Abstract
Much is known about the physiological impairments that can cause muscle fatigue. It is known that fatigue can be caused by many different mechanisms, ranging from the accumulation of metabolites within muscle fibres to the generation of an inadequate motor command in the motor cortex, and that there is no global mechanism responsible for muscle fatigue. Rather, the mechanisms that cause fatigue are specific to the task being performed. The development of muscle fatigue is typically quantified as a decline in the maximal force or power capacity of muscle, which means that submaximal contractions can be sustained after the onset of muscle fatigue. There is even evidence that the duration of some sustained tasks is not limited by fatigue of the principal muscles. Here we review experimental approaches that focus on identifying the mechanisms that limit task failure rather than those that cause muscle fatigue. Selected comparisons of tasks, groups of individuals and interventions with the task-failure approach can provide insight into the rate-limiting adjustments that constrain muscle function during fatiguing contractions.
Although it is not difficult to know when one is fatigued, it is entirely another matter to be able to identify the physiological mechanisms responsible for this condition. Despite the accumulation of a substantial literature on the topic since the seminal work of Angelo Mosso in the late 1800s (Di Giulio et al. 2006), few principles have emerged to characterize the phenomenon of muscle fatigue. Although progress has been made in the study of muscle fatigue (Nybo & Secher, 2004; Cairns, 2006; Nordstrom et al. 2007; Nybo & Rasmussen, 2007), we are largely unable to state with certainty why an individual becomes fatigued under various conditions. The purpose of this topical review is to examine three issues that constrain a more complete understanding of muscle fatigue and its impact on muscle function: the diversity of measures that have been used to quantify fatigue, the specificity of the impairments that cause fatigue and the lack of knowledge on the mechanisms that limit performance. The discussion will focus on what is muscle fatigue, why does it happen and how does it influence muscle function.
Quantifying fatigue
What is muscle fatigue? In contrast to the fatigue encountered in clinical settings (Bailey et al. 2007; Friedman et al. 2007; Hacker & Ferrans, 2007), the term muscle fatigue is used to denote a transient decrease in the capacity to perform physical actions. The following excerpts characterize the range of effects ascribed to muscle fatigue:
‘Intensive activity of muscles causes a decline in performance, known as fatigue…’ (Allen & Westerblad, 2001).
‘Performing a motor task for long periods of time induces motor fatigue, which is generally defined as a decline in a person's ability to exert force.’ (Lorist et al. 2002).
‘…CNS administration of caffeine increased treadmill run time to fatigue…’ (Davis et al. 2003).
‘…a fatiguing task was performed with the muscles of the left hand until the muscles were exhausted.’ (Edgley & Winter, 2004).
‘Fatigue is known to be reflected in the EMG signal as an increase of its amplitude and a decrease of its characteristic spectral frequencies.’ (Kallenberg et al. 2007).
‘…the sensation of fatigue is the conscious awareness of changes in subconscious homeostatic control systems…’ (St Clair Gibson et al. 2003).
‘The primary purpose of the study was to use functional magnetic resonance imaging (fMRI) to determine the association between feelings of mental fatigue and blood oxygen level dependent (BOLD) brain responses during a mentally fatiguing cognitive task.’ (Cook et al. 2007).
Muscle fatigue, it seems, can refer to a motor deficit, a perception or a decline in mental function, it can describe the gradual decrease in the force capacity of muscle or the endpoint of a sustained activity, and it can be measured as a reduction in muscle force, a change in electromyographic activity or an exhaustion of contractile function. Such broad usage is problematic, however, because fatigue in this context can encompass several phenomena that are each the consequence of different physiological mechanisms, which reduces the likelihood that the cause of muscle fatigue can be identified. To circumvent this limitation, most investigators invoke a more focused definition of muscle fatigue as an exercise-induced reduction in the ability of muscle to produce force or power whether or not the task can be sustained (Bigland-Ritchie & Woods, 1984; Søgaard et al. 2006).
A critical feature of this definition is the distinction between muscle fatigue and the ability to continue the task. Accordingly, muscle fatigue is not the point of task failure or the moment when the muscles become exhausted. Rather, muscle fatigue is a decrease in the maximal force or power that the involved muscles can produce, and it develops gradually soon after the onset of the sustained physical activity. A common protocol used to quantify the development of muscle fatigue is to interrupt the fatiguing exercise with brief maximal contractions (voluntary or electrically evoked) to estimate the decline in the maximal force capacity (Merton, 1954; Bigland-Ritchie et al. 1986b; Hunter et al. 2004b; Søgaard et al. 2006). Similarly, the amount of muscle fatigue caused by an intervention can be quantified as the decline in the maximal force or power measured immediately after the fatiguing contraction (Taylor et al. 1996; Griffin et al. 2001; Hunter et al. 2004a; Lévénez et al. 2005; McNeil et al. 2006).
Specificity of the impairments
Why does fatigue occur? The simple answer is that one or several of the physiological processes that enable the contractile proteins to generate a force become impaired. A more complete answer, however, also acknowledges that the site of impairment depends on the task being performed. This effect is known as the task dependency of muscle fatigue and is one of the principles to have emerged in this field over the last 100 years (Asmussen, 1979; Enoka & Stuart, 1992; Bigland-Ritchie et al. 1995). According to this principle, there is no single cause of muscle fatigue and the dominant mechanism is specific to those processes that are stressed during the fatiguing exercise (Cairns et al. 2005). This concept is analogous to the principle of specificity that characterizes the adaptations evoked by several weeks of physical training (Kraemer et al. 2002; Aagaard & Bangsbo, 2006).
