Abstract
Exogenous melatonin is increasingly used for its phase shifting and soporific effects. We generated a three pulse phase response curve (PRC) to exogenous melatonin (3 mg) by administering it to free-running subjects. Young healthy subjects (n = 27) participated in two 5 day laboratory sessions, each preceded by at least a week of habitual, but fixed sleep. Each 5 day laboratory session started and ended with a phase assessment to measure the circadian rhythm of endogenous melatonin in dim light using 30 min saliva samples. In between were three days in an ultradian dim light (< 150 lux)–dark cycle (LD 2.5 : 1.5) during which each subject took one pill per day at the same clock time (3 mg melatonin or placebo, double blind, counterbalanced). Each individual's phase shift to exogenous melatonin was corrected by subtracting their phase shift to placebo (a free-run). The resulting PRC has a phase advance portion peaking about 5 h before the dim light melatonin onset, in the afternoon. The phase delay portion peaks about 11 h after the dim light melatonin onset, shortly after the usual time of morning awakening. A dead zone of minimal phase shifts occurred around the first half of habitual sleep. The fitted maximum advance and delay shifts were 1.8 h and 1.3 h, respectively. This new PRC will aid in determining the optimal time to administer exogenous melatonin to achieve desired phase shifts and demonstrates that using exogenous melatonin as a sleep aid at night has minimal phase shifting effects.
The master mammalian circadian clock, contained in the suprachiasmatic nuclei (SCN) in the hypothalamus, controls circadian rhythms in a range of physiological and behavioural functions, including the secretion of melatonin from the pineal gland (Moore, 1978). Typically, melatonin levels begin to increase a few hours before sleep, peak in the early hours of the morning and decrease to daytime levels around the time of waking. The endogenous melatonin rhythm is considered a reliable marker of the human master circadian clock (Lewy et al. 1999; Klerman et al. 2002). However, melatonin secretion is acutely suppressed by light (Lewy et al. 1980) and so melatonin must be measured in dim light to accurately reflect the timing of the circadian clock.
It has been shown that exogenous melatonin can phase shift the circadian clock in rats (Redman et al. 1983), and in humans (Arendt et al. 1985). Exogenous melatonin probably produces these phase shifts by binding to melatonin receptors in the SCN (Reppert et al. 1988). To date, three phase response curves (PRCs) to exogenous melatonin in humans have been published. In one PRC 20 μg of melatonin was intravenously administered for 3 h (Zaidan et al. 1994), but the marker of circadian phase, the endogenous melatonin rhythm, may have been masked by light (< 150 lux before 23.00 h) and the infusion of exogenous melatonin. Furthermore, as exogenous melatonin is usually administered orally, this PRC is less relevant to the typical use of melatonin. A second PRC was to a 5 mg pill of melatonin, but as the authors themselves state, mathematical demasking of the temperature rhythm may have led to inaccuracies (Arendt et al. 1999). The most commonly cited PRC to exogenous melatonin is a four pulse PRC generated in six entrained humans (1 m, 5 f) to a 0.5 mg immediate release dose of melatonin (Lewy et al. 1998). Each subject maintained his or her habitual sleep schedule at home for 2 weeks and three times at weekly intervals they visited the laboratory to have their dim light melatonin onset (DLMO) assessed. Subjects were instructed to take a pill once per day, at the same time of day when they slept at home. In one week the pill was always placebo, in the other week the last four pills were melatonin. Each subject repeated the protocol an impressive 12 times; taking the pills at one of 12 clock times spread evenly in 2 h intervals across the 24 h day. The resulting PRC indicates that phase delays are produced when exogenous melatonin is taken in the later hours of sleep and in the morning. Phase advances result when exogenous melatonin is taken in the afternoon and early evening.
This important PRC has informed studies that have used exogenous melatonin to entrain free-running blind people (Lockley et al. 2000; Sack et al. 2000), treat delayed sleep phase syndrome (Nagtegaal et al. 1998; Mundey et al. 2005) and facilitate adjustment to night shift work (Sharkey & Eastman, 2002) and eastward jet travel (Revell et al. 2006). Nonetheless, there are limitations to this PRC. These include that there was a large age range in the subjects (27–77 years) and there was no objective confirmation that subjects slept according to their usual times at home. Additionally, light levels at home were uncontrolled and some or all subjects may have received light during the night when they were required to wake up to take the pill. As even dim light can phase shift the human circadian clock (Zeitzer et al. 2000), this light exposure may have confounded the observed phase shifts, particularly phase delays, to the melatonin. Finally, the effect of the placebo pill in each individual subject was not calculated into the resulting PRC. These issues and the failure of a single dose of exogenous melatonin to phase delay human circadian rhythms (Wirz-Justice et al. 2002) has led to some questioning of the accuracy of the phase delay portion of this melatonin PRC. Additionally, as different durations of light produce differently shaped PRCs (Johnson, 1992; Comas et al. 2006), it is reasonable to assume that different doses of melatonin will produce differently shaped PRCs. Thus the PRC of Lewy et al. (1998) generated with 0.5 mg melatonin may not indicate the best times to take higher doses of melatonin for maximum phase advances and phase delays.
