Abstract
Inhibitory circuits are crucial in modulating corticospinal output in the primary motor cortex (M1). Relatively little is known about how these inhibitory circuits interact. Here we measured three forms of inhibition in M1 by paired-pulse transcranial magnetic stimulation: short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI) and short-interval interhemispheric inhibition (SIHI). We specifically tested their interactions under pharmacological challenge with a single oral dose of diazepam, a positive allosteric modulator of the γ-aminobutyric acid type A receptor (GABAAR), or baclofen, a specific agonist at the GABA type B receptor (GABABR). Motor evoked potentials were recorded bilaterally from the first dorsal interosseous muscle in eight right-handed healthy volunteers. Diazepam enhanced SICI, and baclofen produced a trend towards enhanced LICI, corroborating the view that SICI reflects inhibition mediated by the GABAAR, and LICI very likely reflects inhibition mediated by the GABABR. The pharmacology of SIHI was inconclusive and warrants further investigation. Findings strongly suggest that SICI, LICI and SIHI recruit three distinct inhibitory circuits in the human M1. The interactions between SIHI and SICI, LICI and SIHI, and LICI and SICI were all negative, that is SIHI suppressed SICI, and LICI suppressed both SIHI and SICI. Diazepam partially restored SICI in the presence of LICI, while all other interactions remained unaffected by diazepam or baclofen. It will be argued that the negative interactions between SIHI and SICI, LICI and SIHI, and LICI and SICI are most likely due to presynaptic GABABR-mediated autoinhibition.
At least three inhibitory processes can be studied non-invasively by means of paired-pulse transcranial magnetic stimulation (TMS) in the intact human primary motor cortex (M1): short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI) and short-interval interhemispheric inhibition (SIHI). SICI and LICI are measured with paired-pulse TMS involving a subthreshold (SICI) and suprathreshold (LICI) conditioning stimulus (CS) applied ipsilateral to the test stimulus (TS) over M1 at an interstimulus interval (ISI) of 1–5 ms (SICI) (Kujirai et al. 1993) or 50–200 ms (LICI) (Valls-Sole et al. 1992), respectively. SIHI is determined by delivering a CS to the contralateral M1 preceding the TS by 6–15 ms (Ferbert et al. 1992).
SICI and LICI most likely represent local intracortical inhibitory circuits in M1 (Valls-Sole et al. 1992; Kujirai et al. 1993). SICI is thought to be mediated via the γ-aminobutyric acid type A receptor (GABAAR) (Kujirai et al. 1993; Ziemann et al. 1996a; Ilic et al. 2002), while LICI very likely reflects neurotransmission through the GABA type B receptor (GABABR) (McDonnell et al. 2006). Conversely, SIHI is very likely due to activation of an excitatory transcallosal pathway by the contralateral CS, which then activates local inhibitory interneurons in the test hemisphere (Ferbert et al. 1992; Meyer et al. 1995; Lee et al. 2007). Yet, the pharmacology and detailed physiology of interhemispheric inhibition (IHI) are still unclear. Whereas long-interval IHI (LIHI) at ISIs of ≥20 ms seems to depend on GABABR-mediated neurotransmission (Irlbacher et al. 2007), the available data about the pharmacology of SIHI at ISIs <20 ms are inconclusive (Ziemann et al. 1996a; Irlbacher et al. 2007). The pharmacology data clearly indicate that SICI and LICI are mediated by different inhibitory interneuronal circuits. In addition, mainly on the grounds of CS and TS intensity curves and effects of voluntary contraction, it was suggested that the circuits mediating SICI and SIHI also differ, while those mediating SIHI and LICI are similar and overlapping (Sanger et al. 2001; Daskalakis et al. 2002). Identification and differentiation of the neuronal populations mediating SICI, LICI and SIHI is of great potential importance because it would allow measurement of specific inhibitory cortical circuits and their associated functions at the systems level of the human cortex non-invasively by means of paired-pulse TMS.
The knowledge on these inhibitory circuits, in particular whether they are the same or different, can be further advanced by studying their interactions. Recent first reports showed negative interactions between these inhibitory circuits, that is SIHI and LICI inhibit SICI (Sanger et al. 2001; Daskalakis et al. 2002), and LICI suppresses SIHI (Daskalakis et al. 2002). It was suggested that SIHI and LICI suppress SICI by presynaptic GABABR-mediated inhibition (Sanger et al. 2001; Daskalakis et al. 2002). The negative interaction between LICI and SIHI was suggested to be, at least partly, due to a GABABR-mediated autoinhibition within the neuronal circuit mediating both LICI and SIHI (Daskalakis et al. 2002). Here, we tested the effects of a single oral dose of diazepam, a positive allosteric modulator of the GABAAR, and of baclofen, a specific GABABR agonist, in a randomised, placebo-controlled, double-blinded crossover study on these inhibitory processes and their interactions. With this novel approach, we sought to test, in particular, the hypothesis that the negative interactions between the three forms of inhibition are driven by presynaptic autoinhibition through the GABABR.
Methods
Subjects
Eight subjects (3 female) aged 21–42 years (mean age, 27.6 ± 2.5 years) participated in the study. Written informed consent was obtained prior to participation. None of the subjects had a history of neurological disease or was on CNS-active drugs at the time of the experiments. All subjects completed the adult safety screen questionnaire (Keel et al. 2001) prior to the study. The experiments conformed to the Declaration of Helsinki and were approved by the ethics committee of the hospital of the Johann Wolfgang Goethe-University of Frankfurt am Main, Germany. All subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971).
EMG recordings
Subjects were seated in a comfortable reclining chair with their arms and hands lying relaxed on the armrests. Motor evoked potentials (MEPs) were recorded from the right and left first dorsal interosseous (FDI) muscles by surface electromyography (EMG), using Ag–AgCl cup electrodes in a belly tendon montage. The EMG raw signal were amplified and band-pass filtered (20 Hz to 2 kHz; Counterpoint Mk2 electromyograph; Dantec, Skovlunde, Denmark), digitised at an A/D rate of 5 kHz per channel (CED Micro 1401; Cambridge Electronic Design, Cambridge, UK) and stored in a laboratory computer for online visual display and later offline analysis using customised data collection and conditional averaging software (Spike 2 for Windows, Version 3.05, CED). All measurements except for determination of active motor threshold (AMT, see below) were conducted during complete muscle relaxation which was monitored audio-visually by high-gain EMG (50 μV division−1). Trials contaminated by EMG activity were discarded from further analysis.
Stimulation procedures
Focal transcranial magnetic stimulation (TMS) of the left primary motor cortex (M1) was performed with a figure-of-eight coil (diameter of each wing, 70 mm) and three Magstim 200 magnetic stimulators (Magstim Company, Carmarthenshire, Wales, UK) with monophasic current waveforms connected via two Bistim modules (Magstim). The output of two magnetic stimulators was connected to one Bistim module. The output of this Bistim module and the third magnetic stimulator were connected to the second Bistim module, which in turn was connected to the TMS coil. This experimental setup allowed us to deliver up to three TMS pulses of different stimulus intensity at very short interstimulus intervals, through the same TMS coil. The optimal coil position over the hand area of the left M1 for eliciting MEPs in the right FDI was determined as the site where TMS at a slightly suprathreshold intensity consistently produced the largest MEPs. This site was marked with a soft-tipped pen in order to assure a constant placement of the coil throughout the session. The coil was held tangential to the scalp with the handle pointing backwards and 45 deg away from the midline. This orientation induced a posterior to anterior current in the brain, activating the corticospinal system preferentially trans-synaptically via horizontal cortico-cortical connections (Werhahn et al. 1994; Di Lazzaro et al. 2004). Resting motor threshold (RMT) was defined to the nearest 1% of maximum stimulator output (MSO) as the lowest stimulus intensity which elicited small MEPs (>50 μV) in at least five out of ten consecutive trials. AMT was obtained during a slight isometric contraction (∼10% of the maximum voluntary contraction, monitored by audio-visual feedback of the EMG raw signal) of the right FDI and was defined to the nearest 1% MSO as the lowest stimulus intensity which elicited a mean MEP > 100 μV when averaged across five consecutive trials.
TMS of the right M1 was performed with a second figure-of-eight coil (diameter of each wing, 70 mm) and a Magstim Rapid magnetic stimulator (Magstim) with a biphasic current waveform. The coil was placed at the optimal position over the hand area of the right M1 for eliciting MEPs in the left FDI. The coil orientation was tangential to the scalp with the handle pointing laterally or slightly forward in order to assure placement of both coils at their optimal positions which – due to the size of the coils – would not have been possible in most subjects if the handle had pointed backwards and 45 deg away from the midline. This coil orientation is equally appropriate to produce SIHI, as previous studies have found no significant directional preference for SIHI, i.e. SIHI is not significantly influenced by the orientation of the conditioning coil (Chen et al. 2003). RMT and AMT of the right M1 were determined in the left FDI to the nearest 1% of MSO as described above.
