Abstract
Tendon properties contribute to the complex interaction of the central nervous system, muscle–tendon unit and bony structures to produce joint movement. Until recently limited information on human tendon behaviour in vivo was available; however, novel methodological advancements have enabled new insights to be gained in this area. The present review summarizes the progress made with respect to human tendon and aponeurosis function in vivo, and how tendons adapt to ageing, loading and unloading conditions. During low tensile loading or with passive lengthening not only the muscle is elongated, but also the tendon undergoes significant length changes, which may have implications for reflex responses. During active loading, the length change of the tendon far exceeds that of the aponeurosis, indicating that the aponeurosis may more effectively transfer force onto the tendon, which lengthens and stores elastic energy subsequently released during unloading, in a spring-like manner. In fact, data recently obtained in vivo confirm that, during walking, the human Achilles tendon provides elastic strain energy that can decrease the energy cost of locomotion. Also, new experimental evidence shows that, contrary to earlier beliefs, the metabolic activity in human tendon is remarkably high and this affords the tendon the ability to adapt to changing demands. With ageing and disuse there is a reduction in tendon stiffness, which can be mitigated with resistance exercises. Such adaptations seem advantageous for maintaining movement rapidity, reducing tendon stress and risk of injury, and possibly, for enabling muscles to operate closer to the optimum region of the length–tension relationship.
That force generated by the contractile machinery is transferred to bones via tendons to produce moments about joints is intuitive. It is also well known that tendons are not inextensible, but that they exhibit important elastic and time-dependant characteristics that may influence the function of the overall muscle–tendon complex. These viscoelastic properties of tendon allow for a dynamic interaction between the muscle and tendon (Lieber et al. 2000), which can influence not only force transmission (Reeves et al. 2003a), but also energy storage and return during locomotion (Alexander, 1991; Biewener & Baudinette, 1995; Voigt et al. 1995; Fukunaga et al. 2001; Maganaris & Paul, 2002; Ishikawa et al. 2005; Lichtwark & Wilson, 2005), spinal reflexes responses and the way that joint position and movement accuracy are controlled (Rack et al. 1983; Hoffer et al. 1989; Loram et al. 2004, 2005a,b). Tendons also provide protection from muscle fibre tensile injury (Griffiths, 1991; Lieber et al. 2000). Although it is recognized that tendon properties contribute to the complex interaction between the central nervous system, muscle–tendon unit and bony structures to produce joint movement, there is limited information on human tendon behaviour, in vivo. In this article we review recent advances in the understanding of how human tendons function, in vivo.
Mechanical properties of tendon and muscle during passive stretch
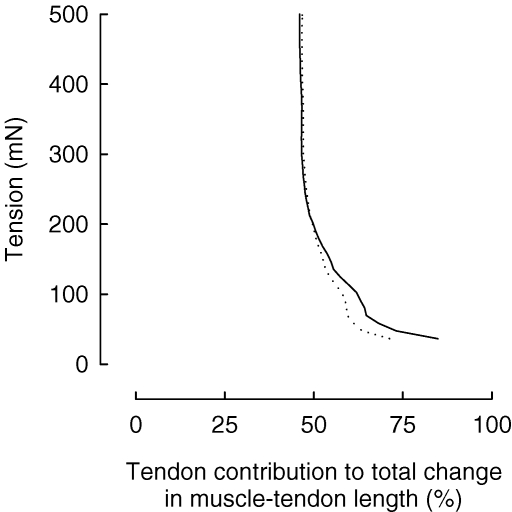
The importance of the mechanical properties of the muscle–tendon unit during contraction for human locomotion is largely appreciated, while those properties in the passive muscle–tendon unit have received considerably less attention. The limited data on humans warrants an examination of animal data. It was recently reported on the history dependence of the passive mechanical properties of the medial gastrocnemius muscle–tendon complex of the cat, in situ (Whitehead et al. 2001). The data show that passive tension was more dependant on prior muscle contractions at long lengths (beyond optimum) than at short lengths, and the authors propose that this history dependence be attributed to intramuscular structures, such as titin, and the detachment of stable cross-bridges and their reformation. Others (Herbert & Crosbie, 1997) have measured the length–tension properties of the rabbit soleus muscle fascicles and tendon during passive stretches at low tensions. The data show that strain of the muscle fascicle (23.3%) surpasses tendon strain (5.6%) by up to four times. However, because the resting length of the tendon far exceeds that of the muscle fascicles, changes in tendon length accounted for nearly half of the total change in muscle–tendon unit length. Moreover, at very low tensions, the tendon accounted for the majority of the overall length change of the muscle–tendon unit (Fig. 1), and these data were comparable to the previously calculated strain values on frog semitendinosis (Trestik & Lieber, 1993). Based on their results, the authors concluded that the tendon is responsible for a large part of the compliance of rabbit soleus muscle–tendon units at physiological resting tensions (Herbert & Crosbie, 1997).
Figure 1. The contribution of the rabbit soleus tendon to changes in muscle–tendon unit length during passive extension.
Continuous line is tendon in series with proximal muscle fascicle, and dotted line is tendon in series with distal muscle fascicle. From Herbert & Crosbie (1997) with kind permission of Springer Science and Business Media.
