Abstract
In late pregnancy maternal hypothalamo-pituitary-adrenal (HPA) axis responses to emotional and physical stressors are attenuated. This is expected to minimize the detrimental programming effects of glucocorticoid exposure on the fetuses. We have utilized a model of immune challenge, systemic administration of interleukin-1β (IL-1β), to investigate the underlying mechanisms. Intravenous IL-1β activates corticotropin-releasing hormone (CRH) neurones in the parvocellular division of the paraventricular nucleus (pPVN) via noradrenergic (A2 cell group) neurones in the nucleus tractus solitarii (NTS). Despite comparable activation of these brainstem neurones by IL-1β in virgin and in late pregnant rats, pPVN CRH neurones are activated only in virgin rats. As a consequence IL-1β fails to evoke ACTH and corticosterone secretion in late pregnant rats, in contrast to virgin rats. Suppressed responsiveness of the CRH neurones, and hence the HPA axis, following IL-1β in late pregnancy is explained by presynaptic inhibition of noradrenaline release in the pPVN, due to increased endogenous enkephalin and μ-opioid receptor production in brainstem NTS neurones. The factor that signals to the brain the pregnancy status of the animal and stimulates opioid production in the brainstem is allopregnanolone, a neurosteroid metabolite of progesterone. The supporting evidence for these mechanisms is discussed.
Exposure to stressful events (stressors) results in a series of orchestrated responses which act to preserve or restore homeostasis and involve complex behavioural, autonomic and endocrine adaptations (for review see Habib et al. 2001). The key neuroendocrine response to stress is activation of the hypothalamo-pituitary-adrenal (HPA) axis which triggers a cascade of events, the final outcome of which is increased production and secretion of glucocorticoids from the adrenal cortex. The principal actions of glucocorticoids are to promote mobilization of glucose and fat stores, stimulate gluconeogenesis, regulate immune system activity, modulate neural activity in coping circuits, and restrain the effectors of the stress response through their negative feedback actions.
Stress and the HPA axis
Corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) are synthesized in parvocellular neurones of the paraventricular nucleus (pPVN). When these neurones are excited (e.g. by stressors) CRH and AVP are released from axon terminals in the median eminence into the hypothalamo-hypophysial portal system to act synergistically on anterior pituitary corticotrophs, stimulating adrenocortiocotrophic hormone (ACTH) secretion. ACTH in turn stimulates secretion of glucocorticoid (cortisol in humans, corticosterone in rodents) from the adrenal cortex. Stressors that activate the HPA axis are divided into two broad categories. Psychological stressors (e.g. restraint, immobilization, noise, predator odour) represent perceived threats to homeostasis and are mainly processed by forebrain limbic circuitry, whereas physical stressors (e.g. cold exposure, haemorrhage, infection) represent a real physiological threat to homeostasis and are rapidly signalled directly to the PVN via hindbrain regions, bypassing cognitive processing regions (for review see Herman et al. 2003).
Exposure to glucocorticoids during development can have detrimental programming effects on the offspring making them more susceptible to disease in adulthood, particularly to affective disorders and cardiovascular and metabolic disease (Fride & Weinstock, 1988; Barker et al. 1993; Lindsay et al. 1996; Levitt et al. 1996, 2000; Welberg et al. 2000; Barker, 2002). Defence mechanisms are in place to protect the young in utero: the placenta expresses the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which serves as a barrier, converting active corticosterone into inactive 11-dehydrocorticosterone (or cortisol to cortisone). This barrier is about 90% effective (Benediktsson et al. 1997) and while acute stress can up-regulate placental 11β-HSD2 activity, this does not occur after chronic stress (Welberg et al. 2005). Another line of defence is achieved by changes in maternal HPA axis responsiveness. During the last week of pregnancy HPA axis responses to stress are progressively reduced (Neumann et al. 1998) (Fig. 1) and by the end of pregnancy HPA axis responses to a range of psychological and physical stressors are markedly suppressed and in some cases completely abolished (da Costa et al. 1996; Neumann et al. 1998; Johnstone et al. 2000; Brunton & Russell, 2003; Brunton et al. 2005, 2006). The mechanisms, as currently understood, are discussed below.
