Abstract
Older (‘Masters’) athletes strive to maintain or even improve upon the performance they achieved at younger ages, but declines in athletic performance are inevitable with ageing. In this review, we describe changes in peak endurance exercise performance with advancing age as well as physiological factors responsible for those changes. Peak endurance performance is maintained until ∼35 years of age, followed by modest decreases until 50–60 years of age, with progressively steeper declines thereafter. Among the three main physiological determinants of endurance exercise performance (i.e. maximal oxygen consumption 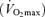 , lactate threshold and exercise economy), a progressive reduction in
, lactate threshold and exercise economy), a progressive reduction in 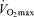 appears to be the primary mechanism associated with declines in endurance performance with age. A reduction in lactate threshold, i.e. the exercise intensity at which blood lactate concentration increases significantly above baseline, also contributes to the reduction in endurance performance with ageing, although this may be secondary to decreases in
appears to be the primary mechanism associated with declines in endurance performance with age. A reduction in lactate threshold, i.e. the exercise intensity at which blood lactate concentration increases significantly above baseline, also contributes to the reduction in endurance performance with ageing, although this may be secondary to decreases in 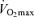 . In contrast, exercise economy (i.e. metabolic cost of sustained submaximal exercise) does not change with age in endurance-trained adults. Decreases in maximal stroke volume, heart rate and arterio-venous O2 difference all appear to contribute to the age-related reductions in
. In contrast, exercise economy (i.e. metabolic cost of sustained submaximal exercise) does not change with age in endurance-trained adults. Decreases in maximal stroke volume, heart rate and arterio-venous O2 difference all appear to contribute to the age-related reductions in 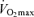 in endurance-trained athletes. Declines in endurance exercise performance and its physiological determinants with ageing appear to be mediated in large part by a reduction in the intensity (velocity) and volume of the exercise that can be performed during training sessions. Given their impressive peak performance capability and physiological function capacity, Masters athletes remain a fascinating model of ‘exceptionally successful ageing’ and therefore are highly deserving of our continued scientific attention as physiologists.
in endurance-trained athletes. Declines in endurance exercise performance and its physiological determinants with ageing appear to be mediated in large part by a reduction in the intensity (velocity) and volume of the exercise that can be performed during training sessions. Given their impressive peak performance capability and physiological function capacity, Masters athletes remain a fascinating model of ‘exceptionally successful ageing’ and therefore are highly deserving of our continued scientific attention as physiologists.
The demographics of age are changing dramatically. According to current population forecasts, the number of elderly will increase worldwide from 6.9% of the population in 2000 to a projected 19.3% by 2050. In parallel with this overall increase in older adults, the number of middle-aged and older (‘Masters’) athletes is expected to increase as well. This is an interesting sub-demographic of adults because many individuals in this group express a highly unique physiological phenotype that could be termed ‘exceptionally successful ageing’. Masters athletes strive to maintain and, in some cases, improve upon the performance they have achieved at younger ages. Indeed, the peak exercise performance of Masters athletes continues to increase each year. However, declines in athletic performance are inevitable and the underlying reasons are important to understand. This raises a number of intriguing questions, including: How does peak exercise performance change with advancing age? What physiological mechanisms and modulating factors are responsible for the age-related decline in exercise performance? Why do these mechanisms and factors change with ageing? The aim of the present review is to address these issues in the context of endurance exercise performance, for which a relatively large body of empirical evidence is available.
Endurance exercise performance with ageing
Peak athletic performance has improved dramatically in the past 100 years, although the age at which peak performance is achieved in Olympic track and field (athletics), swimming, baseball, tennis and golf has remained constant over this period (Schulz & Curnow, 1988). However, as the number of older adults participating in competitive events has increased (at a much greater rate than young adults), and training and nutritional practices have evolved, Masters athletes have achieved impressive improvements in peak exercise performance (Ericsson, 1993). For example, in 2005 Kozo Haraguchi of Japan set a new age-group record in the 100 m dash of 21.69 s at the age of 95. In 2003, Ed Whitlock of Canada became the oldest person to break 3 h in marathon at the age of 73. In some athletic events (e.g. marathon running), Masters athletes over 70 years of age have surpassed the winning time at the first Olympic games held in Athens (Table 1). These exceptional individual athletic achievements are fascinating not only to the general public, but also to those of us who study the effects of ageing on physiological functional capacity. It also highlights the broad question of how endurance exercise performance changes with age in healthy adults.
Table 1.
Comparison of 1896 Olympic winning times in running events and current Masters records that surpass those winning times (from ESPN and World Masters Records)
| Running events | 1896 Olympic winning time (from the first Olympic games in Athens) | Current age-group records that surpass 1896 Olympic times and age at which these records were achieved |
|---|---|---|
| 100 m (s) | 12.0 | 11.7 (61 years) |
| 200 m (s) | 22.2 | 22.1 (46 years) |
| 400 m (s) | 54.2 | 53.9 (63 years) |
| 800 m (min:s) | 2:11.0 | 2:10.4 (60 years) |
| 1500 m (min:s) | 4:33:2 | 4:27:7 (60 years) |
| Marathon (h:min:s) | 2:58:50 | 2:54:5 (73 years) |
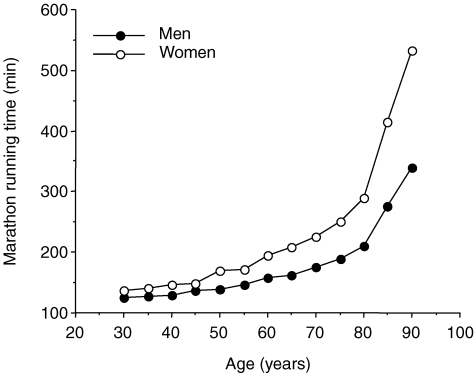
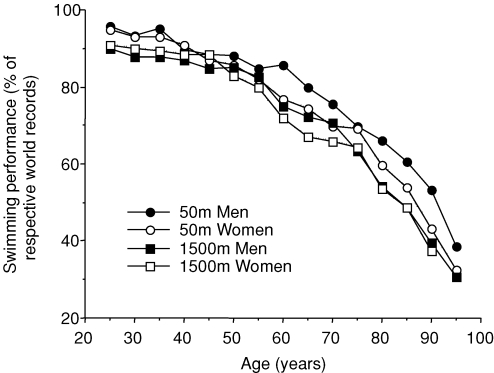
As illustrated in Fig. 1, endurance performance in running events decreases with age in a curvilinear fashion. In general, peak endurance running performance is maintained until ∼35 years of age, followed by modest decreases until 50–60 years of age, with progressively steeper reductions thereafter (Tanaka & Seals, 2003). The pattern appears to be similar for both non-elite and elite endurance athletes (Joyner, 1993). In general, the magnitude of decline in endurance running performance with age is greater in women than in men (Joyner, 1993; Tanaka & Seals, 1997; Donato et al. 2003) (Fig. 1). However, interpretation of this apparent widening of sex differences with advancing age is confounded by the relatively smaller number of female versus male runners in the older groups. Indeed, such increasing sex differences with age are absent in the endurance swimming events, where approximately equal number of men and women compete throughout the age range (Donato et al. 2003) (Fig. 2).