In the left M1 we tested two different intracortical inhibitory circuits: SICI and LICI. SICI was recorded using a paired-pulse TMS paradigm (Kujirai et al. 1993) at an ISI of 3 ms. At this particular interval, conditioning of the test response results in clear inhibition, which is thought to reflect GABAAR-mediated cortical inhibition (Kujirai et al. 1993; Ziemann et al. 1996c, 1998; Hanajima et al. 2003). CS3 denotes the subthreshold conditioning stimulus (CS) that was delivered 3 ms prior to the test stimulus (TS) in the left M1. LICI was tested by using a suprathreshold CS that was delivered 100 ms (CS100) prior to the TS in the left M1 (Valls-Sole et al. 1992). CS100 effectively suppresses test responses at a cortical level (Nakamura et al. 1997) and is thought to measure GABABR-mediated cortical inhibition (McDonnell et al. 2006). To test the interhemispheric inhibitory effect of the right M1 on the left M1, SIHI was measured by a suprathreshold CS delivered to the right M1, followed by the TS delivered to the left M1 with an ISI of 12 ms (Ferbert et al. 1992). This CS will be referred to as CCS12 (contralateral conditioning stimulus).
At baseline (before drug intake), 14 different stimulus conditions were tested, which are listed as conditions A–N in Table 1. The intensity of the TS was adjusted to produce MEPs of different target amplitudes in conditions A–D (TS intensity ‘1mV’), E–H (TS intensity ‘1mVCCS12’) and I–N (TS intensity ‘1mVCS100’) (for definition and nomenclature of TS intensities, see below). The CS3 intensity was initially set at 90% AMT and was then reduced until it produced ∼50% inhibition of the test response (SICI, ratio of conditions B/A in Table 1). Similarly, CS100 intensity was initially set at an intensity that produced MEPs of, on average, 1 mV in peak-to-peak amplitude, and was then adjusted to produce ∼50% inhibition of the test response (LICI, ratio of conditions D/A). CCS12 intensity was initially set at 130% RMT and then adjusted to also produce ∼50% inhibition of the test response (IHI, ratio of conditions C/A). The level of 50% inhibition for SICI, LICI and SIHI was chosen as it provides the largest possible modification range for both increases and decreases of SICI, LICI and SIHI. These CS intensities were used throughout all baseline measurements (i.e. for conditions A–N).
Table 1.
Stimulus conditions
| Condition | CS100 | CCS12 | CS3 | TS |
|---|---|---|---|---|
| A | — | — | — | 1mV |
| B | — | — | 0.9 AMT | 1mV |
| C | — | 1.3 RMT | — | 1mV |
| D | 1 mV | — | — | 1mV |
| E | — | — | — | 1mVCCS12 |
| F | — | — | 0.9 AMT | 1mVCCS12 |
| G | — | 1.3 RMT | — | 1mVCCS12 |
| H | — | 1.3 RMT | 0.9 AMT | 1mVCCS12 |
| I | — | — | — | 1mVCS100 |
| J | — | — | 0.9 AMT | 1mVCS100 |
| K | — | 1.3 RMT | — | 1mVCS100 |
| L | 1 mV | — | — | 1mVCS100 |
| M | 1 mV | — | 0.9 AMT | 1mVCS100 |
| N | 1 mV | 1.3 RMT | — | 1mVCS100 |
CS100, ipsilateral conditioning stimulus delivered 100 ms before test stimulus (TS) over the left hemisphere; CCS12, contralateral conditioning stimulus delivered 12 ms before TS; CS3, ipsilateral conditioning stimulus delivered 3 ms before TS; RMT, resting motor threshold; AMT, active motor threshold. See Methods for TS intensity nomenclature.
Experimental design
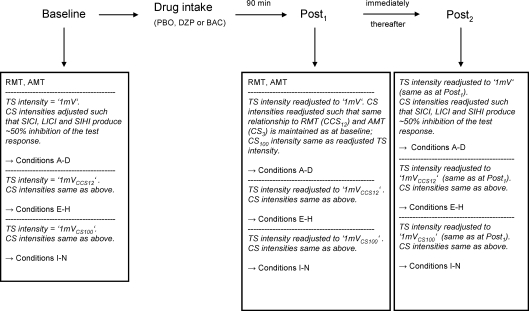
We sought to explore the effects of diazepam (DZP) and baclofen (BAC) on SICI, LICI, SIHI and their interactions. To address this question, we employed a randomised, placebo (PBO)-controlled, double-blinded crossover study design. All subjects participated in three sessions at least one week apart to exclude crossover effects between sessions. Figure 1 illustrates the time line of experiments in each session. Following the baseline measurements, subjects were given either a single oral dose of PBO, DZP (20 mg, Diazepam-ratiopharm, ratiopharm GmbH) or BAC (50 mg, Lioresal, Novartis Pharma). The order of drug allocation was randomised and balanced across subjects. Post-drug measurements started 90 min after drug intake. Drug doses and delay to post-drug measurements were selected according to previous reports which showed a significant enhancement of SICI under 20 mg DZP (Ilic et al. 2002; Di Lazzaro et al. 2006) and of LICI under 50 mg BAC (McDonnell et al. 2006). These effects were significant 90 min after drug intake in line with the pharmacokinetics of the study drugs (Shader et al. 1984; McDonnell et al. 2006).
Figure 1. Time line of experiments.
After baseline measurements subjects were given a single oral dose of either PBO, DZP or BAC. Note that for measurements of drug effects on SICI, LICI and SIHI alone at the Post1 measurement, CS intensities were readjusted differently as compared to at the Post2 measurement for evaluation of drug effects on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI. For details also see Methods.
Each session of the study involved two parts (see Fig. 1). At the Post1 measurement, the drug effects on SICI, LICI and SIHI were examined. At the Post2 measurement, the drug effects on the interactions SIHI–SICI (SICI in the presence of SIHI), LICI–SIHI (SIHI in the presence of LICI) and LICI–SICI (SICI in the presence of LICI) were tested.
Effects of DZP and BAC on SICI, LICI and SIHI
At the Post1 measurement in each session (see Fig. 1) we studied the effects of DZP and BAC on SICI, LICI and SIHI alone. At baseline (before drug intake), stimulus conditions A–N (Table 1) were tested in three separate blocks (block 1: conditions A–D, block 2: conditions E–H, block 3: conditions I–N). Each run consisted of 10 trials each of all four (40 trials, blocks 1 and 2) or six (60 trials, block 3) conditions, respectively. Within each block conditions were randomised. The intertrial interval was on average 5 s (random intertrial interval variation of 25% in order to reduce anticipation). In block 1 the TS intensity was adjusted to produce MEPs of, on average, 1 mV in peak-to-peak amplitude (condition A; TS intensity ‘1mV’). In blocks 2 and 3 the TS intensity was adjusted to produce MEPs of 1 mV amplitude in the presence of CCS12 (condition G; TS intensity ‘1mVCCS12’) or in the presence of CS100 (condition L; TS intensity ‘1mVCS100’), respectively. The CS intensities were adjusted to produce ∼50% inhibition of the unconditioned test response as described above. SICI, LICI and SIHI were quantified by the ratio of the mean conditioned to the mean unconditioned MEP amplitude, i.e. for SICI the ratios of MEP amplitudes in conditions B/A (TS intensity ‘1mV’), F/E (‘1mVCCS12’) and J/I (‘1mVCS100’) were calculated, and accordingly, for LICI the MEP ratios in conditions D/A (‘1mV’) and L/I (‘1mVCS100’), and for SIHI the MEP ratios in conditions C/A (‘1mV’), G/E (‘1mVCCS12’) and K/I (‘1mVCS100’).
To evaluate the effects of DZP and BAC on SICI, LICI and SIHI, one run of conditions A–N was repeated starting 90 min after drug intake (Post1 measurement, Fig. 1). For this purpose, first RMT and AMT were determined again as described above. It is well known that both CS and TS intensities affect the amount of SICI, LICI and SIHI (Ferbert et al. 1992; Valls-Sole et al. 1992; Kujirai et al. 1993; Ilic et al. 2002). Thus, in the next step the TS intensity ‘1mV’ (block 1) was readjusted to produce MEPs of ∼1 mV peak-to-peak-amplitude in order to assure comparable excitation of the excitatory pathways underlying the test response. Finally, CS intensities were readjusted in order to best as possible control for a potential drug effect on the excitability of the inhibitory circuits being tested. CS intensities were readjusted so as to maintain exactly the same relationship to RMT (CCS12) and AMT (CS3) as in the baseline measurements; the readjusted TS intensity ‘1mV’ was also used as CS100 intensity.