The question was recently addressed in a human in vivo model by Herbert et al. (2002). These authors used ultrasonography to measure tibialis anterior and gastrocnemius muscle fascicle length during passive movement compared with changes in length of the whole muscle–tendon unit calculated from joint angles and anthropometric data. In the tibialis anterior nearly half of the total change in muscle–tendon length could be attributed to elongation of the tendon, while in the gastrocnemius muscle ∼27% of the total change in muscle–tendon length was transmitted to muscle fascicles. Niether pre-stretch nor contraction history did appeared to influence the results. It was concluded that when joints are moved passively, muscle fascicle length changes are smaller than those imposed on the entire muscle–tendon unit because the tendon undergoes significant length changes (Herbert et al. 2002). These observations appear to confirm earlier work in animals by the same authors (Herbert & Crosbie, 1997), and have important consequences for reflex responses, as small passive joint movements may simply go undetected by the muscle spindle (Rack et al. 1983), particularly in conditions where tendon compliance is increased, as in disuse and ageing (Reeves et al. 2005b; Narici, 2005).
Mechanical properties of tendon and aponeurosis during muscle contraction
Contractile force is transmitted to the free tendon via the fibrous sheet or flat expanded tendon called the aponeurosis. Morphologically, the aponeurosis differs from the free tendon in that there is a marked gradient in the thickness so that it becomes thicker more distal before it merges with the free tendon (Scott & Loeb, 1995). In the frog gastrocnemius muscle it has been shown that the free tendon and aponeurosis have similar mechanical properties during passive loading and were therefore considered as one functional unit (Trestik & Lieber, 1993). Similarly, others (Scott & Loeb, 1995) have demonstrated that the free tendon and aponeurosis of the cat triceps surae have comparable mechanical properties during an isometric contraction.
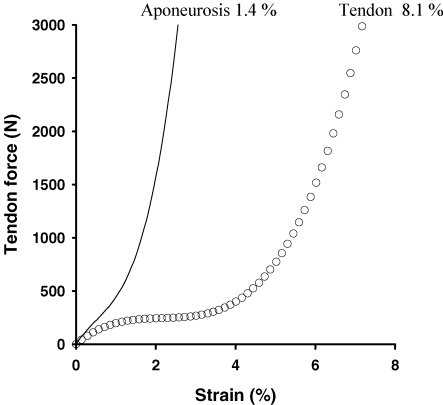
Several recent reports have used ultrasonography to differentiate, in vivo, aponeurosis and free tendon strain, in humans. Maganaris (Maganaris & Paul, 2000a) used ultrasonography to examine strain in the human tibialis anterior muscle–tendon unit, in vivo, during maximum voluntary contraction. The data showed that tendon strain was 3.2%, while aponeurosis strain was 6.5%. Similar results were obtained when the authors achieved muscle contraction with percutaneous tetanic stimulation (Maganaris & Paul, 2000b). Together, these results suggest that, if the tensile force on the tendon and the aponeurosis is the same, the aponeurosis of the anterior tibialis, which is a muscle–tendon complex not subjected to large loads during locomotion, is substantially more compliant than the free tendon. In a recent attempt to separate the mechanical behaviour of the aponeurosis from that of the free tendon, a thin needle, that could be visualized as a landmark during contraction while using ultrasonography, was placed in the free tendon (Magnusson et al. 2003b). These data showed that during near-maximal plantarflexion, the human Achilles free tendon (defined as that distal to the soleus muscle fibre) underwent a strain of ∼8.0% while the aponeurosis showed a strain of 1.4% (Fig. 2) (Magnusson et al. 2003b).
Figure 2. The displacement for the free tendon and separate aponeurosis is expressed as strain (%).
Note the considerable difference between the strain of the aponurosis and free tendon. From Magnusson et al. 2003b and used with permission.
Using cine phase-contrast magnetic resonance imaging, it was recently demonstrated that the Achilles tendon strain during voluntary contractions (40% of MVC) was 4.7% while that of the mid-region aponeurosis was 2.2% (Finni et al. 2003). Together, these studies suggest that the free Achilles tendon is more compliant than the aponeurosis, which is in apparent contrast with the above data on the human tibialis anterior muscle and also with other authors' data on the gastrocnemius tendon and aponeurosis (Arampatzis et al. 2005). However, in the latter study aponeurosis deformation was assessed only in one location of the tendon plate and this may have led to an overestimation of the aponeurosis displacement given the heterogeneous deformation of this structure upon loading.
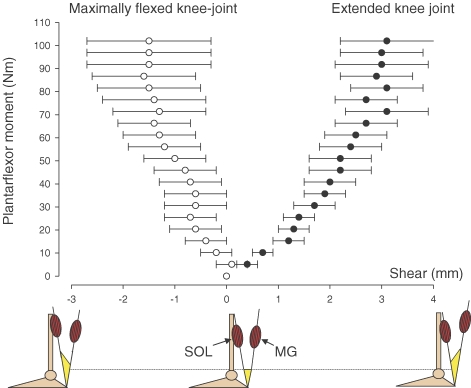
The tendon and aponeurosis of the human triceps surae is of particular interest because of its substantial loading during locomotion, running in particular, during which peak tendon forces up to 11 kN cm−2 have been measured in vivo (Komi et al. 1987, 1992) and the fact that the rather high risk of injury to both tendon and myotendinous junction remain clinical challenges. However, in addition to the potential complexity of muscle activation and/or aponeurosis tissue properties influencing stiffness in various regions of the aponeurosis, the anatomical configuration of the triceps surae represents a challenge in itself: three separate muscle compartments that merge via their aponeuroses into a common tendon. This unique anatomical configuration leaves the possibility of three muscles contributing to the load seen by the free Achilles tendon. In fact, differences in medial and lateral forces in cadaver Achilles tendon has been observed when separate muscles of the triceps surae were loaded (Arndt et al. 1999), which may theoretically result in intratendinous shear strain and cause sliding between planes of tissue layers parallel to the acting forces. As the triceps surae includes the gastrocnemii muscles that cross both the ankle and knee joints, and the soleus muscle that crosses the ankle joint alone, the relative contribution of these muscles to the free tendon force will be influenced by the relative physiological cross-sectional area of each muscle (Narici et al. 1992; Morse et al. 2005) as well as by the degree of knee flexion (Cresswell et al. 1995). It was recently observed that during maximal isometric contractions with the plantar flexor muscles, a differential displacement existed between the soleus and gastrocnemius aponeuroses proximal to the junction of the Achilles tendon (Fig. 3) (Bojsen-Møller et al. 2004).