Figure 1.
Changes in men's and women's marathon running times with advancing age (from USA Track & Field and World Masters Athletics).
Figure 2.
Men's and women's swimming performance with advancing age (from Federation Internationale de Natation).
The overall reduction in peak exercise performance with age tends to be greater in endurance than in ‘sprint’ events (Tanaka & Seals, 1997, 2003) (Fig. 2). It is not known why or how the duration of the athletic event influences the age-associated decline in peak performance. One possibility is that endurance and sprint events rely on different energy-producing pathways to sustain muscle activity. For example, findings from several cross-sectional studies indicate that the declines in maximal aerobic exercise capacity with age are considerably greater than those observed in ‘anaerobic’ muscle power (Larsson et al. 1979; Grimby & Saltin, 1983; Tanaka & Seals, 1997). This appears contradictory to the well-established preferential loss of type II muscle fibres (one of the determinants of anaerobic muscle power) with advancing age in healthy non-physically conditioned adults (Larsson et al. 1979; Grimby & Saltin, 1983; Tanaka & Seals, 1997). However, these fibre type changes are relatively small or non-existent in trained athletes (Coggan et al. 1990; Trappe et al. 1995).
Physiological determinants of endurance exercise performance
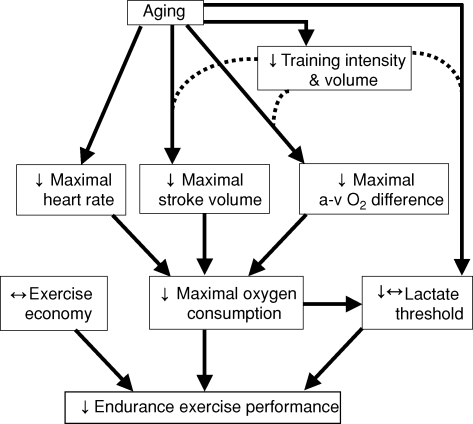
Based largely on studies in young endurance-trained athletes, the three main physiological determinants of endurance performance are believed to be maximal oxygen consumption, exercise economy and the exercise intensity at which a high fraction of the maximal oxygen consumption can be sustained, as typically defined by the ‘lactate threshold’ (Hagberg & Coyle, 1983; Joyner, 1993). In the following section, we review the available information on how changes in these determinants may contribute to age-related declines in endurance exercise performance (Fig. 3).
Figure 3.
Factors and physiological mechanisms contributing to reductions in endurance exercise performance with advancing age in healthy adults.
(a) Exercise economy
Exercise economy is measured as the steady-state oxygen consumption while exercising at a specific submaximal exercise intensity below the lactate threshold (see below). Among endurance athletes, exercise economy is an important determinant of endurance performance (Morgan et al. 1989), particularly in groups that are more homogeneous than heterogeneous in 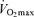 (Morgan et al. 1989). Starting with the findings of the dissertation study of Professor Sid Robinson published in 1938 (Robinson, 1938), cross-sectional observations made by numerous independent laboratories indicate that exercise economy does not change with advancing age (Astrand, 1960; Allen et al. 1985; Wells et al. 1992; Evans et al. 1995). For example, in a cross-sectional study of male endurance runners, there was no difference in running economy between young and older athletes (Allen et al. 1985). Similarly, no significant association was found between running economy and age among highly trained and competitive female runners aged 35–70 years (Wells et al. 1992). We found that in healthy female athletes, running economy explained little of the variance in age-related decreases in endurance running performance after accounting for differences in
(Morgan et al. 1989). Starting with the findings of the dissertation study of Professor Sid Robinson published in 1938 (Robinson, 1938), cross-sectional observations made by numerous independent laboratories indicate that exercise economy does not change with advancing age (Astrand, 1960; Allen et al. 1985; Wells et al. 1992; Evans et al. 1995). For example, in a cross-sectional study of male endurance runners, there was no difference in running economy between young and older athletes (Allen et al. 1985). Similarly, no significant association was found between running economy and age among highly trained and competitive female runners aged 35–70 years (Wells et al. 1992). We found that in healthy female athletes, running economy explained little of the variance in age-related decreases in endurance running performance after accounting for differences in 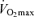 and lactate threshold, which explained 85% of the variability in 10K performance (Evans et al. 1995). The results of several longitudinal studies have confirmed that running economy does not change with age in endurance-trained Masters athletes (Robinson et al. 1976; Trappe et al. 1996). Thus, available data in this area indicate that reductions in exercise economy do not contribute significantly to the decreases in endurance exercise performance observed with advancing age.
and lactate threshold, which explained 85% of the variability in 10K performance (Evans et al. 1995). The results of several longitudinal studies have confirmed that running economy does not change with age in endurance-trained Masters athletes (Robinson et al. 1976; Trappe et al. 1996). Thus, available data in this area indicate that reductions in exercise economy do not contribute significantly to the decreases in endurance exercise performance observed with advancing age.
There are a number of physiological factors that determine exercise economy. Among them, the percentage of type I muscle fibres is positively associated with exercise economy in cyclists (Horowitz et al. 1994). In this context, well-trained Masters athletes have a similar muscle fibre distribution to performance-matched younger runners (Coggan et al. 1990). Consistent with this, a 20 year longitudinal study showed that with maintenance of strenuous endurance training, muscle fibre type distribution did not change with age in highly trained Masters athletes (Trappe et al. 1995). Therefore, maintenance of muscle fibre type with ageing could contribute to the preserved exercise economy of Masters athletes.