As there is no direct read-out for the efficacy of the magnetic stimulus to activate inhibitory interneurons, there is no ideal way of adjusting conditioning stimulus intensities for a drug effect on the excitability of the inhibitory interneuronal circuits underlying SICI, LICI and SIHI. However, for SICI it has been shown that its magnitude and threshold can be predicted from AMT (Orth et al. 2003). SIHI is very likely due to activation of an excitatory transcallosal pathway by the contralateral CS which then activates local inhibitory interneurons in the test hemisphere (Ferbert et al. 1992; Meyer et al. 1995; Lee et al. 2007). This excitatory transcallosal pathway seems to differ from the excitatory neuronal circuit underlying MEP generation (Lee et al. 2007). Thus, the readjustments of CCS12 (for SIHI) and CS100 (for LICI) are somewhat limited, as changes in the excitability of inhibitory interneuronal circuits out of proportion to those in excitatory circuits cannot be entirely excluded.
Finally, the TS intensities ‘1mVCCS12’ (block 2) and ‘1mVCS100’ (block 3) were also readjusted, such that they produced MEPs of, on average, 1 mV peak-to-peak amplitude in the presence of CCS12 (condition G) and in the presence of CS100 (condition L), respectively. These readjustments of CS and TS intensities allowed us to interpret any changes in SICI, LICI or SIHI in the post-drug as compared to the pre-drug measurements, as effects specifically induced by drug action.
Effects of DZP and BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI
At the Post2 measurement in each session (see Fig. 1) we examined the effects of DZP and BAC on the interaction between SIHI and SICI (suppression of SICI in the presence of SIHI) (Daskalakis et al. 2002), on the interaction between LICI and SIHI (suppression of SIHI in the presence of LICI) (Daskalakis et al. 2002), and on the interaction between LICI and SICI (suppression of SICI in the presence of LICI) (Sanger et al. 2001). The same 14 conditions A–N (Table 1; Fig. 1 Baseline) served as the baseline measurement for Post1 and Post2 measurements (Fig. 1). In order to investigate the interaction between two inhibitory systems we employed a triple stimulation approach: SICI alone as determined by the pulse pair CS3–TS (condition F) was compared to SICI in the presence of SIHI as determined by the pulse triple CCS12–CS3–TS (condition H). As CCS12 itself was designed to reduce the test response by ∼50% (see above), the increased TS intensity ‘1mVCCS12’– producing MEPs of ∼1 mV amplitude in the presence of CCS12 (condition G) – in conditions E–H assured that SICI operated on a system that was comparable to the unconditioned situation (condition A, TS intensity ‘1mV’). To quantify the interaction between SIHI and SICI, SICI in the presence of SIHI (ratio of MEP amplitudes of conditions H/G; ‘SIHI–SICI’) was compared with SICI in the absence of SIHI matched for TS intensity (conditions F/E; ‘SICISTIM’) or matched for test MEP amplitude (conditions B/A; ‘SICIMEP’). Similarly, to investigate interactions between LICI and SIHI and between LICI and SICI, respectively, we matched the test MEP amplitude of the CS100–TS pulse pair (condition L) to the unconditioned case (condition A), by increasing TS intensity accordingly (TS intensity ‘1mVCS100’). This assured that SIHI and SICI, respectively, operated on a system comparable to the unconditioned situation. To quantify the interaction between LICI and SIHI, SIHI in the presence of LICI (conditions N/L; ‘LICI–SIHI’) was compared with SIHI in the absence of LICI matched for TS intensity (conditions K/I; ‘SIHISTIM’) or matched for test MEP amplitude (conditions C/A; ‘SIHIMEP’). For the quantification of the interaction between LICI and SICI, SICI in the presence of LICI (condition M/L; ‘LICI–SICI’) was compared with SICI in the absence of LICI matched for TS intensity (conditions J/I; ‘SICISTIM’) or matched for test MEP amplitude (conditions B/A; ‘SICIMEP’).
To evaluate the effect of DZP and BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI, another run of conditions A–N was repeated after drug intake, starting immediately after the Post1 measurement (Post2 measurement, Fig. 1). For this purpose, we used the TS intensities ‘1mV’ (conditions A–D), ‘1mVCCS12’ (conditions E–H) and ‘1mVCS100’ (conditions I–N) of the Post1 measurement which had already been readjusted after drug intake (see above). As the interactions SIHI–SICI, LICI–SIHI and LICI–SICI most likely depend on the magnitude of SICI, LICI and SIHI alone, at the Post2 measurement we additionally readjusted the CS intensities such that the magnitudes of SICI, LICI and SIHI alone were reset to match the ∼50% inhibition of the test response during baseline recordings. Only these careful adjustments allowed us to evaluate drug effects specifically on the interaction between these inhibitory processes.
Data analysis and statistics
For each subject and each condition the 10 single trial MEPs were ranked off-line according to their peak-to-peak amplitude. The lowest and highest value was rejected to exclude potential outliers. Only the remaining eight values were averaged to calculate the mean MEP amplitude per condition. SICI, LICI and SIHI were expressed as the ratio of the mean conditioned to the mean unconditioned MEP amplitudes in each subject. Ratios <1.0 indicate inhibition, and ratios >1.0 indicate facilitation.
To test for the effects of DZP and BAC on RMT (left and right hemisphere) and AMT (left and right hemisphere), a two-way repeated-measures analysis of variance (ANOVARM) was used with the independent within-subjects factors ‘drug’ (PBO, DZP, BAC) and ‘time’ (pre, post). To test for the effects of DZP and BAC on SICI, LICI and SIHI, a three-way ANOVARM was used with the independent within-subjects factors ‘drug’ (PBO, DZP, BAC), ‘time’ (pre, post) and ‘TS intensity’ (‘1mV’, ‘1mVCCS12’and ‘1mVCS100’ for SICI and SIHI; ‘1mV’ and ‘1mVCS100’ for LICI). To test for the effects of DZP and BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI, a three-way ANOVARM was computed with the independent within-subjects factors ‘drug’ (PBO, DZP, BAC), ‘time’ (pre, post) and ‘condition’ (SIHI–SICI, SICISTIM, SICIMEP for the interaction between SIHI and SICI; LICI–SIHI, SIHISTIM, SIHIMEP for the interaction between LICI and SIHI; LICI–SICI, SICISTIM, SICIMEP for the interaction between LICI and SICI).
Post hoc Student's two-tailed paired t tests were conducted if one of the main effects or their interaction was significant. In all tests, the level of statistical significance was set at P < 0.05. All data are expressed as mean ± 1 standard error of the mean (s.e.m.).
Results
All subjects reported mild adverse effects after administration of DZP and BAC, most commonly sedation and light-headedness. These effects did not interfere with the ability of the subjects to comply with all requirements of the study. Subjects were asked to rate their level of alertness on a visual-analog scale (0 being extremely sleepy, 5 being normally alert, 10 being maximally alert) at baseline, 90 min after drug intake, and at the end of the experiment. On average, subjects rated alertness as 5/5/5 in the PBO session, as 5/3/3 in the DZP session and as 5/5/3 in the BAC session.
Effects of DZP and BAC on RMT and AMT
Following drug administration there was a slight but significant increase of RMT in the left M1, as demonstrated by a significant effect of time (F1,7= 7.5; P < 0.05). Post hoc testing showed that this effect was explained by a slight increase of RMT after administration of DZP (P < 0.05; Table 2). There was no significant difference of RMT in the right M1 or of AMT in either M1 due to drug, time or the interaction between drug and time (Table 2). At baseline, there was no significant difference of RMT or AMT in either M1 between the three sessions (i.e. PBO, DZP, BAC; P > 0.4 for all comparisons; Table 2). Both RMT and AMT were significantly higher in the right M1 compared to the left M1 (P < 0.001 for all comparisons; Table 2; all subjects being right-handed). Several reasons account for this latter finding: First, excitability in M1 was shown to be asymmetric with, in right-handed subjects, the dominant left M1 being generally more excitable than the non-dominant right M1 (Macdonell et al. 1991; Triggs et al. 1994; Ilic et al. 2004). Second, AMT in the right M1 is sensitive to the coil orientation during magnetic stimulation such that it is lowest for anterior-medially (AM)-directed induced currents and higher for posterior-medially (PM) induced currents (Chen et al. 2003). As described in Methods, for technical reasons we applied a coil orientation for right M1 stimulation which induced PM rather than AM currents in the brain. Third, motor thresholds are higher with a biphasic than monophasic waveform of the magnetic stimulus (Kammer et al. 2001). While we used a single Magstim Rapid magnetic stimulator with a biphasic pulse waveform for right M1 stimulation, left M1 stimulation was performed with Magstim 200 stimulators with a monophasic pulse waveform.