In this study we generated a new PRC to a different dose of exogenous melatonin using a different protocol. We administered pills of 3 mg of immediate release melatonin to free-running subjects. This dose and formulation of exogenous melatonin is the most commonly available over the counter in the US, and is often used as a sleep aid. In generating this new PRC we aimed to confirm that exogenous melatonin can phase delay the human circadian clock and determine the best times to take this dose of exogenous melatonin for phase advances and for phase delays. We also aimed to determine if any phase shift will occur when this dose is taken close to habitual bedtime, when it is used as a sleep aid.
Methods
Ethical approval
The protocol conformed to the standards set by the Declaration of Helsinki and was approved by the Rush University Medical Center Institutional Review Board. All subjects gave written informed consent prior to participation. Subjects were reimbursed for their participation.
Subjects
Twenty-seven healthy young subjects participated (14 men, 13 women, mean age ± s.d. 28.8 ± 6.9 years, mean body mass index ± s.d. 24.3 ± 2.8 kg m−2). To restrict the dose of exogenous melatonin (mg kg−1), we excluded individuals who weighed more than 100 kg. Subjects accepted into the study were non-smokers, did not habitually consume large caffeine (≤ 300 mg day−1) or alcohol doses (≤ 2 drinks per day) and reported no medical, psychiatric or sleep disorders as assessed from a telephone interview, in-person interview, medical history and several screening questionnaires: Minnesota Multiphasic Personality Inventory-2 (Butcher et al. 1989), Beck Depression Inventory (Beck et al. 1961), Pittsburgh Sleep Quality Index (Buysse et al. 1989), and part of a general health questionnaire (Tasto et al. 1978). All subjects were free of prescription medication, except three females who used hormonal birth control. A urine drug screen confirmed that all subjects were free of common drugs of abuse. None reported taking melatonin supplements. No subject had worked night shifts or flown across more than two time zones in the month preceding the study. Morningness–eveningness was assessed (Horne & Ostberg, 1976) prior to the start of the study and there were four moderate morning types, 22 of neither type and one moderate evening type.
Protocol
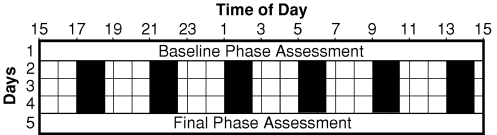
The protocol consisted of two 5 day laboratory sessions during which subjects lived in the laboratory and did not go outside (Fig. 1). Each laboratory session had a baseline phase assessment, 3 days of a 4 h ultradian light–dark (LD) cycle, and then a final phase assessment. Each subject slept at home during 7 days before the first laboratory session and during 9 days in between the two laboratory sessions (more details below). The study was a within-subjects, counterbalanced design; in one laboratory session subjects took placebo pills and in the other they took melatonin pills. The melatonin pills contained 3.00 mg of melatonin as verified with HPLC analysis (3 mg, immediate release, Ecological Formulas, Concord, CA, USA). Subjects participated in groups of four, and each group received their pills at the same time of day, but two subjects received melatonin during the first laboratory session and placebo during the second laboratory session, while the other two subjects received the pills in reverse order. The pills were administered double blind and were given immediately after subjects were awakened from a 1.5 h sleep/dark episode. Subjects gave a saliva sample 1 h and also 4.3 h after taking each pill. These samples were later assayed and confirmed that subjects had received placebo or melatonin pills as planned. The samples taken 1 h after the melatonin pill were typically > 300 pg ml−1. Laboratory staff continuously monitored all subjects throughout each laboratory session.
Figure 1. Diagram of a laboratory session.