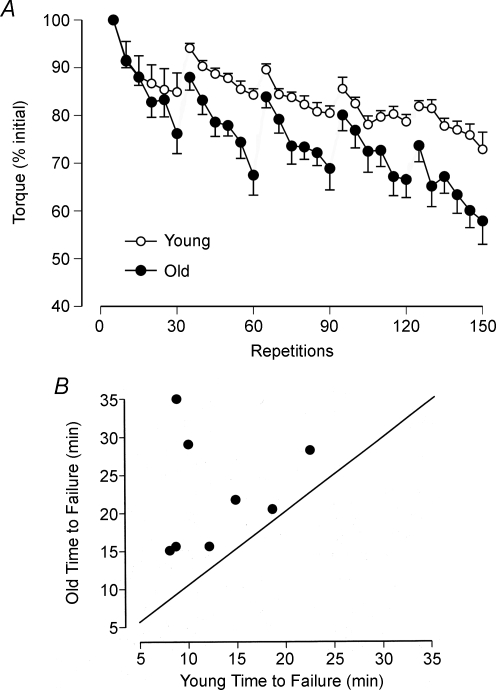
Due to the specificity of the impairments that occur during fatiguing contractions, there are no general answers to such questions as are old adults more fatigable than young adults, are women less fatigable than men, can the nervous system sustain an adequate activation of muscle during fatiguing contractions, and are there differences between muscles? The difficulty in answering such questions becomes obvious when the results obtained in selected studies are compared. First, consider the influence of age on fatigability. Baudry et al. (2007) measured the decline in torque when young (mean ± s.d.; 30.5 ± 2.5 years) and old (77.2 ± 1.4 years) men and women performed maximal shortening and lengthening contractions with the dorsiflexor muscles. The task was to perform five sets of 30 maximal contractions at a rate of one contraction every 3.5 s, and with each contraction comprising a 30 deg range of motion and the speed controlled by a motor at 50 deg s−1. There was a 60 s pause between each set of contractions. The shortening and lengthening contractions were performed on separate days. The young adults were stronger than the old adults as indicated by a greater peak torque during a maximal isometric contraction (38.3 ± 3.1 and 28.6 ± 1.3 Nm, respectively). Nonetheless, the decline in peak torque during both tasks was greater for the old adults. Figure 1A shows the data for the lengthening contractions in which the final peak torque declined by 27.1% for the young adults and by 42.1% for the old adults. The decrease in peak torque after 150 maximal shortening contractions was 40.9% for the young adults and 50.2% for the old adults. Furthermore, the decline in peak torque during each set of 30 maximal contractions was greater for the old adults for both the shortening and lengthening contractions (Fig. 1A). The fatigue experienced by both groups of subjects was associated with changes in the control of excitation–contraction coupling by Ca2+ and, for the old adults only, impairment of neuromuscular propagation. These results indicate therefore that the old adults were more fatigable than the young adults when performing maximal shortening and lengthening contractions with the dorsiflexor muscles.
Figure 1. Differences in fatigability between young and old adults.
A, average torque exerted by young and old adults during five sets of 30 maximal lengthening contractions with the dorsiflexor muscles. Each data point indicates the mean ± s.e.m. of five successive contractions for 16 subjects in each group. Adapted with permission from Baudry et al. (2007). B, each data point denotes the time to failure for the one young man and one old man who were matched for strength. The task was to sustain an isometric contraction with the elbow flexor muscles at 20% of maximum for as long as possible. The time to failure was longer for the older man of each pair. Adapted from Hunter et al. (2005).
In contrast to these results with maximal anisometric contractions, Hunter et al. (2005) found that old men (71.3 ± 2.9 years) could sustain a submaximal isometric contraction with the elbow flexor muscles for a longer duration than young men (21.5 ± 4.4 years). The task was to sustain a net muscle torque that was 20% of the maximal voluntary contraction (MVC) torque for as long as possible. Each subject was required to match the exerted torque to a target that was displayed on a monitor. The task was terminated when the torque exerted by the subject declined by 10% of the target torque or greater for at least 5 s. Eight pairs of men were matched for MVC torque (young: 65.9 ± 8.0 Nm; old: 65.4 ± 8.7 Nm), which meant that they exerted a similar net muscle torque during the fatiguing contraction. The time that the old men could sustain the submaximal isometric contraction was longer (22.6 ± 7.4 min) than that for the young men (13.0 ± 5.2 min) as indicated by all data points lying above the line of identity in Fig. 1B. The decrease in MVC torque at task failure, which indicates the amount of fatigue that occurred during the fatiguing contraction, was similar for the two groups of men (−31.4 ± 10.6%). The briefer time to failure for the young men was associated with more rapid increases in the amplitude of the surface EMG, the frequency of bursts of activity in the EMG signal, the rating of perceived exertion, mean arterial pressure and heart rate. Hence, the rate at which fatigue developed during the submaximal isometric contraction was more gradual for the old men, which contrasts with the results reported by Baudry et al. (2007) for maximal anisometric contractions with the dorsiflexor muscles.
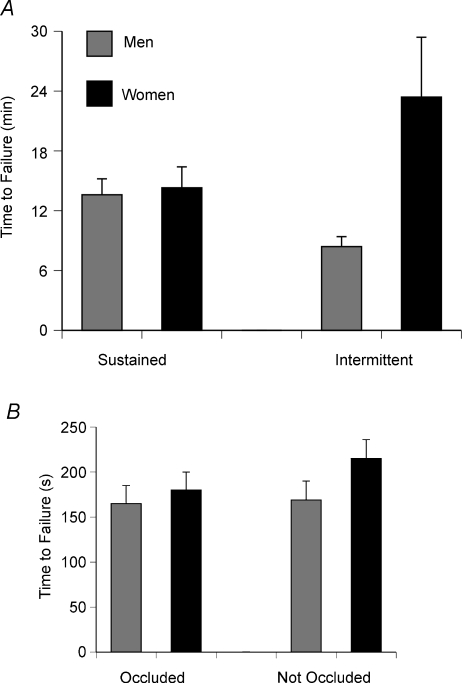
The second example on the specificity of the impairments during fatiguing contractions involves the sex difference that is often observed during such tasks. Women are usually able to sustain a contraction for a longer duration, especially at lower contraction intensities (Hicks et al. 2001; Clark et al. 2005; Hunter et al. 2006) but not maximal contractions (Baudry et al. 2007). The most common explanations for this sex difference are the greater muscle mass activated by men and a lesser reliance on glycolytic metabolism by women. As men are typically stronger than women, they must activate a larger muscle mass to exert the same relative force (% maximum) as women, which will be accompanied by larger intramuscular pressures and a greater occlusion of blood flow (de Ruiter et al. 2007). The influence of strength on the duration that submaximal contractions could be sustained was examined by comparing the performance of men and women who were matched for strength. Subjects were matched for MVC torque, which meant that the target torque (20% of maximum) required the same absolute torque (men: 12.6 ± 1.6 Nm; women: 12.8 ± 1.6 Nm) by the elbow flexor muscles (Hunter et al. 2004a). There was no difference in the duration that the men and women could sustain the contraction (819 ± 306 and 864 ± 391 s, respectively) and the decline in MVC force (−31 ± 9%) at task failure (Fig. 2A). Accordingly, there were no differences between the men and women in the rates of increase for the amplitude of the surface EMG, the frequency of bursts of activity in the EMG signal, the rating of perceived exertion, mean arterial pressure or heart rate. Furthermore, Clark et al. (2005) found that the duration an isometric contraction could be sustained at 25% MVC force with the knee extensor muscles was similar for men (165 ± 20 s) and women (180 ± 20 s) only when blood flow was occluded (Fig. 2B), even though the peak force that the men could exert (905 ± 34 N) was greater than that for the women (722 ± 34 N).
Figure 2. Times to task failure for men and women.
A, mean (+s.e.m.) time to task failure for sustained and intermittent isometric contractions performed by strength-matched men and women with the elbow flexor muscles. The target torque was 20% of MVC torque for the sustained contractions and 50% of MVC torque for the intermittent contractions. Reprinted with permission from Hunter et al. (2004b). B, mean (± s.e.m.) time to task failure for sustained isometric contractions performed by men and women with the knee extensor muscles with the blood flow to the limb either occluded or not occluded. The men were stronger than the women in this study. The target was 25% of MVC torque. Data from Clark et al. (2005).