Figure 3. Anterior–posterior shear between medial gastrocnemius and soleus aponeuroses.
From Bojsen-Møller et al. 2004 used with permission.
When the knee joint was extended, displacement of the medial gastrocnemius aponeurosis exceeded that of the soleus aponeurosis, whereas the converse occurred when the knee joint was flexed. These differences in aponeurosis displacement created a ‘shear’ effect with a direction that was governed by the knee joint position. It remains to be established if these muscle-specific and joint-position-governed aponeurosis shear result in regional differences in strains in the free tendon. These observations may have implications for tendon injury and/or rehabilitation, but also add to the challenge of studying the aponeurosis and tendon of the human triceps surae.
The effect of repeated loading on the mechanical properties of tendon
Tendons respond in a non-linear fashion when stretched, with an initial curvilinear toe region followed by an approximately linear region (Viidik, 1973; Butler et al. 1978). It is also well accepted that in addition to the magnitude of tensile loading, the history and rate of loading will affect the tendon properties. This history dependency is readily observed during repeated tensile loading of isolated tendon as the stress–strain curve is shifted to the right, in particular with the initial loading cycles (Rigby et al. 1959; Viidik, 1973). Such an acute response of tendon material may have significant physiological implications since it would influence the overall length–tension curve of the muscle–tendon complex.
With respect to laboratory measurement procedures, such a rightward shift of the load–deformation curve demands so-called pre-conditioning to obtain reproducible data (Rigby et al. 1959; Viidik, 1973). However, tendons are composite materials, and it is therefore a considerable challenge to obtain repeatable mechanical test results, and the existence of a natural ‘steady state’, which at best can be achieved during certain specific loading conditions, has even been questioned (Fung, 1973). Nevertheless, the rightward shift is noticeable with the initial loading cycles, and pre-conditioning is therefore required to obtain reproducible data (Rigby et al. 1959; Viidik, 1973; Fung, 1973). Although conditioning is a recognized creep-related phenomenon, its importance with respect to human, in vivo, conditions has, until recently, not been investigated, and mechanical testing of isolated tendon may not necessarily represent human in vivo conditions, where concerns regarding tissue storage, clamping technique, perfusion and pressure can be avoided.
Kubo et al. (2001a) used ultrasonography to investigate the changes in the elastic properties of human in vivo vastus lateralis tendon and aponeurosis following 50 consecutive 3 s maximal isometric contractions. The average compliance of the combined tendon and aponeurosis in this study increased by 22.7%. Although there was no distinction between the tendon and aponeurosis, these were some of the first findings that suggest that human tendinous tissue, in vivo, display history-dependant properties. Because of the viscous, time-dependant properties of tendon, it is likely that the duration of each repeated tensile loading event will in some way influence the load–deformation properties of the tendon. The issue of history dependency in the mechanical behaviour of human tendons in vivo was addressed by Maganaris who also used ultrasonography to examine the effect of 10 repeated 4 s isometric plantarflexion contractions to 80% of MVC on the gastrocnemius tendon elongation during contraction and residual deformation after the relaxation phase in each contraction (Maganaris, 2003). The loading pattern caused tendon elongation to increase by ∼5 mm from the first to the fifth contraction, without any significant changes thereafter. A similar pattern and magnitude of changes were found in the tendon residual deformation after relaxation. These changes clearly demonstrate a ‘conditioning’ effect.
The mechanism for this alteration of tendon properties with repeated loading remains obscure. Temperature is perhaps one possible explanation; however, it is unlikely that the first few of several contractions would induce a large and rapid enough temperature increase in the tendon that would influence its properties. The lack of a significant temperature effect is further supported by the fact that the elevation in intramuscular temperature associated with 40 min of running does not appear to influence the passive resistance of the human muscle–tendon complex (Magnusson et al. 2000). Fluid shifts within the tendon are another possible explanation since it has been shown that in humans, in vivo, there is a marked negative tissue pressure in the peritendinous space around the Achilles tendon during exercise (Langberg et al. 1999a).
It appears that human tendons, in vivo, display time-dependant conditioning properties when the effect of prolonged and repeated loading on elongation is examined. Theoretical analyses of the data of Maganaris (Maganaris et al. 2002; Maganaris, 2003) indicate that the associated transient extra muscle shortening caused by tendon conditioning would shift the length–tension relationship of the muscle counter-clockwise, to an extent that the gastrocnemius muscle would produce 10% less force (Maganaris et al. 2002). In experimental contractions, such large force reductions could be mistaken for evidence of neuromuscular fatigue. On the other hand, in a physiological situation involving repeated loading of the gastrocnemius tendon after a period of unloading, e.g. in the first steps taken after awakening in the morning, the extra tendon stretch needed to make the tendon taut and capable for force transmission could be obtained by further dorsiflexing the ankle and/or further extending the knee at push-off, by ∼6 deg (Maganaris, 2003). It must be considered, however, that the time period during which the tendons are loaded during locomotion is much shorter than the loading duration of 3–10 s in each load-cycle in the above studies. For instance, the stance phase is in the order of 0.4 s in normal walking and 0.1 s in sprinting (Fukunaga et al. 2001). Whether such short durations of loading can induce time-dependant conditioning properties in the tendons remains unknown.
