Abstract
Peripheral nerve inflammation can cause axons conducting through the inflamed site to become mechanically sensitive. Axonal mechanical sensitivity (AMS) of intact axons may explain symptoms in a diverse number of conditions characterized by radiating pain evoked by movements of the affected nerve. Because nerve inflammation also disrupts axoplasmic transport, we hypothesized that the disruption of axoplasmic transport by nerve inflammation could cause the cellular components responsible for mechanical transduction to accumulate and become inserted at the inflamed site, causing AMS. This was tested by examining AMS in C-fibre nociceptors following the application of axoplasmic transport blockers (colchicine and vinblastine) to the sciatic nerve. Both 10 mm colchicine and 0.1 mm vinblastine caused AMS to develop in 30.6% and 33.3% of intact axons, respectively (P < 0.05 compared to sham treatment). Since high doses of colchicine (> 50 mm) can damage axons, and inflammation is involved in the removal of axonal debris, experiments were performed to assess conduction across the treatment site as well as signs of inflammation. Results indicated minimal axonal loss (95% of A- and C-fibres conducting), consistent with the normal microscopic appearance of the colchicine treatment site and absence of ED1-positive (recruited) macrophages. In a separate series of experiments, the block of axoplasmic transport proximal to a localized neuritis significantly reduced inflammation-induced AMS (15.6% compared to 55.6%; P < 0.05), further supporting that the components necessary for AMS are moved by anterograde transport. In summary, nerve inflammation that causes the disruption of axoplasmic transport in patients with painful conditions may result in the accumulation and insertion of mechanosensitive elements at the inflamed site.
Localized peripheral nerve inflammation can cause intact axons passing through the inflamed site to become mechanically sensitive, responding to both pressure and stretch (Eliav et al. 2001; Bove et al. 2003; Dilley et al. 2005; Dilley & Bove, 2007). Such changes in axonal physiology occur following a relatively minor insult to the nerve, contrasting with observations of mechanical sensitivity from the cut tips of axons following peripheral nerve transection or crush (Howe et al. 1977; Scadding, 1981; Michaelis et al. 1995; Tal et al. 1999). The development of mechanical sensitivity along an intact axon following a minor insult is consistent with the pattern of radiating pain in many patients with conditions such as back pain, repetitive strain injuries (non-specific arm pain), compressive neuropathies, and complex regional pain syndrome. In these conditions, symptoms can often be reproduced either by mechanically tensioning or applying direct pressure to the affected nerve. Furthermore, there are rarely signs of an overt nerve injury on standard clinical testing.
Recent studies of nerve inflammation, or neuritis, have shown that both myelinated (A-) and unmyelinated (C-) axons can develop axonal mechanical sensitivity (AMS; Eliav et al. 2001; Bove et al. 2003; Dilley et al. 2005), and that slowly conducting axons innervating deep structures are more susceptible (Bove et al. 2003). However, the mechanisms underlying the development of AMS still remain to be determined. It has been shown that inflammation (Armstrong et al. 2004), and more specifically histamine (Amano et al. 2001), can disrupt axoplasmic transport. Since the components necessary for mechanical transduction are transported by fast axoplasmic transport (Koschorke et al. 1994), we hypothesized that the disruption of axoplasmic transport by localized nerve inflammation could cause these components to accumulate at the inflamed site and become inserted into the axonal membrane.
To determine whether the disruption of axoplasmic transport is sufficient to cause AMS, we have examined the effects of two agents known to block axoplasmic transport (10 mm colchicine and 0.1 mm vinblastine) on the mechanical sensitivity of intact C-fibre axons. Both colchicine and vinblastine are anti-mitotic agents that act by either inhibiting microtubule polymerization or suppressing microtubule dynamics, depending upon their concentration (reviewed in Jordan & Wilson, 2004). At low doses, their topical application can effectively block axoplasmic transport in both myelinated and unmyelinated axons without causing axonal damage (< 10 mm for colchicine: Jackson & Diamond, 1977; Tiedt et al. 1977; Vergara et al. 1993; Kingery et al. 1998; and < 0.15 mm for vinblastine: Fitzgerald et al. 1984; Katoh et al. 1992; Kashiba et al. 1992; Zhuo et al. 1995). At these doses, the local application of colchicine or vinblastine can reduce ongoing activity from a neuroma (Devor & Govrin-Lippmann, 1983) and prevent the recovery of nerve conduction following chronic demyelination (Liverant & Meiri, 1990), suggesting that these agents are effective at inhibiting channel delivery by axoplasmic transport. Colchicine can be neurotoxic at high doses (> 50 mm:Jackson & Diamond, 1977; Liverant & Meiri, 1990; Colburn & DeLeo, 1999), and the inflammatory processes associated with the removal of damaged axons can cause AMS (Dilley & Bove, 2007). To confirm that our methods did not cause axonal damage or inflammation, we also examined the electrical continuity (through-conduction) of axons passing through the treatment site, and evaluated the treated nerves microscopically for signs of pathology.
A further aim of this study was to determine whether the development of AMS following localized nerve inflammation (neuritis) is in fact dependent upon axoplasmic transport. If so, then blocking axoplasmic flow should also block AMS from developing at an inflamed, distal section of the nerve. To test this possibility, nerves were treated with vinblastine, and then complete Freund's adjuvant was applied at a more distal location to induce neuritis. In these experiments, AMS of intact C-fibre axons was assessed at the inflamed site as performed previously (Bove et al. 2003).
This study was designed to test the hypothesis that disrupted axoplasmic transport along intact C-fibre axons is a possible mechanism for the development of AMS. We show that axonal transport blockade using colchicine and vinblastine leads to AMS without causing signs of inflammation or axonal damage. We also show that blocking axoplasmic transport prevents the development of AMS that occurs during nerve inflammation.
Methods
Ethical approval
All experiments were approved by the Animal Care and Use Committee of Beth Israel Deaconess Medical Center, and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. These experiments necessitate survival surgeries. Because immune-mediated inflammation induces AMS (Bove et al. 2003; Dilley et al. 2005; Dilley & Bove, 2007), it was important not to interfere with the immune response in these studies. Therefore, as in previous studies, no post-operative pain medication was provided. Our collective experience with this model does not support substantial pain following this surgery (i.e. the rats ambulate and feed normally within an hour of surgery, and do not lose weight). At the end of each experiment, animals were killed by overdose (5% isoflurane) followed by a thoracotomy. A total of 34 animals were used for this study.