Nonetheless, strength cannot explain all of the sex differences in fatigability. For example, women can perform a greater number of intermittent contractions than men even when the two groups are matched for strength. Hunter et al. (2004b) matched the MVC torque of the elbow flexors for men (64.8 ± 9.2 Nm) and women (62.2 ± 7.9 Nm) and found that the women (1408 ± 1133 s) could perform the intermittent contraction (6 s contraction, 4 s rest) to a target of 50% MVC torque for a longer duration than the men (513 ± 194 s) (Fig. 2A). Despite the contractions requiring the same absolute torque, the rate of decline in MVC torque was greater for the men (3.0 Nm min−1) compared with the women (0.97 Nm min−1). In contrast, the decrease in MVC torque for the dorsiflexor muscles after performing 24 MVCs in 4 min was similar for men (−79 ± 4%) and women (−78 ± 4%) when blood flow was occluded, but the decline in MVC torque differed (men: −34 ± 3%; women: −21 ± 4%) when blood flow was not occluded (Russ & Kent-Braun, 2003). As the supply and demand of ATP is altered during the occlusion of blood flow (Lanza et al. 2006), the lower rate of fatigue development observed in women during intermittent contractions may be attributable to a sex difference in the relative contributions of the metabolic pathways that supply ATP during fatiguing contractions (Russ et al. 2005).
The third example on the specificity of the impairments during fatiguing contractions concerns the potential contribution to the decrease in force by a reduction in the activation of muscle by the nervous system. If activation is inadequate, a few electrical stimuli delivered to the motor nerve during an MVC will evoke additional force from the muscle (Merton, 1954). The deficit in activation is quantified by normalizing the amplitude of the force increment to the force that can be elicited by the same stimulus when the muscle is at rest; the ratio is expressed as an index of voluntary activation (Hales & Gandevia, 1988; Kent-Braun & Le Blanc, 1996). Voluntary activation (% maximum) during brief MVCs varies among individuals, across days, and between trials (Allen et al. 1995), and among muscles (Belanger & McComas, 1981; McKenzie et al. 1992; Klass et al. 2007). A decrease in voluntary activation, as can occur during long-lasting contractions, can contribute to the development of fatigue.
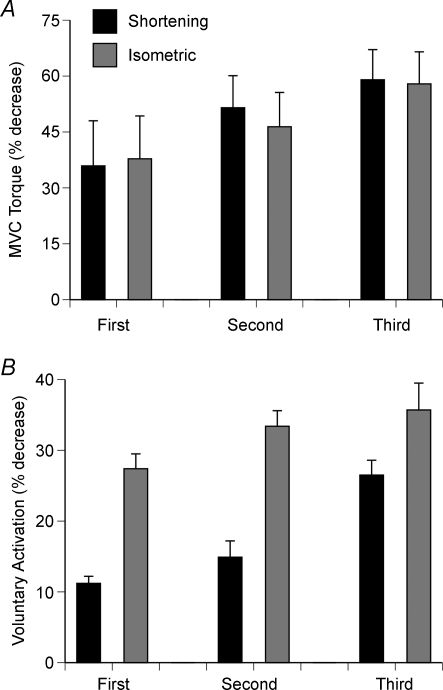
The magnitude of the decline in voluntary activation during fatiguing contractions ranges from minimal to substantial. When subjects sustained an MVC with the elbow flexor muscles for 3 min, Gandevia et al. (1996) found that the 74.1 ± 8.6% decline in force was accompanied by only a 10% decrease in voluntary activation of biceps brachii. In contrast, Babault et al. (2006) observed a more pronounced decrement in voluntary activation when subjects performed sets of maximal isometric and shortening contractions with the knee extensor muscles. Three sets of maximal shortening contractions reduced MVC torque by 59.0 ± 8.1% and voluntary activation by 26.5 ± 2.1%, whereas three matched isometric contractions decreased MVC torque by 57.9 ± 8.6% and voluntary activation by 35.7 ± 3.8%. The time course of the decrease differed in the two tasks (Fig. 3). Similarly, Søgaard et al. (2006) reported that a submaximal isometric contraction (25% MVC torque) sustained with the elbow flexor muscles for 43 min decreased MVC torque by 41.4 ± 13.2% and voluntary activation measured in response to stimulation of the motor nerve from 98.0 ± 5.1% prior to the fatiguing contraction to 71.9 ± 38.9% (27% reduction) at the end of task. Smith et al. (2007) estimated that two-thirds of the 28% decrease in MVC torque after a 70 min isometric contraction sustained at 5% MVC torque with the elbow flexor muscles was due to a decline in voluntary activation. These studies suggest that some of the fatigue experienced during repetitive maximal contractions and long-duration submaximal contractions can be caused by an inadequate activation of muscle by the nervous system. Furthermore, the insufficiency of the activation can be exacerbated by such conditions as a decline in the levels of blood glucose and exercise in hot environments (Nybo & Nielsen, 2001; Nybo, 2003; Todd et al. 2005).
Figure 3. Decreases in MVC torque and voluntary activation during a fatiguing contraction.
A, decrease in MVC torque after three sets of maximal shortening contractions (60 deg s−1 over a 75 deg range of motion) and three matched maximal isometric contractions with the knee extensor muscles. The two tasks were performed on separate days and the isometric contractions were sustained to produce the same relative decrease in MVC torque. The data correspond to mean +s.e.m. for the shortening contractions and isometric contractions for the first, second and third sets of shortening contractions and the three isometric contractions. Adapted with permission from Babault et al. (2006). B, corresponding decreases in voluntary activation as assessed with paired stimuli (10 ms apart) during selected maximal contractions. Initial values for voluntary activation were 88.3 ± 3.0% for the session in which the shortening contractions were performed and 89.4 ± 3.1% in the session for the isometric contractions. Data from Babault et al. (2006).
The final example on the specificity of the impairments examines some of the differences that have been observed across muscles. Since the early observations on the differences in the contractile properties of red and white muscle and the classification of motor units based on fatigability (Buller et al. 1960; Edström & Kugelberg, 1968; Burke et al. 1973), the endurance capacity of muscle, at least in experimental animals with electrically evoked contractions, can depend on the proportion of its muscle fibre types (Cairns et al. 2005). When similar stimulation protocols are applied to motor units in human muscle, however, it is not possible to identify comparable groups of motor units based on differences in contractile properties, including fatigability (Bigland-Ritchie et al. 1998). Furthermore, human muscles are comprised of a mixture of fibre types that blurs potential differences in fatigability attributable to the proportions of the various fibre types in a muscle, and all voluntary actions involve multiple muscles.