Subjects participated in two laboratory sessions. They slept on a fixed sleep schedule tailored to their habitual sleep times for 7 days before the first laboratory session and for 9 days in between the two laboratory sessions. During the phase assessments, on days 1 and 5, saliva was sampled every 30 min in dim light (< 5 lux) to measure the entire melatonin profile. There were three days of an ultradian light–dark cycle during which subjects were permitted to sleep in the dark (0 lux) for 1.5 h (represented by black bars) and were kept awake for 2.5 h in room light (< 150 lux). Each day subjects were given a pill at the start of one of the awake episodes, and thus at the same time of day for 3 days in each laboratory session. During one laboratory session the pill was melatonin (3 mg) and during the other session it was placebo, counterbalanced. Different groups of subjects received the pills at the start of different awake episodes in order to cover all 24 h. The phase shift (free-run) during the placebo session was subtracted from the phase shift during the melatonin session to generate the PRC.
Circadian phase assessments
The circadian rhythm of salivary melatonin was used as the marker of circadian phase. Non-steroidal anti-inflammatory drugs were not permitted for at least 3 days prior to saliva collection as these drugs suppress endogenous melatonin (Murphy et al. 1996). Similarly, subjects were not permitted to consume alcohol or caffeine for 4 days before each phase assessment. Subjects were breathalysed on arrival to ensure their blood alcohol concentration was zero. Subjects were then seated in recliners in dim light (< 5 lux, at the level of the subjects' eyes, in the direction of gaze, Minolta TL-1 light meter, Ramsey, NJ, USA). Subjects gave a saliva sample every 30 min using Salivettes (Sarstedt, Newton, NC, USA). The final phase assessments started at least 12.5 h after the last pill administration to allow for pill ‘wash out’ prior to saliva collection. The baseline phase assessments were at least 20 h long, and the final phase assessments were 24 h long. Toothpaste and mouthwash were not allowed during the phase assessments. Small snacks and fluids were permitted, except in the 10 min before each sample, and subjects were required to rinse and brush their teeth with water while remaining seated 10 min before each sample if they had consumed food or drink. The saliva samples were centrifuged immediately after collection and frozen. The samples were later shipped in dry ice to Pharmasan Laboratories (Osceola, WI, USA) and were radioimmunoassayed for endogenous melatonin levels. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg ml−1, and intra- and interassay coefficient of variabilities were 12.1 and 13.2%, respectively.
Ultradian light–dark cycle
The ultradian LD cycle consisted of 1.5 h dark episodes alternating with 2.5 h wake episodes in room lighting. During the 1.5 h dark episodes subjects lay on cots (194 cm × 76 cm) and were permitted to sleep. The four cots were spread around a large windowless room. During their 2.5 h awake times in room light, subjects were not allowed to sleep but were permitted to eat and drink ad lib, read, play games, use a computer, talk on their cell phones and watch TV/videos. Subjects typically sat around a large round table. Light levels were < 150 lux, on average 35.4 ± 10.6 (s.d.) lux (measured periodically at the level of the subjects' eyes, in the direction of gaze, Minolta TL-1 light meter, Ramsey, NJ, USA). The light was generated from three ceiling fixtures, each of which contained three fluorescent tubes, controlled by a dimmer switch locked to a low setting.
Home portions of the study
Each subject was assigned a home sleep schedule of 8–9 h in duration with the average assigned bedtime and wake times being 23.40 and 08.00 h, respectively. Sleep times were chosen to be similar to each subject's habitual sleep schedule. The fixed sleep schedule was designed to stabilize circadian phase and ensure subjects were not significantly sleep deprived prior to each laboratory session. Subjects also slept at home after each laboratory session ended (returning home via a taxi). On the first day after the first laboratory session, subjects were permitted to nap at home until 16 h after the midpoint of their assigned baseline sleep schedule. During their scheduled sleep times at home, subjects were instructed to lie in bed in the dark and try to sleep. They were not permitted to read, watch TV, listen to music or talk on the telephone at this time. To ensure compliance subjects were required to call the laboratory voice mail (time and date stamped) before turning out their lights and at their wake time. Subjects also completed sleep logs and wore a waterproof wrist actigraphy monitor with light sensor (Actiwatch-L, Mini-Mitter, Bend, OR, USA) throughout the study which recorded their activity and light exposure every minute and was downloaded and inspected in their presence every 2–3 days. Subjects also completed a daily log noting the time and dose of any caffeinated beverages, alcohol, and medications they consumed that day. Subjects were permitted to consume up to a maximum of 100 mg of caffeine in the first 3 h after their wake time and up to two standard drinks of alcohol per day, except in the 4 days prior to each laboratory session.