(b) Lactate threshold
The ability to sustain a high fraction of one's maximal oxygen consumption during submaximal exercise typically is evaluated using the blood lactate threshold. Numerous criteria, techniques and nomenclature for the lactate threshold have been used in the past. It is generally defined as the exercise intensity at which blood lactate concentration increases significantly above baseline (Allen et al. 1985; Coyle, 1995). In young endurance-trained adults, the lactate threshold predicts exercise performance in distance events ranging from 2 miles to the marathon (Hagberg & Coyle, 1983; Allen et al. 1985; Joyner, 1993), whereas power output at the lactate threshold is the best laboratory predictor of time-trial performance among competitive female Masters cyclists (Nichols et al. 1997). Absolute work rate or running speed at lactate threshold declines with advancing age in endurance athletes (Iwaoka et al. 1988; Maffulli et al. 1994; Evans et al. 1995; Wiswell et al. 2000). However, lactate threshold does not appear to change with increasing age when expressed relative to the percentage of 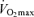 (Iwaoka et al. 1988; Maffulli et al. 1994; Evans et al. 1995). The latter finding suggests that the contribution of decreases in lactate threshold to reductions in endurance exercise performance with ageing may be secondary to decreases in
(Iwaoka et al. 1988; Maffulli et al. 1994; Evans et al. 1995). The latter finding suggests that the contribution of decreases in lactate threshold to reductions in endurance exercise performance with ageing may be secondary to decreases in 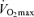 . Indeed, a recent longitudinal study of 51 male and 23 female Masters runners reported that the change in lactate threshold over a mean follow-up period of 6 years was not predictive of a corresponding change in running performance when it was expressed as a percent of
. Indeed, a recent longitudinal study of 51 male and 23 female Masters runners reported that the change in lactate threshold over a mean follow-up period of 6 years was not predictive of a corresponding change in running performance when it was expressed as a percent of 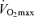 (Marcell et al. 2003).
(Marcell et al. 2003).
(c) Maximal aerobic capacity
Maximal oxygen consumption establishes the upper limit of maximal energy production through oxidative phosphorylation and is generally considered to be a primary determinant of endurance exercise performance among young endurance-trained athletes (Joyner, 1993; Coyle, 1995). 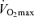 declines approximately 10% per decade after age 25–30 years in healthy sedentary adults of both sexes (Heath et al. 1981; Buskirk & Hodgson, 1987; FitzGerald et al. 1997; Tanaka et al. 1997; Eskurza et al. 2002; Pimentel et al. 2003). Early investigations suggested that the rate of decline in
declines approximately 10% per decade after age 25–30 years in healthy sedentary adults of both sexes (Heath et al. 1981; Buskirk & Hodgson, 1987; FitzGerald et al. 1997; Tanaka et al. 1997; Eskurza et al. 2002; Pimentel et al. 2003). Early investigations suggested that the rate of decline in 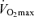 with advancing age was as much as 50% smaller in endurance exercise-trained athletes than in sedentary adults (Heath et al. 1981; Kasch et al. 1990). However, we and others subsequently established that when expressed as per cent decrease from early adulthood, the rate of decline in
with advancing age was as much as 50% smaller in endurance exercise-trained athletes than in sedentary adults (Heath et al. 1981; Kasch et al. 1990). However, we and others subsequently established that when expressed as per cent decrease from early adulthood, the rate of decline in 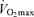 with age is not reduced in healthy adults who habitually perform aerobic exercise (Hodgson & Buskirk, 1977; FitzGerald et al. 1997; Tanaka et al. 1997; Wilson & Tanaka, 2000; Eskurza et al. 2002; Pimentel et al. 2003; Fleg et al. 2005). In fact, endurance exercise-trained men and women demonstrate greater ‘absolute’ (ml kg−1 min−1) rates of decline in
with age is not reduced in healthy adults who habitually perform aerobic exercise (Hodgson & Buskirk, 1977; FitzGerald et al. 1997; Tanaka et al. 1997; Wilson & Tanaka, 2000; Eskurza et al. 2002; Pimentel et al. 2003; Fleg et al. 2005). In fact, endurance exercise-trained men and women demonstrate greater ‘absolute’ (ml kg−1 min−1) rates of decline in 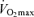 with age than healthy sedentary adults (FitzGerald et al. 1997; Tanaka et al. 1997; Eskurza et al. 2002; Pimentel et al. 2003), probably as a result of greater baseline levels of
with age than healthy sedentary adults (FitzGerald et al. 1997; Tanaka et al. 1997; Eskurza et al. 2002; Pimentel et al. 2003), probably as a result of greater baseline levels of 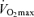 as young adults and greater reductions in habitual exercise with ageing compared with sedentary adults (FitzGerald et al. 1997; Eskurza et al. 2002). It is interesting to note that the greater absolute rate of decline in
as young adults and greater reductions in habitual exercise with ageing compared with sedentary adults (FitzGerald et al. 1997; Eskurza et al. 2002). It is interesting to note that the greater absolute rate of decline in 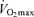 with age in endurance-trained vs sedentary subjects also been observed in rats (Zimmerman et al. 1993) and has been reviewed in detail elsewhere (Tanaka & Seals, 2003).
with age in endurance-trained vs sedentary subjects also been observed in rats (Zimmerman et al. 1993) and has been reviewed in detail elsewhere (Tanaka & Seals, 2003).
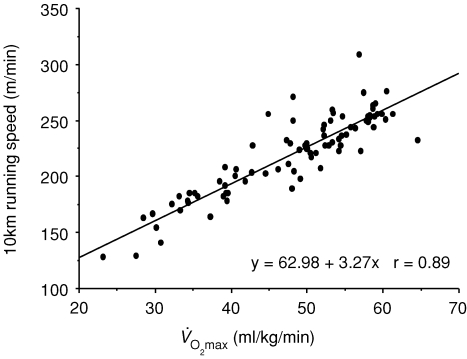
Endurance performance and 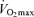 are strongly and positively related in groups of highly trained and competitive distance runners varying in age (Fig. 4). Moreover, reductions in endurance exercise performance with age are closely associated with corresponding decreases in
are strongly and positively related in groups of highly trained and competitive distance runners varying in age (Fig. 4). Moreover, reductions in endurance exercise performance with age are closely associated with corresponding decreases in 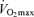 (Fuchi et al. 1989; Marcell et al. 2003). Indeed,
(Fuchi et al. 1989; Marcell et al. 2003). Indeed, 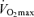 was the best predictor of age-related changes in endurance exercise performance in 51 male and 23 female Masters runners who were followed longitudinally for a period of 6 years (Marcell et al. 2003). Thus, a progressive reduction in
was the best predictor of age-related changes in endurance exercise performance in 51 male and 23 female Masters runners who were followed longitudinally for a period of 6 years (Marcell et al. 2003). Thus, a progressive reduction in 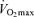 appears to be a key physiological mechanism associated with declines in endurance performance with advancing age.
appears to be a key physiological mechanism associated with declines in endurance performance with advancing age.
Figure 4.
Relation between 10 km running speed and maximal oxygen consumption 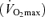 among competitive female distance runners varying widely in age (H. Tanaka and D. R. Seals, unpublished observations).
among competitive female distance runners varying widely in age (H. Tanaka and D. R. Seals, unpublished observations).