Nonetheless, there are other differences between muscles that can influence the adjustments during fatiguing contractions, such as the afferent feedback delivered by group III–IV afferents that are activated by the metabolic by-products of the muscle contraction. One strategy that has been used to identify a contribution by group III–IV afferents to the development of fatigue is to compare the recovery of muscle function when blood flow is normal and when it is impeded. When blood flow is occluded, the metabolites that accumulate in the fatigued muscle will continue to provide a stimulus that sustains the discharge of group III–IV afferents (Hayes et al. 2006). With this approach, Bigland-Ritchie et al. (1986a) found that the depression in discharge rate of motor units in biceps brachii after a sustained MVC did not recover during the 3 min when blood flow was occluded but did recover to control values within 3 min when blood flow was not occluded. Such results and those of Duchateau & Hainaut (1993) suggest that a peripheral reflex mediated by group III–IV afferents from the fatigued muscle contributed to the decrease in discharge rate during this type of exercise. In contrast, others found that feedback delivered by afferents that are sensitive to an occlusion of blood flow did not contribute to the decrease in voluntary activation after a 2 min MVC with the elbow flexor muscles (Taylor et al. 2000; Butler et al. 2003).
Subsequent studies, however, have demonstrated that the contribution of group III–IV afferents to the development of fatigue can vary across muscles. Martin et al. (2006) examined the contribution of feedback by group III–IV afferents to the fatigue experienced during sustained 2 min MVCs with the elbow flexor and extensor muscles. The protocols involved comparing the amplitude of potentials evoked in muscle with transmastoid stimulation of the corticospinal tract to assess the excitability of motor neurones during MVCs and during recovery when blood flow to the muscles was occluded and when it was intact. The influence of these afferents on motor neurone excitability was estimated by comparing the amplitude of the evoked potentials in the presence and absence of ischaemia that altered the feedback delivered by group III–IV afferents. When the fatiguing contraction was performed with the triceps brachii muscle, the amplitude of the evoked response decreased during the MVC and remained depressed while blood flow was occluded, but recovered within 15 s with the restoration of blood flow. The amplitude of the potentials evoked in triceps brachii also decreased after the fatiguing contraction was performed with the elbow flexor muscles. In contrast, the amplitude of the potentials evoked in biceps brachii increased after a fatiguing contraction with triceps brachii. These results indicate that group III–IV afferents depressed the excitability of triceps brachii motor neurones, but facilitated those that innervate biceps brachii. Given the known differences in central connections between muscles that can exert different actions (Pierrot-Deseilligny & Burke, 2005), it seems likely that fatiguing contractions can involve other differences in adjustments between motor neurone pools.
These four examples underscore the specificity of the impairments that contribute to the development of muscle fatigue and thereby make it impossible to identify a single causal mechanism for muscle fatigue. Furthermore, the extensive literature on the specificity of the impairments demonstrates that it is possible to impair any of the physiological processes involved in the activation of the contractile proteins and the force that they can produce. However, relatively little is known about the functional relevance of these impairments and the conditions that can trigger a change in the prevailing mechanisms.
Identifying rate-limiting impairments
How does fatigue influence muscle function? By definition, a muscle begins to experience fatigue as soon as its maximal force or power capacity starts to decline. When the task involves sustaining a maximal contraction, the decline in performance parallels the increase in fatigue. When the task requires a submaximal contraction, however, the onset of fatigue is probably not associated with the termination of the task. As most activities of daily living involve submaximal forces, the onset of fatigue may not limit the ability of an individual to perform a task and, furthermore, task failure may not be caused by fatigue of the principal muscles involved in the task.
Primary muscles involved in the task
The association between task failure and muscle fatigue has been studied in protocols that required subjects to inspire against various resistive loads. The force capacity of the inspiratory muscles can be estimated from the maximal inspiratory pressure an individual can generate, which McKenzie et al. (1997) measured as subjects inspired for 20 min against resistive loads that ranged from 35 to 90% of maximal inspiratory pressure. Task failure occurred when subjects came off the mouthpiece or when they could not reach the target pressure throughout inspiration for three consecutive breaths. All subjects experienced task failure before 20 min with the high loads (75–90%). Task failure was associated with the development of hypercapnia and dyspnoea (hunger for air) and not either muscle fatigue or an impairment of voluntary activation. Gorman et al. (1999) used a similar protocol with a load that was 80% of maximal inspiratory pressure and defined task failure as an intolerable discomfort of breathing. The duration that breathing could be sustained was related to the accumulation of CO2 and not fatigue of the inspiratory muscles. Similarly, although Rohrbach et al. (2003) could detect fatigue in the diaphragm and rib cage muscles when subjects inspired against a load that was 67% of maximal inspiratory pressure, the duration that breathing could be sustained was not related to the amount of fatigue in these muscles, which suggested that such factors as the accumulation of CO2 and a decrease in arterial oxygen saturation probably caused task failure.
Task failure has also been studied in limb muscles. One approach has been to compare the performance of two similar tasks and to identify the adjustments that limit the duration of the more difficult task (Hunter et al. 2004c; Maluf & Enoka, 2005). In one task, referred to as the force task, the limb was attached to a restraint and the subject was required to maintain a constant force for as long as possible while viewing force feedback on a monitor. The other task, referred to as the position task, required the subject to support an inertial load that was equivalent to the force exerted during the force task, and to maintain a constant joint angle for as long as possible while viewing position feedback on a monitor. Although both tasks involve sustained isometric contractions and required the same net muscle torque for each subject, stretch reflexes evoked during such tasks suggest that the neural strategy underlying the same motor output differs (Doemges & Rack, 1992a,b; Dietz et al. 1994). Furthermore, the recruitment thresholds of motor units in biceps brachii differ for tasks that involve maintaining a position or exerting a force (Tax et al. 1989).