Data analysis
Each subject generated four melatonin profiles which were smoothed with a locally weighted least squares (LOWESS) curve (Cleveland, 1979; Chambers et al. 1983) generated by Prism (GraphPad, Inc., San Diego, CA, USA), using the ‘fine’ setting. A threshold for each melatonin profile was calculated as the mean of five low consecutive daytime values (raw data points) plus twice the standard deviation of these points (Voultsios et al. 1997). This method yields low thresholds that are typically close to the physiological onset of endogenous melatonin secretion. The highest threshold was then applied to all four melatonin profiles for that individual subject. The mean (± s.d.) threshold was 3.1 ± 1.0 pg ml−1. The DLMO for each profile was the point in time (as determined with linear interpolation) when the smooth melatonin curve exceeded the threshold. The dim light melatonin offset (DLMOff) for each profile was the point in time when the smooth melatonin curve fell below the threshold. The phase shift during each laboratory session was the final DLMO minus the baseline DLMO.
Each individual's phase shift to exogenous melatonin was corrected by subtracting their phase shift during the placebo session (effect of laboratory session), to calculate the phase shift due to exogenous melatonin alone. For example, if a subject advanced 2 h during the laboratory session with exogenous melatonin, but advanced 0.5 h during the laboratory session with placebo, then the net shift (due to exogenous melatonin alone) was calculated to be an advance of 1.5 h. Each individual's net shift was then plotted against the time of the pill administration relative to their baseline circadian phase (DLMO) in the laboratory session during which exogenous melatonin was administered. Thus each subject contributed one point to the PRC. A dual harmonic sinusoidal regression was fit to the raw data of the PRC as in Khalsa et al. (2003). We did not calculate a PRC to the DLMOff because unlike the DLMO, the DLMOff is influenced by individual differences in the metabolism and clearance of melatonin from the circulation, and thus we believe it is a less reliable marker of the circadian clock.
Results
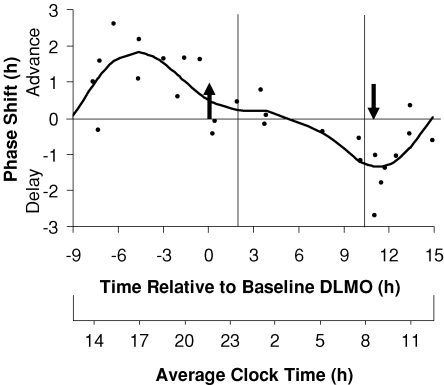
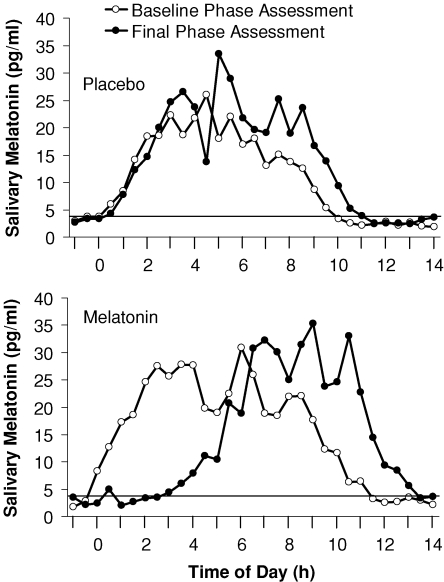
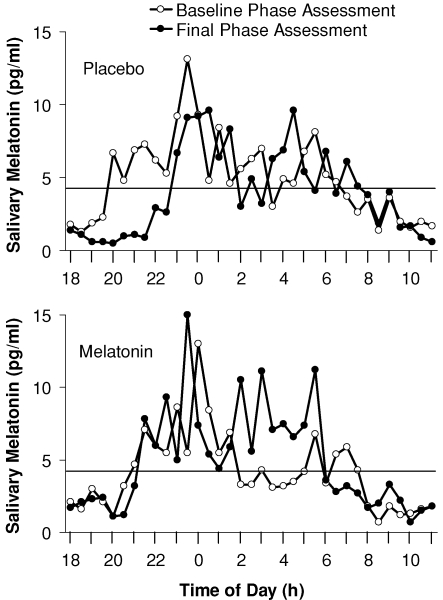
The resulting three pulse PRC (Fig. 2) shows distinct phase advance and phase delay portions. The peak of the fitted phase advance portion occurred 4.6 h before the DLMO, in the afternoon. The peak of the fitted delay portion occurred 11.1 h after the DLMO, at about the time of the DLMOff and shortly after the usual time of morning awakening. The fitted maximum phase advance shift was 1.8 h, and the fitted maximum phase delay shift was 1.3 h, although of course larger phase shifts occurred in some individuals. The endogenous melatonin profiles from the subject that showed the largest phase delay (2.7 h) is shown in Fig. 3, and the endogenous melatonin profiles from the subject that showed the largest phase advance (2.6 h) is shown in Fig. 4. The endogenous melatonin profiles from a subject that showed a minimal phase shift to exogenous melatonin is shown in Fig. 5. For this subject, the free-run during the melatonin and placebo sessions was about the same; melatonin did not alter the course of the free-run. Figure 2 shows that the phase advance portion of the fitted PRC was slightly larger than the phase delay portion. There was a dead zone of minimal phase shift (less than 0.5 h) starting at about the DLMO and continuing through the first half of sleep. There was a crossover point from delays to advances about 9 h before the DLMO.