In spite of the strong association between 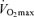 and endurance exercise performance, other factors may contribute to age-associated reductions in endurance performance. Although the comparison is not straightforward because of different scaling and units, the fact that the rate of decline in endurance performance with age appears to be smaller than the corresponding fall in
and endurance exercise performance, other factors may contribute to age-associated reductions in endurance performance. Although the comparison is not straightforward because of different scaling and units, the fact that the rate of decline in endurance performance with age appears to be smaller than the corresponding fall in 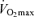 (Joyner, 1993) is consistent with this idea. It may be that other putative determinants of endurance performance (e.g. ‘anaerobic’ muscular power, muscle capillary density) may decline to a lesser extent with advancing age (Grimby & Saltin, 1983; Coggan et al. 1992; Coyle, 1995), thus offsetting the effects of the decrease in
(Joyner, 1993) is consistent with this idea. It may be that other putative determinants of endurance performance (e.g. ‘anaerobic’ muscular power, muscle capillary density) may decline to a lesser extent with advancing age (Grimby & Saltin, 1983; Coggan et al. 1992; Coyle, 1995), thus offsetting the effects of the decrease in 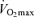 . Furthermore, reductions in endurance performance with age are typically curvilinear, whereas the decrease in
. Furthermore, reductions in endurance performance with age are typically curvilinear, whereas the decrease in 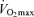 typically is presented as a linear function of age. On the other hand, recent evidence indicates that the rate of decline in
typically is presented as a linear function of age. On the other hand, recent evidence indicates that the rate of decline in 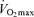 over the entire adult age range actually may be curvilinear, rather than linear, with the rate accelerating with advancing age (Fleg et al. 2005). This concept, originally described by Buskirk & Hodgson in 1987 (Buskirk & Hodgson, 1987), would be more consistent with the notion that a decrease in maximal oxygen consumption is the primary mechanism causing age-related reductions in endurance exercise performance.
over the entire adult age range actually may be curvilinear, rather than linear, with the rate accelerating with advancing age (Fleg et al. 2005). This concept, originally described by Buskirk & Hodgson in 1987 (Buskirk & Hodgson, 1987), would be more consistent with the notion that a decrease in maximal oxygen consumption is the primary mechanism causing age-related reductions in endurance exercise performance.
Declines in the exercise training ‘stimulus’ with advancing age
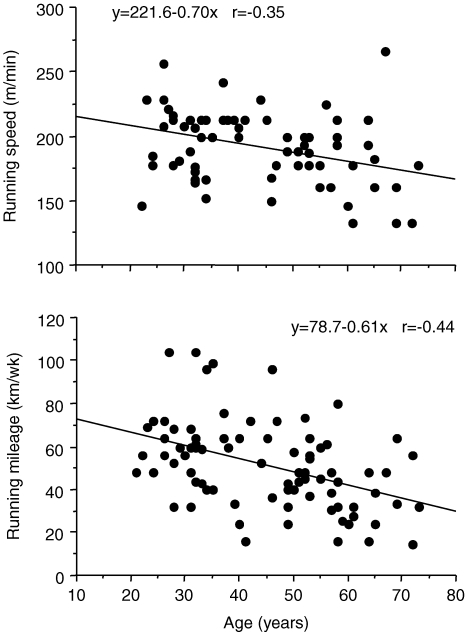
The factors that contribute to reductions in 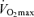 with age in Masters endurance athletes are incompletely understood. Available evidence points to the seemingly inevitable consequence of an overall reduction in the exercise training ‘stimulus’ (i.e. exercise-training intensity, session duration and weekly frequency) with advancing age (Pollock et al. 1997; Tanaka et al. 1997; McGuire et al. 2001; Eskurza et al. 2002) (Fig. 5). As early as 1967, Dill et al. suggested that highly trained distance runners who become sedentary exhibit a greater than normal decrease in maximal aerobic capacity with advancing age (Dill et al. 1967). Similarly, the results of longitudinal studies suggest that
with age in Masters endurance athletes are incompletely understood. Available evidence points to the seemingly inevitable consequence of an overall reduction in the exercise training ‘stimulus’ (i.e. exercise-training intensity, session duration and weekly frequency) with advancing age (Pollock et al. 1997; Tanaka et al. 1997; McGuire et al. 2001; Eskurza et al. 2002) (Fig. 5). As early as 1967, Dill et al. suggested that highly trained distance runners who become sedentary exhibit a greater than normal decrease in maximal aerobic capacity with advancing age (Dill et al. 1967). Similarly, the results of longitudinal studies suggest that 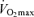 can be fairly well maintained over phases of middle-age lasting up to 10 years in men and women who continue to train vigorously (Kasch & Wallace, 1976; Pollock et al. 1987). However, there is no evidence that exercise training intensity and volume (and
can be fairly well maintained over phases of middle-age lasting up to 10 years in men and women who continue to train vigorously (Kasch & Wallace, 1976; Pollock et al. 1987). However, there is no evidence that exercise training intensity and volume (and 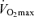 ) can be maintained for longer periods, especially at older ages (Dill et al. 1967; Pollock et al. 1997). Increases in job- and family-related responsibilities may impinge on the availability of time and energy for the intensive training required to remain competitive. Increased prevalence of exercise training-associated injuries among Masters athletes also probably contributes to their reduced training intensity and volume (Kallinen & Markku, 1995). Moreover, the motivation to train may be reduced with advancing age among Masters athletes similar to the declines in compliance observed in older patients participating in cardiac rehabilitation programs (Cooper et al. 2002). The goals underlying the motivation to train also may shift somewhat from achieving personal records in younger athletes to health benefits in older athletes (Ogles & Masters, 2000); the latter would probably accommodate reductions in exercise intensity with age. The ‘intrinsic drive’ to exercise or be physically active may decline with ageing as well, as rodents given lifelong access to running wheels demonstrate marked reductions in noctural running behaviour with advancing age (Valentinuzzi et al. 1997). In summary, it appears that at least in healthy adults, the ability to maintain the overall exercise-training stimulus contributes to the rate of decline in
) can be maintained for longer periods, especially at older ages (Dill et al. 1967; Pollock et al. 1997). Increases in job- and family-related responsibilities may impinge on the availability of time and energy for the intensive training required to remain competitive. Increased prevalence of exercise training-associated injuries among Masters athletes also probably contributes to their reduced training intensity and volume (Kallinen & Markku, 1995). Moreover, the motivation to train may be reduced with advancing age among Masters athletes similar to the declines in compliance observed in older patients participating in cardiac rehabilitation programs (Cooper et al. 2002). The goals underlying the motivation to train also may shift somewhat from achieving personal records in younger athletes to health benefits in older athletes (Ogles & Masters, 2000); the latter would probably accommodate reductions in exercise intensity with age. The ‘intrinsic drive’ to exercise or be physically active may decline with ageing as well, as rodents given lifelong access to running wheels demonstrate marked reductions in noctural running behaviour with advancing age (Valentinuzzi et al. 1997). In summary, it appears that at least in healthy adults, the ability to maintain the overall exercise-training stimulus contributes to the rate of decline in 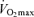 and therefore endurance exercise performance with advancing age.
and therefore endurance exercise performance with advancing age.