The role of the mechanical properties of tendon on muscle–tendon function
The fact that tendon tissue has elastic properties has significant implications for the interaction between muscle and tendon, and the central nervous system. The importance of this muscle–tendon interaction during locomotion has been elegantly shown in animal models. For example, in the medial gastrocnemius muscle in walking cat it has been shown that while the muscle fibres shortened during the stance phase, the tendon was actually elongated and subsequently recoiled (Griffiths, 1991). In running turkeys it has been demonstrated that the stretch and recoil of tendon of the lateral gastrocnemius supply considerable mechanical work while active muscle fibres produce high forces during running (Roberts et al. 1997). These studies show that tendon stretch and subsequently recoil upon release can provide considerable elastic energy to the energy cost of locomotion. For obvious reasons such a separation of the human, in vivo, tendon and muscle behaviour during locomotion has been difficult to investigate, and has therefore largely been limited to modelling and simulation. Nevertheless, the recent technical advances provided by the use of ultrasonography have enabled studies on the in vivo interaction between muscle and tendon during locomotor activities that include a stretch–shortening cycle.
During isolated plantar flexion movement in a toe-standing position at a slow and fast speed that included a counter-movement, it was shown that going from dorsi- to plantar flexion, the muscle fascicle length was shorter in the fast than slow exercise, which suggests that the elongation of tendon structures was greater in the fast than in the slow exercise (Kubo et al. 2000). Further, in the initial stage of fast plantar flexion, the length of the muscle fascicle length remained essentially constant while the tendon shortened. These observations suggest that during isolated tasks that include stretch–shortening the muscle fibres work almost isometrically, while the tendon can store and release elastic energy.
The muscle and tendon interaction has also been examined during more dynamic functional tasks. Fukunaga et al. (2001) examined in vivo length changes in the fascicles and tendon of the human medial gastrocnemius muscle during treadmill walking. The data show the muscle contracted with little or no length change in the stance phase, while the tendon underwent a significant stretch during single support, and recoiled during push-off (Fig. 4).
Figure 4. Typical data from one subject in one step cycle.
a, changes in qastrocnemis medialis fascicular length (thick line), musculotendon length (dashed line) and tendon length (thin line). Positive and negative values indicate elongation and shortening, respectively. All values are given relative to heel-strike. b, EMG recordings from the GM muscle. c, ankle-joint (thick line) and knee-joint (thin line) angles. The 0 deg joint position corresponds to the anatomically neutral ankle- and full knee-extension positions. For the ankle joint, plantarflexion and dorsiflexion positions are indicated by positive and negative values, respectively. d, vertical component of ground reaction force. From Fukunaga et al. (2001) and used with permission.
Based on these findings, the authors suggested that the lack of muscle fascicle length change enables the muscle to operate near its highest force region of the force–velocity curve, which serves to support the body weight economically instead of performing mechanical work, and that the behaviour of the tendon indicates storage and release of elastic-strain energy (Fukunaga et al. 2001). These and similar findings during counter-movement exercise involving human jumping (Kawakami et al. 2002; Kurokawa et al. 2003) further support the notion that the human, in vivo, gastrocnemius tendon can store and release elastic energy, which is in accordance with previous work in animal models. It has been estimated that human tendons provide 52–60% of the total work during locomotion (Voigt et al. 1995), and that the elastic strain energy in the Achilles tendon of a man running at 3.9 m s−1 is ∼42 J (Alexander & Bennet-Clark, 1977). Direct measures in human, in vivo, gastrocnemius tendinous tissue show that it may release ∼1.3 J during walking, which corresponds to 6% of the respective total external work (Maganaris & Paul, 2002), ∼4 J during two-leg vertical jump (Kurokawa et al. 2003) and 38 J during one-leg vertical jump, which corresponds to 16% of the respective total external work (Lichtwark & Wilson, 2005), with the elastic strain energy increasing with increasing dropping height (Ishikawa & Komi, 2004). However, it must be noted that the above energy storage/release capacity of the tendon depends on the mechanical hysteresis, i.e. how much energy is lost as heat in the tendon stretch–recoil cycle.