Figure 2. The three pulse phase response curve (PRC) to 3 mg of exogenous melatonin generated from subjects free-running during an ultradian LD cycle.
Phase shifts of the DLMO are plotted against the time of administration of the melatonin pill relative to the baseline DLMO (top x-axis). The average baseline DLMO is represented by the upward arrow, the average baseline DLMOff by the downward arrow, and the average assigned baseline sleep times from before the laboratory sessions are enclosed by the vertical lines. Each dot represents the phase shift of an individual subject, calculated by subtracting the phase shift during the placebo session (free-run) from the phase shift during the melatonin session. The curved line illustrates the dual harmonic curve fit. The average clock time axis (bottom x-axis) corresponds to the average baseline sleep times. This PRC can be applied to people with different sleep schedules by moving the average clock time axis until the vertical lines align with the individual's sleep schedule.
Figure 3. Raw endogenous melatonin profiles from the individual subject who showed the largest phase delay to exogenous melatonin.
The horizontal line represents the threshold used to calculate the DLMO and DLMOff using the crossings of the smoothed melatonin profiles (not shown here). The DLMO delayed by 3.1 h in the melatonin laboratory session and 0.4 h in the placebo laboratory session. Therefore, the net phase shift plotted in the PRC was a delay of 2.7 h.
Figure 4. Raw endogenous melatonin profiles from the individual subject who showed the largest phase advance to exogenous melatonin.
The horizontal line represents the threshold used to calculate the DLMO and DLMOff using the crossings of the smoothed melatonin profiles (not shown here). The DLMO delayed by 0.2 h in the melatonin laboratory session and 2.8 h in the placebo laboratory session. Therefore, the net phase shift plotted in the PRC was an advance of 2.6 h.
Figure 5. Raw endogenous melatonin profiles from an individual subject who showed a minimal phase shift to exogenous melatonin.
The horizontal line represents the threshold used to calculate the DLMO and DLMOff using the crossings of the smoothed melatonin profiles (not shown here). The DLMO delayed by 1.9 h in the melatonin laboratory session and 2.0 h in the placebo laboratory session. Therefore, the net phase shift plotted in the PRC was an advance of 0.1 h.
The average phase shift during the 5 day laboratory sessions with placebo was a delay of 1.1 ± 0.8 h. The average baseline DLMO and DLMOff during the laboratory sessions with exogenous melatonin were 21.37 h and 08.34 h, respectively, and the average DLMO–DLMOff interval was 11.0 ± 1.4 h.
Discussion
We have generated a new PRC to exogenous melatonin by administering a single daily dose to free-running subjects for three consecutive days. The resulting three pulse PRC shows that maximum phase advances to 3 mg of melatonin occur when it is taken about 5 h before the DLMO, and maximal phase delays occur when it is taken about 11 h after the DLMO, at about the time of the DLMOff. Although the phase delay portion is somewhat smaller than the phase advance portion, the PRC clearly shows that multiple doses of exogenous melatonin can produce phase delays. Thus, while a single dose of 5 mg of melatonin given at 07.00 h failed to phase delay the DLMO (Wirz-Justice et al. 2002), it may be that multiple doses of exogenous melatonin are needed to produce significant phase delays in the majority of subjects, as reported previously in mice (Benloucif & Dubocovich, 1996). Our PRC also indicates that if 3 mg of exogenous melatonin is taken just prior to usual bedtime, as a sleep aid, there are minimal phase advances of less than 0.5 h in 3 days. However, exogenous melatonin taken near the end of sleep could inadvertently delay the circadian clock.
The magnitude of phase shifts in our melatonin PRC are consistent with other reports of phase shifts in response to exogenous melatonin (Deacon & Arendt, 1995; Krauchi et al. 1997; Lewy et al. 2002; Sharkey & Eastman, 2002; Rajaratnam et al. 2003; Lewy et al. 2005; Emens et al. 2006). However, it is difficult to compare the phase shifts among the various studies and ours because they differ in so many ways, such as in (1) the dose of melatonin, (2) the use of an immediate release or sustained release dose, (3) the number of consecutive days of melatonin administration, and (4) whether the subjects were entrained to a 24 h day or free-running or subjected to an abrupt shift of the sleep/dark schedule. The 0.5 mg PRC of Lewy et al. shows maximum phase shifts of 1.5 h or less over 4 days, which are smaller than what we observed in our PRC. There are at least two potential reasons for this. First, we used a larger dose of exogenous melatonin. Second, the phase shifts to exogenous melatonin in the Lewy PRC may have been constrained by the fixed sleep schedule that subjects were asked to follow at home: the LD cycle and other 24 h zeitgebers may have opposed or constrained the phase shift to exogenous melatonin.