Figure 5.
Progressive declines in running (training) mileage and speed with age in female endurance-trained runners. (Modified from Tanaka et al. (1997); used with permission).
Physiological mechanisms responsible for age-related declines in 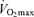 in the endurance-trained athletes
in the endurance-trained athletes
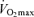 has an exact physiological definition that is expressed by the Fick equation: maximal cardiac output × maximal arterio-venous O2 difference. Within this physiological context, there is some controversy regarding the mechanisms responsible for the reduction in
has an exact physiological definition that is expressed by the Fick equation: maximal cardiac output × maximal arterio-venous O2 difference. Within this physiological context, there is some controversy regarding the mechanisms responsible for the reduction in 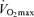 with age in endurance athletes. In particular, the exact contribution of central (i.e. cardiac) and peripheral (i.e. oxygen extraction) factors to the reduced
with age in endurance athletes. In particular, the exact contribution of central (i.e. cardiac) and peripheral (i.e. oxygen extraction) factors to the reduced 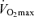 in Masters compared with young adult endurance athletes is unclear. However, it appears that decreases in both maximal cardiac output and maximal arterio-venous O2 difference may play a role (Table 2 and Fig. 3). Attempts to determine the effects of ageing on the Fick determinants of
in Masters compared with young adult endurance athletes is unclear. However, it appears that decreases in both maximal cardiac output and maximal arterio-venous O2 difference may play a role (Table 2 and Fig. 3). Attempts to determine the effects of ageing on the Fick determinants of 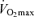 in endurance athletes have relied almost exclusively on cross-sectional studies comparing young and Masters endurance-trained athletes. Longitudinal studies clearly are needed to fully address this question.
in endurance athletes have relied almost exclusively on cross-sectional studies comparing young and Masters endurance-trained athletes. Longitudinal studies clearly are needed to fully address this question.
Table 2.
Oxygen consumption and its determinants at maximal exercise in endurance exercise-trained men
| Young men (28 years) | Older men (60 years) | Age-related change (%) | |
|---|---|---|---|
| Oxygen consumption (ml kg−1 min−1) | 68.2 | 49.4 | 28 |
| Cardiac output (l min−1) | 27.0 | 21.7 | 20 |
| Stroke volume (ml beat−1) | 147 | 132 | 10 |
| Heart rate (beats min−1) | 184 | 165 | 10 |
| a–v O2 difference (ml (100 ml)−1) | 16.7 | 15.2 | 8 |
The data were compiled from four studies in which values for all of the variables were reported in groups of young and older groups (Grimby et al. 1966; Hagberg et al. 1985; Rivera et al. 1989; Ogawa et al. 1992).
(a) Central factors
Maximal cardiac output. Although it has been reported that maximal cardiac output is maintained with advancing age (Rodeheffer et al. 1984), the majority of the evidence supports the idea that maximal cardiac output decreases with advancing age in healthy sedentary adults (Julius et al. 1967; Saltin, 1986; Rivera et al. 1989; Ogawa et al. 1992; Hunt et al. 1998) in proportion to the decline in maximal oxygen consumption (Grimby et al. 1966; Proctor et al. 1998). Maximal cardiac output also is reduced in older Masters (60–70 years) compared with young (20–30 years) endurance-trained athletes as a result of reductions in both maximal stroke volume and maximal heart rate (Rivera et al. 1989; Ogawa et al. 1992) (Table 2).
Maximal heart rate
Historically, maximal heart rate has been viewed as the primary mechanism mediating age-related reductions in maximal cardiac output and 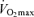 , particularly in endurance exercise-trained athletes (Heath et al. 1981; Hagberg et al. 1985). Starting from early adulthood, maximal heart rate declines with age at a rate of ∼0.7 beats min−1 year−1 in healthy sedentary, recreationally active and endurance exercise-trained adults (Tanaka et al. 2001). A slower conduction velocity, a reduced responsiveness of the sinoatrial node to β-adrenergic stimulation (Fleg et al. 1994) and a decreased intrinsic heart rate (Jose & Collison, 1970) are among the mechanisms believed to contribute to the reduction in maximal heart rate with ageing.
, particularly in endurance exercise-trained athletes (Heath et al. 1981; Hagberg et al. 1985). Starting from early adulthood, maximal heart rate declines with age at a rate of ∼0.7 beats min−1 year−1 in healthy sedentary, recreationally active and endurance exercise-trained adults (Tanaka et al. 2001). A slower conduction velocity, a reduced responsiveness of the sinoatrial node to β-adrenergic stimulation (Fleg et al. 1994) and a decreased intrinsic heart rate (Jose & Collison, 1970) are among the mechanisms believed to contribute to the reduction in maximal heart rate with ageing.
Maximal stroke volume
In older endurance exercise-trained adults, maximal stroke volume is reduced modestly to 80–90% of that observed in young endurance-trained adults (Ogawa et al. 1992) (Table 2). There is very limited information as to how changes in the major determinants of stroke volume (e.g. preload, afterload and intrinsic contractility of the heart) contribute to the age-related reduction in maximal stroke volume in endurance-trained adults. It is unclear if a reduction in left ventricular filling is involved. Results of studies indicating that left ventricular preload, as expressed as left ventricular end-diastolic dimension, area or volume, is not related to age in healthy relatively active adults (Fleg, 1986) do not support such a role. In young adults, total blood volume exerts an important influence on maximal stroke volume and maximal oxygen consumption (Convertino, 1991). However, total blood volume appears to be preserved in older endurance-trained athletes (Jones et al. 1997). We cannot exclude the possibility that other determinants of the cardiac preload, including LV end-diastolic pressure, diastolic filling time, venomotor tone, myocardial compliance and/or a combination of these factors, contribute to the age-related decline in maximal stroke volume in endurance exercise-trained adults (Schulman et al. 1992; Arbab-Zadeh et al. 2004).