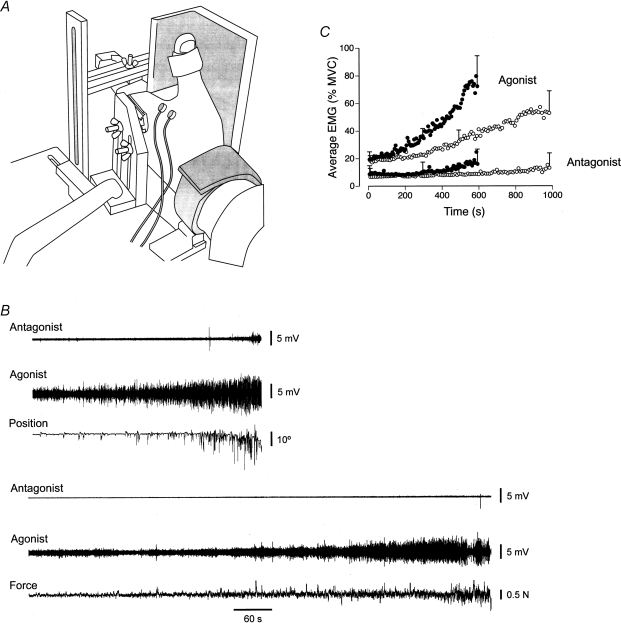
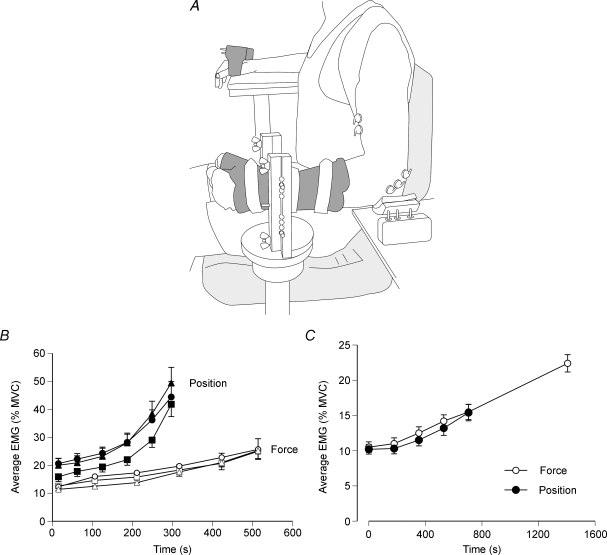
Maluf et al. (2005) compared the performance of the first dorsal interosseus muscle on the force and position tasks when the hand was placed in the position shown in Fig. 4A. The extended index finger exerted an abduction force (20% of maximum) against a force transducer for the force task and supported an inertial load while maintaining a constant angle at the metacarpophalangeal joint for the position task. The force task was terminated when the subject was unable to maintain the abduction force within 1.5% of the target force, whereas the position task was ended when the finger could not be held within 10 deg of the target angle. The fluctuations in the abduction force and the joint angle during the two tasks are shown in Fig. 4B. The duration that the position task could be sustained (593 ± 212 s) was substantially less than that for the force task (983 ± 328 s). The briefer duration for the position task was associated with a more rapid increase in the amplitude of the surface EMG for first dorsal interosseus (Fig. 4B and C). Due to minimal EMG activity in the antagonist muscle (second palmar interosseus), the greater rate of increase in EMG amplitude for the agonist suggested a more rapid recruitment of the motor unit pool during the position task, even though the net muscle torque was similar for the two tasks. To examine this possibility, Maluf et al. (2005) compared the two tasks at a target force that corresponded to the upper limit of motor unit recruitment for first dorsal interosseus (60% MVC force). Consistent with the explanation that the position task involved a more rapid recruitment of motor units when the target force was 20% of MVC force, there was no difference in the time to failure for the force (93 ± 41 s) and position tasks (86 ± 31 s) when all motor units were recruited at the onset of each contraction.
Figure 4. Characteristics of the force and position tasks performed to failure.
A, the two tasks were performed with the first interosseus muscle with the hand in the posture shown. The index finger pushed up against a rigid restraint for the force task and supported an inertial load for the position task. B, representative data for the position (top) and force (bottom) tasks as performed by one individual. For each task, the data comprise the EMG for the antagonist (second palmar interosseus) and agonist (first dorsal interosseus) muscles, and either the joint angle (position task) or the abduction force (force task). Adapted with permission from Maluf et al. (2005). C, group data (n = 20) for EMG amplitude for the agonist and antagonist muscles during the force and position tasks. EMG amplitude was calculated for each 1% interval of the time to failure and averaged across subjects. 95% confidence intervals are shown for the first, middle and last data point of each condition. Adapted from Maluf et al. (2005).
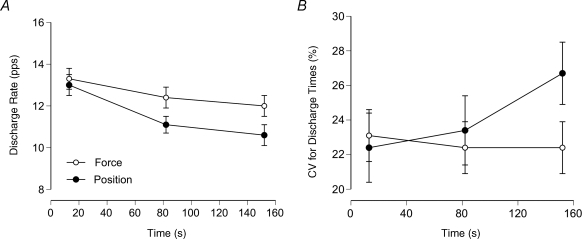
Motor unit recordings confirmed this conclusion. Mottram et al. (2005) recorded the discharge of the same motor unit (n = 32) when subjects performed the two tasks with the same load (∼20% MVC force) for 161 ± 96 s. The contractions with the elbow flexor muscles were not performed to task failure so that it was possible to record the discharge of the same motor unit in a single experimental session. The two tasks were performed in a randomised order and sufficient rest was given so that there was no statistical difference in MVC force at the start of each task. The average discharge rate (13.1 pps) and coefficient of variation for interspike interval (22.8%) were similar at the beginning of the two contractions, but discharge rate decreased more and the coefficient of variation for discharge times increased more during the position task (Fig. 5). The greater increase in the coefficient of variation during the position task suggests a more rapid rise in synaptic noise during this task (Stein et al. 2005). Despite the decline in discharge rate, the synaptic input to the motor neurone pool increased during the two contractions and recruited additional units. The subcutaneous recordings detected the recruitment of the same 26 units during the two tasks, but another 6 units that were recruited only during the force task and another 20 units that were activated only during the position tasks. The enhanced recruitment during the position task was accompanied by more rapid increases in both the perceived exertion and mean arterial pressure. Even though each subject exerted the same net muscle torque during the two tasks, the motor output was sustained by different motor unit activity.
Figure 5. Discharge characteristics of single motor units in biceps brachii during the force and position tasks.
A, mean ± s.e.m. discharge rate at the beginning, middle and end of the force and position tasks for the 32 motor units. The decrease in mean discharge rate was significantly greater during the position task (13.1–10.6 pps) than during the force task (13.3–12.0 pps). B, the coefficient of variation for interspike interval did not change from the beginning (23.1 ± 7.8%) to the end (22.4 ± 8.6%) of the force task, whereas it increased significantly during the position task (22.4 ± 10.4 to 26.7 ± 9.2%). Data from Mottram et al. (2005).
Insight into the rate-limiting mechanisms can be obtained by comparing performances across individuals and with practice of the two tasks. The duration that the force and position tasks can be sustained at the same relative intensity varies widely among individuals. For example, the time to failure for the elbow flexor muscles when performing the position task while supporting a load equivalent to 20% MVC force (17.3 ± 3.7 Nm) ranged from 176 to 1411 s (567 ± 353 s) for 20 subjects (Rudroff et al. 2007b). Based on a multiple regression analysis, 78% of the variability in the time to failure could be explained by three variables: the frequency of EMG bursts for the long head of biceps brachii during the first 20% of the contraction, the amplitude of the EMG bursts for brachioradialis during the first 20% of the contraction and the target torque. Individuals with longer times to failure had greater EMG burst frequencies for the long head of biceps brachii, lower EMG burst amplitudes for brachioradialis and lower target torques. As bursts of activity in the surface EMG correspond to the transient recruitment of higher threshold motor units, individuals with longer times to failure exerted a lower muscle force and transiently recruited higher threshold motor units more frequently in one primary muscle (long head of biceps brachii) and intermittently recruited units with smaller amplitude action potentials in the surface EMG of another primary muscle (brachioradialis).