Lewy et al. previously implied differently shaped PRCs to different doses of exogenous melatonin (Lewy et al. 1998). Specifically, they proposed that the largest phase shifts occur when exogenous melatonin in the circulation overlaps with the endogenous melatonin profile. Thus, the longer the duration of the combined melatonin profile, the larger the phase shift. This concept can help explain the timing of the phase advance peak in our PRC. Results from a previous pharmacokinetic study suggest that our 3 mg melatonin pills were likely in the circulation for about 8 h (DeMuro et al. 2000). Thus, when melatonin is administered around the time of the DLMO or usual start of sleep, it most likely does not increase the duration of melatonin in the circulation. However, when melatonin is administered earlier in the evening, slightly before the DLMO, the duration of melatonin will be extended, perhaps simulating an earlier dusk, and phase advances result. As melatonin is administered earlier and earlier it will extend the duration more and more until a maximum phase advance is obtained at about 5 h before the DLMO. At this point, given our average 11 h interval between the DLMO and DLMOff, the artificially extended melatonin duration will be about 16 h. As the melatonin is administered even earlier, and the profile is extended beyond 16 h, the magnitude of the phase advance decreases, presumably because these earlier doses of exogenous melatonin do not optimally overlap with the endogenous melatonin profile.
The same concept can be applied to aid understanding of the peak time of the phase delay portion of our PRC. The fitted peak of the phase delay portion occurred about 11 h after the DLMO, close to the timing of the DLMOff (and habitual wake time). If exogenous melatonin is administered at this time when endogenous melatonin levels are tapering off, it would produce the optimal overlap, maximally extending the melatonin profile into the morning, perhaps simulating a later dawn and producing phase delays. Thus, as we observed in our PRC, it would be expected that if exogenous melatonin were administered later in time, smaller phase delays would be observed.
This potential explanation of the peak times of our PRC is further strengthened when we compare our PRC to the PRC of Lewy et al.. The largest phase advances to 0.5 mg in the PRC of Lewy et al. occurred 2–3 h before the DLMO (see their Fig. 1, the DLMO = circadian time 14). This would be expected, as the smaller dose of 0.5 mg would have to be administered later in time than a 3 mg dose, in order to overlap with the endogenous melatonin profile. The timing of the peak of the delay portion in the PRC of Lewy et al. is more difficult to determine because of the large scatter of points, and likely occurs 6–11 h after the DLMO. If optimal overlap with the endogenous melatonin profile is indeed important to the size of the resulting phase shift, we would predict that both the 0.5 mg and 3 mg dose would produce maximal phase delays when administered around the time of the DLMOff. Furthermore, as the 3 mg dose would further extend the endogenous melatonin profile, it would produce larger phase delays. In summary, we expect the peak of the advance portions of the melatonin PRCs to vary with the dose and shift earlier as the dose is increased. In contrast, we expect the peak of the delay portion to remain at about the same time.
The dose of melatonin probably influences the shape of the PRC relative to the circadian. Therefore, a specific clock time of administration may be optimal for one dose of melatonin but not for another, and this may explain the apparent dose–response relationship previously reported. For example, in an early study, 0.05 mg, 0.5 mg and 5 mg of exogenous melatonin were administered at 17.00 h, producing phase advances of 0.4, 0.7 and 1.4 h, respectively, in the endogenous melatonin onset (Deacon & Arendt, 1995). As subjects had a DLMO of about 21.00 h, the 17.00 h administration time was probably closer to the optimal time for the large 5 mg dose, but too early for the 0.5 mg dose and earlier still for the 0.05 mg dose. In another study (Sharkey & Eastman, 2002), 3.0 and 0.5 mg doses were both given 7.5 h before bedtime. Our PRC (Fig. 2) shows that this was an ideal time for the 3.0 mg dose, but we expect a later time would have been better for the 0.5 mg dose, as shown by the PRC of Lewy et al. Thus, in both studies the administration of the lower doses of exogenous melatonin at suboptimal times may have produced the apparent dose–response relationship.