The large elastic arteries stiffen with advancing age and lead to an increase in aortic input impedance as well as vascular afterload, thereby impeding the ejection of blood from the left ventricle during systole and, consequently, stroke volume during exercise (Chen et al. 1999). Although the degree of arterial stiffening with advancing age is attenuated in endurance-trained compared with sedentary adults, endurance athletes nevertheless demonstrate large elastic artery stiffening with age (Tanaka et al. 2000). This could contribute to the reduction in maximal stroke volume seen in older endurance-trained adults via increases in the left ventricular afterload and aortic input impedance (Mazzaro et al. 2005). On the other hand, no differences in left ventricular afterload, as indirectly assessed by mean arterial pressure or total peripheral resistance, have been reported in young and older endurance-trained men (Rivera et al. 1989).
It is, especially difficult to evaluate the contractility of the left ventricle in intact human subjects during exercise because of the complex and concurrent actions of multiple influencing factors. Moreover, there is no satisfactory index of contractility that is completely independent of left ventricular preload and afterload (Fleg, 1986; Giada et al. 1998). Animal studies using the isolated perfused heart preparation find that contractility declines significantly with advancing age in endurance-trained rats and that the magnitude and the rate of the decline is similar to sedentary rats (Starnes & Rumsey, 1988). The similarly lower ejection fractions at maximal exercise observed in both older sedentary and older endurance-trained athletes compared with young men are consistent with these observations in experimental animals (Schulman et al. 1992).
(b) Peripheral factors
Maximal arterio-venous O2 difference reflects the capacity of (primarily) active skeletal muscles and the respiratory muscles to extract and consume oxygen from the blood for ATP production during maximal exercise. In sedentary adults, maximal arterio-venous O2 difference clearly declines with advancing age, consistent with the marked reductions in capillary density and mitochondrial enzyme activities observed with ageing in this group (Coggan et al. 1992). Reductions in peripheral oxygen extraction during maximal exercise also appears to contribute to the decline in 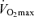 with age in endurance exercise-trained adults (Table 2), as maximal arterio-venous O2 difference declines modestly (5–10%) over a span of ∼30 years in this group (Hagberg et al. 1985; Saltin, 1986; Rivera et al. 1989). It remains to be determined if the reduction in maximal arterio-venous O2 difference with ageing in endurance athletes reflects reductions in maximal oxygen delivery to or extraction by the active muscles. However, older endurance-trained athletes can oxygeneate blood in the lungs to a similar extent as young athletes, and their contracting muscles are capable of extracting oxygen as much as their younger counterparts (Saltin, 1986). Furthermore, muscle oxidative enzyme activities and capillarization (expressed per area or per fibre) are similar between young and older endurance-trained adults (Coggan et al. 1992; Proctor et al. 1995). Thus, it is likely that maximal oxygen delivery, rather than oxygen extraction, is a major contributor to the age-related reduction in maximal arterio-venous O2 difference in endurance-trained adults.
with age in endurance exercise-trained adults (Table 2), as maximal arterio-venous O2 difference declines modestly (5–10%) over a span of ∼30 years in this group (Hagberg et al. 1985; Saltin, 1986; Rivera et al. 1989). It remains to be determined if the reduction in maximal arterio-venous O2 difference with ageing in endurance athletes reflects reductions in maximal oxygen delivery to or extraction by the active muscles. However, older endurance-trained athletes can oxygeneate blood in the lungs to a similar extent as young athletes, and their contracting muscles are capable of extracting oxygen as much as their younger counterparts (Saltin, 1986). Furthermore, muscle oxidative enzyme activities and capillarization (expressed per area or per fibre) are similar between young and older endurance-trained adults (Coggan et al. 1992; Proctor et al. 1995). Thus, it is likely that maximal oxygen delivery, rather than oxygen extraction, is a major contributor to the age-related reduction in maximal arterio-venous O2 difference in endurance-trained adults.
As skeletal muscle mass is closely related to maximal aerobic capacity among healthy humans across the adult age range (Fleg & Lakatta, 1988), a decline in maximal arterio-venous O2 difference may be secondary to an age-related loss of muscle mass. However, 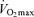 remains lower in older compared with young endurance-trained athletes after correcting for muscle mass (Proctor & Joyner, 1997). Rather, we find that among healthy men varying in age, fat-free mass exerts its permissive influence on
remains lower in older compared with young endurance-trained athletes after correcting for muscle mass (Proctor & Joyner, 1997). Rather, we find that among healthy men varying in age, fat-free mass exerts its permissive influence on 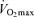 via an effect on central circulatory function involving blood volume, stroke volume and cardiac output (Hunt et al. 1998).
via an effect on central circulatory function involving blood volume, stroke volume and cardiac output (Hunt et al. 1998).
Summary and conclusions
In summary, Masters endurance athletes are capable of remarkable athletic and physiological functional performance, thereby representing a uniquely positive example of ‘exceptional ageing’. Nevertheless, endurance exercise performance decreases during middle-age and declines at an even more rapid rate in older age. The available data indicate that decreases in 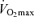 are the most clear and consistent contributor to these declines in performance. Reductions in the lactate threshold also may contribute, whereas submaximal exercise economy is preserved with ageing in endurance athletes. The age-associated decreases in
are the most clear and consistent contributor to these declines in performance. Reductions in the lactate threshold also may contribute, whereas submaximal exercise economy is preserved with ageing in endurance athletes. The age-associated decreases in 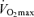 in endurance exercise-trained adults are mediated by reductions in maximal cardiac output and maximal arterio-venous O2 difference, with reductions in both maximal stroke volume and heart rate contributing to the former. The decreases in endurance exercise performance and
in endurance exercise-trained adults are mediated by reductions in maximal cardiac output and maximal arterio-venous O2 difference, with reductions in both maximal stroke volume and heart rate contributing to the former. The decreases in endurance exercise performance and 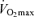 with ageing in endurance exercise-trained athletes are associated most closely with reductions in exercise training intensity and volume, probably as a consequence of changes in a number of physical and behavioural factors (e.g. increased prevalence of injuries, and reductions in energy, time and motivation to train). The ‘Masters athlete model’ continues to be a rich source of insight into our ability (or lack thereof) to maintain peak physical performance and physiological function with human ageing.
with ageing in endurance exercise-trained athletes are associated most closely with reductions in exercise training intensity and volume, probably as a consequence of changes in a number of physical and behavioural factors (e.g. increased prevalence of injuries, and reductions in energy, time and motivation to train). The ‘Masters athlete model’ continues to be a rich source of insight into our ability (or lack thereof) to maintain peak physical performance and physiological function with human ageing.