Consistent with this result, Hunter & Enoka (2003) previously found that the capacity to alter the bursts of EMG activity enabled some subjects to prolong the time to failure with practice. Subjects performed the force task with the elbow flexor muscles at a target force of 20% MVC force on three occasions with at least 2 weeks between each performance. A cluster analysis classified the subjects into two groups: those who increased time to failure (responders) and those who did not (non-responders). The responders increased time to failure from session 1 to session 3 by > 15% (60 ± 28%; range: 25–115%), whereas the non-responders did not experience any change in the time to failure across sessions (−3 ± 11%; range: −12–15%). MVC force was similar for the responders (289 ± 117 N) and non-responders (290 ± 114 N) and decreased by a similar amount (31.3 ± 16.6 and 35.9 ± 18.1%, respectively) at task failure. The most significant adaptation associated with the prolongation of the time to failure was a delay in the onset of the bursts of activity in the surface EMG for the elbow flexor muscles. Thus, some individuals were able to improve performance by delaying the transient recruitment of higher threshold motor units. As the net muscle torque was the same across the three sessions, varying the number of activated motor units influences the relative force contributed by the involved muscle fibres. The increase in the time to failure exhibited by the responders therefore indicates that task failure in session 1 was not attributable to a decline in the force capacity of the activated muscle fibres, but rather was a consequence of the strategy used to perform the task.
Synergist and accessory muscles
Although activity in postural muscles can influence the duration a task can be sustained (Le Bozec & Bouisset, 2004), few studies have examined the influence of synergist and accessory muscles on the performance of fatiguing contractions (Caffier et al. 1992; Sacco et al. 1997). When the force and position tasks were performed with the elbow flexor muscles with the arm in the position shown in Fig. 6A, the ratio of the times to failure for the two tasks at the same relative intensity (20% MVC force) differed from that observed for the hand muscle. The time to failure for the position task (702 ± 582 s) was 51 ± 26% of the duration for the force task (1402 ± 728 s) for the elbow flexor muscles, compared with 63 ± 28% for first dorsal interosseus. Subjects reported that performance of the position task with the elbow flexor muscles when the arm was abducted from the trunk by about 0.4 rad (Fig. 6A) imposed an additional demand on shoulder muscles that was not experienced during the force task. The lesser ratio for the elbow flexor muscles with the arm in this posture indicates the significant influence that postural activity can have on limiting task performance. Accordingly, Rudroff et al. (2007a) found that the rate of increase in the amplitude of the EMG activity for selected shoulder and trunk muscles was much greater during the position task compared with the force task (Fig. 6B).
Figure 6. EMG activity of the elbow flexor muscles during the force and position tasks.
A, the two tasks were performed with the elbow joint at a right angle and the upper arm abducted from the trunk by about 0.4 rad (23 deg). B, the average rectified EMG (mean ± s.e.m.) for infraspinatus (circles), supraspinatus (squares) and teres minor (triangles) during the force and position tasks. The target force was 20% MVC force. The EMGs were normalized to the peak values observed during the MVCs and averaged over 30 s epochs at 20% intervals during each task. Adapted with permission from Rudroff et al. (2007a). C, rectified EMG averaged over 30 s epochs at 25% intervals for the duration of the position task and at the end of the force task across the biceps brachii, brachialis and brachioradialis muscles during the two tasks. The target force was 15% MVC force. The EMGs were normalized to the peak values observed during the MVCs. Data from Hunter et al. (2002).
The relative times to failure for the force and position tasks when performed with the elbow flexor muscles are also influenced by the orientation of the forearm. When the arm was rotated forward so that the upper arm was horizontal and not abducted from the trunk, there was no difference in the time to failure for the force (476 ± 246 s) and position tasks (468 ± 270 s) when the forearm was in a neutral position, whereas the position task (477 ± 276 s) was briefer than the force task (609 ± 250 s) when the forearm was supinated (Rudroff et al. 2005, 2007a). Despite the influence of limb posture on the relative times to failure for the two tasks, the rate of increase in the amplitude of the EMG activity was similar for the two tasks in both postures (Fig. 6C). In contrast to the more rapid increase in EMG amplitude during the position task for first dorsal interosseus (Fig. 4B), the comparable rates of increase in EMG amplitude for the elbow flexor muscles suggest that the briefer times to failure for the position task are due to an inability to increase the EMG as much as during the force task (Fig. 6C). As the activity of motor units in biceps brachii does differ when the arm posture facilitates a briefer duration for the position task (Mottram et al. 2005), another mechanism appears to constrain the increase in EMG activity during the position task. A likely candidate is the reflex connections between the elbow flexor muscles. Although these muscles all contribute to the flexor torque, they produce opposing actions about the pronation–supination axis of the forearm (Zhang et al. 1998) and there are inhibitory pathways between some of them (Naito et al. 1996, 1998).
Potential mechanisms limiting the position task
The strategy used by the nervous system to perform the force and position tasks probably involves heightened activation of the stretch reflex pathway during the position task. The amplitude of the stretch reflex, for example, is enhanced when a limb acts against a compliant load compared with a rigid restraint (Akazawa et al. 1983; De Serres et al. 2002). An increase in the stretch reflex response suggests that the task involves greater net excitation from spinal and supraspinal sources, which results in the earlier recruitment of the motor unit pool and thereby premature task failure (Maluf et al. 2005; Mottram et al. 2005). Although the more rapid recruitment of the motor unit pool can be explained by an increase in the descending drive to the motor neurone pool (Löscher et al. 1996), the greater decrease in mean discharge rate and increase in discharge variability during the position task suggests the involvement of other mechanisms also (Mottram et al. 2005). As these effects were observed in the same motor unit during the two tasks, the different adjustments can probably be attributed to variation in peripheral synaptic input to the motor neurones rather than to changes in the intrinsic properties of the motor neurones.
Accordingly, the amplitude of the Hoffmann reflex in biceps brachii decreased sooner during the position task compared with the force task, whereas the amplitude of the compound muscle potential evoked in biceps brachii by transcranial magnetic stimulation increased at a similar rate during the two tasks (Duchateau et al. 2007). These observations suggest that the difference in the synaptic input received by the motor neurone pool probably involved presynaptic rather than postsynaptic mechanisms (Maluf et al. 2007). This interpretation is consistent with the observation that the application of muscle vibration during a sustained fatiguing contraction, an intervention that enhances the excitatory input from Ia afferents to the motor neurone pool, can transiently restore the discharge rate of motor units (Bongiovanni & Hagbarth, 1990; Griffin et al. 2001). Taken together, these data suggest that the duration of the position task may be limited by spinal mechanisms, presumably due to a reduction in the peripheral excitatory input to the motor neurone pool.
