Abstract
Immune-independent diabetes often occurs via pancreatic β cell dysfunction. However, the role of the tumour suppressor p53 that regulates cellular life and death in multiple tissues, in pancreatic cell death and diabetes has not been clarified. We have therefore utilized an established mouse model for diabetes in which the MHC class I antigen is overexpressed in pancreatic β cells under the rat insulin promoter, to investigate the role of p53. We show that pancreatic β cell death, as determined by TUNEL staining, is elevated in transgenic mice compared to wild-type mice. However, there was no increase in immuno-reactivity towards anti-p53 antibodies in the pancreas of transgenic mice over the course of diabetes formation and β cell death, suggesting that p53 may not be involved in these processes. Interestingly, p53 expression was also not induced in pancreas upon γ-irradiation, which resulted in a massive increase in the number of TUNEL-positive cells, suggesting that the p53 pathway may not be causally involved in pancreatic cell death. To further confirm these findings, we generated MHC class I transgenic mice lacking p53 expression. Absence of p53 did not result in any significant changes in pancreatic morphology or affect cell death levels. Importantly, p53 absence did not rescue the diabetic phenotype of the transgenic mice. The results therefore demonstrate that p53 may not be causally involved in pancreatic β cell death, and suggests that the classical cell death pathway dependent on p53 may not be operating in pancreatic β cells.
Both insulin-dependent (type-1) and insulin-independent (type-2) diabetes arise due to defects in pancreatic β cells (Faideau et al. 2002; Ozcan et al. 2004; Chakravarthy & Semenleovoch, 2007; Laybutt et al. 2007). In the former, there is a strong influence of the immune system, leading to β cell destruction by multifactorial immune factors (Faideau et al. 2002). The latter, however, does not involve the immune system, and is often due to reduced insulin production because of pancreatic β cell death occurring by other mechanisms (Ozcan et al. 2004; Chakravarthy & Semenleovoch, 2007; Laybutt et al. 2007). In between both of these types of diabetes is the ketosis-prone type-2 diabetes, which is a variant type-2 diabetes – also known as atypical diabetes – that also occurs eventually due to β cell dysfunction (Umpierrez, 2006). Comparison of pancreatic β cell death mechanisms between type-1 and type-2 diabetes shows many differences with few similarities (Cnop et al. 2005; Donath et al. 2005). Essentially, pancreatic β cell death occurs via the Nuclear factor-kappa B (NF-κB)-dependent, caspase-3-mediated apoptotic pathway in type-1 diabetes, in contrast to a NF-κB-independent pathway, involving both apoptosis and necrosis in type-2 diabetes (Jörns et al. 2002; Cnop et al. 2005). Multiple mechanisms have been proposed for β cell destruction in type-2 diabetes. The foremost among them is due to an elevated level of endoplasmic reticulum (ER) stress (Ozcan et al. 2004; Laybutt et al. 2007). Consistently, many regulators of ER stress have been shown to be up-regulated in type-2 diabetes, together with elevated pancreatic β cell death (Oyadomari et al. 2002; Rhodes, 2005; Riggs et al. 2005; Laybutt et al. 2007). However, the mechanisms of pancreatic β cell death in atypical diabetes have not been clarified.
Many rodent models for diabetes have been generated and utilized (Buschard, 1996). For example, the NOD mice and the BB rat are the commonly used models for type-1 diabetes. In addition, injection of streptozotocin (STZ) also promotes type-1 diabetes, leading to β cell dysfunction dependent on the activation of the immune system (Zhang et al. 2007). Similarly, the obese rat, the GK rat, the db/db mice, the ob/ob mice, etc. are classical models used to study type-2 diabetes (Movassat et al. 1995; Shafrir, 2001; Allen et al. 2004). Genetically modified mice have also increasingly been used to evaluate the mechanisms of pancreatic β cell death. For example, the role of caspase-3, PARP and NF-κB in β cell death in the type-1 models has been confirmed using the respective knockout mice (Hassa & Hottiger, 2002; Lamhamedi-Cherradi et al. 2003; Liadia et al. 2005). Similarly, deletion of genes involved in ER stress, leptin receptors, etc. have been used to confirm their role in β cell death and dysfunction in type-2 diabetic models (Oyadomari et al. 2002; Uchida et al. 2005).
One model that was generated a long time ago is the RIP-H-2kb transgenic mouse model, where the MHC class I molecule was overexpressed in pancreatic β cells (Allison et al. 1988). This led to the rapid onset of diabetes that was reliably reproducible over many generations (Allison et al. 1988). Though this model was generated to resemble type-1 diabetes, lack of immune infiltration and its involvement indicated that this may not be an ideal model for type-1 diabetes (Allison et al. 1988; Mandel et al. 1991). Moreover, obesity was not associated with this model, though the diabetic phenotype was ascribed to pancreatic dysfunction. Therefore, this may be a good model for atypical diabetes. However, the mechanisms regulating pancreatic β cell death in this model have not been studied.