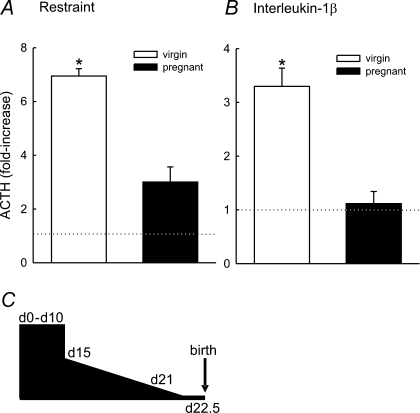
Figure 1. Responsiveness of the HPA axis in late pregnancy.
The mean increase in ACTH secretion (expressed relative to basal (dotted line) plasma concentrations). A, 15 min after the onset of a 30 min period of restraint (a psychological stressor) in conscious virgin (n = 4) and day 21 pregnant (n = 5) rats. B, 15 min after administration of interleukin-1β (a physical stressor; 500 ng kg−1i.v.) in conscious virgin (n = 8) and day 21 pregnant (n = 7) rats. Values are group means ± s.e.m.*P < 0.001 Student's t test. Blood was collected from rats via a chronic jugular vein cannula implanted 5 days prior to the experiment under inhalation of halothane anaesthesia (2–3% in 600 ml min−1 each of oxygen and nitrous oxide) in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and local (University of Edinburgh) ethics guidelines. Data in B from Brunton et al. (2005). C, schematic diagram representing the timescale of the change in HPA axis responsiveness during pregnancy. In early pregnancy (days 0–10) ACTH secretory responses to stressors (elevated plus maze, forced swimming, restraint) are indistinguishable from those observed in virgin rats. Between days 11 and 15 the mechanisms that restrain the HPA axis in late pregnancy emerge resulting in a progressive reduction in HPA axis responsiveness until term when ACTH responses are minimal.
Immune signalling and the HPA axis
A bi-directional relationship exists between the immune system and the HPA axis. Following immune system activation (e.g. by infection), macrophages increase the production of cytokines (e.g. interleukins). As well as regulating immunity and inflammation, cytokines also inform the brain that an immune response is occurring and stimulate HPA axis activity (Rivier, 1993). Glucocorticoids have immunosuppressive actions and their release following HPA axis activation by immune challenge modulates the immune response.
Systemic administration of interleukin-1β (IL-1β) is a potent activator of the HPA axis (Besedovsky et al. 1991; Harbuz et al. 1992). IL-1β given peripherally activates neurones in the nucleus tractus solitarii (NTS; A2 cell group) and ventrolateral medulla (VLM; A1 cell group) (Ericsson et al. 1994) which represent rich sources of afferent catecholaminergic innervation to the PVN (Sawchenko & Swanson, 1981), resulting in increased noradrenaline release in the PVN (Brunton et al. 2005) (Fig. 2). These inputs play an important role in mediating HPA axis responses to IL-1β, hence surgical or pharmacological lesions of these noradrenergic projections abolish HPA axis responses to IL-1β, and adrenoreceptor antagonists suppress IL-1β-induced ACTH secretion and pPVN neurone activation (Weidenfeld et al. 1989; Ericsson et al. 1994; Parsadaniantz et al. 1995; Li et al. 1996; Buller et al. 2001).
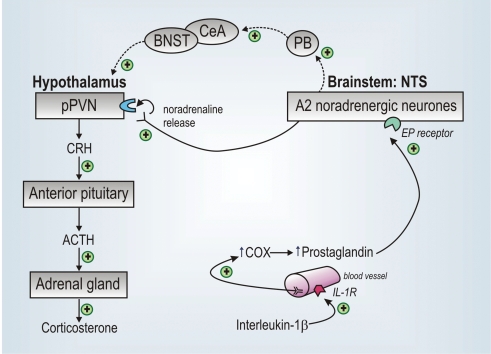
Figure 2. How circulating IL-1β signals to the HPA axis.