Conclusion
The study of task failure affords a strategy to identify the functional significance of physiological adjustments that occur during fatiguing contractions. The rate-limiting adjustments can be identified by comparing the performance of one group of subjects on two similar tasks, two groups of subjects sustaining the same task, and one group of subjects performing the same task before and after an intervention. Although relatively few studies have adopted this approach, it appears capable of relating the specificity of the impairments that occur during fatiguing contractions to many of the tasks performed during activities of daily living. These studies underscore the significant contributions of synergist, antagonist and postural muscle activity in limiting the performance of many different physical activities.
References
- Aagaard P, Bangsbo J. The muscular system: design, function, and performance relationships. In: Tipton CM, editor. ACSM's Advanced Exercise Physiology. Philadelphia: Lippincott, Williams & Wilkins; 2006. pp. 144–160. [Google Scholar]
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol. 1983;49:16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve. 1995;18:593–600. doi: 10.1002/mus.880180605. [DOI] [PubMed] [Google Scholar]
- Asmussen E. Muscle fatigue. Med Sci Sports. 1979;11:313–321. [PubMed] [Google Scholar]
- Babault N, Desbrosses K, Fabre MS, Michaut A, Pousson M. Neuromuscular fatigue development during maximal concentric and isometric knee extensions. J Appl Physiol. 2006;100:780–785. doi: 10.1152/japplphysiol.00737.2005. [DOI] [PubMed] [Google Scholar]
- Bailey A, Channon S, Beaumont JG. The relationship between subjective and cognitive fatigue in advanced multiple sclerosis. Mult Scler. 2007;13:73–80. doi: 10.1177/1352458506071162. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–526. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Dawson NJ, Johansson RS, Lippold OCJ. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986a;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Fuglevand AJ, Thomas CK. Contractile properties of human motor units: Is man a cat? Neuroscientist. 1998;4:240–249. [Google Scholar]
- Bigland-Ritchie B, Furbush F, Woods JJ. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol. 1986b;61:421–429. doi: 10.1152/jappl.1986.61.2.421. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Rice CL, Garland SJ, Walsh ML. Task-dependent factors in fatigue of human voluntary contractions. In: Gandevia SC, Enoka RM, Stuart DG, Thomas CK, editors. Fatigue: Neural & Muscular Mechanisms. New York: Plenum Press; 1995. pp. 361–380. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- Bongiovanni LG, Hagbarth KE. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J Physiol. 1990;423:1–14. doi: 10.1113/jphysiol.1990.sp018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., III Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffier G, Rehfeldt H, Kramer H, Mucke R. Fatigue during sustained maximal voluntary contraction of different muscles in humans: dependence on fibre type and body posture. Eur J Appl Physiol. 1992;64:237–243. doi: 10.1007/BF00626286. [DOI] [PubMed] [Google Scholar]
- Cairns SP. Lactic acid and exercise performance. Culprit or friend? Sports Med. 2006;36:279–291. doi: 10.2165/00007256-200636040-00001. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Knicker AJ, Thompson MW, Sjøgaard G. Evaluation of models used to study neuromuscular fatigue. Exerc Sport Sci Rev. 2005;33:9–16. [PubMed] [Google Scholar]
- Clark BC, Collier SR, Manini TM, Ploutz-Snyder LL. Sex differences in muscle fatigability and activation patterns of the human quadriceps femoris. Eur J Appl Physiol. 2005;94:196–206. doi: 10.1007/s00421-004-1293-0. [DOI] [PubMed] [Google Scholar]
- Cook DB, O'Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndromes patients and controls. Neuroimage. 2007;36:108–122. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Davis JM, Zhao Z, Stock HS, Mehl KA, Buggy J, Hand GA. Central nervous system effects on caffeine and adenosine on fatigue. Am J Physiol Regul Integr Comp Physiol. 2003;284:R399–R404. doi: 10.1152/ajpregu.00386.2002. [DOI] [PubMed] [Google Scholar]
- de Ruiter CJ, Goudsmit JF, Van Tricht JA, de Haan A. The isometric torque at which knee-extensor muscle reoxygenation stops. Med Sci Sports Exerc. 2007;39:443–453. doi: 10.1249/mss.0b013e31802dd3cc. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Bennett DJ, Stein RB. Stretch reflex gain in cat triceps surae muscles with compliant loads. J Physiol. 2002;545:1027–1040. doi: 10.1113/jphysiol.2002.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephal Clin Neurophysiol. 1994;93:49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Di Giulio C, Daniele F, Tipton CM. Angelo Mosso and muscular fatigue: 116 years after the first congress of physiologists: IUPS Commemoration. Adv Physiol Educ. 2006;30:51–57. doi: 10.1152/advan.00041.2005. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks. J Physiol. 1992a;447:563–573. doi: 10.1113/jphysiol.1992.sp019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol. 1992b;447:575–585. doi: 10.1113/jphysiol.1992.sp019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. J Physiol. 1993;471:787–799. doi: 10.1113/jphysiol.1993.sp019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Klass M, Lévénez M, Enokâ RM. Cortico-spinal excitability differs with load type during a contraction performed to task failure. Proceedings of the IBRO World Congress of Neuroscience Satellite Meeting, Darwin, Australia. 2007:48. [Google Scholar]
- Edgley SA, Winter AP. Different effects of fatiguing exercise on corticospinal and transcallosal excitability in human hand area motor cortex. Exp Brain Res. 2004;159:530–536. doi: 10.1007/s00221-004-1978-y. [DOI] [PubMed] [Google Scholar]
- Edström L, Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiat. 1968;31:424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS, Marsh L, Nail L, Shulman L, Taylor CB. Fatigue in Parkinson's disease: a review. Movement Disorders. 2007;22:297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman RB, McKenzie DK, Gandevia SC. Task failure, breathing discomfort and CO2 accumulation without fatigue during inspiratory resistive loading in humans. Respir Physiol. 1999;115:273–286. doi: 10.1016/s0034-5687(99)00010-9. [DOI] [PubMed] [Google Scholar]