Table 2.
TMS measures of motor cortical excitability before (pre) and after (post) a single oral dose of PBO, DZP or BAC
| Measure | pre-PBO | post-PBO | pre-DZP | post-DZP | pre-BAC | post-BAC |
|---|---|---|---|---|---|---|
| RMT left M1 (%) | 38.4 ± 2.2 | 38.0 ± 2.0 | 37.9 ± 1.2* | 40.3 ± 1.5* | 39.9 ± 2.5 | 41.0 ± 2.9 |
| RMT right M1 (%) | 53.8 ± 3.2 | 53.4 ± 2.7 | 54.5 ± 2.9 | 55.3 ± 2.8 | 54.9 ± 3.1 | 55.0 ± 3.1 |
| AMT left M1 (%) | 30.9 ± 2.3 | 30.9 ± 2.4 | 30.5 ± 1.6 | 31.5 ± 2.0 | 31.9 ± 2.3 | 32.4 ± 2.2 |
| AMT right M1 (%) | 46.4 ± 3.5 | 45.5 ± 3.0 | 47.4 ± 3.7 | 47.4 ± 3.3 | 48.3 ± 3.4 | 48.9 ± 3.2 |
All values are given as mean ± 1 s.e.m. (n = 8). Both RMT and AMT are given as a percentage of maximum stimulator output. Note that coil orientations and stimulator setup were different for the left and right M1 (see Methods), resulting in higher thresholds over the right M1 in all three sessions (Student's paired two-tailed t tests, P < 0.001 for all comparisons).
Indicates significant differences between pre and post measurements (Student's paired two-tailed t test, P < 0.05).
Effects of DZP and BAC on SICI, LICI and SIHI
At baseline, the TS intensity ‘1mV’ (conditions A–D) was similar in all three sessions (48.6 ± 4.0% MSO, PBO; 48.8 ± 3.4%, DZP; 49.1 ± 3.7%, BAC; P > 0.9 for all comparisons) as was the TS intensity ‘1mVCCS12’(conditions E–H; 54.1 ± 4.6% MSO, PBO; 55.1 ± 4.8%, DZP; 53.8 ± 4.3%, BAC; P > 0.7 for all comparisons) and the TS intensity ‘1mVCS100’ (conditions I–N; 52.8 ± 4.1% MSO, PBO; 53.8 ± 3.8%, DZP; 53.8 ± 3.7%, BAC; P > 0.7 for all comparisons). In all three sessions the TS intensities ‘1mVCCS12’ and ‘1mVCS100’ were significantly higher as compared to the TS intensity ‘1mV’ (P < 0.05 for all comparisons). These TS intensities produced similar MEP amplitudes when compared across sessions (P > 0.1 for all comparisons, Table 3), with MEP amplitudes at TS intensities ‘1mVCCS12’ (condition E in Table 1) and ‘1mVCS100’ (condition I in Table 1) being significantly higher than at the TS intensity ‘1mV’ (condition A in Table 1) in all three sessions (P < 0.05; Table 3). Furthermore, the CS intensity ‘CS100’ was similar in all three sessions (46.5 ± 3.8% MSO, PBO; 46.9 ± 4.0%, DZP; 46.8 ± 3.6%, BAC; P > 0.9 for all comparisons) as was the CS intensity ‘CCS12’(63.5 ± 3.5% MSO, PBO; 62.1 ± 2.3%, DZP; 62.5 ± 3.0%, BAC; P > 0.6 for all comparisons) and the CS intensity “CS3” (26.3 ± 1.8% MSO, PBO; 25.8 ± 1.5%, DZP; 25.6 ± 2.0%, BAC; P > 0.6 for all comparisons) at baseline. Importantly, with these CS intensities SICI, LICI and SIHI were well adjusted to produce ∼50% inhibition of the test response in all three sessions at baseline (P > 0.3 for all comparisons, Table 3).
Table 3.
Matching of MEP amplitudes and SICI, LICI, SIHI before (pre) and after (post) a single oral dose of PBO, DZP or BAC
| Measure | pre-PBO | post-PBO | pre-DZP | post-DZP | pre-BAC | post-BAC |
|---|---|---|---|---|---|---|
| MEP amplitude (condition A) | 1.2 ± 0.2* | 1.3 ± 0.2* | 1.0 ± 0.1* | 1.1 ± 0.2* | 1.3 ± 0.2* | 1.4 ± 0.2* |
| MEP amplitude (condition E) | 1.9 ± 0.2* | 1.9 ± 0.2* | 1.7 ± 0.2* | 1.6 ± 0.3* | 1.9 ± 0.3* | 2.2 ± 0.5* |
| MEP amplitude (condition I) | 1.8 ± 0.2* | 1.8 ± 0.2* | 1.9 ± 0.2* | 1.5 ± 0.4* | 1.8 ± 0.2* | 2.3 ± 0.5* |
| MEP amplitude (condition G) | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.3 |
| MEP amplitude (condition L) | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.4 ± 0.2 |
| SICI | 0.45 ± 0.06 | 0.49 ± 0.06 | 0.48 ± 0.05 | 0.52 ± 0.07 | 0.45 ± 0.02 | 0.40 ± 0.05 |
| LICI | 0.51 ± 0.08 | 0.60 ± 0.08 | 0.46 ± 0.09 | 0.51 ± 0.10 | 0.50 ± 0.04 | 0.48 ± 0.06 |
| SIHI | 0.49 ± 0.07 | 0.46 ± 0.07 | 0.50 ± 0.06 | 0.50 ± 0.10 | 0.55 ± 0.04 | 0.50 ± 0.04 |
All values are given as mean ± 1 s.e.m. (n = 8). MEP amplitude (stimulation condition given in brackets) values are in mV; SICI, LICI and SIHI (given for TS intensity ‘1mV’) are given as the ratio of the conditioned to the unconditioned MEP amplitude. MEP amplitudes in conditions E (TS intensity ‘1mVCCS12’) and I (TS intensity ‘1mVCS100’) were significantly higher than those in condition A (TS intensity ‘1mV’) in all three sessions
(Student's paired two-tailed t tests, P < 0.05). Note that data for MEP amplitudes in conditions A, E and I are derived from the Post1 measurement, in which the drug effect on SICI, LICI and SIHI was measured. In contrast, data for MEP amplitudes in conditions G and L as well as data for SICI, LICI and SIHI post-drug intake are derived from the Post2 measurement, in which SICI, LICI and SIHI were matched to produce ∼50% inhibition of the test response similar to baseline measurements in order to test for a specific drug effect on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI. See also Table 1 for definition of conditions. Within sessions there were no significant differences pre- versus post-drug intake for any of the measurements (Student's paired two-tailed t test, all P > 0.05).
Importantly, CS and TS intensities were successfully readjusted after drug intake at the Post1 measurement in all three sessions (see Methods for readjustment of CS and TS intensities). Test MEP amplitudes elicited by the TS alone (conditions A, E and I in Table 1) at the Post1 measurement were comparable to those at baseline in all three sessions (MEP amplitudes for conditions A, E and I in Table 3, P > 0.1 for all comparisons). Therefore, any changes in SICI, LICI and SIHI in the post-drug measurement as compared to the pre-drug measurement can be attributed specifically to a drug effect.
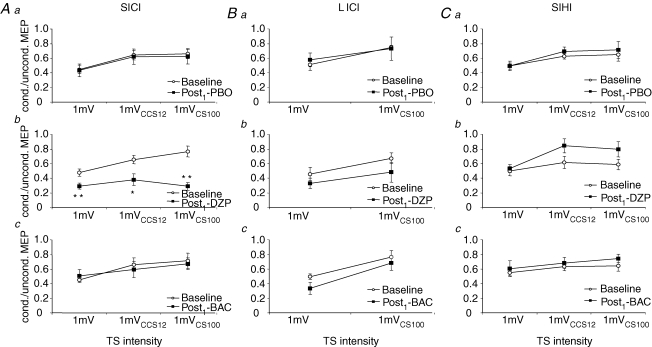
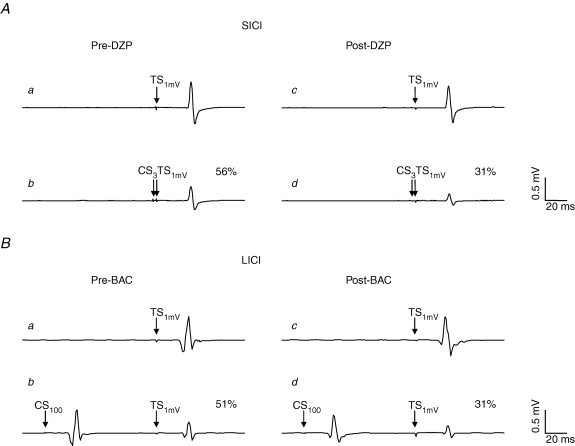
For SICI, there was a significant effect of time (F1,7= 13.8; P < 0.01), TS intensity (F2,14= 18.2; P < 0.001) and the interaction drug with time (F2,14= 9.2; P < 0.01), whereas the effect of drug and all other interactions were not significant. Post hoc testing revealed that SICI was significantly less at TS intensities of ‘1mVCCS12’ and ‘1mVCS100’ than at the TS intensity ‘1mV’ (P < 0.001 each; Fig. 3A). The effect of time and the interaction drug with time was explained by a significant increase of SICI at all TS intensities after administration of DZP (P < 0.01, TS intensity ‘1mV’; P = 0.02, TS intensity ‘1mVCCS12’; P < 0.001, TS intensity ‘1mVCS100’; Figs 2A and 3A).