Circulating interleukin-1β (IL-1β) binds to IL-1 receptors (IL-1R) on the endothelium of brain blood vessels, stimulating (+) cyclo-oxygenase (COX) and local production of prostaglandins. Prostaglandins activate A2 noradrenergic neurones (via EP receptors) in the nucleus tractus solitarii (NTS) which project directly to the parvocellular division of the paraventricular nucleus (pPVN). Noradrenaline released from the nerve terminals in the pPVN acts via α-adrenoreceptors to activate CRH neurones. CRH released at the median eminence enters the hypothalamo-hypophysial portal system and acts on CRH type 1 receptors on anterior pituitary corticotropes to trigger ACTH secretion, which in turn stimulates corticosterone secretion from the cortex of the adrenal glands. Other neural circuitry involving the parabrachial nucleus (PB), central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST) is also involved in signalling to the CRH neurones following systemic IL-1β.
IL-1β does not act directly on brainstem catecholamine neurones to exert its effects: few of these neurones express IL-1 receptors, and given systemically IL-1β is unlikely to cross the blood–brain barrier (Rivest et al. 2000). Instead, IL-1β relies on prostaglandins to mediate its effects. IL-1β binds to IL-1 receptors located on the endothelium of brain blood vessels (Ericsson et al. 1995) to stimulate cyclo-oxygenase (COX) (Cao et al. 1996; Lacroix & Rivest, 1998) and hence local production of prostaglandin (Komaki et al. 1992), which activates NTS neurones via EP4 receptors (Zhang & Rivest, 1999; Rivest et al. 2000) (Fig. 2). Hence, COX inhibitors and antisera against various prostaglandins block IL-1β-induced HPA activity in vivo and CRH release from hypothalamic explants (Watanobe et al. 1995; Niimi et al. 1996; Ericsson et al. 1997; Buller et al. 1998). Peripherally administered IL-1β also activates neurones in the central nucleus of the amygdala (CeA) (Xu et al. 1999), an action mediated via the parabrachial nucleus, which receives inputs from activated NTS and VLM neurones following systemic IL-1β (Buller et al. 2004). Neurones in the CeA relay information via the bed nucleus of the stria terminalis (BNST) to the pPVN and lesions of this pathway result in attenuated HPA axis responses to IL-1β (Xu et al. 1999; Crane et al. 2003).
Mechanisms of suppressed HPA axis responses to IL-1β in late pregnancy
We have employed a model of immune challenge (intravenous administration of IL-1β) to tease out the mechanisms underlying attenuated HPA axis responses to inflammatory stress in late pregnant rats. Systemic IL-1β administration fails to evoke ACTH (Fig. 1B) and corticosterone secretion in late pregnancy (Brunton et al. 2005). This is a result of reduced CRH drive by the pPVN, as up-regulation of CRH mRNA expression is not stimulated by IL-1β in late pregnant rats, in contrast to non-pregnant rats (Brunton et al. 2005). Furthermore, IL-1β increases pro-opiomelanocortin (POMC) mRNA expression in the anterior pituitary in virgin rats (Brunton et al. 2005), indicating corticotroph exposure to CRH; however, this was not observed in late pregnant rats, consistent with studies where centrally administered IL-1β was shown to deplete CRH content in the median eminence in virgin, but not pregnant rats (Nakamura et al. 1998). The pPVN CRH neurones are prevented from responding to IL-1β in late pregnancy as a consequence of reduced drive from the brainstem noradrenergic neurones. COX-2, the inducible form of this enzyme, is involved in prostaglandin generation and subsequent activation of brainstem noradrenergic neurones following systemic IL-1β. We have found a significant increase in COX-2 mRNA expression in the NTS after IL-1β in virgin rats, but not late pregnant rats (P. J. Brunton & J. A. Russell, unpublished observations), consistent with other studies comparing COX-2 induction by endotoxin in the hypothalamus in mid- and late-pregnancy (Mouihate et al. 2002). Despite these differences in COX-2 induction, brainstem neurones in the A2 cell region of the NTS are similarly activated (they express Fos protein) in virgin and late pregnant rats following IL-1β (Brunton et al. 2005), suggesting that in pregnancy COX-1 (the constitutive form) effectively generates prostaglandins that activate the NTS. In addition to the NTS, the number of neurones in the CeA activated by systemic IL-1β is comparable in virgin and late pregnant rats, indicating that the NTS projection via the parabrachial nucleus to the CeA is functioning in late pregnancy (P. J. Brunton & J. A. Russell, unpublished observations). Hence, suppressed HPA axis responsiveness to IL-1β in late pregnancy is not the result of a global suppression of the ascending NTS noradrenergic projections, but may arise from selective inhibition of noradrenergic transmission at the level of the PVN, in particular at terminals that innervate the CRH neurones.