References
- Allen WK, Seals DR, Hurley BF, Ehsani AA, Hagberg JM. Lactate threshold and distance-running performance in young and older endurance athletes. J Appl Physiol. 1985;58:1281–1284. doi: 10.1152/jappl.1985.58.4.1281. [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Cirulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand. 1960;49:1–92. [PubMed] [Google Scholar]
- Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Federation Proc. 1987;46:1824–1829. [PubMed] [Google Scholar]
- Chen CH, Nakayama M, Talbot M, Nevo E, Fetics B, Gerstenblith G, Becker LC, Kass DA. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol. 1999;33:1602–1609. doi: 10.1016/s0735-1097(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, Rogers MA, King DS, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol. 1990;68:1896–1901. doi: 10.1152/jappl.1990.68.5.1896. [DOI] [PubMed] [Google Scholar]
- Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc. 1991;23:1338–1348. [PubMed] [Google Scholar]
- Cooper AF, Jackson G, Weinman J, Horne R. Factors associated with cardiac rehabilitation attendance: a systematic review of the literature. Clin Rehabil. 2002;16:541–552. doi: 10.1191/0269215502cr524oa. [DOI] [PubMed] [Google Scholar]
- Coyle EF. Integration of the physiological factors determining endurance performance ability. In: Holloszy JO, editor. Exercise and Sport Sciences Reviews. Vol. 23. Baltimore, MD: Williams & Wilkins; 1995. pp. 25–63. [PubMed] [Google Scholar]
- Dill DB, Robinson S, Ross JC. A longitudinal study of 16 champion runners. J Sports Med. 1967;7:4–27. [PubMed] [Google Scholar]
- Donato AJ, Tench K, Glueck DH, Seals DR, Eskurza I, Tanaka H. Declines in physiological functional capacity with age: a longitudinal study in peak swimming performance. J Appl Physiol. 2003;94:764–769. doi: 10.1152/japplphysiol.00438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson KA. Peak performance and age: an examination of peak performance in sports. In: Baltes PB, Baltes MM, editors. Successful Aging: Perspectives from the Behavioral Sciences. Cambridge, UK: Cambridge University Press; 1993. pp. 164–196. [Google Scholar]
- Eskurza I, Donato AJ, Moreau KL, Seals DR, Tanaka H. Changes in maximal aerobic capacity with age in endurance-trained women: 7-year follow-up. J Appl Physiol. 2002;92:2303–2308. doi: 10.1152/japplphysiol.01124.2001. [DOI] [PubMed] [Google Scholar]
- Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol. 1995;78:1931–1941. doi: 10.1152/jappl.1995.78.5.1931. [DOI] [PubMed] [Google Scholar]
- FitzGerald MD, Tanaka H, Tran ZV, Seals DR. Age-related decline in maximal aerobic capacity in regularly exercising vs sedentary females: a meta-analysis. J Appl Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- Fleg JL. Alterations in cardiovascular structure and function with advancing age. Am J Cardiol. 1986;57:33C–44C. doi: 10.1016/0002-9149(86)91025-8. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2max. J Appl Physiol. 1988;65:1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Schulman S, O'Connor F, Becker LC, Gerstenblith G, Clulow JF, Renlund DG, Lakatta EG. Effects of acute β-adrenergic receptor blockade on age-associated changes in cardiovascular performance during dynamic exercise. Circulation. 1994;90:2333–2341. doi: 10.1161/01.cir.90.5.2333. [DOI] [PubMed] [Google Scholar]
- Fuchi T, Iwaoka K, Higuchi M, Kobayashi S. Cardiovascular changes associated with decreased aerobic capacity and aging in long-distance runners. Eur J Appl Physiol. 1989;58:884–889. doi: 10.1007/BF02332223. [DOI] [PubMed] [Google Scholar]
- Giada F, Bertaglia E, De Piccoli B, Franceschi M, Sartori F, Raviele A, Pascotto P. Cardiovascular adaptations to endurance training and detraining in young and older athletes. Int J Cardiol. 1998;65:149–155. doi: 10.1016/s0167-5273(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Grimby G, Nilsson NJ, Saltin B. Cardiac output during submaximal and maximal exercise in active middle-aged athletes. J Appl Physiol. 1966;21:1150–1156. doi: 10.1152/jappl.1966.21.4.1150. [DOI] [PubMed] [Google Scholar]
- Grimby G, Saltin B. The ageing muscle. Clin Physiol. 1983;3:209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Allen WK, Seals DR, Hurley BF, Ehsani AA, Holloszy JO. A hemodynamic comparison of young and older endurance athletes during exercise. J Appl Physiol. 1985;58:2041–2046. doi: 10.1152/jappl.1985.58.6.2041. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Coyle EF. Physiological determinants of endurance performance as studied in competitive racewalkers. Med Sci Sports Exerc. 1983;15:287–289. doi: 10.1249/00005768-198315040-00006. [DOI] [PubMed] [Google Scholar]
- Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol. 1981;51:634–640. doi: 10.1152/jappl.1981.51.3.634. [DOI] [PubMed] [Google Scholar]
- Hodgson JL, Buskirk ER. Physical fitness and age, with emphasis on cardiovascular function in the elderly. J Am Geriatr Soc. 1977;25:385–392. doi: 10.1111/j.1532-5415.1977.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Sidossis LS, Coyle EF. High efficiency of type I muscle fibers improves performance. Int J Sports Med. 1994;15:152–157. doi: 10.1055/s-2007-1021038. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Davy KP, Jones PP, DeSouza CA, Van Pelt RE, Tanaka H, Seals DR. Role of central circulatory factors in the fat-free mass-maximal aerobic capacity relation across age. Am J Physiol Heart Circ Physiol. 1998;275:H1178–H1182. doi: 10.1152/ajpheart.1998.275.4.H1178. [DOI] [PubMed] [Google Scholar]
- Iwaoka K, Fuchi T, Higuchi M, Kobayashi S. Blood lactate accumulation during exercise in older endurance runners. Int J Sports Med. 1988;9:253–256. doi: 10.1055/s-2007-1025016. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, DeSouza CA, van Pelt RE, Seals DR. Absence of age-related decline in total blood volume in physically active females. Am J Physiol Heart Circ Physiol. 1997;272:H2534–H2540. doi: 10.1152/ajpheart.1997.272.6.H2534. [DOI] [PubMed] [Google Scholar]
- Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4:160–167. doi: 10.1093/cvr/4.2.160. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Physiological limiting factors and distance running: influence of gender and age on record performances. In: Holloszy JO, editor. Exercise and Sport Science Reviews. Vol. 21. Baltimore, MD: Williams & Wilkins; 1993. pp. 103–133. [PubMed] [Google Scholar]
- Julius S, Amery A, Whitlock LS, Conway J. Influence of age on the hemodynamic response to exercise. Circulation. 1967;36:222–230. doi: 10.1161/01.cir.36.2.222. [DOI] [PubMed] [Google Scholar]