Imaging techniques for human tendon elongation, in vivo
Currently, the most widely used technique to investigate human tendon displacement, in vivo, during muscle contractions is B-mode ultrasonography, which was developed in the mid 90s for measurements in the whole tendon–aponeurosis complex (Fukashiro et al. 1995) and late 90s for measurements in isolated tendon (Maganaris & Paul, 1999). Although the technique is very attractive, and many limitations have been successfully addressed, several details have to be considered. The method accounts for only two dimensions of structural deformation (i.e. in the sagittal plane), and cannot account for deformation in three dimensions, which may be of importance with respect to the aponeurosis (see discussion above). The technique was originally based on the displacement of intramuscular fascicular structures that can be observed on the ultrasound image, and therefore the resulting deformation does not represent that of the tendon per se, but rather the total deformation of the combined tendon and aponeurosis distal to the measurement site. This can, in some muscle–tendon complexes but not all, be circumvented by identifying the very junction between the aponeurosis and free tendon (the myotendinous junction), or alternatively by introducing a visible landmark such as a needle. For the patellar tendon the problem can be overcome by having two bony landmarks (tibia and patella) (Hansen et al. 2006). The technique is most commonly applied during ‘isometric’ conditions in which small amounts of joint rotation or body movement may take place that can affect the displacement measurements, which need to be accounted for. A true resting length of the tendon (i.e. 0% strain) may be difficult to obtain in vivo, but may be defined as that corresponding to zero net joint moment. Another limitation is that, unlike isolated material testing, the tendon elongations obtained in vivo depend not only on the tensile force applied, but also on the length of the in-series contractile machinery: For given tendon length and sarcomeric shortening, the greater the number of serial sarcomeres the greater the absolute shortening in the entire muscle and the lengthening of the tendon on isometric contraction. To account for this effect and avoid misinterpretation of results from studies across different tendons, differences in the ratio of tendon length/muscle fascicle length should be considered (Zajac, 1989; Trestik & Lieber, 1993).
Adaptations of tendons to chronic regimes of loading, unloading and ageing, new findings and perspectives
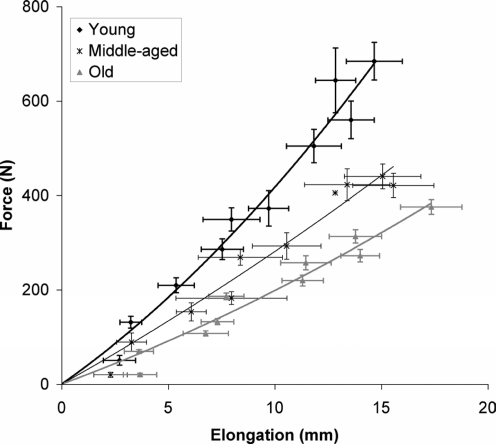
In contrast to previous beliefs, it has recently been shown that human tendinous tissue is metabolically rather active in response to activity. In fact, using the microdialysis technique it has been shown that an acute bout of exercise immediately reduces human tendinous collagen synthesis followed by a dramatic increase in the subsequent days (Langberg et al. 1999b). Chronic loading appears to increase synthesis and degradation, although the latter occurs primarily in the initial phase of a period in persons subjected to increased physical activity (Langberg et al. 2001). More recently, positron emission tomography (PET) has been used to demonstrate increase glucose uptake in tendon tissue after an acute bout of exercise (Hannukainen et al. 2005; Kalliokoski et al. 2005), further demonstrating the metabolic activity of tendon tissue, which may result in structural changes (Magnusson et al. 2002). In addition, several components of the extracellular matrix, such as proteoglycans, glycosaminoglycans and cross-links, appear to be influenced by physical activity (Kjaer, 2004). Loading of tendons imposes strain of the fibroblast and may thus influence mechanical transduction (Arnoczky et al. 2002). However, the influence of the various components of the extracellular matrix or the collagen fibril size, number or density, on the mechanical properties of tendon has not been established. Moreover, up to recently, whether the influence of various forms of physical activity, ageing and a combination of these, would influence the mechanical properties of human tendons was mostly unknown. However, new findings, based on the above-described ultrasound techniques, show that the mechanical properties of human tendons, assessed in vivo, undergo substantial changes both with ageing and disuse. In both conditions, a decrease in tendon stiffness has been found (Fig. 5) (Reeves et al. 2003a; Karamanidis & Arampatzis, 2005; Narici et al. 2005; Reeves et al. 2005b; Maganaris et al. 2006).
Figure 5. Gastrocnemius tendon force–elongation properties in young, middle-aged and older individuals.
From Onambele et al. 2006 and used with permission.
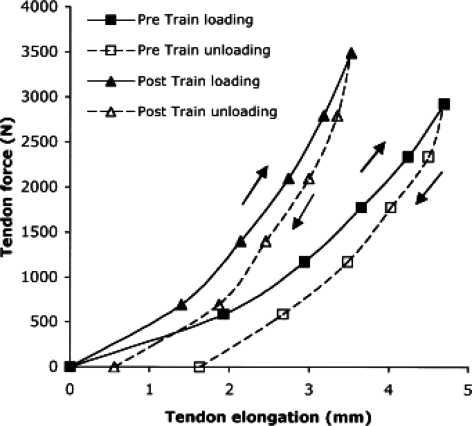
The results suggest that the decrease in tendon stiffness is at least partly due to tendon material deterioration, caused by factors such as decreased collagen turnover and packing density (Naresh & Brodsky, 1992), increased collagen molecules crimp angle (Patterson-Kane et al. 1997) and/or decreased water content (Ippolito et al. 1980). Tendons of individuals with a spinal cord injury were recently found to be 17% thinner than in age-, height- and body mass-matched controls (Maganaris et al. 2006). Of course, it ought to be borne in mind that changes in tendon dimensions are unlikely to occur ceteris paribus and that changes in the ‘quality’ of connective tissue must also be considered. As a matter of fact, in vitro studies have shown that older tendon tissue displays: (1) an increase in collagen cross-linking; (2) a reduction in collagen fibril crimp angle; (3) an increase in elastin content; (4) a reduction in extracellular water and mucopolysacharide content; and (5) an increase in type V collagen (Kjaer, 2004). Despite the reduction in tendon stiffness with ageing, resistive loading has been shown to significantly reverse/mitigate these alterations (Reeves et al. 2003a,b, 2005a). Recently, in fact, in a group of septuagenarian males, tendon stiffness was increased by 65% after only 14 weeks of resistive training (Reeves et al. 2003a,b), an increase probably explained by changes in tendon material properties since tendon dimensions were unchanged. As a consequence, Young's modulus increased by 69%; furthermore a 22% reduction in mechanical hysteresis was found indicating that training made the tendon more capable of returning stored strain energy (Fig. 6).