Surgery
The sciatic nerves of adult male Wistar rats (225–250 g; Charles River, USA) were treated with colchicine (n = 12) or vinblastine (n = 9). Animals were anaesthetized and maintained on isoflurane (1.75%) in oxygen. The left sciatic nerve was exposed in the mid-thigh by blunt dissection and a 7–8 mm length was carefully freed from its surrounding connective tissue. A strip of Parafilm (6 mm × 20 mm) was positioned under the nerve to prevent leakage of the agent onto the surrounding tissue. A 5 mm × 5 mm × 10 mm piece of absorbable gelatin sponge (Gelfoam, Pharmacia & UpJohn, USA) saturated in either 10 mm colchicine or 0.1 mm vinblastine (diluted in synthetic interstitial fluid (SIF); Bretag, 1969) was wrapped around the nerve (Fig. 1A). The concentrations used were determined from previous studies (refer to Introduction) as well as from our preliminary experiments that confirmed axonal toxicity at higher doses, indicated by conduction block through the treatment site. After 15 min, the Gelfoam and Parafilm were removed and the nerve was rinsed with SIF. The location of the treatment site was carefully noted. The muscle and skin were closed using 4/0 monofilament sutures (Webster Veterinary, USA). In six ‘sham’ animals, the same surgical procedure was followed, except that the nerve was exposed to SIF alone. In one colchicine-treated animal, a sham surgery consisting of the identical procedure without the colchicine or vinblastine was also performed on the contralateral side. This animal was used for immunohistochemistry.
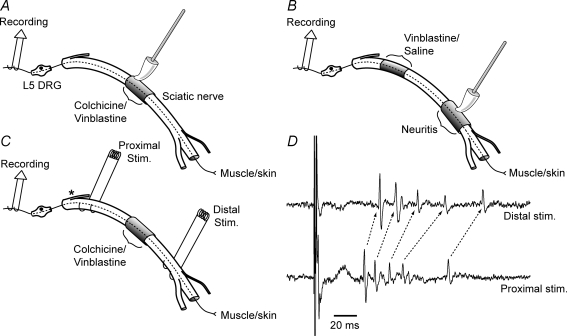
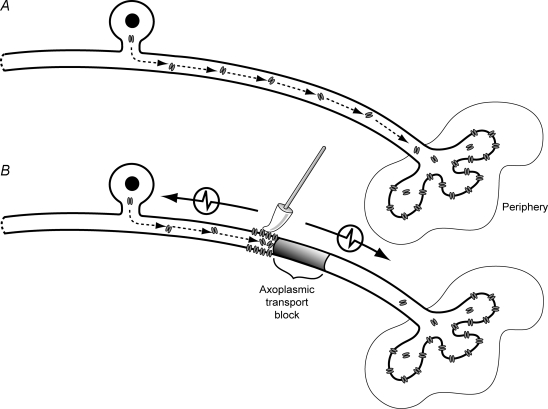
Figure 1. Schematic diagram of methods.
A–C represent the experimental setups for examining the effects of local colchicine and vinblastine treatment on the development of axonal mechanical sensitivity (AMS). Each diagram shows the sciatic nerve in the thigh, with the branch to the hamstrings (near the sciatic notch; asterisk in C), and the trifurcation of the nerve (behind the knee). The recording site at the L5 dorsal root is also shown, along with the probe used for AMS testing. A single intact C-fibre axon is drawn in each of the figures passing from the L5 dorsal root to the periphery (continuous/dashed line). A, colchicine or vinblastine was applied to the nerve and AMS tested using a soft probe. B, vinblastine was applied to the proximal sciatic nerve and then a CFA-neuritis was induced distally. AMS was tested at the neuritis site. C, stimulating electrodes were positioned on both sides of the treatment site at the sciatic notch and close to the trifurcation of the sciatic nerve to assess through-conduction. The asterisk shows the close proximity of the proximal stimulating electrodes to the branch to the hamstrings. D, recording from a filament stimulated at the proximal and distal sites. This particular filament had five C-fibre axons that conducted through the treatment site. Note the shift in latency between stimulating sites, the identical waveforms indicated by arrows.
In an additional group of animals, a 7–8 mm segment of nerve was dissected in the proximal thigh, and exposed for 15 min to either 0.1 mm vinblastine (n = 4) or SIF alone (n = 3), as described above. Care was taken not to disturb the connective tissue surrounding the more distal sciatic nerve segment, thereby preventing contact with the vinblastine. Following thorough washing with SIF, a further 7–8 mm length of the nerve was dissected distally, and freed of its surrounding tissue (Fig. 1B). Inflammation (neuritis) of this more distal segment was induced as previously described (Bove et al. 2003; Dilley & Bove, 2007). A 5 mm × 5 mm × 10 mm piece of Gelfoam saturated in complete Freund's adjuvant (CFA; approximately 150 μl; diluted 50% with SIF) was wrapped around the nerve. The subcutaneous and skin incisions were closed using 4/0 sutures. In a single animal used for immunohistochemistry, neuritis was induced along the sciatic nerve at the mid thigh.
Electrophysiology
To evaluate the effect of axoplasmic flow blockade on AMS, animals were electrophysiologically examined on days post surgery at which colchicine and vinblastine are considered to have their maximum effect (Krikorian et al. 1980; Fitzgerald et al. 1984; Yamamoto & Yaksh, 1993). Single unit recordings from intact C-fibre axons with identified receptive fields were performed on animals 3–8 days following colchicine treatment (n = 7), 3–5 days following vinblastine treatment (n = 6), and in sham animals 3–7 days following treatment with SIF alone (n = 5). Similar recordings were also performed on animals with neuritis (3–4 days post surgery) following treatment of the sciatic nerve at a more proximal location with either vinblastine (n = 4) or SIF (n = 3; refer to Fig. 1A–C for diagrams). To perform the recordings, animals were anaesthetized and maintained on isoflurane (1.75%) in oxygen. After tracheal cannulation, rats were ventilated (1 cm3 at 40–50 strokes min−1) for the duration of the experiment. The body temperature was monitored by a rectal thermistor probe and maintained at 37°C using a heated pad (FHC, USA). The heart rate and end-tidal CO2 levels were also monitored throughout the experiment, and were maintained by anaesthesia depth at 350–450 beats min−1 and 4–5%, respectively. A lumbar laminectomy was performed from L2 to L5 to expose the contents of the spinal canal. The surrounding skin was stitched to a metal ring to form a pool that was filled with mineral oil. The dura mater was opened and the left L5 dorsal root was cut close to the dorsal root entry zone. The cut end of the root was positioned on a small black glass platform (10 mm × 6 mm) for recording. Bipolar stimulating electrodes were placed under the dorsal root, distal to the platform.
