;8:343–350. doi: 10.3758/bf03196171. [DOI] [PubMed] [Google Scholar]
- Destrebecqz A, Peigneux P, Laureys S, Degueldre C, Del Fiore G, Aerts J, Luxen A, Van Der Linden M, Cleeremans A. Maquet P. The neural correlates of implicit and explicit sequence learning: interacting networks revealed by the process dissociation procedure. Learn Mem. 2005;12:480–490. doi: 10.1101/lm.95605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaut H. Etude electrocorticographique de la reactivite des rhythmes rolandiques. Rev Neurol. 1952;87:176–182. [PubMed] [Google Scholar]
- Gualberto Cremades J. The effects of imagery perspective as a function of skill level on alpha activity. Int J Psychophysiol. 2002;43:261–271. doi: 10.1016/s0167-8760(01)00186-6. (March. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Sauseng P, Doppelmayr M, Schabus M, Klimesch W. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophysiol Biofeedback. 2005;30:1–10. doi: 10.1007/s10484-005-2169-8. [DOI] [PubMed] [Google Scholar]
- Hatfield BD, Landers DM, Ray WJ. Cognitive processes during self paced motor performance: an electroencephalographic profile of skilled marksmen. J Sport Psychol. 1984;6:42–59. [Google Scholar]
- Kazennikov O, Hyland B, Wicki U, Perrig S, Rouiller EM, Wiesendanger M. Effects of lesions in the mesial frontal cortex on bimanual co-ordination in monkeys. Neuroscience. 1998;85:703–716. doi: 10.1016/s0306-4522(97)00693-3. (August. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory. EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Landers DM, Han M, Salazar W, Petruzzello SJ, Kubitz KA. Effects of learning on electroencephalographic and electrocardiographic patterns in novice archers. Int J Sport Psychol. 1994;25:313–330. [Google Scholar]
- Lim SH, Dinner DS, Pillay PK, Lüders H, Morris HH, Klem G, Wyllie E, Awad IA. Functional anatomy of the human supplementary sensorimotor area: results of extraoperative electrical stimulation. Electroencephalogr Clin Neurophysiol. 1994;91:179–193. doi: 10.1016/0013-4694(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Loze GM, Collins D, Holmes PS. Pre-shot EEG alpha-power reactivity during expert air-pistol shooting: a comparison of best and worst shots. Sports Sci. 2001;19:727–733. doi: 10.1080/02640410152475856. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Babiloni F, Carducci F, Cincotti F, Remondini E, Rossini PM, Salinari S, Babiloni C. Computerized processing of EEG-EOG-EMG artifacts for multi-centric studies in EEG oscillations and event-related potentials. Int J Psychophysiol. 2003;47:199–216. doi: 10.1016/s0167-8760(02)00153-8. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Freudenthaler HH. Ultradian rhythms in cognitive performance: no evidence for a 1. 5-H. Rhythm Biol Psychol. 1995;40:281–298. doi: 10.1016/0301-0511(95)05121-p. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Penfield W, Welch K. Instability of response to stimulation of the sensorimotor cortex of man. J Physiol. 1949;109:358–365. doi: 10.1113/jphysiol.1949.sp004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr Clin Neurophysiol. 1979;46:138–146. doi: 10.1016/0013-4694(79)90063-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva FH. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage. 2006;31:153–159. doi: 10.1016/j.neuroimage.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett. 1994;174:93–96. doi: 10.1016/0304-3940(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. J Psychophysiol. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol. 2000;111:1873–1879. doi: 10.1016/s1388-2457(00)00428-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Woertz M, Krausz G, Neuper C. Distinction of different fingers by the frequency of stimulus induced beta oscillations in the human EEG. Neurosci Lett. 2001;307:49–52. doi: 10.1016/s0304-3940(01)01924-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Meulenbroek RJ, Vaughan J, Jansen C. Posture-based motion planning: applications to grasping. Psychol Rev. 2001;108:709–734. doi: 10.1037/0033-295x.108.4.709. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Vaughan J, Barnes HJ, Jorgensen MJ. Time course of movement planning: selection of handgrips for object manipulation. J Exp Psychol Learn Mem Cogn. 1992;18:1058–1073. doi: 10.1037//0278-7393.18.5.1058. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Liang F, Babalian A, Moret V, Wiesendanger M. Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas: a multiple tracing study in macaque monkeys. J Comp Neurol. 1994;345:185–213. doi: 10.1002/cne.903450204. [DOI] [PubMed] [Google Scholar]
- Salazar W, Landers DM, Petruzzello SJ, Han M, Crews DJ, Kubitz KA. Hemispheric asymmetry, cardiac response, and performance in elite archers. Res Q Exerc Sport. 1990;61:351–359. doi: 10.1080/02701367.1990.10607499. [DOI] [PubMed] [Google Scholar]
- Shaw JC. Electroencephalographic measures of attentional patterns prior to golf putt. Med Sci Sports Exerc. 1993;25:1084–1085. [PubMed] [Google Scholar]
- Shaw JC. Intention as a component of the alpha-rhythm response to mental activity. Int J Psychophysiol. 1996;24:7–23. doi: 10.1016/s0167-8760(96)00052-9. [DOI] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalogr Clin Neurophysiol. 1994;93:380–389. doi: 10.1016/0168-5597(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Wertheim AH. Occipital alpha activity as a measure of retinal involvement in oculomotor control. Psychophysiology. 1981;18:432–439. doi: 10.1111/j.1469-8986.1981.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Rouiller EM, Kazennikov O, Perrig S. Is the supplementary motor area a bilaterally organized system? Adv Neurol. 1996;70:85–93. [PubMed] [Google Scholar]
- Wiesendanger M, Wise SP. Current issues concerning the functional organization of motor cortical areas in nonhuman primates. Adv Neurol. 1992;57:117–134. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The golf green simulator used for the present study.
Electroencephalographic (EEG) electrode montage.