Systemic IL-1β evokes noradrenaline release in the PVN of virgin rats; however, this response is absent in late pregnant rats even though the cell bodies in the A2 region of the NTS are similarly excited (Brunton et al. 2005). This is explained by endogenous opioid inhibition of noradrenaline release in the PVN. In males and non-pregnant females opioids have excitatory actions on HPA axis activity (Buckingham & Cooper, 1986; Buller et al. 2005); however, in late pregnancy opioids have a net inhibitory effect, such that systemic naloxone (an opioid receptor antagonist) reinstates HPA axis responses to IL-1β (Brunton et al. 2005). In our model opioids are evidently acting at the level of the noradrenergic nerve terminals in the PVN to inhibit noradrenaline release evoked by IL-1β since naloxone retro-dialysed into the PVN restores a noradrenaline response in late pregnant rats (Brunton et al. 2005). Expression of mRNAs for proenkephalin-A and μ-opioid receptor is increased in the NTS at the end of pregnancy (Brunton et al. 2005). It is not known whether the NTS neurones expressing these opioid genes are the same set of neurones that also produce noradrenaline, project to the PVN and are activated by systemic IL-1β. If this is the case it provides a mechanism by which up-regulated enkephalin could act on the up-regulated opioid receptor at the A2 nerve terminals in the PVN to auto-inhibit release of noradrenaline (and other co-peptides) in late pregnancy (Fig. 3). Whether endogenous opioids also have postsynaptic actions on the CRH cell bodies is as yet unknown.
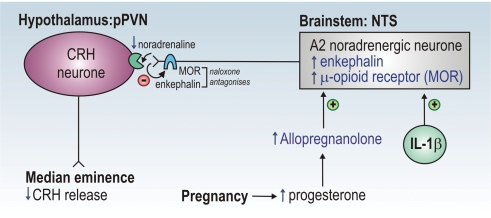
Figure 3. Proposed mechanism of suppressed HPA axis responses in late pregnancy.
Central and circulating levels of allopregnanolone are increased (↑) in pregnancy. Allopregnanolone increases the expression of mRNA for proenkephalin-A (and perhaps for μ-opioid receptor) in the A2 cell region of the nucleus tractus solitarii (NTS). NTS noradrenergic A2 neurones innervate CRH neurones in the parvocellular division of the paraventricular nucleus (pPVN) and are activated (+) following systemic administration of interleukin-1β (IL-1β; see Fig. 2). However, in late pregnancy, increased opioid (enkephalin) inhibition prevents IL-1β from stimulating noradrenaline release in the PVN. Enkephalin acts presynaptically on μ-opioid receptors (MOR) to inhibit noradrenaline release from nerve terminals in the PVN. The opioid receptor antagonist, naloxone, restores HPA axis responses to IL-1β when administered systemically, and when infused directly into the PVN reinstates noradrenaline release evoked by IL-1β. Induction of this inhibitory (−) opioid mechanism by allopregnanolone in late pregnancy prevents activation of the CRH neurones and consequently HPA axis responses to IL-1β are suppressed (↓).
What pregnancy-related factor triggers these adaptations in HPA axis responsiveness?
Circulating concentrations of oestrogen and progesterone are greatly increased in late pregnancy implicating these sex steroids as candidate mediators in signalling to the brain to induce adaptations in HPA axis responsiveness. We have previously found that prolonged exposure of virgin rats to pregnancy levels of oestrogen and progesterone induces inhibitory opioid tone over stimulated oxytocin secretion, as is found in late pregnancy, but does not alter HPA axis responses to stress (Douglas et al. 2000).