Indeed, when we recently tested two doses of exogenous melatonin, combined with bright morning light and a gradually advancing sleep/dark schedule (a phase advancing protocol), we attempted to administer the doses at their respective optimal times. The 3 mg dose was administered on average 4.8 h before the DLMO and the 0.5 mg dose of melatonin an average of 2.4 h before the DLMO. The magnitude of the phase advances with the two doses was similar; no dose–response relationship was observed (Revell et al. 2006). This suggests that the timing but not the amplitude of the phase advance portion of the PRCs differs with dose.
On a final note, the results from studies of free-running blind people suggest that very high doses of exogenous melatonin, such as 10 mg, may actually produce smaller phase advances than lower doses of exogenous melatonin, such as 0.5 mg (Lewy et al. 2002). Lewy et al. explain this result by referring to their 0.5 mg melatonin PRC. They propose that the very high doses of melatonin ‘spillover’ onto the phase delay portion of the 0.5 mg melatonin PRC, thereby reducing the overall phase advance (Lewy et al. 2002). Another explanation is that the 10 mg PRC probably differs in shape from the 0.5 mg PRC and may have little or no phase advance portion, just as in mice long duration light pulses produce PRCs with little or no phase advance portion (Comas et al. 2006).
We believe that the best way to use exogenous melatonin to facilitate circadian phase shifts in sighted humans is to gradually shift the timing of administration concurrently with the timing of sleep. In this way the exogenous melatonin can continue to hit the optimal time on the melatonin PRC as it shifts, and the shift in the LD cycle (produced by the shift in sleep/dark) augments rather than opposes the shift to the exogenous melatonin. This approach can phase shift the clock without causing circadian misalignment between the clock and the sleep/wake schedule and thus avoids the associated jet-lag type symptoms and negative consequences. In previous studies we found that 3 days of advancing sleep 1 h per day in dim light (< 60 lux) produced a phase advance of 0.6 h (Burgess et al. 2003). Adding morning intermittent bright light pulses (> 3000 lux) increased the phase advance to 1.5–1.7 h (Burgess et al. 2003; Revell et al. 2006). Adding afternoon exogenous melatonin to the advancing sleep schedule and morning bright light, increased the phase advance even further to 2.5–2.6 h (Revell et al. 2006). As mentioned previously, there was no difference in the phase advance between the 3 mg and 0.5 mg doses. Thus, we recommend the lower dose of melatonin in order to avoid the soporific effects of higher doses. Therefore, when phase advances are needed, such as for delayed sleep phase type, jet travelling east or early morning shifts, we continue to recommend the combination of a slowly shifting sleep/dark schedule, morning bright light and a low dose of melatonin taken 4–6 h before bedtime.
When a classical Type I PRC, such as to brief light pulses, is generated in free-running laboratory animals, the phase shift between the free-running segments before and after the light pulse is measured (Aschoff, 1965). Thus the free-run itself is essentially subtracted out. When generating our PRC, each individual's phase shift to exogenous melatonin was corrected by subtracting their phase shift due to the free-run during the placebo session. Although the light intensity during the light portions of the ultradian LD cycle should affect the free-running period, the intensity was the same in both the melatonin and placebo sessions. Thus, by subtracting the placebo shift we obtain the shift due to melatonin regardless of light intensity. Even if we assume that the subjects did not free-run during the placebo session, the phase shift during this session represents the appropriate control, revealing phase shifts induced by the laboratory session that should be subtracted from the phase shifts due to exogenous melatonin. As we used each individual's placebo shift during the placebo session to calculate the net shift to exogenous melatonin, our PRC does not have a horizontal line indicating the average phase shift to be used as a corrected x-axis, as do some light PRCs (Khalsa et al. 2003; Kripke et al. 2007). We believe that correcting the PRC for each individual's phase shift is more precise than correcting a PRC for an average phase shift.
One question is whether our PRC meets the definition of a PRC. A PRC is classically defined as being derived by measuring phase shifts from a single exposure to a zeitgeber or stimulus in free-running animals. We did apply the zeitgeber of exogenous melatonin to free-running subjects, but we repeated the dose of exogenous melatonin across three days instead of just one. This was done in part to produce larger, more detectable phase shifts and also because multiple doses of exogenous melatonin are more typically used in the field. Nonetheless, the term ‘PRC’ has more recently been expanded to include graphs generated from the administration of multiple pulses of a zeitgeber (Czeisler et al. 1989; Lewy et al. 1998), from entrained humans (Lewy et al. 1998) and even from re-entrainment protocols (Czeisler et al. 1989; Khalsa et al. 2003). Thus, we believe our protocol fits within this broader definition of a PRC.