- Griffin L, Garland SJ, Ivanova T, Gossen ER. Muscle vibration sustains motor unit firing rates during submaximal isometric fatigue in humans. J Physiol. 2001;535:929–936. doi: 10.1111/j.1469-7793.2001.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33:267–275. doi: 10.1016/j.jpainsymman.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Hales JP, Gandevia SC. Assessment of maximal voluntary contraction with twitch interpolation: an instrument to measure twitch responses. J Neurosci Meth. 1988;25:97–102. doi: 10.1016/0165-0270(88)90145-8. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29:109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol. 2006;101:1036–1044. doi: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol. 2005;99:890–897. doi: 10.1152/japplphysiol.00243.2005. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol. 2004a;96:195–202. doi: 10.1152/japplphysiol.00893.2003. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004b;96:2125–2132. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Duchateau RM, Enoka RM. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev. 2004c;32:44–49. doi: 10.1097/00003677-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Enoka RM. Changes in muscle activation can prolong the endurance time of a submaximal isometric contraction in humans. J Appl Physiol. 2003;94:108–118. doi: 10.1152/japplphysiol.00635.2002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega J, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol. 2002;88:3087–3096. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- Kallenberg LAC, Schulte E, Disselhorst-Klug C, Hermens HJ. Myoelectric manifestations of fatigue at low contraction levels in subjects with and without chronic pain. J Electromyogr Kinesiol. 2007;17:264–274. doi: 10.1016/j.jelekin.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Stone MH, Ratamess NA, Triplett-McBride T. Amercian College of Sports Medicine Position Stand on progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bozec S, Bouisset S. Do bimanual isometric push efforts in humans stop as a consequence of postural exhaustion? Neurosci Lett. 2004;356:61–65. doi: 10.1016/j.neulet.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Lévénez M, Kotzamanidis C, Carpentier A, Duchateau J. Spinal reflexes and coactivation of ankle muscles during a submaximal fatiguing contraction. J Appl Physiol. 2005;99:1182–1188. doi: 10.1152/japplphysiol.00284.2005. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Kernell D, Meijman TF, Zijdewind I. Motor fatigue and cognitive task performance in humans. J Physiol. 2002;545:313–319. doi: 10.1113/jphysiol.2002.027938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Central fatigue during a long-lasting submaximal contraction of the triceps surae. Exp Brain Res. 1996;108:305–314. doi: 10.1007/BF00228103. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Allen GM, Butler JE, Gandevia SC. Task failure with lack of diaphragm fatigue during inspiratory resistive loading in human subjects. J Appl Physiol. 1997;82:2011–2019. doi: 10.1152/jappl.1997.82.6.2011. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Bigland-Ritchie B, Gorman RB, Gandevia SC. Central and peripheral fatigue of human diaphragm and limb muscles by twitch interpolation. J Physiol. 1992;454:643–656. doi: 10.1113/jphysiol.1992.sp019284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Murray BJ, Rice CL. Differential changes in muscle oxygenation between voluntary and stimulated isometric fatigue of human dorsiflexors. J Appl Physiol. 2006;100:890–895. doi: 10.1152/japplphysiol.00921.2005. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Barry BK, Riley ZA, Enoka RM. Reflex responsiveness of a human hand muscle when controlling isometric force and joint position. Clin Neurophysiol. 2007;118:2063–2071. doi: 10.1016/j.clinph.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluf KS, Enoka RM. Task failure and the fatiguing contractions performed by humans. J Appl Physiol. 2005;99:389–396. doi: 10.1152/japplphysiol.00207.2005. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Shinohara M, Stephenson JL, Enoka RM. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res. 2005;167:165–177. doi: 10.1007/s00221-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JN, Semmler JG, Enoka RM. Motor-unit activtity deffers with load type during a fatigning contraction. J Neurophysiol. 2005;93:1381–1392. doi: 10.1152/jn.00837.2004. [DOI] [PubMed] [Google Scholar]
- Naito A, Shindo M, Miyasaka T, Sun YJ, Momoi H, Chishima M. Inhibitory projection from pronator teres to biceps brachii motoneurones in human. Exp Brain Res. 1998;121:99–102. doi: 10.1007/s002210050441. [DOI] [PubMed] [Google Scholar]
- Naito A, Shindo M, Miyasaka T, Sun YJ, Morita H. Inhibitory projection from brachioradialis to biceps brachii motoneurones in human. Exp Brain Res. 1996;111:483–486. doi: 10.1007/BF00228739. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Gorman RB, Laouris Y, Spielmann JM, Stuart DG. Does motoneuron adaptation contribute to muscle fatigue? Muscle Nerve. 2007;35:135–158. doi: 10.1002/mus.20712. [DOI] [PubMed] [Google Scholar]
- Nybo L. CNS fatigue and prolonged exercise: effect of glucose supplementation. Med Sci Sports Exerc. 2003;35:589–594. doi: 10.1249/01.MSS.0000058433.85789.66. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev. 2007;35:110–118. doi: 10.1097/jes.0b013e3180a031ec. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher NH. Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol. 2004;72:223–261. doi: 10.1016/j.pneurobio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Rohrbach M, Perret C, Kayser B, Boutellier U, Spengler CM. Task failure from inspiratory resistive loaded breathing: a role for inspiratory muscle fatigue? Eur J Appl Physiol. 2003;90:405–410. doi: 10.1007/s00421-003-0871-x. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Barry BK, Stone AL, Barry CJ, Enoka RM. Accessory muscle activity contributes to the variation in time to task failure for different arm postures and loads. J Appl Physiol. 2007a;102:1000–1006. doi: 10.1152/japplphysiol.00564.2006. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Christou EA, Poston B, Bojsen-Møller J, Enoka RM. Time to failure of a sustained contraction is predicted by target torque and initial electromyographic bursts in elbow flexor muscles. Muscle Nerve. 2007b;35:657–666. doi: 10.1002/mus.20752. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Poston B, Shin IS, Bojsen-Møller J, Enoka RM. Net excitation of the motor unit pool varies with load type during fatiguing contractions. Muscle Nerve. 2005;31:78–87. doi: 10.1002/mus.20241. [DOI] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94:2414–2422. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
- Russ DW, Lanza IR, Rothman D, Kent-Braun JA. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve. 2005;32:647–655. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- Sacco P, Newberry R, McFadden L, Brown T, McComas AJ. Depression of human electromyographic activity by fatigue of a synergistic muscle. Muscle Nerve. 1997;20:710–717. doi: 10.1002/(sici)1097-4598(199706)20:6<710::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- St Clair Gibson A, Baden DA, Lambert MI, Lambert V, Harley YXR, Hampson D, Russell VA, Noakes TD. The conscious perception of the sensation of fatigue. Sports Med. 2003;33:167–176. doi: 10.2165/00007256-200333030-00001. [DOI] [PubMed] [Google Scholar]
- Smith JL, Martin PG, Gandevia SC, Taylor JL. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol. 2007;103:560–568. doi: 10.1152/japplphysiol.00220.2007. [DOI] [PubMed] [Google Scholar]
- Søgaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573:511–523. doi: 10.1113/jphysiol.2005.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nature Rev Neurosci. 2005;6:389–397. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Gielen CC, van den Tempel CM. Differences in the activation of m. biceps brachii in the control of slow isotonic movements and isometric contractions. Exp Brain Res. 1989;76:55–63. doi: 10.1007/BF00253623. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE, Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol. 2000;525:793–801. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Butler J, Nishida T, Nuber G, Huang H, Rymer WZ. In vivo determination of the direction of rotation and moment-angle relationship of individual elbow muscles. J Biomech Eng. 1998;120:625–633. doi: 10.1115/1.2834754. [DOI] [PubMed] [Google Scholar]