To allow access for mechanical stimuli, the sciatic nerves were re-exposed in the thigh, at the treatment site. In neuritis experiments, the sciatic nerve was dissected only at the site of the neuritis (not at the vinblastine or SIF treatment site) and the Gelfoam carefully removed. A plastic platform (9 mm × 5 mm), notched to accommodate the nerve, was positioned under the nerve with the treatment or neuritis site in its centre. To prevent the exposed tissue from drying out between mechanosensitivity testing, it was temporarily covered with gauze saturated in SIF.
Recordings were made from fine filaments (6–10 μm) that were teased from the cut end of the dorsal root, using finely sharpened forceps. Filaments were split until single action potentials could be evoked using electrical stimulation of the dorsal root (square wave pulses: 0.5–0.9 ms duration and 30–40 V amplitude). Only filaments with clearly identifiable waveforms were studied. The latency of the action potential and the conduction distance were used to calculate the conduction velocity of the axon. Conduction velocities below 1.5 m s−1 were considered C-fibres (Perl & Burgess, 1973).
Receptive fields for isolated neurons were searched below the knee using mechanical stimuli. Most receptive fields were located by squeezing the periphery, using either fingers or forceps, or by probing the skin with blunt forceps. The loose property of the skin was exploited to carefully discriminate cutaneous versus deep fields (Bove et al. 2003). Cutaneous neurons had receptive fields that remained associated with the skin regardless of the skin excursion. In contrast, deep neurons were identified by moving the skin and repeating the effective stimulus to the same underlying spot. Neurons were only included for further study if they had mechanically sensitive receptive fields in the lower limb located distal to the exposed part of the sciatic nerve, indicating that their axons passed through the treatment site. Since AMS develops predominantly in neurons with deep receptive fields (Bove et al. 2003) and following vinblastine treatment (see Results), only those neurons with deep receptive fields were examined in the neuritis experiments.
After determining the location of the receptive field, we confirmed that the electrically and mechanically evoked responses were from the same neuron by stimulating the receptive field mechanically whilst stimulating the dorsal root electrically. If the electrical stimulus occurred during the relative refractory period of the neuron to mechanical stimuli, the action potential was not initiated or was delayed (see Bove et al. 2003).
Since the axons of all L5 dorsal root neurons innervating the distal lower limb pass through the sciatic nerve in the mid thigh, i.e. through the inflamed site, it was not considered necessary to use electrical stimulation distal to the neuritis as previously described (Bove et al. 2003). If, however, there was any uncertainty as to whether an axon passed through the treatment site, for example, if the receptive field of a neuron was close to the knee, bipolar stimulating electrodes were positioned distal to the treatment site and the neuron was again identified electrically and compared to the evoked potentials from both dorsal root stimuli and mechanical stimulation.
Ongoing activity was not focused upon in this study, since there is no way to find deep receptive fields without using damaging stimuli, especially to more superficial structures. This causes frank inflammation evidenced by swelling, and almost certainly sensitizes nociceptors (see Bove & Light, 1995). There is some evidence for ongoing activity following neuritis although this evidence is conflicting (Bove et al. 2003; Dilley et al. 2005; Dilley & Bove, 2007).
All data were collected in raw format at 20 kHz for offline analysis using Spike 2 software (Cambridge Electronic Designs, UK).
Mechanosensitivity testing at the treatment site
Mechanical stimulation of the nerve was tested manually as previously described (Bove et al. 2003). It was critical to avoid axonal damage, and therefore the mechanical stimulus was applied using a soft silicone tapered probe, which provides a more gentle and controllable pressure than a rigid probe. When applied to an electronic scale, this probe delivered forces up to 40 cN, although in these experiments, forces at or below 20 cN were sufficient to activate mechanically sensitive axons (Dilley & Bove, 2007). The mechanical stimulus was applied successively along the length of sciatic nerve that was supported by the platform (i.e. at the treatment/neuritis site) and adjacent areas. In the neuritis experiments, the nerve was only mechanically tested along the distal length of nerve at the neuritis site, and not along the segment treated with either vinblastine or SIF. During testing of adjacent areas beyond the length of the platform, the back of the nerve was carefully supported using a 5 mm wide flat metal spatula. The duration of each mechanical stimulus was 1–2 s. If one or more action potentials were initiated during this period, the axon was considered to be mechanically sensitive. Using the probe in this manner does not interrupt the conduction of action potentials through the probed nerve site, which was confirmed by stimulation of the distal receptive field after probing the nerve. A continued response from the receptive field indicated through-conduction and therefore a lack of axonal damage.
Conduction across the treatment site
If colchicine or vinblastine damaged axons, conduction through the treatment site would be impaired. To assess this possibility, we examined through-conduction of both unmyelinated and myelinated axons across the treatment site, 3–8 days following colchicine (n = 4) and 3–5 days following vinblastine (n = 4) treatment. Recordings were performed at the dorsal root, as described above. In the thigh, the sciatic nerve was dissected from the sciatic notch to the trifurcation at the knee. The surrounding thigh skin was stitched to a metal ring to form a pool that was filled with mineral oil. Bipolar stimulating electrodes were positioned both proximal (close to the sciatic notch at the pelvis) and distal to the treatment site (close to the trifurcation of the sciatic nerve, see Fig. 1C). The peroneal and tibial branches of the sciatic nerve were crushed to prevent muscular movement of the limb during electrical stimulation. Recordings were made from L5 dorsal root filaments as previously described, except that multi-fibre filaments were examined (1–10 action potentials per filament). The long conduction distance (50–60 mm from the proximal stimulating site) allowed good separation of action potentials that were evoked by electrical stimulation. Both C-fibre and A-fibre (myelinated) axons were identified by their conduction velocities. Conduction velocities above 1.5 m s−1 were considered A-fibres. Electrical stimulation necessary to evoke A-fibres was 1–10 V amplitude and 0.5 ms, compared to 30–90 V amplitude and 0.5–0.9 ms duration for C-fibres. The stimulus intensity was increased until the maximum number of action potentials could be identified for each filament. This was repeated in response to distal stimulation, and the percentage of through-conducting axons was calculated. The conduction velocity across the treatment site (from the distal to proximal stimulating electrodes) was determined from the latency and conduction distance for all action potentials that could be identified (by both conduction velocity and shape) on both proximal and distal stimulation, and compared to the conduction velocity along the proximal nerve segment (from the proximal stimulating electrodes to the recording electrodes) (Fig. 1D). Peripheral receptive fields were not determined in these experiments.