p53 is the most important tumour suppressor gene product regulating cellular life and death (Oren, 2003; Slee et al. 2004). Absence of functional p53 often leads to defective apoptosis in many cell types and increased proliferation, both culminating in tumour progression (Sigal & Rotter, 2000; Petitjean et al. 2007). Besides regulating carcinogenesis, absence of p53 has also been shown to affect many physiological and developmental processes (Bargonetti & Manfredi, 2002; Braithwaite & Prives, 2006). For example, lack of p53 was shown to affect neural tube closure, a process involving massive apoptosis that needs to be coordinated (Sah et al. 1995; Pani et al. 2006; Trovato et al. 2007). In the pancreas, absence of p53 was shown to lead to polyploid pancreatic cells at early stages of life (Ramel et al. 1995; Sphyris & Harrison, 2005). Nonetheless, whether p53 has any regulatory role in directly controlling pancreatic β cell death during diabetes formation and progression has not been clarified.
We have therefore investigated the role of p53 in diabetes and pancreatic cell death using the RIP-H-2kb transgenic mouse model. The data presented here demonstrate the interesting fact that pancreatic cells do not up-regulate p53, even when undergoing cell death due to γ-irradiation. Furthermore, we did not notice any up-regulation of p53 in the transgenic mice in which there were significant levels of pancreatic apoptosis. Finally, by crossing these mice to p53 null mice, we show that absence of p53 does not rescue any of the phenotypes, thus excluding any role for the tumour suppressor protein in pancreatic cell death and diabetes.
Methods
Mice
The RIP-H-2kb transgenic mice have been previously described and were kindly provided by Dr Janette Allison (St Vincent's Institute, Fitzroy, Australia) (Allison et al. 1988). Only male transgenic mice were used for expanding the colony by breeding with wild-type and p53+/− female mice of the B6/129 background. The RIP-H-2kb;p53+/− mice were further crossed with p53+/− mice to obtain RIP-H-2kb;p53−/– mice. Pups aged 2–3 weeks were weaned, males and females were separated, and tail biopsies were taken for genotyping purpose. Mice were housed in cages of a maximum of five animals with free access to food and water. All experimental mice carried a single copy of the transgene. Genotyping was performed for the RIP-H-2Kb transgene with the following primers: sense, 5′-CGAGTGGGCTATGGGTTTGT-3′; antisense, 5′-TTGGCTTTCTGTGTCTCCCG-3′; and for p53 as described (Jacks et al. 1994).
Pancreases were harvested 24 h after γ-irradiating wild-type mice at 1, 5 or 15 Gy. Spleen and thymus were harvested 24 h after irradiating at 0.25 Gy.
Mice were monitored at least once a week for signs of illness and obvious signs of distress. Transgenic animals between 16 and 24 weeks of age, which showed continued diabetes or distress, were killed by CO2 inhalation. All animal experiments were carried out with the approval of the institutional animal care and use committee.
Blood glucose, insulin and glucagon measurements
Two-microlitre blood samples were taken once a week from the tail vein of mice by nipping, and glucose levels were measured weekly from 2- to 10-week-old mice using a compact glucose analyser (Accu-chek; Roche, Basel, Switzerland). Potassium permanganate crystals were applied to the wounded tail to stop the bleeding and for disinfection. At least five mice per group for males and females were analysed for each time point.
To harvest blood for serum analysis, mice were killed by CO2 inhalation and cardiac puncture was performed later to collect the whole blood. Serum insulin and glucagon measurement was carried out using the insulin radio-immunoassay (RIA) kit and the glucagon RIA kit (Linco Research, St Charles, MO, USA), as per manufacturer's recommendations. Briefly, 50 or 100 μl of serum was used for measurement of insulin or glucagon, respectively. The concentration in samples was determined based on glucagon and insulin standards provided by the manufacturer.
Tissue samples and immunohistochemistry
Pancreatic sections were obtained from mice between 2 and 10 weeks of age. Thymus and spleen sections were obtained from wild-type mice of 10 weeks of age. Freshly removed tissues were fixed in formalin solution (4% formaldehyde in PBS) for 12–24 h, 4°C, and were subsequently embedded in paraffin for histological analysis. Tissue sections of 5 μm thick were deparaffinized in xylene and re-hydrated progressively in decreasing concentrations of ethanol. Thereafter the slides were placed in water and subjected to immunostaining or H and E staining, which was done by incubating the slides in haematoxylin for 5 min, followed by eosin for 2 min.
Immunostaining for insulin and glucagon were done strictly according to the manufacturer's protocol (histomouse-max kit from Zymed Laboratories, San Francisco, CA, USA). Antigen retrieval was performed by boiling the tissue sections in 10 μm citrate buffer antibodies for 15 min. The sections were incubated with insulin (rabbit anti-insulin, clone Z006, Zymed Laboratories) or glucagon (rabbit anti-glucagon, Zymed Laboratories) for 1 h at room temperature. The slides were rinsed with washing buffer and incubated with anti-mouse HRP-conjugated IgG. Tissues sections were washed, after which DAB-chromogen substrate mixture was applied. Analysis of the tissue sections was done by light microscopy.