Figure 3. Effects of DZP and BAC on SICI, LICI and SIHI.
Data are from eight subjects. Aa–c, effects of PBO (Aa), DZP (Ab) and BAC (Ac) on SICI. SICI was quantified by the ratio of the conditioned to the unconditioned MEP amplitude, i.e. conditions B/A for TS1mV, F/E for TS1mVCCS12 and J/I for TS1mVCS100. DZP increased SICI significantly at all TS intensities (Student's paired two-tailed t tests; *P < 0.05; **P < 0.01). Note that SICI was significantly less at the TS intensities ‘1mVCCS12’ and ‘1mVCS100’ than at the TS intensity ‘1mV’ pre- (P < 0.001 each) but not post-DZP intake (P > 0.1 for SICI at TS intensity ‘1mV’versus at TS intensity ‘1mVCCS12’; P > 0.9 for SICI at TS intensity ‘1mV’versus at TS intensity ‘1mVCS100’). Ba–c, effects of PBO (Ba), DZP (Bb) and BAC (Bc) on LICI. LICI was quantified as the ratio of MEP amplitudes in conditions D/A for TS1mV and L/I for TS1mVCS100. BAC produced a trend towards enhanced LICI at the TS intensity ‘1mV’ (Student's paired two-tailed t test; P = 0.09), but had no effect at the higher TS intensity ‘1mVCS100’. Ca–c, effects of PBO (Ca), DZP (Cb) and BAC (Cc) on SIHI. SIHI was quantified as the ratio of MEP amplitudes in conditions C/A for TS1mV, G/E for TS1mVCCS12 and K/I for TS1mVCS100. DZP produced a trend towards less SIHI at the higher TS intensities ‘1mVCCS12’ and ‘1mVCS100’ (Student's paired two-tailed t tests; P = 0.07 and P = 0.18, respectively), but had no effect at the TS intensity ‘1mV’. Note that all data are derived from the Post1 measurement. Error bars, s.e.m.
Figure 2. Effects of DZP on SICI and BAC on LICI in representative subjects.
Each trace represents the average of eight trials. All traces are recordings from the right FDI muscle. A, effect of DZP on SICI. a, response pre-DZP to TS with intensity ‘1mV’ (TS1mV) alone (condition A in Table 1). b, response pre-DZP to the CS3–TS1mV pulse pair (condition B in Table 1): the MEP amplitude is suppressed to 56% of its unconditioned value (SICI). c and d, DZP increased SICI significantly, resulting in an MEP amplitude in the conditioned situation (d) of only 31% of the unconditioned situation (c). B, effect of BAC on LICI. a, response pre-BAC to TS1mV alone. b, response pre-BAC to the CS100–TS1mV pulse pair (condition D in Table 1): the MEP amplitude is suppressed to 51% of its unconditioned value (LICI). c and d, BAC increased LICI significantly, resulting in an MEP amplitude in the conditioned situation (d) of only 31% of the unconditioned situation (c). Note that MEP amplitudes to TS1mV alone post-drug intake (c) were matched to those pre-drug intake (a) in A and B.
For LICI, there was a significant effect of TS intensity (F1,7= 18.7; P < 0.01), whereas all other effects or their interactions were not significant. The effect of TS intensity was explained by LICI being significantly weaker at the TS intensity ‘1mVCS100’ as compared to the TS intensity ‘1 mV’ (P < 0.01; Fig. 3B). Previous studies strongly suggested that LICI is mediated by the GABABR (Werhahn et al. 1999; McDonnell et al. 2006). In this study, BAC produced a trend towards stronger LICI at the TS intensity ‘1mV’ (P = 0.09), but had no effect at the higher TS intensity ‘1mVCS100’ (P = 0.36) (Figs 2B and 3B).
For SIHI, TS intensity was a significant main effect (F2,14= 8.8; P < 0.01), whereas all other effects or their interactions were not significant. Post hoc analyses showed that SIHI was significantly less at TS intensities of ‘1mVCCS12’ and ‘1mVCS100’ than at the TS intensity ‘1 mV’ (P < 0.01 each; Fig. 3C). DZP produced a trend towards less SIHI at the higher TS intensities ‘1mVCCS12’ (P = 0.07) and ‘1mVCS100’ (P = 0.18), but had no effect at the TS intensity ‘1mV’ (P > 0.7) (Fig. 3C).
Effects of DZP and BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI
The TS intensities ‘1mVCCS12’ and ‘1mVCS100’ were designed to produce MEPs of ∼1 mV peak-to-peak amplitude in the presence of the CCS12 or CS100 pulse, respectively (see Methods). At baseline, a TS with intensity ‘1mVCCS12’ in the presence of CCS12 (condition G in Table 1) and a TS with intensity ‘1mVCS100’in the presence of CS100 (condition L in Table 1) resulted in MEP amplitudes that were not significantly different from those in the unconditioned situation (TS alone with intensity ‘1 mV’, condition A in Table 1) (P > 0.2 for all comparisons; Table 3), indicating good matching of MEP amplitudes across conditions.
Importantly, test MEP amplitudes in conditions G and L (TS intensities ‘1mVCCS12’ and ‘1mVCS100’) were successfully readjusted after drug intake at the Post2 measurement to ∼1 mV peak-to-peak amplitude in all three sessions (MEP amplitudes for conditions G and L in Table 3, P > 0.06 for all comparisons). Equally important, SICI, LICI and SIHI were readjusted after drug intake at the Post2 measurement to produce ∼50% inhibition of the test response (P > 0.1 for all comparisons; Table 3). With these readjustments, any change in the interactions SIHI–SICI, LICI–SIHI and LICI–SICI in the post-drug measurement as compared to the pre-drug measurement can most likely be attributed specifically to a drug effect.
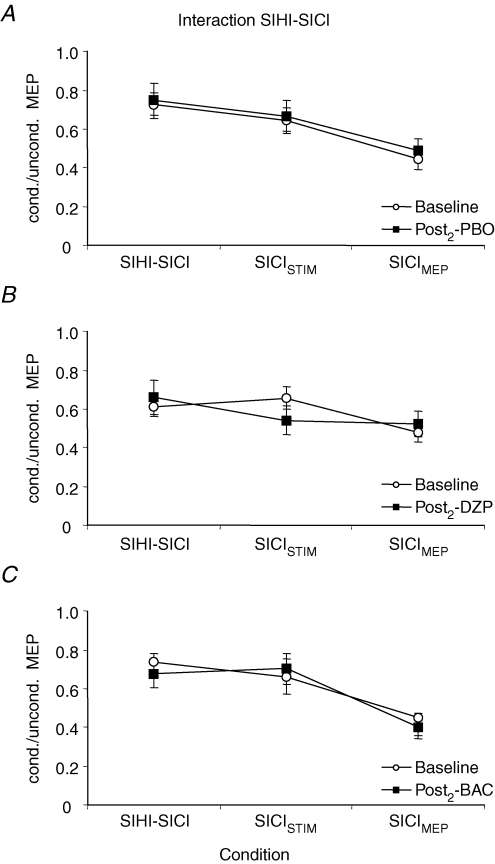
For the interaction SIHI–SICI, there was a significant effect of condition (F2,14= 26.0; P < 0.0001). All other effects and all interactions were not significant, indicating that neither PBO, DZP, nor BAC significantly changed the interaction SIHI–SICI (Figs 4 and 5). Post hoc analyses revealed that SICI was significantly less in the presence of SIHI (SIHI–SICI) than in the absence of SIHI when matched for test MEP amplitude (SICIMEP) (P < 0.0001; Fig. 5). SICI in the absence of SIHI was significantly less in the TS intensity-matched condition (SICISTIM) as compared to the test MEP amplitude-matched condition (SICIMEP) (P < 0.0001; Fig. 5).