Histology
The colchicine-treated nerve segment and the equivalent contralateral nerve segment were removed from the animals used in the conduction experiments (n = 4) for histological processing. The tissue was fixed overnight by submersion (4% paraformaldehyde in 0.1 m phosphate-buffered saline) and stored in phosphate buffer. To visualize myelin sheaths, nerves were osmicated, dehydrated, and embedded in epoxy resin. Transverse semi-thin sections (1 μm) were cut and stained with toluidine blue. Sections were viewed under a light microscope (Nikon, Japan) and photographed using a SPOT digital camera (USA). The nerve sections were examined for signs of pathology, such as axonal degeneration, demyelination, cellular infiltration, and oedema.
Immunohistochemistry
At 4 days post surgery, a colchicine-treated animal (that was also treated with SIF on the contralateral side) and a vinblastine-treated animal were examined for signs of inflammation using an antibody to ED1, which labels recruited macrophages. As a positive control, a 4 day CFA-neuritis animal was also examined. The animals were anaesthetized with an overdose of sodium pentobarbital (200 mg kg−1, i.p.) and perfused transcardially with heparinized 0.1 m phosphate-buffered saline (pH 7.4). Nerve segments from the colchicine, SIF, and neuritis treatment sites, and an untreated segment of nerve from a more proximal location on the contralateral side of the colchicine-treated animal, were removed and aligned in embedding medium, and flash frozen in dry-ice-chilled 2-methylbutane. Cross-sections were cut at 8 μm with a cryostat, thaw-mounted on slides, and fixed with 4% paraformaldehyde in 0.1% phosphate-buffered saline for 7 min. Slides were dried for 1 h, rinsed with dH2O for 10 min, and processed for immunohistochemistry. The primary anti-ED1 (Serotec, USA) was used at 1 : 250. A goat anti-mouse secondary antibody was used at 1 : 500 (Jackson Laboratories, USA). Product visualization was achieved with the ABC Elite Kit (Vector Laboratories, USA), followed by DAB (Vector Laboratories, USA). Immunohistochemical controls were performed using no primary antibody. Sections were rinsed in distilled water for 10 min, counterstained with haematoxylin and eosin (Thermo-Shandon, USA), dehydrated with ascending concentrations of alcohol, cleared with xylene, and coverslipped with DPX (Biochemika, Switzerland). Slides were viewed and photographed using a Nikon microscope fitted with a SPOT digital camera.
Statistical analysis
All comparisons of proportions were made using multiple χ2 tests with Yates correction for continuity or, when expected frequencies were less than 5, Fisher's exact tests. Paired comparisons were against the sham group unless stated. Conduction velocity data were not normally distributed (determined by Kolmogorov–Smirnov test) and therefore comparisons of the conduction velocity across the treatment site with the proximal nerve segment were made using Wilcoxon signed rank test for paired samples.
Results
Colchicine and vinblastine induce axonal mechanical sensitivity
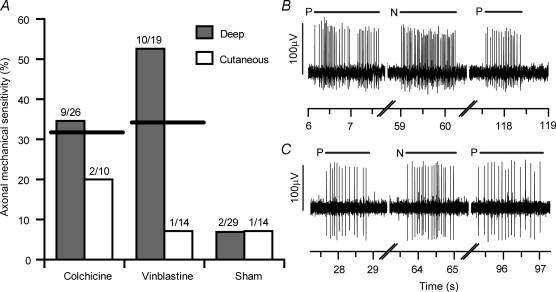
A total of 112 neurons with C-fibres (conduction velocity < 1.5 m s−1) and high threshold mechanically sensitive receptive fields in the lower limb were examined in 18 experiments. None of the neurons responded to light touch at their receptive field, suggesting that most were probably nociceptors. Forty-three neurons that were intact across the treatment site (i.e. they had distal receptive fields) were studied in sham-operated animals 3–7 days following surgery. Twenty-nine innervated deep structures and 14 were cutaneous. Axonal mechanical sensitivity was observed at the treatment site in 7.0% (3/43) of these neurons (Fig. 2A). Following treatment of the sciatic nerve with either colchicine or vinblastine, there was a substantial increase in the number of intact neurons with AMS. Of the 36 neurons that were examined 3–8 days following a 15 min exposure to 10 mm colchicine, 30.6% (11/36) developed AMS (P = 0.01 compared to axons in the sham group, χ2 test; Fig. 2A and B). Similarly, of the 33 neurons studied 3–5 days following a 15 min exposure to 0.1 mm vinblastine, 33.3% (11/33) developed AMS (P = 0.008, χ2 test; Fig. 2A and C). The majority of axonal responses occurred from mechanical stimulation of small ‘hotspots’, 59% (13/22) of which were clearly localized 1–2 mm proximal to the treatment site. The remaining 41% were localized to the lesion site. There were no hotspots located distal to the treatment site. All of the axonal responses were repeatable and there was no sustained discharge following removal of the mechanical stimulus, indicating that the responses were not due to overt axonal damage (Tal et al. 1999). Stimulation of the distal receptive fields following testing at the treatment site confirmed that the axons remained in continuity.
Figure 2. Axonal mechanical sensitivity following blockade of axoplasmic transport.