Immunohistochemistry of p53 (CM5, Novocastra, Newcastle upon Tyne, UK) was performed according to the protocol used for insulin and glucagon. p53 antibody was diluted in primary antibody dilution buffer (5% serum, 0.5% Tween-20/PBS).
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) staining was carried out with an apoptosis detection kit (Roche) on the paraffin-embedded tissue sections, according to the manufacturer's protocol. Sixty to 250 nuclei were counted for each islet, and 2–4 islets were counted per section. Three to five sections were counted per mouse using at least four independent mice per time point.
Statistical analysis
Results were expressed as mean ± standard error of the mean (s.e.m.). ANOVA and Student's t test were used to determine if the observed difference was statistically significant. Significance was defined as P < 0.05.
Results
Overexpression of MHC class I H-2Kb results in elevated cell death in pancreatic β cells
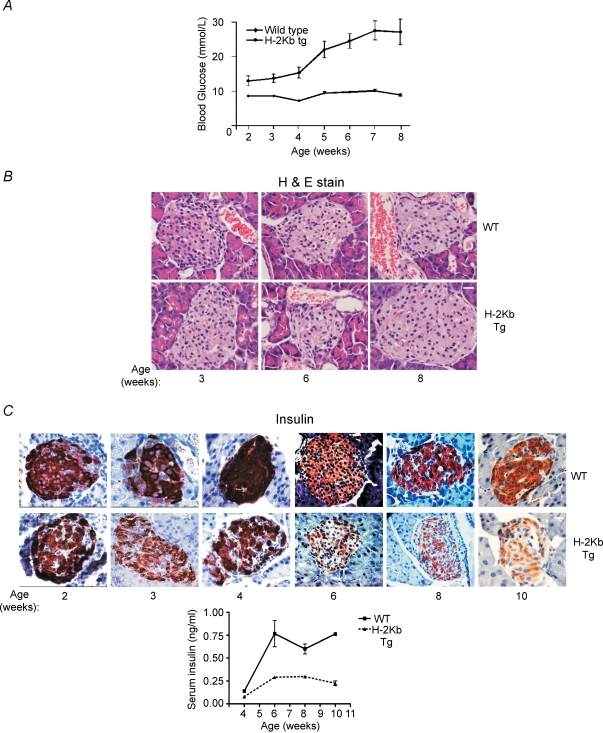
We determined the blood glucose levels to confirm the diabetic phenotype of the RIP-H-2Kb transgenic mice, which has been previously generated (Allison et al. 1988). Whereas blood glucose levels were basal (below 10 mm) throughout the 10-week monitoring period in non-transgenic mice, it was elevated in the RIP-H-2Kb transgenic mice, starting from 2 weeks after birth (about 13 mm) (Fig. 1A). The levels increased rapidly starting from about 4 weeks of age and reached to about 30 mm around the seventh week after birth (Fig. 1A), confirming previous data on the diabetic phenotype of these transgenic mice. Mice gradually died between about 16 and 24 weeks due to continued diabetes (data not shown).
Figure 1. Elevated pancreatic cell death and diabetes in RIP-H-2kb transgenic mice.
A, glucose levels were determined weekly from peripheral blood of RIP-H-2kb transgenic mice and their wild-type littermates from the ages of 2–8 weeks. N≥ 5 mice per time point. Mean values and standard deviation are shown. B, representative H&E staining of pancreatic sections, focusing primarily on the islets from wild-type and RIP-H-2kb transgenic mice are shown. Magnification: ×40. Scale bar represents 20 μm. C and D, insulin (C) and glucagon (D) staining of pancreatic sections from RIP-H-2kb transgenic mice and their wild-type littermates. Representative sections are shown. Magnification: ×20. Scale bar represents 40 μm. N = 2 mice per time point. Lower panel in each case shows quantification of serum insulin or glucagon levels, at various ages. At least 2 mice per group were used. E, tissue sections of wild-type and RIP-H-2kb transgenic pancreas were TUNEL stained to determine apoptotic cell. Representative sections are shown. Magnification: ×20, scale bar represents 40 μm (upper panel). Lower middle panel shows sections from mice of 6 and 8 weeks at a higher magnification (×40, scale bar represents 20 μm). N = 4 mice per time point per genotype. Lower right-most panel shows the ratio of TUNEL-positive over unstained nuclei in the pancreatic islets. A total of about 60–250 nuclei were counted from each islet, and from nine individual islets from independent sections from the four mice. Lower left panel shows sections stained with and without the transferase enzyme (e/z), to show background staining. Arrowheads show TUNEL-positive cells in pancreatic islets.