Progesterone is metabolized by the enzyme 5α-reductase to dihydroprogesterone, which is converted to allopregnanolone by 3α-hydroxysteroid dehydrogenase. Circulating and central levels of allopregnanolone increase during pregnancy, peaking around 2 days prior to parturition (Concas et al. 1998). Allopregnanolone is a neuroactive steroid and it acts allosterically on GABAA receptors to prolong the opening time of the activated GABAA receptor chloride channel, hence enhancing inhibitory GABA neurotransmission (Brussaard et al. 1999). Furthermore, allopregnanolone attenuates stress-induced HPA axis activity in male rats (Patchev et al. 1996). We have investigated a role for allopregnanolone in the suppressed HPA axis responses to IL-1β in late pregnancy by blocking its production with finasteride, a 5α-reductase inhibitor (P. J. Brunton & A. J. Russell, unpublished observations). Finasteride had little effect on the HPA axis response to IL-1β in non-pregnant rats; however, in late pregnant rats, blocking allopregnanolone production substantially restored an ACTH response. This effect of finasteride in pregnant rats was reversed by simultaneous allopregnanolone treatment, and allopregnanolone treatment suppressed IL-1β-induced ACTH secretion in virgin rats. However, the same effect was not achieved with either progesterone or dihydroprogesterone treatment, suggesting that the activity of one or both of the progesterone-converting enzymes is up-regulated centrally in pregnancy.
In late pregnant rats pre-treated with finasteride and then naloxone we observed ACTH responses to IL-1β similar to those measured when either drug was administered alone. The inference that allopregnanolone might induce opioid inhibition of HPA axis responses to IL-1β was confirmed by finding that allopregnanolone treatment induced inhibitory opioid tone over HPA axis responses to IL-1β in virgin rats, and furthermore that allopregnanolone induced proenkephalin-A mRNA expression in the NTS of virgin rats, as found at the end of pregnancy (Brunton et al. 2005). The mechanism of this neurosteroid–opioid connection needs further investigation.
Conclusions
In late pregnancy HPA axis responses to IL-1β are essentially absent. This is a consequence of a lack of excitatory noradrenergic drive to the CRH neurones in the PVN. Up-regulated opioid peptide and receptor expression, originating from NTS neurons, act presynaptically on noradrenergic terminals in the PVN to inhibit noradrenaline release in response to IL-1β in late pregnancy. In pregnancy, increased concentrations of central allopregnanolone induce the changes in opioid expression. Whether the collective effects of allopregnanolone and opioids reported here represent a global mechanism acting to restrain HPA axis responses to other types of stress remains to be elucidated.
Acknowledgments
The authors would like to thank Helen Cameron for her contribution to the work presented here. Financial support was provided by the Biotechnology and Biological Sciences Research Council (BBSRC).
References
- Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CRW, Seckl JR. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol. 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, Del Rey A, Klusman I, Furukawa H, Monge Arditi G, Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40:613–618. doi: 10.1016/0960-0760(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Bales J, Russell JA. Neuroendocrine stress but not feeding responses to centrally administered neuropeptide Y are suppressed in pregnant rats. Endocrinology. 2006;147:3737–3745. doi: 10.1210/en.2006-0048. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci. 2005;25:5117–5126. doi: 10.1523/JNEUROSCI.0866-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Hypothalamic-pituitary- adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J Neuroendocrinol. 2003;15:633–637. doi: 10.1046/j.1365-2826.2003.01045.x. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Effects of naloxone on hypothalamo-pituitary-adrenocortical activity in the rat. Neuroendocrinology. 1986;42:421–426. doi: 10.1159/000124481. [DOI] [PubMed] [Google Scholar]