A, proportion of AMS in C-fibre axons following 10 mm colchicine treatment, 0.1 mm vinblastine treatment, and in sham-operated animals. The thick horizontal lines indicate the combined percentage of axons with mechanical sensitivity for cutaneous and deep-innervating neurons. The total number of neurons sampled and the number responding to mechanical stimulation at the test site are shown for each group. B and C are representative responses to mechanical stimuli of the axons of neurons with deep receptive fields following colchicine treatment (B) and vinblastine treatment (C). Short horizontal lines above the traces represent the duration of the mechanical stimuli. For each neuron, the peripheral receptive field was mechanically stimulated initially (P). This was followed by mechanical stimulation of the nerve at the test site (N). Mechanical responses from the periphery after stimulating the nerve demonstrated that the mechanical stimulus did not adversely affect the conduction of the axon.
There was a significantly larger proportion of neurons with deep receptive fields that developed AMS following vinblastine treatment (52.6% (10/19)) compared to those neurons with cutaneous receptive fields (7.1% (1/14); P = 0.009; Fisher's Exact test; Fig. 2A). This was not observed following colchicine treatment (34.6% (9/26) of neurons with deep receptive fields compared to 20.0% (2/10) with cutaneous receptive fields; P = 0.46; Fisher Exact test).
In the colchicine group, there was no significant difference in the proportion of neurons that developed AMS in animals examined early (3–5 days; n = 4/8) or late (6–8 days; n = 7/28) following exposure (P = 0.21; χ2 test).
Conduction through the treatment site
Axonal conduction through the treatment site was examined following colchicine and vinblastine exposure. Animals were examined at similar time points to AMS experiments (colchicine group: 3–8 days post treatment; vinblastine group: 3–5 days). In the colchicine group, 95.3% (102/107) of C-fibre axons and 94.2% (113/120) of A-fibre axons conducted through the treatment site (Table 1). In the vinblastine group, 95.3% (102/107) of C-fibre axons and 94.9% (74/78) of A-fibre axons conducted through the treatment site.
Table 1.
Summary of the conduction data
| Median conduction velocity (m s−1) | ||||
|---|---|---|---|---|
| Percentage conduction (n) | Proximal (IQR) | Treatment site (IQR) | ||
| Colchicine | C | 95.3% | 0.72 | 0.64* |
| (102/107) | (0.63–0.85) | (0.49–0.87) | ||
| A | 94.2% | 26.8 | 27.3 | |
| (113/120) | (19.5–33.7) | (19.5–36.8) | ||
| Vinblastine | C | 95.3% | 0.79 | 0.74* |
| (102/107) | (0.67–0.92) | (0.52–0.86) | ||
| A | 94.9% | 32.0 | 26.5* | |
| (74/78) | (22.9–36.7) | (21.8–34.6) | ||
Percentage conduction represents the percentage of C- and A-fibre axons conducting through the treatment site. Note that following treatment with either colchicine or vinblastine, the majority of axons conducted through the treatment site. The median conduction velocity is shown for the proximal nerve segment (between the proximal stimulating electrodes and the recording electrodes) and across the treatment site. There was a significant reduction in C-fibre conduction velocity through the colchicine and vinblastine treatment site and also a significant reduction in A-fibre conduction velocity through the vinblastine treatment site.
P < 0.05 compared to proximal conduction velocity (Wilcoxon signed rank test for paired samples). IQR, interquartile range.
The conduction velocity through the treatment site was compared to the conduction velocity along the proximal nerve segment (from the proximal stimulating electrodes to the recording electrodes) for each axon (Table 1). Following vinblastine treatment, there was a median reduction of 10.2% in C-fibre (n = 98) and 11.8% in A-fibre (n = 62) conduction velocity across the treatment site, differences that were both significant (P < 0.001; Wilcoxon signed rank test for paired samples). Following colchicine treatment, there was a median reduction of 14.0% in C-fibre conduction velocity across the treatment site (n = 96), a difference that was also significant (P < 0.0001). There was no significant change in A-fibre conduction velocity across the treatment site (P = 0.19; n = 92). Similar changes in conduction velocity across the treatment site have been observed in unmyelinated axons following neuritis (Dilley et al. 2005). Therefore, the decrease in conduction velocity in both the colchicine and vinblastine groups may be due to minor inflammation induced during surgical exposure of the sciatic nerve. Since inflammation disrupts axoplasmic transport (Amano et al. 2001; Armstrong et al. 2004), the mechanisms are likely to be identical.
Histology
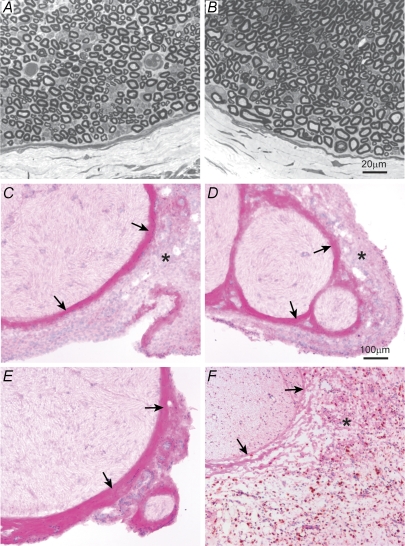
In the colchicine-treated animals (n = 4), the light microscopic appearance of the treatment site was examined following electrophysiological assessment. Consistent with the electrophysiological findings, myelinated profiles appeared normal with no obvious signs of Wallerian degeneration or demyelination. Apart from some signs of oedema, each treated nerve was normal in appearance (Fig. 3A and B). The only difference compared to the contralateral side was the slight swelling that was attributed to the fact that these nerves were used in the conduction experiments and had been dissected free of connective tissue and suspended over stimulating electrodes for 3–4 h prior to harvest. Swelling was not apparent in nerves used for immunohistochemical staining.
Figure 3. Microscopic appearance of the treatment site.
Toluidine blue-stained sections of the colchicine treatment site at 7 days (A) and a similar nerve segment on the contralateral side (B) reveal no abnormalities other than signs of very mild swelling in the colchicine-treated side. C–F are haematoxylin and eosin-stained sections immuno-reacted against an ED1 macrophage antibody 4 days following colchicine treatment (C), synthetic interstitial fluid (SIF) treatment (D), in an untreated nerve (E) and 4 days following neuritis induction using CFA (F). Arrows mark the perineurium and in E, the epi-perineurium. Asterisks mark the epineurium. There was no ED1-positive immunoreactivity in the colchicine- or SIF-treated, or in untreated nerves. Following neuritis, there was considerable ED1-positive staining in the epineurium (dark stain) indicating significant inflammation. Note the apparent thinning (or breaking up) of the perineurium following neuritis (arrows in F) and also the substantial thickening and swelling of the epineurium. Following colchicine and SIF treatment, the epineurium appears thicker than in the untreated nerve. This thickening is the fibrotic tissue associated with healing of the nerve following its surgical isolation during treatment. The scale bar in B applies to panels A and B and the scale bar in D applies to panels C–F.