Histological analysis indicated that the size of islets was not significantly affected in the transgenic mice, and macroscopic analysis at higher magnifications did not show major distortions in the architecture of the islets (Fig. 1B). Immunohistochemical analysis indicated that insulin levels started decreasing in the islets of transgenic mice, observable from 3 weeks after birth and progressively declining over time. At 8–10 weeks of age, there were hardly any cells with insulin-positive stain, in contrast to control wild-type mice (Fig. 1C). Quantification of serum insulin by radio-immunoassays indicated that the levels increased in wild-type mice starting from 4 weeks, whereas, the levels were much lower in the transgenic mice (wild-type vs. transgenic at 4, 6, 8 and 10 weeks: 0.140 vs. 0.078, 0.767 vs. 0.292, 0.600 vs. 0.297 and 0.764 vs. 0.225) (Fig. 1C, lower panel). Consistent with a decline in the number of insulin-producing β cells, there was an infiltration of the glucagon-producing pancreatic α cells. These α cells, which are normally found along the periphery of the islets in wild-type mice (Allison et al. 1988), were seen infiltrating into the islets, which was visible from weeks 2–3 after birth and progressing over time (Fig. 1D). Notably, there was a slight but consistent increase in the levels of glucagon in the transgenic mice at all ages (wild-type vs. transgenic at 4, 6 and 8 weeks: 3.12 vs. 19.95, 59.26 vs. 68.88 and 25.00 vs. 35.39) (Fig. 1D, lower panel).
As it has been reported that there is no T cell infiltration in this mouse model (Allison et al. 1988); (Fig. 1B), ruling out the role of T, NK and macrophage cells in β cell destruction, we evaluated if the β cells were undergoing programmed cell death by utilizing the conventional TUNEL assay analysis. There was a basal level of TUNEL positivity in wild-type pancreas, but this was significantly elevated in transgenic mice at 6 and 8 weeks of age (Fig. 1E, top panel). The levels of TUNEL-positive cells were much higher at these time points (ratio of TUNEL-positive to total cells, WT vs. transgenic: 0.38 vs. 0.59 at 6 weeks, P = 0.0066 and 0.36 vs. 0.56 at 8 weeks, P = 0.0028) (Fig. 1E, lower middle and right panels). Control analysis without the transferase enzyme indicated that the staining observed was indeed specific (Fig. 1E, lower left-most panel). These data together suggest that the diabetic phenotype of the RIP-H-2Kb transgenic mice correlates with increased cell death and decreased insulin production.
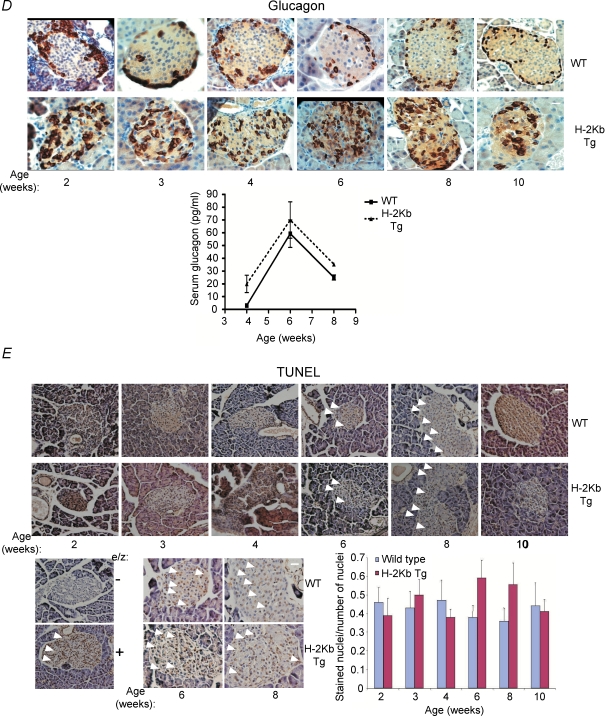
p53 is not up-regulated in pancreatic β cells of H-2Kb transgenic mice
As p53 is a regulator of cell death (Oren, 2003; Slee et al. 2004), we examined if cell death in pancreatic β cells of the RIP-H-2Kb transgenic mice is mediated by p53. To this end, we evaluated the expression of p53 in wild-type and transgenic pancreas over a time course after birth. To our surprise, there was no significant immuno-staining of p53 visible in both wild-type and transgenic pancreatic sections (Fig. 2A). To rule out the possibility that the anti-p53 antibody was not working, we used thymus and spleen sections from wild-type mice that were γ-irradiated to induce p53 expression, and compared them to sections from p53 null mice. As shown in Fig. 2B, both wild-type spleen and thymus were positively stained for p53, in contrast to the sections from the p53 null mice, ruling out that the antibody was defective.
Figure 2. Lack of p53 immunoreactivity in pancreatic cells undergoing cell death.
A, pancreatic sections from wild-type and RIP-H-2kb transgenic mice of 2–10 weeks of age were stained with anti-p53 antibody. Magnification: ×40. Scale bar represents 20 μm. N≥ 5 mice per time point. B, p53 immunostaining was performed on thymic and splenic sections from wild-type and p53−/– mice which were γ-irradiated at 0.25 Gy. Tissues were harvested after 24 h. Magnification: ×40. Scale bar represents 20 μm. N = 3 mice per time point. C, pancreas sections from irradiated wild-type mice were stained for p53 (upper panels) and TUNEL staining (lower panels). Mice were γ-irradiated at 1, 5 and 15 Gy and pancreases were harvested after 24 h. Magnification: ×40. Scale bar represents 20 μm. Islets from ≥ 5 mice were analysed for each time point.