Figure 6. Patella tendon force–elongation relations before and after strength training for 14 weeks.
The arrows indicate the loading and unloading directions. From Reeves et al. 2003b.
Resistive loading exercise has also been found to counteract the decrease in tendon stiffness induced by prolonged inactivity (bed rest) (Reeves et al. 2005a). Performing high-intensity flywheel exercise every third day over a 90 day bed rest period decreased tendon stiffness by 37%, whereas in a non-exercise control group, stiffness decreased by 58% (Fig. 7).
Figure 7. Gastrocnemius tendon stress–strain relation for the bed rest and bed rest + exercise groups.
From Reeves et al. 2005a and used with permission.
Animal data show that tendon may undergo either qualitative (Buchanan & Marsh, 2001; Viidik, 1967) or hypertrophic changes (Woo et al. 1982; Birch et al. 1999), or both (Woo et al. 1982) in response to endurance-type exercise. In humans, cross-sectional data suggest that habitual long distance running (≥ 5 years) is associated with a markedly greater cross-sectional area (22%) of the Achilles tendon compared with that of non-runners (Rosager et al. 2002; Magnusson & Kjaer, 2003; Kongsgaard et al. 2005). Similar adaptive responses of tendons to training are also observed in thoroughbred horses which show greater tendon cross-sectional areas in comparison with untrained age-matched horses (Kasashima et al. 2002; Firth et al. 2004). An increased tendon cross-sectional area would reduce the average stress of the tendon thereby decreasing the risk for accute tensile tendon rupture. Surpisingly, however, a total training stimulus of ∼9 months of running in previously untrained subjects did not result in tendon hypertrophy of the Achilles tendon (Hansen et al. 2003). At the same time it has been shown that resistance training for 3 months induced marked changes in the material properties of human tendon in the absence of any tendon hypertrophy (Reeves et al. 2003a,b). It is possible that these apparent discrepancies may be explained by the training mode, namely endurance versus resistance training. On the other hand, perhaps qualitative changes in the extracellular components preceed any hypertrophic response since metabolic activity increases with an acute bout of loading (Langberg et al. 1999b; Kalliokoski et al. 2005; Miller et al. 2005; Bojsen-Moller et al. 2006), and there is a lack of hypertrophy with loading for months, while the material changes (Young's modulus) are apparent fairly soon (Reeves et al. 2003a,b). Furthermore, what remains to be established is whether the tendon hypertrophy induced by chronic training represents a physiological response to reduce tissue stress or a tissue repair response to damage induced by repeated loading (Rosager et al. 2002).
In conclusion, new evidence exists to support the notion that, in vivo, human tendons are metabolically active, especially during exercise, and exhibit visco-elastic properties that enable them to interact with the contractile element and mediate the outcome of muscle contraction and whole body performance. These tendon properties are worsened by chronic disuse and ageing, but training can partly mitigate these changes highlighting the plasticity of tendons to variations in mechanical loading. Future studies should investigate the time-course and the exact mechanisms of these changes.
References
- Alexander RM. Energy-saving mechanisms in walking and running. J Exp Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature. 1977;265:114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Stafilidis S, DeMonte G, Karamanidis K, Morey-Klapsing G, Bruggemann GP. Strain and elongation of the human gastrocnemius tendon and aponeurosis during maximal plantarflexion effort. J Biomech. 2005;38:833–841. doi: 10.1016/j.jbiomech.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Arndt AN, Bruggemann GP, Koebke J, Segesser B. Asymmetrical loading of the human tricpes surae. I. Mediolateral force difference in the Achilles tendon. Foot Ankle Int. 1999;20:445–449. doi: 10.1177/107110079902000709. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, Lavagnino M, Whallon JH, Hoonjan A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20:29–35. doi: 10.1016/S0736-0266(01)00080-8. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Baudinette RV. In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (Macropus eugenii) J Exp Biol. 1995;198:1829–1841. doi: 10.1242/jeb.198.9.1829. [DOI] [PubMed] [Google Scholar]
- Birch HL, McLaughlin L, Smith RK, Goodship AE. Treadmill exercise-induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Vet J Suppl. 1999;30:222–226. doi: 10.1111/j.2042-3306.1999.tb05222.x. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller J, Hansen P, Aagaard P, Svantesson U, Kjaer M, Magnusson SP. Differential displacement of the human soleus and medial gastrocnemius aponeuroses during isometric plantar flexor contractions in vivo. J Appl Physiol. 2004;97:1908–1914. doi: 10.1152/japplphysiol.00084.2004. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller J, Kalliokoski KK, Seppanen M, Kjaer M, Magnusson SP. Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles tendon. J Appl Physiol. 2006;101:196–201. doi: 10.1152/japplphysiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol. 2001;90:164–171. doi: 10.1152/jappl.2001.90.1.164. [DOI] [PubMed] [Google Scholar]
- Butler DL, Grood ES, Noyes FR. Biomechanics of ligaments and tendons. In: Hutton RS, editor. Exercise and Sports Sciences Reviews. Philadelphia: Franklin Institute; 1978. pp. 125–181. [PubMed] [Google Scholar]
- Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol. 2003;95:829–837. doi: 10.1152/japplphysiol.00775.2002. [DOI] [PubMed] [Google Scholar]