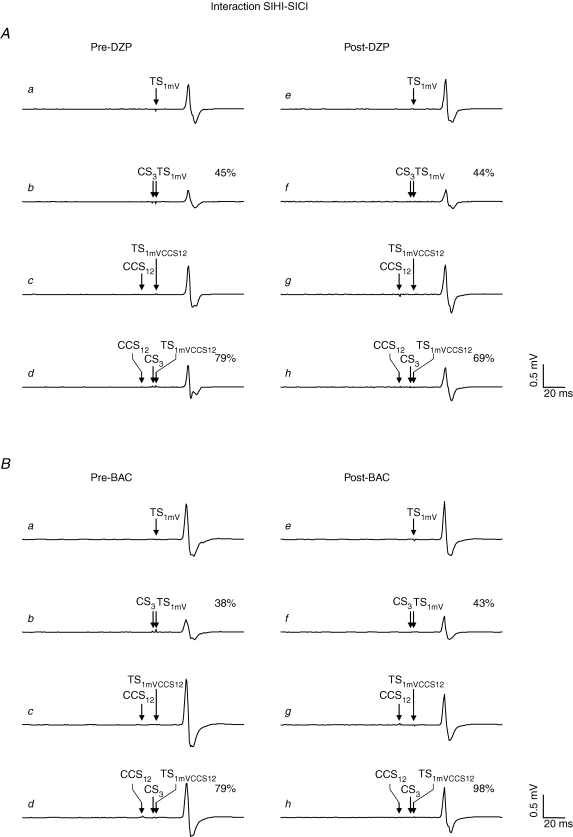
Figure 4. Effects of DZP and BAC on the interaction SIHI–SICI in representative subjects.
Each trace represents the average of eight trials. All traces are recordings from the right FDI muscle. A, effect of DZP on the interaction SIHI–SICI. a, response pre-DZP to TS1mV alone (condition A in Table 1). b, response pre-DZP to the CS3–TS1mV pulse pair (condition B in Table 1) with suppression of the test response to 45% (SICI). c, response pre-DZP to the TS in the presence of CCS12 (condition G in Table 1): the increased test stimulus intensity ‘1mVCCS12’ compensated for the CCS12-induced SIHI of the test response, resulting in a test MEP amplitude of the CCS12–TS1mVCCS12 pulse pair of comparable size as in the unconditioned situation (a). d, triple pulse stimulation pre-DZP (condition H in Table 1): SICI in the presence of SIHI. The response to the CCS12–CS3–TS1mVCCS12 pulse triple is 79% of the CCS12–TS1mVCCS12 pulse pair (c), indicating less SICI in the presence of SIHI as compared to SICI alone matched for test MEP amplitude. e–h, DZP did not change this negative interaction between SIHI and SICI. B, effect of BAC on the interaction SIHI–SICI. BAC did not change the interaction SIHI–SICI. Note that MEP amplitudes to TS1mV alone and CCS12–TS1mVCCS12 as well as the amount of SICI alone post-drug intake were matched to those pre-drug intake in A and B.
Figure 5. Effects of DZP and BAC on the interaction SIHI–SICI.
Data are from eight subjects. A–C, SICI in the absence of SIHI was quantified as the ratio of MEP amplitudes in conditions F/E (‘SICISTIM’) and B/A (‘SICIMEP’). SICI in the presence of SIHI was quantified as the ratio of MEP amplitudes in conditions H/G (‘SIHI–SICI’). SICI was significantly less in the presence of SIHI than in the absence of SIHI matched for test MEP amplitude (SICIMEP) in all three sessions (Student's paired two-tailed t test; P < 0.0001). SICI in the absence of SIHI was significantly less in the TS intensity-matched condition (SICISTIM) as compared to the test MEP amplitude-matched condition (SICIMEP) (Student's paired two-tailed t test; P < 0.0001). PBO, DZP and BAC had no effect on the interaction between SIHI and SICI. Note that all data are derived from the Post2 measurement where SICI and SIHI alone were readjusted to baseline after drug intake (see Methods and Fig. 1). Error bars, s.e.m.
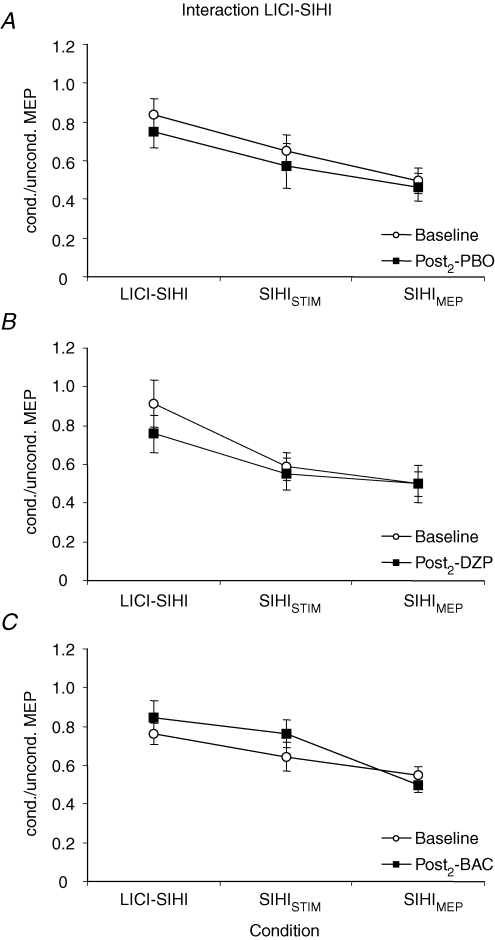
For the interaction LICI–SIHI, there was a significant effect of condition (F2,14= 148.4; P < 0.0001), whereas all other effects and all interactions were not significant. Thus, PBO, DZP and BAC did not significantly change the interaction LICI–SIHI (Figs 6 and 7). The significant main effect of condition was explained by SIHI being significantly less in the presence of LICI (LICI–SIHI) than in the absence of LICI matched for TS intensity (SIHISTIM) and test MEP amplitude (SIHIMEP) (P < 0.0001 each; Fig. 7). SIHI in the absence of LICI was significantly less in the TS intensity-matched condition (SIHISTIM) as compared to the test MEP amplitude-matched condition (SIHIMEP) (P < 0.0001; Fig. 7).
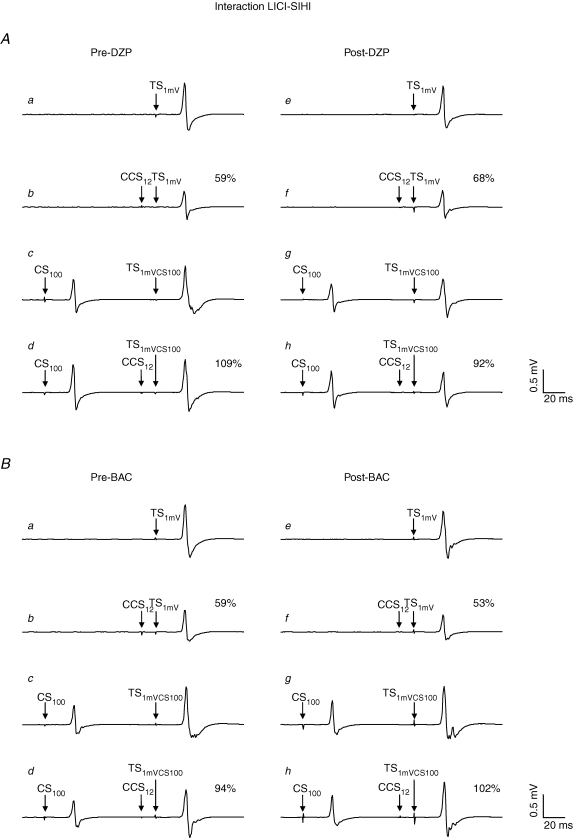
Figure 6. Effects of DZP and BAC on the interaction LICI–SIHI in representative subjects.
Each trace represents the average of eight trials. All traces are recordings from the right FDI muscle. A, effect of DZP on the interaction LICI–SIHI. a, response pre-DZP to TS1mV alone (condition A in Table 1). b, response pre-DZP to the CCS12–TS1mV pulse pair (condition C in Table 1) with suppression of the test response to 59% (SIHI). c, response pre-DZP to the TS in the presence of CS100 (condition L in Table 1): the increased test stimulus intensity ‘1mVCS100’ compensated for the CS100-induced LICI of the test response, resulting in a test MEP amplitude of the CS100–TS1mVCS100 pulse pair of comparable size as in the unconditioned situation (a). d, triple pulse stimulation pre-DZP (condition N in Table 1): SIHI in the presence of LICI. The response to the CS100–CCS12-TS1mVCS100 pulse triple is 109% of the CS100–TS1mVCS100 pulse pair (c), showing no longer SIHI but even some facilitation of the test response in the presence of LICI. e–h, DZP did not change this negative interaction between LICI and SIHI. B, effect of BAC on the interaction LICI–SIHI. BAC did not change the interaction LICI–SIHI. Note that MEP amplitudes to TS1mV alone and CS100–TS1mVCS100 as well as the amount of SIHI alone post-drug intake were matched to those pre-drug intake in A and B.