Hence, we tested if p53 is inducible during γ-irradiation (IR)-induced cell death of pancreatic cells, by whole-body irradiation with various doses of IR. Whereas irradiation resulted in a significant increase in the number of TUNEL-positive cells correlating with the IR dose, there was no significant p53 immuno-staining observed even at the highest IR dose (Fig. 2C). Similar results were obtained when analysis was performed at various time-points after irradiation (data not shown). Since the data suggest that p53 is not inducible even in TUNEL-positive pancreatic cells after irradiation, it is likely that the cell death occurring in pancreatic β cells of the RIP-H-2Kb transgenic mouse is also probably not due to p53.
Absence of p53 expression does not rescue the diabetic phenotype of RIP-H-2Kb transgenic mice
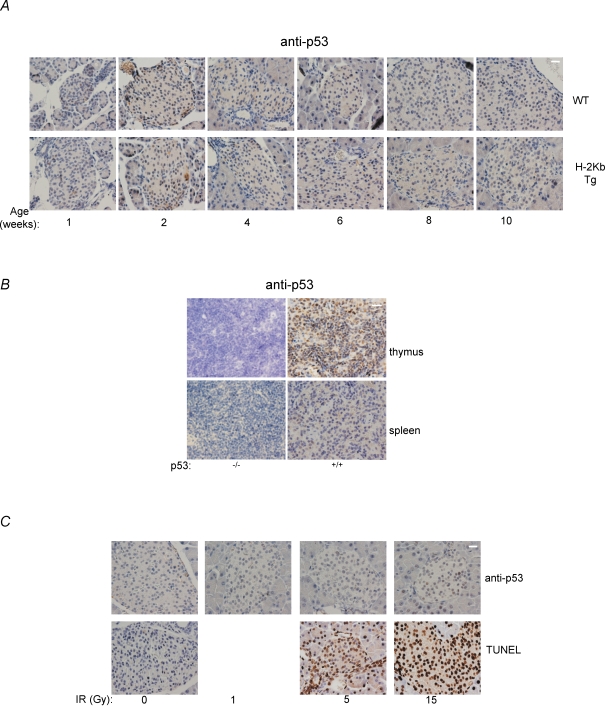
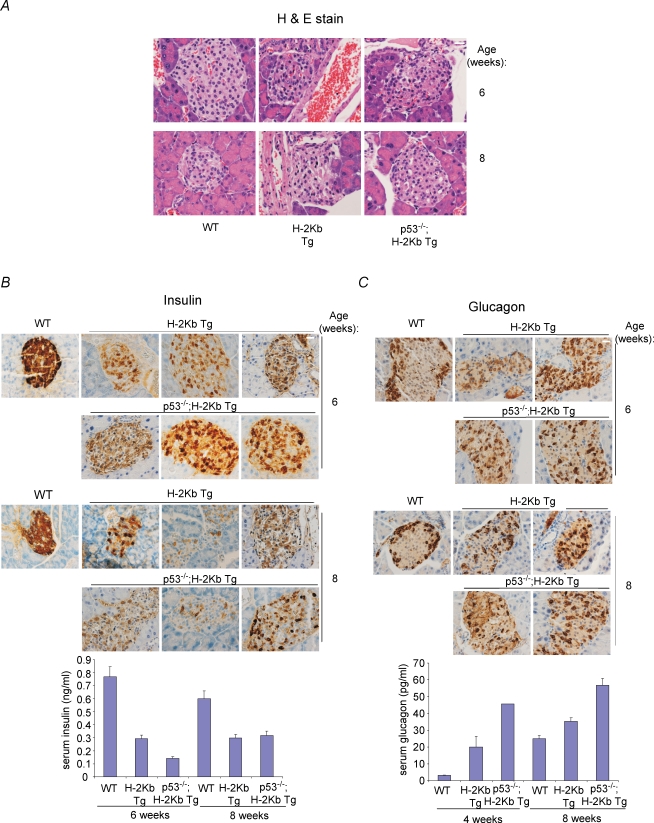
To genetically prove that p53 may not be required for pancreatic β cell death and the diabetes phenotype in RIP-H-2Kb transgenic mice, we crossed these mice with p53 null mice to obtain RIP-H-2Kb transgenic mice in the p53+/+, p53+/− and p53−/– backgrounds. We did not notice any aberrations in the expected Mendelian frequencies of the various genotypes (data not shown). Histological analysis revealed that the absence of p53 did not affect the architecture of the pancreatic islets (Fig. 3A). Similarly, insulin production that was defective in RIP-H-2Kb transgenic mice was not rescued in H-2Kb;p53−/– mice at various ages after birth (Fig. 3B, showing 6 and 8 weeks, and data not shown). Quantification of serum insulin confirmed these findings (wild-type vs. transgenic vs. p53−/– transgenic at 6 and 8 weeks: 0.767 vs. 0.292 vs. 0.140 and 0.600 vs. 0.297 vs. 0.319) (Fig. 3B, lower panel). Consistently, glucagon-expressing pancreatic α cells were seen infiltrating the pancreatic islets in RIP-H-2Kb;p53−/– mice similar to the control transgenic mice, and the glucagon levels were higher both in the presence and absence of p53 in the transgenic mice (wild-type vs. transgenic vs. p53−/– transgenic at 4 and 8 weeks: 3.12 vs. 19.95 vs. 45.61, 25.00 vs. 35.39 vs. 56.64) (Fig. 3C, lower panel).