- Buller KM, Allen T, Wilson LD, Munro F, Day TA. A critical role for the parabrachial nucleus in generating central nervous system responses elicited by a systemic immune challenge. J Neuroimmunol. 2004;152:20–32. doi: 10.1016/j.jneuroim.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Buller KM, Hamlin AS, Osborne PB. Dissection of peripheral and central endogenous opioid modulation of systemic interleukin-1β responses using c-fos expression in the rat brain. Neuropharmacology. 2005;49:230–242. doi: 10.1016/j.neuropharm.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Buller KM, Xu Y, Day TA. Indomethacin attenuates oxytocin and hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1β. J Neuroendocrinol. 1998;10:519–528. doi: 10.1046/j.1365-2826.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Buller KM, Xu Y, Dayas CV, Day TA. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1β-induced HPA axis responses. Neuroendocrinology. 2001;73:129–138. doi: 10.1159/000054629. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1β: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1β. J Comp Neurol. 2003;467:232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- da Costa APC, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 1996;742:177–184. doi: 10.1016/s0006-8993(96)00962-6. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone H, Brunton P, Russell JA. Sex-steroid induction of endogenous opioid inhibition on oxytocin secretory responses to stress. J Neuroendocrinol. 2000;12:343–350. doi: 10.1046/j.1365-2826.2000.00460.x. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: Distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Stephanou A, Sarlis N, Lightman SL. The effects of recombinant human interleukin (IL)-1α, IL-1β or IL-6 on hypothalamo-pituitary-adrenal axis activation. J Endocrinol. 1992;133:349–355. doi: 10.1677/joe.0.1330349. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, Russell JA. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: Changes in feedforward and feedback mechanisms. J Neuroendocrinol. 2000;12:811–822. doi: 10.1046/j.1365-2826.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- Komaki G, Arimura A, Koves K. Effect of intravenous injection of IL-1beta on PGE2 levels in several brain areas as determined by microdialysis. Am J Physiol Endocrinol Metabol. 1992;262:E246–E251. doi: 10.1152/ajpendo.1992.262.2.E246. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young south african adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;85:4611–4618. doi: 10.1210/jcem.85.12.7039. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci U S A. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: Studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Clerget-Froidevaux MS, Nakamura K, Negishi M, Wallace JL, Pittman QJ. Suppression of fever at near term is associated with reduced COX-2 protein expression in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2002;283:R800–R805. doi: 10.1152/ajpregu.00258.2002. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Seto T, Hatta K, Matsuzaki I, Nagase H, Yoshida M, Ogino K. Central administration of interleukin-1β reduces natural killer cell activity in non-pregnant rats, but not in pregnant rats. Psychoneuroendocrinology. 1998;23:651–659. doi: 10.1016/s0306-4530(98)00037-7. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi M, Sato M, Wada Y, Takahara J, Kawanishi K. Effect of central and continuous intravenous injection of interleukin-1beta on brain c-fos expression in the rat: involvement of prostaglandins. Neuroimmunomodulation. 1996;3:87–92. doi: 10.1159/000097232. [DOI] [PubMed] [Google Scholar]
- Parsadaniantz SM, Gaillet S, Malaval F, Lenoir V, Batsche E, Barbanel G, Gardier A, Terlain B, Jacquot C, Szafarczyk A, Assenmacher I, Kerdelhue B. Lesions of the afferent catecholaminergic pathways inhibit the temporal activation of the CRH and POMC gene expression and ACTH release induced by human interleukin-1beta in the male rat. Neuroendocrinology. 1995;62:586–595. doi: 10.1159/000127054. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AHS, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Neuroendocrine effects of cytokines in the rat. Rev Neurosci. 1993;4:223–237. doi: 10.1515/revneuro.1993.4.3.223. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Sasaki S, Takebe K. Role of prostaglandins E1, E2 and F2α in the brain in interleukin 1β-induced adrenocorticotropin secretion in the rat. Cytokine. 1995;7:710–712. doi: 10.1006/cyto.1995.0083. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Abramsky O, Avadia H. Evidence for the involvement of the central adrenergic system in interleukin 1-induced adrenocortical response. Neuropharmacology. 1989;28:1411–1414. doi: 10.1016/0028-3908(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR, Holmes MC. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11β-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol. 2005;186:R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamic–pituitary–adrenal axis responses to systemic interleukin-1β administration. Neuroscience. 1999;94:175–183. doi: 10.1016/s0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rivest S. Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. Eur J Neurosci. 1999;11:2651–2668. doi: 10.1046/j.1460-9568.1999.00682.x. [DOI] [PubMed] [Google Scholar]