Figure 7. Effects of DZP and BAC on the interaction LICI–SIHI.
Data are from eight subjects. A–C, SIHI in the absence of LICI was quantified as the ratio of MEP amplitudes in conditions K/I (‘SIHISTIM’) and C/A (‘SIHIMEP’). SIHI in the presence of LICI was quantified as the ratio of MEP amplitudes in conditions N/L (‘LICI–SIHI’). SIHI was significantly less in the presence of LICI than in the absence of LICI matched for TS intensity (SIHISTIM) and test MEP amplitude (SIHIMEP) in all three sessions (Student's paired two-tailed t tests; P < 0.0001). SIHI in the absence of LICI was significantly less in the TS intensity-matched condition (SIHISTIM) as compared to the test MEP amplitude-matched condition (SIHIMEP) (Student's paired two-tailed t test; P < 0.0001). PBO, DZP and BAC had no effect on the interaction between LICI and SIHI. Note that all data are derived from the Post2 measurement where SIHI and LICI alone were readjusted to baseline after drug intake (see Methods and Fig. 1). Error bars, s.e.m.
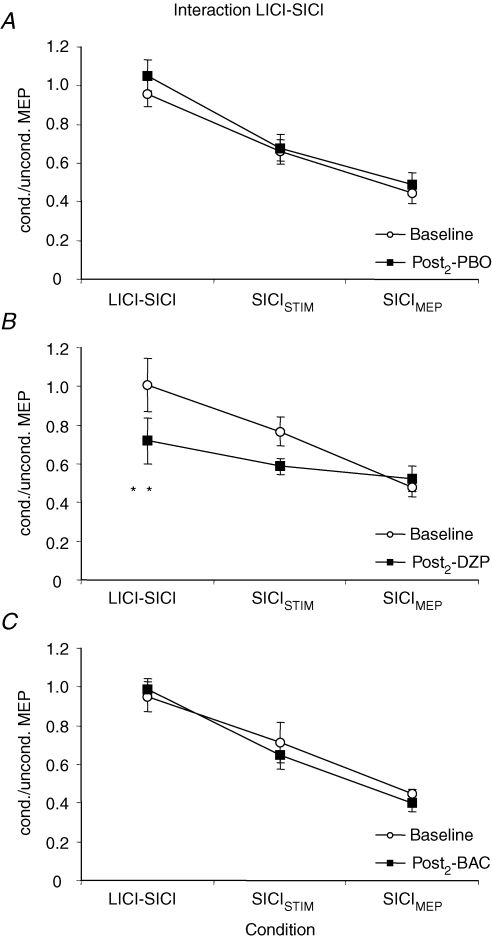
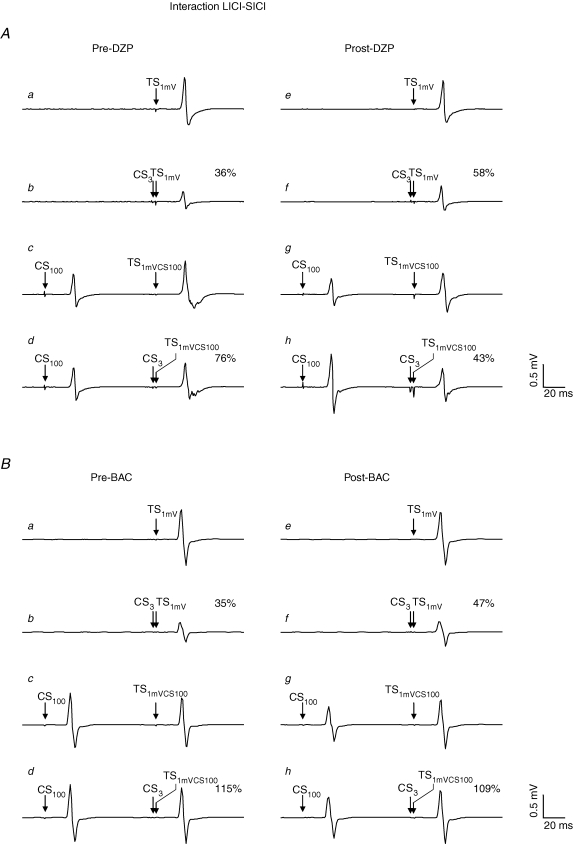
For the interaction LICI–SICI, there was a significant effect of condition (F2,14= 39.0; P < 0.0001) and of the interaction drug with time (F2,14= 5.2; P = 0.02), whereas all other effects and interactions were not significant. Post hoc testing showed that SICI in the presence of LICI was significantly suppressed (in fact, it was almost completely blocked) when compared to SICI in the absence of LICI matched for TS intensity (SICISTIM) and test MEP amplitude (SICIMEP) (P < 0.001 each; Fig. 9). SICI in the absence of LICI was significantly less in the TS intensity-matched condition (SICISTIM) as compared to the test MEP amplitude-matched condition (SICIMEP) (P < 0.01; Fig. 9). The significant effect of the interaction drug with time was explained by a significant increase of SICI in the presence of LICI after administration of DZP (P < 0.01; Fig. 8A and 9B), whereas PBO (P > 0.3) and BAC (P > 0.6) did not significantly change the interaction LICI–SICI (Figs 8B and 9C).
Figure 9. Effects of DZP and BAC on the interaction LICI–SICI.
Data are from eight subjects. A–C, SICI in the absence of LICI was quantified as the ratio of MEP amplitudes in conditions J/I (‘SICISTIM’) and B/A (‘SICIMEP’). SICI in the presence of LICI was quantified as the ratio of MEP amplitudes in conditions M/L (‘LICI–SICI’). SICI was significantly suppressed (in fact, it was almost completely blocked) in the presence of LICI when compared to SICI alone matched for TS intensity (‘SICISTIM’) and test MEP amplitude (‘SICIMEP’) (Student's paired two-tailed t tests; P < 0.001). SICI in the absence of LICI was significantly less in the TS intensity-matched condition (SICISTIM) as compared to the test MEP amplitude-matched condition (SICIMEP) (Student's paired two-tailed t test; P < 0.01). Whereas DZP significantly reinstated SICI in the presence of LICI (**P < 0.01; Student's paired two-tailed t test), PBO and BAC did not have any effect on the interaction between LICI and SICI. Note that all data are derived from the Post2 measurement where SICI and LICI alone were readjusted to baseline after drug intake (see Methods and Fig. 1). Error bars, s.e.m.
Figure 8. Effects of DZP and BAC on the interaction LICI–SICI in representative subjects.
Each trace represents the average of eight trials. All traces are recordings from the right FDI muscle. A, effect of DZP on the interaction LICI–SICI. a, response pre-DZP to TS1mV alone (condition A in Table 1). b, response pre-DZP to the CS3–TS1mV pulse pair (condition B in Table 1) with suppression of the test response to 36% (SICI). c, response pre-DZP to the TS in the presence of CS100 (condition L in Table 1): the increased test stimulus intensity ‘1mVCS100’ compensated for the CS100 induced LICI of the test response, resulting in a test MEP amplitude of the CS100–TS1mVCS100 pulse pair of comparable size as in the unconditioned situation (a). d, triple pulse stimulation pre-DZP (condition M in Table 1): SICI in the presence of LICI. The response to the CS100–CS3-TS1mVCS100 pulse triple is 76% of the CS100–TS1mVCS100 pulse pair (c), indicating less SICI in the presence of LICI as compared to SICI alone matched for test MEP amplitude. e–h, DZP reversed the negative interaction between LICI and SICI in this subject, resulting in more-pronounced SICI in the presence of LICI as compared to SICI in the absence of LICI matched for test MEP amplitude after administration of DZP. B, effect of BAC on the interaction LICI–SICI. On the contrary, BAC did not change the interaction LICI–SICI. Note that MEP amplitudes to TS1mV alone and CS100–TS1mVCS100 as well as the amount of SICI alone post-drug intake were matched to those pre-drug intake in A and B.