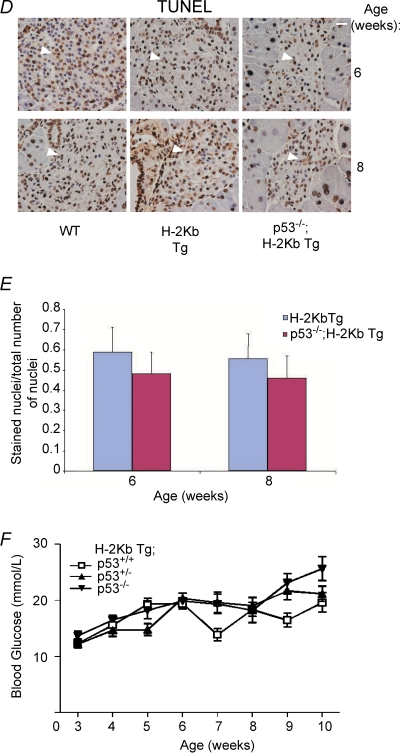
Figure 3. Absence of p53 does not rescue pancreatic cell death and diabetes in RIP-H-2kb transgenic mice.
A–D, H&E (A), insulin (B), glucagon (C) and TUNEL (D) staining were performed on pancreatic sections from wild-type, RIP-H-2kb transgenic and RIP-H-2kb;p53−/– mice of 6 and 8 weeks of age. Magnification: ×40. Scale bar represents 20 μm. N≥ 4 mice for each time point. Two to three independent sections are shown in B and C. Arrowheads show TUNEL-positive cells in islets (D). Lower panels in B and C show quantification of serum insulin and glucagon, respectively, at various ages. At least 1–3 mice per group were used for quantification. E, bar chart showing the ratio of stained over unstained nuclei by TUNEL staining, representing apoptotic cells. Pancreatic islets from ≥ 5 mice were counted for each time point. F, blood glucose levels were determined from RIP-H-2kb transgenic mice of various p53 genotypes (p53+/+, p53+/− and p53−/–), as described. N≥ 5 mice per time point.
Cell death analysis also revealed no reduction in the number of TUNEL-positive cells at 6 and 8 weeks of age in the RIP-H-2Kb;p53−/– mice compared to the RIP-H-2Kb mice (Fig. 3D). Enumeration of the TUNEL-positive cells did not show any statistically significant differences (Fig. 3E). Finally, analysis of blood glucose levels from RIP-H-2Kb mice of all three p53 genotypes showed that there was no significant decrease in the levels of blood glucose (Fig. 3F). Absence of p53 did not reduce or delay the increase of blood glucose levels (Fig. 3E). The data together indicate that p53 is not a regulator of the diabetic phenotype and pancreatic β cell death in the RIP-H-2Kb transgenic mouse model.
Discussion
The data presented here demonstrate that absence of p53 does not ameliorate pancreatic β cell death in the RIP-H-2Kb transgenic mouse model of immune-independent diabetes. Moreover, it highlights the fact that cell death in pancreatic β cells during diabetes disease progression and upon γ-irradiation also occurs in a p53-independent manner.
Immune-independent diabetes is a common form of disease associated with pancreatic β cell death that is not primarily dependent on the immune system (Ozcan et al. 2004; Chakravarthy & Semenleovoch, 2007; Laybutt et al. 2007). In this case, there is an excessive loss of pancreatic β cells through apoptosis and necrosis, leading to the dysfunction of these cells. Although multiple mechanisms have been suggested for pancreatic β cell death independent of the immune system (Ozcan et al. 2004; Chakravarthy & Semenleovoch, 2007; Laybutt et al. 2007), the role of the tumour suppressors has not been clarified either in type-2 diabetes or in atypical diabetes.
The generation of the RIP-H-2Kb transgenic mice almost two decades ago has highlighted the significance of immune-independent β cell death leading to diabetes (Allison et al. 1988). In this mouse model, the animals developed rapid diabetes, making it an easy and convenient system in which to study the disease. This was a good model that reflected diabetes that was not strictly type-1 or -2, as it was neither immune nor obesity dependent. Notable is the lack of infiltration of immune cells, which excluded the possibility also of immune-cell-derived cytokines in pancreatic cell death. However, though it was shown that β cell death in this model occurred due to the misfolding of the H-2Kb molecules that disrupted the insulin secretory pathway and was critical for disease development (Allison et al. 1991), the molecular mechanisms, and in particular, the role of tumour suppressor genes, were not unravelled.