- Firth EC, Rogers CW, Anderson BH. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 4. Morphometric, microscopic and biomechanical properties of the digital tendons of the forelimb. N Z Vet J. 2004;52:285–292. doi: 10.1080/00480169.2004.36441. [DOI] [PubMed] [Google Scholar]
- Fukashiro S, Itoh M, Ichinose Y, Kawakami Y, Fukunaga T. Ultrasonography gives directly but noninvasively elastic characteristic of human tendon in vivo. Eur J Appl Physiol. 1995;71:555–557. doi: 10.1007/BF00238560. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc R Soc Lond B Biol Sci. 2001;268:229–233. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung YC. Biorheology of soft tissues. Biorheology. 1973;10:139–155. doi: 10.3233/bir-1973-10208. [DOI] [PubMed] [Google Scholar]
- Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannukainen J, Kalliokoski KK, Nuutila P, Fujimoto T, Kemppainen J, Viljanen T, Laaksonen MS, Parkkola R, Knuuti J, Kjaer M. In vivo measurements of glucose uptake in human Achilles tendon during different exercise intensities. Int J Sports Med. 2005;26:727–731. doi: 10.1055/s-2005-837458. [DOI] [PubMed] [Google Scholar]
- Hansen P, Aagaard P, Kjaer M, Larsson B, Magnusson SP. Effect of habitual running on human Achilles tendon load-deformation properties and cross-sectional area. J Appl Physiol. 2003;95:2375–2380. doi: 10.1152/japplphysiol.00503.2003. [DOI] [PubMed] [Google Scholar]
- Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon) 2006;21:54–58. doi: 10.1016/j.clinbiomech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Crosbie J. Rest length and compliance of non-immobilised and immobilised rabbit soleus muscle and tendon. Eur J Appl Physiol Occup Physiol. 1997;76:472–479. doi: 10.1007/s004210050277. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Moseley AM, Butler JE, Gandevia SC. Change in length of relaxed muscle fascicles and tendons with knee and ankle movement in humans. J Physiol. 2002;539:637–645. doi: 10.1113/jphysiol.2001.012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer JA, Caputi AA, Pose IE, Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res. 1989;80:75–85. doi: 10.1016/s0079-6123(08)62201-3. [DOI] [PubMed] [Google Scholar]
- Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am. 1980;62:583–598. [PubMed] [Google Scholar]
- Ishikawa M, Komi PV. Effects of different dropping intensities on fascicle and tendinous tissue behavior during stretch-shortening cycle exercise. J Appl Physiol. 2004;96:848–852. doi: 10.1152/japplphysiol.00948.2003. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann GP. Muscle–tendon interaction and elastic energy usage in human walking. J Appl Physiol. 2005;99:603–608. doi: 10.1152/japplphysiol.00189.2005. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Langberg H, Ryberg AK, Scheede-Bergdahl C, Doessing S, Kjaer A, Boushel R, Kjaer M. The effect of dynamic knee-extension exercise on patellar tendon and quadriceps femoris muscle glucose uptake in humans studied by positron emission tomography. J Appl Physiol. 2005;99:1189–1192. doi: 10.1152/japplphysiol.00283.2005. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A. Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol. 2005;208:3907–3923. doi: 10.1242/jeb.01830. [DOI] [PubMed] [Google Scholar]
- Kasashima Y, Smith RK, Birch HL, Takahashi T, Kusano K, Goodship AE. Exercise-induced tendon hypertrophy: cross-sectional area changes during growth are influenced by exercise. Equine Vet J Suppl. 2002;34:264–268. doi: 10.1111/j.2042-3306.2002.tb05430.x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Muraoka T, Ito S, Kanehisa H, Fukunaga T. In vivo muscle fibre behaviour during counter-movement exercise in humans reveals a significant role for tendon elasticity. J Physiol. 2002;540:635–646. doi: 10.1113/jphysiol.2001.013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. 1992;11:521–531. [PubMed] [Google Scholar]
- Komi PV, Salonen M, Jarvinen M, Kokko O. In vivo registration of Achilles tendon forces in man. I. Methodological development. Int J Sports Med. 1987;8(Suppl. 1):3–8. doi: 10.1055/s-2008-1025697. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol. 2005;99:1965–1971. doi: 10.1152/japplphysiol.00384.2005. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Effects of repeated muscle contractions on the tendon structures in humans. Eur J Appl Physiol. 2001;84:162–166. doi: 10.1007/s004210000337. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Takeshita D, Kawakami Y, Fukashiro S, Fukunaga T. In vivo dynamics of human medial gastrocnemius muscle-tendon complex during stretch-shortening cycle exercise. Acta Physiol Scand. 2000;170:127–135. doi: 10.1046/j.1365-201x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Kurokawa S, Fukunaga T, Nagano A, Fukashiro S. Interaction between fascicles and tendinous structures during counter movement jumping investigated in vivo. J Appl Physiol. 2003;95:2306–2314. doi: 10.1152/japplphysiol.00219.2003. [DOI] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Bulow J, Kjaer M. Negative interstitial pressure in the peritendinous region during exercise. J Appl Physiol. 1999a;87:999–1002. doi: 10.1152/jappl.1999.87.3.999. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999b;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM. Effects of series elasticity and activation conditions on muscle power output and efficiency. J Exp Biol. 2005;208:2845–2853. doi: 10.1242/jeb.01710. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Leonard ME, Brown-Maupin CG. Effects of muscle contraction on the load-strain properties of frog aponeurosis and tendon. Cells Tissues Organs. 2000;166:48–54. doi: 10.1159/000016708. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Paradoxical muscle movement in human standing. J Physiol. 