Immunohistochemistry
There was no ED1-positive macrophage staining at the treatment site 4 days following colchicine (n = 1) or vinblastine treatment (n = 1) or in SIF-treated or untreated nerve (Fig. 3C–E). In contrast, there was substantial ED1-positive staining in the epineurium at the treatment site 4 days following neuritis (Fig. 3F).
Effects of axoplasmic transport block on neuritis-induced AMS
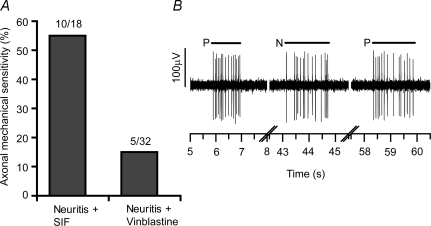
A total of 50 neurons were examined in seven experiments 3–4 days following vinblastine (n = 4) or SIF treatment (n = 3) combined with the induction of a localized neuritis at a more distal location along the sciatic nerve. In the SIF-neuritis group, 18 neurons with deep receptive fields were studied, of which 55.6% (10/18) developed AMS at the neuritis (Fig. 4A and B). In the vinblastine-neuritis group, 32 neurons with deep receptive fields were studied, and only 15.6% (5/32) of these developed AMS at the neuritis, a significant decrease compared to the SIF group (P = 0.008; χ2 test; Fig. 4A).
Figure 4. The effects of vinblastine treatment on inflammation-induced axonal mechanical sensitivity.
A, proportion of deep C-fibre axons responding to mechanical stimulation at the CFA-neuritis site following proximal treatment of the sciatic nerve with either SIF (control) or 0.1 mm vinblastine (also see Fig. 1B). B, representative response to mechanical stimuli of an axon of a deep neuron following SIF treatment proximal to the neuritis. Short horizontal lines above the responses represent the duration of the mechanical stimuli (P and N: peripheral and nerve stimuli, respectively).
Discussion
Axonal mechanical sensitivity following the application of axoplasmic transport blockers
To determine whether the development of AMS was associated with the disruption of axoplasmic transport, the effects of two agents known to effectively block axoplasmic transport (colchicine and vinblastine) were tested. The results show that the transient local application of either of these agents to the sciatic nerve induces AMS in C-fibre axons, supporting the hypothesis that inflammation-induced AMS may be due to the accumulation and insertion of the components responsible for mechano-electrical transduction into an otherwise intact, conducting, axon. These findings expand on previous studies that have examined inflammation-induced AMS (Eliav et al. 2001; Bove et al. 2003; Dilley et al. 2005; Dilley & Bove, 2007), by introducing a possible mechanism for the development of such physiological changes.
A number of strategies were used to confirm that the doses of both colchicine and vinblastine used in the present study did not damage axons at the treatment site or induce inflammation, which can cause AMS (Dilley & Bove, 2007). Conduction through the treatment site following either colchicine or vinblastine exposure was examined to assess axonal continuity. In these experiments, there was only a 5% conduction failure of both unmyelinated and myelinated axons across the treatment site. This small percentage was probably due to stimulation of axons innervating the hamstrings and other proximal muscles in the hind limb that passed through a branch of the sciatic nerve located close to the proximal stimulating electrode and not the treatment site (refer to Fig. 1C). Histological assessment of the treatment site following colchicine exposure did not reveal any signs of pathology, such as axonal degeneration. Consistent with these findings, immunohistochemical staining for ED1-positive macrophages on day 4 following colchicine or vinblastine treatment was comparable to both SIF-treated and untreated control nerves, indicating the absence of inflammation. This was in contrast to the abundance of ED1-positive cells on day 4 following treatment with CFA, which induced a robust inflammation. The lack of an inflammatory response following colchicine treatment is consistent with the anti-inflammatory properties of the agent (see below).
In previous studies examining the neuritis model, AMS was confined to small localized hotspots at the treatment site (Eliav et al. 2001; Bove et al. 2003; Dilley et al. 2005). There was a similar pattern of AMS following treatment with either colchicine or vinblastine, i.e. discrete hotspots, except that the majority of these hotspots were 1–2 mm proximal to the treatment site. As in the neuritis experiments, the hotspots were never distal to the treatment site.
In the present study, there was no measure of the extent of axoplasmic transport blockade by colchicine or vinblastine. The methodology and doses were similar to those used in previous studies (e.g. Jackson & Diamond, 1977; Tiedt et al. 1977; Fitzgerald et al. 1984; Katoh et al. 1992; Vergara et al. 1993; Kashiba et al. 1992; Zhuo et al. 1995; Kingery et al. 1998) and therefore, although not measured, it was expected that the transient exposure of these agents to the sciatic nerve for 15 min would have caused some degree of axoplasmic transport disruption. A greater concern with using these agents, in particular colchicine, is that at higher doses they are neurotoxic, causing axonal loss and even death (Jackson & Diamond, 1977; Liverant & Meiri, 1990; Colburn & DeLeo, 1999). However, the conduction experiments and histological assessment of the treatment site ruled out any neurotoxic effect.
Axoplasmic transport in inflammation-induced AMS
An additional series of experiments were performed to establish whether the development of AMS following neuritis was in fact dependent upon axoplasmic transport. In these experiments, there was a significant decrease (71.9%) in the proportion of C-fibre axons with deep receptive fields that developed AMS at the neuritis site following proximal blockade of axoplasmic transport compared to the sham group. This finding suggests that the components, or factors, necessary for AMS are transported along the axon, consistent with the disruption of axoplasmic transport as a possible mechanism for the development of AMS. Only C-fibre axons with deep receptive fields were examined in these experiments, since axons with cutaneous receptive fields are less likely to develop AMS (Bove et al. 2003).