This prompted us to analyse if increased β cell death in the RIP-H-2Kb transgenic mice leading to diabetes is due to p53-dependent cell death. Our results indicate that although cell death occurs in pancreatic β cells in this mouse model, this is not associated with p53, as its levels were not elevated in the diabetic pancreas. Consistently, elimination of p53 expression by crossing the transgenic mice with p53 null mice did not also rescue the diabetic phenotype or pancreatic cell death. These results suggest that p53 may not be critical for pancreatic β cell death and immune-independent diabetes. On the contrary, the role of p53 has been examined in the STZ-induced type-1 diabetic model. In this case, there was augmentation of the diabetic phenotype as p53 was thought to inhibit the pro-inflammatory cytokine production. In the absence of p53, there was elevated cytokine production leading to exaggeration of the disease (Zheng et al. 2005). However, there are no other reports examining if p53 or its family members regulate pancreatic cell death directly.
In order to further explore if p53 is indeed important for pancreatic cell death induced by other means, we whole-body-irradiated mice to induce apoptosis in all tissues. We noted that though γ-irradiation induced pancreatic cell death leading to the appearance of TUNEL-positive cells, this was not associated with an increase in p53 levels. This was in contrast to cell death and concomitant p53 induction in thymic and splenic tissues. Hence, the results were unexpected, as it is generally thought that many tissues often respond to irradiation by up-regulating p53 and associated death pathway (Bouvard et al. 2000; Fei et al. 2002). However, a literature search indicated the lack of information on pancreatic cell death and p53. Hence, to our knowledge, this is the first report that has evaluated p53 activation in pancreas, and the data suggest that p53 may not be critical in regulating apoptosis in pancreatic cells, similar to hepatocytes (Midgley et al. 1995), at least in mice. Consistently, support for the lack of a role for p53 in pancreatic apoptosis comes from a previous study by Hanahan and colleagues. In their RIP-TAg model of pancreatic cancer in which there was elevated apoptosis, absence of p53 did not lead to reduction of cell death (Naik et al. 1996). Together, the data presented here suggest that p53 may not have an essential role in directly regulating pancreatic cell death induced by multiple means, and hence, also diabetes.
There are many different diabetic models reflecting the various disturbances in the physiological system leading to diabetes. However, pancreatic cell death is a common feature in most cases and has been extensively described. Hence, understanding the mechanisms of pancreatic cell death is crucial in the study of the disease. Whether tumour suppressors have any regulatory role in this process has not been well characterized. The data presented here therefore provide evidence that the major tumour suppressor gene product p53 may not have any significant role in pancreatic cell death, and hence, atypical diabetes.
Acknowledgments
We thank Dr Janette Allison (St Vincent's Institute, Fitzroy, Australia) for kindly providing the RIP-H-2Kb transgenic mice, Dr Amanda Charlton for expert advice on tissue sections and the National Medical Research Council for the generous funding support to K.S.
References
- Allen TJ, Cooper ME, Lan HY. Use of genetic mouse models in the study of diabetic nephropathy. Curr Diab Rep. 2004;4:435–440. doi: 10.1007/s11892-004-0053-1. [DOI] [PubMed] [Google Scholar]
- Allison J, Campbell IL, Morahan G, Mandel TE, Harrison LC. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic β cells. Nature. 1988;333:529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Allison J, Malcolm L, Culvenor J, Bartholomeusz RK, Holmberg K, Miller JF. Overexpression of β2-microglobulin in transgenic mouse islet β cells results in defective insulin secretion. Proc Natl Acad Sci U S A. 1991;88:2070–2074. doi: 10.1073/pnas.88.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargonetti J, Manfredi JJ. Multiple roles of the tumor suppressor p53. Curr Opin Oncol. 2002;14:86–91. doi: 10.1097/00001622-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Zaitchouk T, Vacher M, Duthu A, Canivet M, Choisy-Rossi C, Nieruchalski M, May E. Tissue and cell-specific expression of the p53 target genes: bax, fas, mdm2 and waf1/p21, before and following ionizing irradiation in mice. Oncogene. 2000;19:649–660. doi: 10.1038/sj.onc.1203366. [DOI] [PubMed] [Google Scholar]
- Braithwaite AW, Prives CL. p53: more research and more questions. Cell Death Differ. 2006;13:887–880. doi: 10.1038/sj.cdd.4401938. [DOI] [PubMed] [Google Scholar]
- Buschard K. Diabetic animal models. APMIS. 1996;104:609–614. doi: 10.1111/j.1699-0463.1996.tb04920.x. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Semenleovoch CF. The ABCs of B cell dysfunction in type-2 diabetes. Nat Med. 2007;13:340–347. doi: 10.1038/nm0307-241. [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β-cell death in type-1 and type-2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl. 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Donath MY, Ehses JA, Maedler K, Schumman DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of β-cell death in type-2 diabetes. Diabetes. 2005;54(Suppl. 2):S108–S113. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- Faideau B, Larger E, Lepault F, Carel JC, Boitard C. Role of β-cells in type-1 diabetes pathogenesis. Diabetes. 2002;54:S87–S96. doi: 10.2337/diabetes.54.suppl_2.s87. [DOI] [PubMed] [Google Scholar]
- Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res. 2002;62:7316–7327. [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. The functional role of poly (ADP-ribose) polymerase 1 as novel coactivator of NF-κB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumour spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jörns A, Tiedge M, Ziv E, Shafrir E, Lenzen S. Gradual loss of pancreatic β-cell insulin, glucokinase and GLUT2 glucose transporter immunoreactivities during the time course of nutritionally induced type-2 diabetes in Psammomys obesus (sand rat) Virchows Arch. 2002;440:63–69. doi: 10.1007/s004280100490. [DOI] [PubMed] [Google Scholar]
- Lamhamedi-Cherradi SE, Zheng S, Hilliard BA, Xu L, Sun J, Alsheadat S, Liou HC, Chen YH. Transcriptional regulation of type I diabetes by NF-κ. J Immunol. 2003;171:4886–4892. doi: 10.4049/jimmunol.171.9.4886. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to β cell apoptosis in type-2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- Liadia N, Murakami K, Eweida M, Elford AR, Sheu L, Gaisano HY, Hakem R, Ohashi PR, Woo M. Caspase-3-dependent β-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol. 2005;25:3620–3629. doi: 10.1128/MCB.25.9.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel TE, Allison J, Cambell IL, Koulmanda M, Malcolm L, Cutri A, Miller JF. Inherent β-cell dysfunction induced by transgenic expression of allogeneic major histocompatibility complex class I antigen in islet cells. Autoimmunity. 1991;9:47–53. doi: 10.3109/08916939108997123. [DOI] [PubMed] [Google Scholar]
- Midgley CA, Owens B, Briscoe C, Thomas DB, Lane CP, Hall PA. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Sci. 1995;108:1843–1848. doi: 10.1242/jcs.108.5.1843. [DOI] [PubMed] [Google Scholar]
- Movassat J, Saulnier C, Portha B. β-cell mass depletion precedes the onset of hyperglycaemia in the GK rat, a genetic model of non-insulin-dependent diabetes mellitus. Diabete Metab. 1995;21:365–370. [PubMed] [Google Scholar]
- Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic β-cells. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type-2 diabetes. Science. 2004;306:451–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3-dependent development and tumorigenesis. Genes Dev. 2006;16:676–680. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- Ramel S, Sanchez CA, Schimke MK, Neshat K, Cross SM, Raskind WH, Reid BJ. Inactivation of p53 and the development of tetraploidy in the elastase-SV40 T antigen transgenic mouse pancreas. Pancreas. 1995;11:213–222. doi: 10.1097/00006676-199510000-00001. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ. Type 2 diabetes – a matter of β-cell life and death? Science. 2005;21:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Riggs AC, Bernal-Mizrachi E, Ohsugi M, Wasson J, Fatrai S, Welling C, Murray J, Schmidt RE, Herrera PL, Permutt MA. Mice conditionally lacking the Wolfram gene in pancreatic islet β cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48:2313–2321. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T. A subset of p53-deficient embryos exhibit exencephaly. Nat Genet. 1995;10:175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- Shafrir E. Albert Renold memorial lecture: molecular background of nutritionally induced insulin resistance leading to type-2 diabetes – from animal models to humans. Int J Exp Diabetes Res. 2001;2:299–319. doi: 10.1155/EDR.2001.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: The demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- Slee EA, O'Connor DJ, Lu X. To die or not to die: how does p53 decide? Oncogene. 2004;23:2809–2818. doi: 10.1038/sj.onc.1207516. [DOI] [PubMed] [Google Scholar]
- Sphyris N, Harrison DJ. p53 deficiency exacerbates pleiotropic mitotic defects, changes in nuclearity and polyploidy in transdifferentiating pancreatic acinar cells. Oncogene. 2005;24:2184–2194. doi: 10.1038/sj.onc.1208249. [DOI] [PubMed] [Google Scholar]
- Trovato M, D'Armiento M, Lavra L, Ulivieri A, Dominia R, Vitarelli E, Groisso M, Vecchione R, Barresi G, Sciacchitano S. Expression of p53/hgf/c-met/STAT3 signal in fetuses with neural tube defects. Vichows Arch. 2007;450:203–210. doi: 10.1007/s00428-006-0356-5. [DOI] [PubMed] [Google Scholar]
- Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KL, White MF, Kasuga M. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med. 2005;11:175–182. doi: 10.1038/nm1187. [DOI] [PubMed] [Google Scholar]
- Umpierrez GE. Ketosis-prone type-2 diabetes: time to revise the classification of diabetes. Diabetes Care. 2006;29:2755–2757. doi: 10.2337/dc06-1870. [DOI] [PubMed] [Google Scholar]
- Zhang B, Lu Y, Campbell-Thompson M, Spencer T, Wasserfall C, Atkinson M, Song S. α1-Antitrypsin protects β-cells from apoptosis. Diabetes. 2007;56:1316–1323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes. 2005;54:1423–1428. doi: 10.2337/diabetes.54.5.1423. [DOI] [PubMed] [Google Scholar]