2004;556:683–689. doi: 10.1113/jphysiol.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, non-spring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? J Physiol. 2005a;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol. 2005b;564:295–311. doi: 10.1113/jphysiol.2004.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN. Tendon conditioning: artefact or property? Proc R Soc Lond B Biol Sci. 2003;270(Suppl. 1):S39–S42. doi: 10.1098/rsbl.2003.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Repeated contractions alter the geometry of human skeletal muscle. J Appl Physiol. 2002;93:2089–2094. doi: 10.1152/japplphysiol.00604.2002. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521:307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendinous tissue stretch upon maximum muscle force generation. J Biomech. 2000a;33:1453–1459. doi: 10.1016/s0021-9290(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Load-elongation characteristics of in vivo human tendon and aponeurosis. J Exp Biol. 2000b;203:751–756. doi: 10.1242/jeb.203.4.751. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech. 2002;35:1639–1646. doi: 10.1016/s0021-9290(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33:85–92. doi: 10.1002/mus.20441. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Hansen J, Kirkeby K, Andersen R, Hansen P, Bojsen-Moller J. Pre-conditioning of human tendon-aponeurosis, in vivo (abstract) Med Sci Sports Exerc. 2003a;35:443. [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003b;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur J Appl Physiol. 2003;90:549–553. doi: 10.1007/s00421-003-0865-8. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, Krogsgaard M, Kjaer M. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–377. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Birch KM, Narici MV. Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand. 2005;183:291–298. doi: 10.1111/j.1365-201X.2004.01404.x. [DOI] [PubMed] [Google Scholar]
- Naresh MD, Brodsky B. X-ray diffraction studies on human tendon show age-related changes in collagen packing. Biochim Biophys Acta. 1992;1122:161–166. doi: 10.1016/0167-4838(92)90319-9. [DOI] [PubMed] [Google Scholar]
- Narici MV. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sport. 2005;15:392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- Narici MV, Landoni L, Minetti AE. Assessment of human knee extensor muscles stress from in vivo physiological cross-sectional area and strength measurements. Eur J Appl Physiol Occup Physiol. 1992;65:438–444. doi: 10.1007/BF00243511. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005;15:392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN, Gladys L. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100:2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane JC, Wilson AM, Firth EC, Parry DA, Goodship AE. Comparison of collagen fibril populations in the superficial digital flexor tendons of exercised and nonexercised thoroughbreds. Equine Vet J. 1997;29:121–125. doi: 10.1111/j.2042-3306.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Rack PM, Ross HF, Thilmann AF, Walters DK. Reflex responses at the human ankle: the importance of tendon compliance. J Physiol. 1983;344:503–524. doi: 10.1113/jphysiol.1983.sp014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol. 2005a;98:2278–2286. doi: 10.1152/japplphysiol.01266.2004. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003a;548:971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Plasticity of dynamic muscle performance with strength training in elderly humans. Muscle Nerve. 2005b;31:355–364. doi: 10.1002/mus.20275. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve. 2003b;28:74–81. doi: 10.1002/mus.10392. [DOI] [PubMed] [Google Scholar]
- Rigby BJ, Hirai N, Spikes JD, Eyring H. The mechanical properties of rat tail tendon. J Gen Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work [see comments] Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12:90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. Mechanical properties of aponeurosis and tendon of the cat soleus muscle during whole-muscle isometric contractions. J Morph. 1995;224:73–86. doi: 10.1002/jmor.1052240109. [DOI] [PubMed] [Google Scholar]
- Trestik CL, Lieber RL. Relationship between Achilles tendon mechanical properties and gastrocnemius muscle function. J Biomech Eng. 1993;115:225–230. doi: 10.1115/1.2895479. [DOI] [PubMed] [Google Scholar]
- Viidik A. The effect of training on the tensile strength of isolated rabbit tendons. Scand J Plastic Reconstructive Surgery. 1967;1:141–147. doi: 10.3109/02844316709022844. [DOI] [PubMed] [Google Scholar]
- Viidik A. Functional properties of collagenous tissues (review) Int Rev Connective Tissue Research. 1973;6:127–215. doi: 10.1016/b978-0-12-363706-2.50010-6. [DOI] [PubMed] [Google Scholar]
- Voigt M, Bojsen-Moller F, Simonsen EB, Dyhre-Poulsen P. The influence of tendon Youngs modulus, dimensions and instantaneous moment arms on the efficiency of human movement. J Biomech. 1995;28:281–291. doi: 10.1016/0021-9290(94)00071-b. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Gregory JE, Morgan DL, Proske U. Passive mechanical properties of the medial gastrocnemius muscle of the cat. J Physiol. 2001;536:893–903. doi: 10.1111/j.1469-7793.2001.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendon and ligaments. The relationship of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon. properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Larsson B, Kjaer M. Passive energy absorption by human muscle-tendon unit is unaffected by increase in intramuscular temperature. J Appl Physiol. 2000;88:1215–1220. doi: 10.1152/jappl.2000.88.4.1215. [DOI] [PubMed] [Google Scholar]