- Kallinen M, Markku A. Aging, physical activity and sports injuries. An overview of common sports injuries in the elderly. Sports Med. 1995;20:41–52. doi: 10.2165/00007256-199520010-00004. [DOI] [PubMed] [Google Scholar]
- Kasch FW, Boyer JL, Camp SPV, Verity LS, Wallace JP. The effect of physical activity and inactivity on aerobic power in older men (a longitudinal study) Phys Sportsmed. 1990;18:73–83. doi: 10.1080/00913847.1990.11710022. [DOI] [PubMed] [Google Scholar]
- Kasch FW, Wallace JP. Physiological variables during 10 years of endurance exercise. Med Sci Sports. 1976;8:5–8. [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Testa V, Capasso G. Anaerobic threshold determination in master endurance runners. J Sports Med Phys Fitness. 1994;34:242–249. [PubMed] [Google Scholar]
- Marcell TJ, Hawkins SA, Tarpenning KM, Hyslop DM, Wiswell RA. Longitudinal analysis of lactate threshold in male and female master athletes. Med Sci Sports Exerc. 2003;35:810–817. doi: 10.1249/01.MSS.0000065002.69572.6F. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study. I. Effect of age on the cardiovascular response to exercise. Circulation. 2001;104:1350–1357. [PubMed] [Google Scholar]
- Mazzaro L, Almasi SJ, Shandas R, Seals DR, Gates PE. Aortic input impedance increases with age in healthy men and women. Hypertension. 2005;45:1101–1106. doi: 10.1161/01.HYP.0000164579.73656.c4. [DOI] [PubMed] [Google Scholar]
- Morgan DW, Martin PE, Krahenbuhl GS. Factors affecting running economy. Sports Med. 1989;7:310–330. doi: 10.2165/00007256-198907050-00003. [DOI] [PubMed] [Google Scholar]
- Nichols JF, Phares SL, Buono MJ. Relationship between blood lactate response to exercise and endurance performance in competitive female master cyclists. Int J Sports Med. 1997;18:458–463. doi: 10.1055/s-2007-972664. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, III, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- Ogles BM, Masters KS. Older vs. younger adult male marathon runners: participative motives and training habits. J Sport Behavior. 2000;23:130–143. [Google Scholar]
- Pimentel AE, Gentile CL, Tanaka H, Seals DR, Gates PE. Greater rate of decline in maximal aerobic capacity with age in endurance-trained vs. sedentary men. J Appl Physiol. 2003;94:2406–2413. doi: 10.1152/japplphysiol.00774.2002. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Foster C, Knapp D, Rod JL, Schmidt DH. Effect of age and training on aerobic capacity and body composition of master athletes. J Appl Physiol. 1987;62:725–731. doi: 10.1152/jappl.1987.62.2.725. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Mengelkoch LJ, Graves JE, Lowenthal DT, Limacher MC, Foster C, Wilmore JH. Twenty-year follow-up of aerobic power and body composition of older track athletes. J Appl Physiol. 1997;82:1508–1516. doi: 10.1152/jappl.1997.82.5.1508. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output–VO2 relationships during submaximal cycle ergometry. J Appl Physiol. 1998;84:599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2 max in trained older subjects. J Appl Physiol. 1997;82:1411–1415. doi: 10.1152/jappl.1997.82.5.1411. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Rivera AM, Pels AE, Sady SP, Sady MA, Cullinane EM, Thompson PD. Physiological factors associated with the lower maximal oxygen consumption of master runners. J Appl Physiol. 1989;66:949–954. doi: 10.1152/jappl.1989.66.2.949. [DOI] [PubMed] [Google Scholar]
- Robinson S. Experimental studies of physical fitness in relation to age. Arbeitsphysiol. 1938;10:251–323. [Google Scholar]
- Robinson S, Dill DB, Robinson RD, Tzankoff SP, Wagner JA. Physiological aging of champion runners. J Appl Physiol. 1976;41:46–51. doi: 10.1152/jappl.1976.41.1.46. [DOI] [PubMed] [Google Scholar]
- Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- Saltin B. The aging endurance athlete. In: Sutton JR, Brock RM, editors. Sports Medicine for the Mature Athlete. Indianapolis, IN: Benchmark Press; 1986. pp. 59–80. [Google Scholar]
- Schulman SP, Lakatta EG, Fleg JL, Lakatta L, Becker LC, Gerstenblith G. Age-related decline in left ventricular filling at rest and exercise. Am J Physiol Heart Circ Physiol. 1992;263:H1932–H1938. doi: 10.1152/ajpheart.1992.263.6.H1932. [DOI] [PubMed] [Google Scholar]
- Schulz R, Curnow C. Peak performance and age among superathletes: track and field, swimming, baseball, tennis, and golf. J Gerontol. 1988;43:113–120. doi: 10.1093/geronj/43.5.p113. [DOI] [PubMed] [Google Scholar]
- Starnes JW, Rumsey WL. Cardiac energetics and performance of exercised and food-restricted rats during aging. Am J Physiol Heart Circ Physiol. 1988;254:H599–H608. doi: 10.1152/ajpheart.1988.254.3.H599. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol. 1997;83:1947–1953. doi: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Age and gender interactions in physiological functional capacity: insight from swimming performance. J Appl Physiol. 1997;82:846–851. doi: 10.1152/jappl.1997.82.3.846. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Dynamic exercise performance in Masters athletes: insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol. 2003;95:2152–2162. doi: 10.1152/japplphysiol.00320.2003. [DOI] [PubMed] [Google Scholar]
- Trappe SW, Costill DL, Fink WJ, Pearson DR. Skeletal muscle characteristics among distance runners: a 20-yr follow-up study. J Appl Physiol. 1995;78:823–829. doi: 10.1152/jappl.1995.78.3.823. [DOI] [PubMed] [Google Scholar]
- Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol. 1996;80:285–290. doi: 10.1152/jappl.1996.80.1.285. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Wells CL, Boorman MA, Riggs DM. Effect of age and menopausal status on cardiorespiratory fitness in masters women runners. Med Sci Sports Exerc. 1992;24:1147–1154. [PubMed] [Google Scholar]
- Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. Am J Physiol Heart Circ Physiol. 2000;278:H829–H834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- Wiswell RA, Jaque SV, Marcell TJ, Hawkins SA, Tarpenning KM, Constantino N, Hyslop DM. Maximal aerobic power, lactate threshold, and running performance in master athletes. Med Sci Sports Exerc. 2000;32:1165–1170. doi: 10.1097/00005768-200006000-00021. [DOI] [PubMed] [Google Scholar]
- Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J Appl Physiol. 1993;75:1670–1674. doi: 10.1152/jappl.1993.75.4.1670. [DOI] [PubMed] [Google Scholar]