This new PRC further defines the physiological effects of exogenous melatonin. It demonstrates that exogenous melatonin can phase delay as well as phase advance the human circadian clock, and shows the optimal time to administer the pill to achieve a desired phase shift. It also demonstrates that using exogenous melatonin as a sleep aid at night has minimal phase shifting effects, but that taking it near the end of the sleep episode may inadvertently phase delay the circadian clock. In future studies we plan to use exactly the same protocol to generate a PRC to 0.5 mg of exogenous melatonin. This will enable the direct comparison of the phase shifting efficacy and optimal administration times of two different doses of exogenous melatonin. We also plan to generate PRCs to bright light using various light boxes, and indeed a preliminary PRC has been presented (Revell & Eastman, 2005). Thus, we will be able to directly compare the phase shifting efficacy of exogenous melatonin to that of bright light.
Acknowledgments
This work was made possible by grants from the National Institutes of Health including R01 NR007677 and R01 HL086934. We thank the following people for their assistance with data collection: Daniel Alderson, Stephanie Crowley, Erin Cullnan, Valerie Ellois, Clifford Gazda, Cynthia Hiltz, Clara Lee, Vanessa Meyer, Thomas Molina, Meredith Rathert, Mark Smith and Christine Tseng. We thank our medical director Keith Callahan, MD. We also thank Ecological Formulas for donating the melatonin and matching placebo pills.
References
- Arendt J, Bojkowski C, Folkard S, Franey C, Marks V, Minors D, Waterhouse J, Wever RA, Wildgruber C, Wright J. Some effects of melatonin and the control of its secretion in humans. In: Evered D, Clark S, editors. Photoperiodism, Melatonin, and the Pineal. London: Pitman; 1985. pp. 266–283. [DOI] [PubMed] [Google Scholar]
- Arendt J, Middleton B, Stone B, Skene D. Complex effects of melatonin: Evidence for photoperiodic responses in humans? Sleep. 1999;22:625–635. doi: 10.1093/sleep/22.5.625. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian Clocks. Amsterdam: North-Holland; 1965. pp. 95–111. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;11:113–125. doi: 10.1177/074873049601100204. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JN, Dahlstrom WG, Graham JR, Tellegen A, Kaemmer B. MMPI-2 (Minnesota Multiphasic Personality Inventory-2): Manual for Administration and Scoring. Minneapolis: University of Minnesota Press; 1989. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chambers JM, Cleveland WS, Kleiner B, Tukey PA. Graphical Methods for Data Analysis. Boston: Duxbury Press; 1983. [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–837. [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–372. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- DeMuro RL, Nafziger AN, Blask DE, Menhinick AM, Bertino JS. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Yuhas K, Jackman A, Johnson K. Melatonin entrains free-running blind individuals with circadian periods less than 24 hours. Sleep. 2006;29:A62. [Google Scholar]
- Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Johnson CH. Phase response curves: What can they tell us about circadian clocks? In: Hiroshige T, Honma K, editors. Circadian Clocks from Cell to Human. Sapporo: Hokkaido University Press; 1992. pp. 209–249. [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol 549. 2003;3:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Krauchi K, Cajochen C, Mori D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol. 1997;272:R1178–R1188. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR. Melatonin entrains free-running blind people according to a physiological dose–response curve. Chronobiol Int. 2005;22:1093–1106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19:649–658. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- Moore RY. The innervation of the mammalian pineal gland. Prog Reprod Biol. 1978;4:1–29. [Google Scholar]
- Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Nagtegaal JE, Kerkhof GA, Smits MG, Swart ACW, Van der meer YG. Delayed sleep phase syndrome: a placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res. 1998;7:135–143. doi: 10.1046/j.1365-2869.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SMW, Dijk DJ, Middleton B, Stone BM, Arendt J. Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-hour production of reproductive hormones. J Clin Endocrinol Metab. 2003;88:4303–4309. doi: 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91:54–59. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. Cincinnati: NIOSH Publication; 1978. pp. 78–154. [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Werth E, Renz C, Muller S, Krauchi K. No evidence for a phase delay in human circadian rhythms after a single morning melatonin administration. J Pineal Res. 2002;32:1–5. doi: 10.1034/j.1600-079x.2002.10808.x. [DOI] [PubMed] [Google Scholar]
- Zaidan R, Geoffriau M, Brun J, Taillard J, Bureau C, Chazot G, Claustrat B. Melatonin is able to influence its secretion in humans: Description of a phase-response curve. Neuroendocrinology. 1994;60:105–112. doi: 10.1159/000126726. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526. 2000;3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]