Colchicine and possibly vinblastine can suppress inflammation by altering microtubule assembly, which affects the migration of neutrophils and causes the down-regulation of certain cell surface proteins (reviewed in Cronstein & Terkeltaub, 2006). In the present experiments, the anti-inflammatory effects of vinblastine must be considered as a possible explanation for the reduction in AMS at the CFA-neuritis site. However, vinblastine treatment was localized to a short proximal nerve segment only, and extreme care was taken not to disturb the connective tissue surrounding the adjacent nerve segments, thus minimizing agent contact at the neuritis site. Furthermore, the nerve and surrounding tissue were washed prior to neuritis induction. On dissection of the neuritis site during preparation for electrophysiological testing, the surface of the nerve appeared inflamed at the neuritis site (i.e. well vascularized), similar to the appearance of the nerve in other CFA-neuritis experiments (Bove et al. 2003; Dilley et al. 2005).
Disrupted axoplasmic transport as a mechanism for AMS
The present study demonstrated the development of AMS in C-fibre axons following the disruption of axoplasmic transport without inflammation. We hypothesize that AMS was caused by the accumulation and insertion of mechanically sensitive ion channels at the site of blockade (Fig. 5). Both colchicine and vinblastine rapidly disrupt microtubules, which in axons results in the inhibition of axoplasmic transport. By 24 h, transported materials accumulate proximal to the site of blockade (Krikorian et al. 1980; Gamse et al. 1982; Fitzgerald et al. 1984), most likely including the accumulation of ion channels or their components, which are known to be conveyed along the axon by fast axoplasmic transport (Koschorke et al. 1994). One mechanically sensitive channel that may contribute to AMS is the vanilloid receptor subtype 1 (VR1). Carrageenan-induced peripheral inflammation was shown to increase the axoplasmic transport of mRNA for VR1 into the periphery (Tohda et al. 2001). Following neuritis, increased nerve growth factor at the inflamed site (Obata et al. 2002) might aid in both the up-regulation and insertion of mechanically sensitive channels into the axon membrane (Di Castro et al. 2006). Alternatively, AMS might not be due to a mechanosensitive channel that is transported along the axon but instead a neurotrophic factor or enzyme that accumulates at the site of axoplasmic transport block. Correspondingly, certain neurotrophic factors are known to increase the excitability of sensory nerve terminals within the periphery (reviewed in McMahon et al. 2005). The reduction of inflammation-induced AMS by proximal treatment of the nerve trunk with vinblastine further confirmed a role for axoplasmic transport in the development of mechanical sensitivity. This result suggests that an essential component necessary for the development of AMS is conveyed by anterograde transport from the cell body.
Figure 5. Hypothesis for axonal mechanical sensitivity due to the blockade of axoplasmic transport.
A, components required for mechanical sensitivity are transported from the cell body of a single C-fibre neuron to the periphery for insertion at the terminals. B, blocking axoplasmic transport leads to the accumulation and insertion of mechanosensitive components proximal to the site of axoplasmic blockade. Mechanical stimulation of the axon membrane therefore becomes an effective stimulus to generate action potentials that pass in both directions along the axon (denoted by arrows).
Clinical relevance
Axonal mechanical sensitivity of intact peripheral nerve fibres is a likely cause of radiating pain that often accompanies conditions such as back pain, repetitive strain injury, compressive neuropathies, and complex regional pain syndrome. In these patients, symptoms are often triggered by movements that tension or move (i.e. mechanically stimulate) the affected peripheral nerve. Symptoms often persist in the absence of a clinically detectable peripheral nerve injury. We hypothesize that many of these patients have a more minor inflammatory insult sufficient to cause the disruption of axoplasmic transport, leading to AMS.
Patients with radiating pain often describe a ‘deep ache’ as one of their major symptoms (Bove et al. 2005). This observation is consistent with a bias towards the development of AMS in neurons with deep receptive fields following colchicine and vinblastine treatment, a bias that was also observed in our previous studies of AMS during focal neuritis (Bove et al. 2003).
The results from this study are also relevant to the treatment of cancer. Many of the anti-cancer drugs target microtubules, since microtubules are important in the processes of mitosis (Jordan & Wilson, 2004). Although colchicine has been relatively unsuccessful in anti-cancer trials, vinblastine and other Vinca alkaloids are used in the treatment of some neoplastic diseases, such as Hodgkin's and non-Hodgkin's lymphoma, and lymphoblastic leukaemia (Duflos et al. 2002). A major complication with the use of these drugs is the development of painful peripheral neuropathies (Gidding et al. 1999; Topp et al. 2000). Since it is the C-fibre neurons innervating deep structures that are more susceptible to the effects of vinblastine, this subgroup of axons may play a significant role in symptom production in cancer patients with neuropathy following treatment with the Vinca alkaloids.
Acknowledgments
Financial support was provided by National Institutes of Health Grant 5R01AR048925 to G. M. Bove, through the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Center for Complementary and Alternative Medicine. The authors also thank Dr Peter Grigg for reading drafts of this manuscript.
References
- Amano R, Hiruma H, Nishida S, Kawakami T, Shimizu K. Inhibitory effect of histamine on axonal transport in cultured mouse dorsal root ganglion neurons. Neurosci Res. 2001;41:201–206. doi: 10.1016/s0168-0102(01)00275-9. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Lee M, Chhith S, Gomariz RP, Waschek JA. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004;129:93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Bove GM, Light AR. Unmyelinated nociceptors of rat paraspinal tissues. J Neurophysiol. 1995;73:1752–1762. doi: 10.1152/jn.1995.73.5.1752. [DOI] [PubMed] [Google Scholar]
- Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol. 2003;90:1949–1955. doi: 10.1152/jn.00175.2003. [DOI] [PubMed] [Google Scholar]
- Bove GM, Zaheen A, Bajwa ZH. Subjective nature of lower limb radicular pain. J Manipulative Physiol Ther. 2005;28:12–14. doi: 10.1016/j.jmpt.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Bretag AH. Synthetic interstitial fluid for isolated mammalian tissue. Life Sci. 1969;8:319–329. doi: 10.1016/0024-3205(69)90283-5. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA. The effect of perineural colchicine on nerve injury-induced spinal glial activation and neuropathic pain behavior. Brain Res Bull. 1999;49:419–427. doi: 10.1016/s0361-9230(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(Suppl. 1):S3. doi: 10.1186/ar1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Govrin-Lippmann R. Axoplasmic transport block reduces ectopic impulse generation in injured peripheral nerves. Pain. 1983;16:73–85. doi: 10.1016/0304-3959(83)90087-8. [DOI] [PubMed] [Google Scholar]
- Di Castro A, Drew LJ, Wood JN, Cesare P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A. 2006;103:4699–4704. doi: 10.1073/pnas.0508005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley A, Bove GM. Resolution of inflammation induced axonal mechanical sensitivity and conduction slowing in C-fiber nociceptors. J Pain. 2007 doi: 10.1016/j.jpain.2007.10.012. in press. [DOI] [PubMed] [Google Scholar]
- Dilley A, Lynn B, Pang SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain. 2005;117:462–472. doi: 10.1016/j.pain.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflos A, Kruczynski A, Barret JM. Novel aspects of natural and modified Vinca alkaloids. Curr Med Chem Anticancer Agents. 2002;2:55–70. doi: 10.2174/1568011023354452. [DOI] [PubMed] [Google Scholar]
- Eliav E, Benoliel R, Tal M. Inflammation with no axonal damage of the rat saphenous nerve trunk induces ectopic discharge and mechanosensitivity in myelinated axons. Neurosci Lett. 2001;311:49–52. doi: 10.1016/s0304-3940(01)02143-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Woolf CJ, Gibson SJ, Mallaburn PS. Alterations in the structure, function, and chemistry of C fibers following local application of vinblastine to the sciatic nerve of the rat. J Neurosci. 1984;4:430–441. doi: 10.1523/JNEUROSCI.04-02-00430.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R, Petsche U, Lembeck F, Jancso G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982;239:447–462. doi: 10.1016/0006-8993(82)90521-2. [DOI] [PubMed] [Google Scholar]
- Gidding CE, Kellie SJ, Kamps WA, de Graaf SS. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29:267–287. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25–41. doi: 10.1016/0304-3959(77)90033-1. [DOI] [PubMed] [Google Scholar]
- Jackson P, Diamond J. Colchicine block of cholinesterase transport in rabbit sensory nerves without interference with the long-term viability of the axons. Brain Res. 1977;130:579–584. doi: 10.1016/0006-8993(77)90121-4. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Senba E, Kawai Y, Ueda Y, Tohyama M. Axonal blockade induces the expression of vasoactive intestinal polypeptide and galanin in rat dorsal root ganglion neurons. Brain Res. 1992;577:19–28. doi: 10.1016/0006-8993(92)90532-e. [DOI] [PubMed] [Google Scholar]
- Katoh K, Tohyama M, Noguchi K, Senba E. Axonal flow blockade induces α-CGRP mRNA expression in rat motoneurons. Brain Res. 1992;599:153–157. doi: 10.1016/0006-8993(92)90864-6. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Guo TZ, Poree LR, Maze M. Colchicine treatment of the sciatic nerve reduces neurogenic extravasation, but does not affect nociceptive thresholds or collateral sprouting in neuropathic or normal rats. Pain. 1998;74:11–20. doi: 10.1016/S0304-3959(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Koschorke GM, Meyer RA, Campbell JN. Cellular components necessary for mechanoelectrical transduction are conveyed to primary afferent terminals by fast axonal transport. Brain Res. 1994;641:99–104. doi: 10.1016/0006-8993(94)91820-1. [DOI] [PubMed] [Google Scholar]
- Krikorian JG, Guth L, Barrett CP. Transport of acid phosphatase in normal and transected rat sciatic nerve. Exp Neurol. 1980;70:665–674. doi: 10.1016/0014-4886(80)90191-0. [DOI] [PubMed] [Google Scholar]
- Liverant S, Meiri H. Colchicine prevents recovery of nerve conduction at chronic demyelination. Brain Res. 1990;519:50–56. doi: 10.1016/0006-8993(90)90059-k. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Blenk KH, Janig W, Vogel C. Development of spontaneous activity and mechanosensitivity in axotomized afferent nerve fibers during the first hours after nerve transection in rats. J Neurophysiol. 1995;74:1020–1027. doi: 10.1152/jn.1995.74.3.1020. [DOI] [PubMed] [Google Scholar]
- Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, Yonenobu K, Yoshikawa H, Noguchi K. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain. 2002;99:121–132. doi: 10.1016/s0304-3959(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Perl ER, Burgess PR. Classification of afferent dorsal root fibers: mammals. In: Altman PL, Dittmer DS, editors. Biology Data Book. Vol. 2. Bethesda, MD, USA: FASEB; 1973. pp. 1141–1149. [Google Scholar]
- Scadding JW. Development of ongoing activity, mechanosensitivity, and adrenaline sensitivity in severed peripheral nerve axons. Exp Neurol. 1981;73:345–364. doi: 10.1016/0014-4886(81)90271-5. [DOI] [PubMed] [Google Scholar]
- Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Res. 1999;824:218–223. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- Tiedt TN, Wisler PL, Younkin SG. Neurotrophic regulation of resting membrane potential and acetylcholine sensitivity in rat extensor digitorum longus muscle. Exp Neurol. 1977;57:766–791. doi: 10.1016/0014-4886(77)90107-8. [DOI] [PubMed] [Google Scholar]
- Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000;424:563–576. [PubMed] [Google Scholar]
- Vergara C, Ramirez B, Behrens MI. Colchicine alters apamin receptors, electrical activity, and skeletal muscle relaxation. Muscle Nerve. 1993;16:935–940. doi: 10.1002/mus.880160908. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Effects of colchicine applied to the peripheral nerve on the thermal hyperalgesia evoked with chronic nerve constriction. Pain. 1993;55:227–233. doi: 10.1016/0304-3959(93)90151-E. [DOI] [PubMed] [Google Scholar]
- Zhuo H, Lewin AC, Phillips ET, Sinclair CM, Helke CJ. Inhibition of axoplasmic transport in the rat vagus nerve alters the numbers of neuropeptide and tyrosine hydroxylase messenger RNA-containing and immunoreactive visceral afferent neurons of the nodose ganglion. Neuroscience. 1995;66:175–187. doi: 10.1016/0306-4522(94)00561-i. [DOI] [PubMed] [Google Scholar]