Data analyses from the Post1 measurement showed comparable results for the interaction SIHI–SICI (effect of condition, F2,14= 14.9; P < 0.001) and the interaction LICI–SIHI (effect of condition, F2,14= 19.6; P < 0.0001) as data analyses of the Post2 measurements, revealing no drug effect on either interaction (data not shown). For the interaction LICI–SICI, there was a significant effect of condition (F2,14= 15.8; P < 0.001), of the interaction drug with time (F2,14= 4.9; P < 0.05) and of the interaction drug with time and condition (F4,28= 2.9; P < 0.05). However, the significant effect of the interaction drug with time and of the interaction drug with time and condition was explained by a significant increase of SICI matched for TS intensity (SIHISTIM) and SICI matched for test MEP amplitude (SIHIMEP) after administration of DZP (P < 0.01 each), whereas SICI in the presence of LICI was not different pre- and post-administration of DZP (P > 0.7; data not shown). This finding clearly demonstrates that only readjusting single inhibitions post-drug intake to baseline values of ∼50% inhibition of the test response (as done in the Post2 measurement) revealed the DZP effect on the interaction LICI–SICI, which was masked by the DZP-induced enhancement of SICI alone in the Post1 measurement.
Discussion
This study examined the effects of DZP and BAC on SICI, LICI and SIHI and their interactions. Our results corroborate the notion that SICI represents a GABAAR-mediated inhibition, and that LICI very likely represents a GABABR-mediated inhibition. The pharmacology of SIHI remains inconclusive and warrants further investigation. Furthermore, we showed that the interactions SIHI–SICI, LICI–SIHI and LICI–SICI are all negative. BAC did not change any of these interactions, whereas DZP significantly increased SICI in the presence of LICI.
SICI, LICI and SIHI depend on TS intensity
We found that SICI, LICI and SIHI all decreased with increasing TS intensity (see Fig. 3, baseline). The mean MEP amplitudes for the different TS intensities applied were 1.2 ± 0.2 mV (TS intensity ‘1mV’), 1.8 ± 0.2 mV (‘1mVCCS12’) and 1.8 ± 0.2 mV (‘1mVCS100’) when averaged over all three sessions at baseline. The results for LICI and SIHI are in line with previous studies that reported a decrease in LICI and SIHI when test MEP amplitude increased from ∼1 mV to ∼4 mV (Sanger et al. 2001; Daskalakis et al. 2002). The result for SICI is complementary to earlier studies which showed an increase in SICI when test MEP amplitudes increase from ∼0.2 mV to ∼1 mV, while there was no significant effect on SICI if test MEP amplitudes increased further from ∼1 mV to ∼4 mV (Sanger et al. 2001; Daskalakis et al. 2002). Thus, it appears that SICI exhibits a more-complex relation to TS intensity, as was already suggested in one earlier study that systematically investigated the relation of SICI to both CS and TS intensity (Ilic et al. 2002). In summary, findings suggest that those neurons involved in the generation of MEP amplitudes of ∼1 mV are most susceptible to SICI.
Pharmacology of SICI, LICI and SIHI
Successful readjustment of CS and TS intensities at the Post1 measurement after drug intake (see Fig. 1) allowed us to attribute any changes in SICI, LICI and SIHI post-drug intake specifically to a drug effect. The significant increase in RMT of the left M1 after intake of DZP (Table 2) was only minor (on average 2.4% of MSO) and did not interfere with our testing of the effects of DZP on SICI, LICI and SIHI, as no stimulus intensity was normalised to RMT of the left M1 (see Methods and Table 1; CCS12 was set at 130% RMT of right M1).
Our results confirm previous reports that showed that SICI represents a GABAAR-mediated cortical inhibition (Ziemann et al. 1996a; Di Lazzaro et al. 2000; Ilic et al. 2002; Di Lazzaro et al. 2006). In addition, we found that the decrease of SICI with increasing TS intensity (Fig. 3A, Baseline) is suppressed after intake of DZP, i.e. DZP enhanced SICI to a similar level irrespective of TS intensity (Fig. 3A, Post1). There is direct evidence from spinal epidural recordings of the descending corticospinal volley that the SICI mediating neuronal circuit exerts its inhibitory effect primarily by suppressing the late I-waves, and that this suppression of the late I-waves is enhanced after intake of a positive modulator at the GABAAR (Di Lazzaro et al. 1998; Di Lazzaro et al. 2000). The proportion of late I-waves contributing to the MEP increases with increasing TS intensity (Di Lazzaro et al. 1999). This may explain why the enhancement of SICI after intake of DZP became most evident with the high TS intensities. Recently, another study corroborated these findings by showing that intravenous administration of lorazepam decreases MEP input–output-curves only at high TS intensities (Kimiskidis et al. 2006). However, the finding that late I-waves are particularly susceptible to SICI per se (Di Lazzaro et al. 1998) would suggest that SICI increases with increasing TS intensities. In contrast, our results (see Fig. 3) and prior work (Stefan et al. 2002, cf. their Fig. 1) demonstrated that SICI decreases with increasing TS intensities above those necessary to produce MEP amplitudes of ∼1 mV. The reason for this apparent discrepancy is not clear. One possible explanation may be that all late I-waves are already recruited at TS intensities as low as AMT +∼20% AMT, typically producing MEPs < 1 mV, but all late I-waves increase in amplitude when TS intensity is further increased (Di Lazzaro et al. 1999). It is possible that those late I-waves recruited at low TS intensities are particularly susceptible to SICI, while those recruited at higher TS intensities are less susceptible but may also be inhibited if neurotransmission through the GABAAR is enhanced by DZP, thus resulting in a prominent SICI effect.
We did not find any effect of BAC on SICI. Previous studies reported inconsistent results – either no significant change (Ziemann et al. 1996b; McDonnell et al. 2007) or a weak but significant decrease (McDonnell et al. 2006). This latter finding would be in line with the hypothesis that the inhibitory interneurons mediating SICI are controlled by presynaptic GABABR-mediated inhibition (Werhahn et al. 1999; Sanger et al. 2001). In the present study, BAC decreased SICI in four subjects, increased it in three subjects and had no effect in one subject. One possibility to explain this inter-individual variability may be genetic polymorphisms of the GABABR. The resulting amino acid substitutions are located in the N-terminal extracellular domain of the GABABR which is likely to constitute the ligand binding site and thus may account for variable effects of BAC at this receptor (Kaupmann et al. 1997; Sander et al. 1999).
Previous data suggest that LICI is mediated by the GABABR because the GABA reuptake inhibitor tiagabine (Werhahn et al. 1999) and the specific GABABR agonist BAC (McDonnell et al. 2006) enhanced LICI. In this study, BAC produced a trend towards enhanced LICI at the TS intensity ‘1mV’ (with six of eight subjects showing enhanced LICI under BAC), but had no significant effect at the higher TS intensity ‘1mVCS100’(with five of eight subjects showing enhanced LICI under BAC) (Fig. 3B). Even though we found only a trend towards an increase of LICI under BAC that failed to reach significance most likely due to interindividual variability and small sample size, this finding and the work of McDonnell et al. (2006) suggest that BAC enhances LICI at low TS intensities but does not have an effect on LICI at higher TS intensities. The reason for this difference is not entirely clear. LICI, similar to SICI, acts primarily on the late I-waves (Di Lazzaro et al. 2002). However, we do not currently know which I-waves are predominantly modified by BAC. If BAC particularly suppresses the early I-waves, then this could explain why BAC exerted a more conspicuous enhancing effect on LICI at low compared to high TS intensities. Further studies in larger samples are needed to clarify this issue.
Indirect evidence from TMS studies suggested that SIHI is a GABABR-mediated inhibition (Daskalakis et al. 2002). Recently, evidence emerged that different mechanisms are involved in SIHI (tested at ISIs ∼8–15 ms) and LIHI (intervals ∼40 ms) (Chen et al. 2003). Yet, the exact mechanisms and pharmacology of SIHI and LIHI remain to be clarified. Whereas LIHI seems to be GABABR mediated because it is enhanced by BAC (Irlbacher et al. 2007), the results on SIHI are inconclusive. The present study showed a trend towards less SIHI under DZP at high TS intensities, whereas BAC had no effect. This finding is in line with one earlier study that reported a trend towards less SIHI after intake of lorazepam (Ziemann et al. 1996a), while the study of Irlbacher et al. (2007) showed that both DZP and BAC did not affect SIHI. Thus, further investigation is required to elucidate the neuronal circuit that mediates SIHI, but the available data suggest that GABABR-mediated neurotransmission does not significantly contribute to SIHI.
Different neuronal populations mediate SICI, LICI and SIHI
Several lines of evidence suggest that SICI and LICI are mediated by different interneuronal circuits. First, increasing test MEP amplitude from ∼0.2 mV to ∼1 mV had opposite effects on the magnitude of SICI and LICI (Sanger et al. 2001). Second, the magnitudes of SICI and LICI did not correlate with each other (Sanger et al. 2001). Third, SICI and LICI showed different pharmacological profiles. While SICI depends on neurotransmission through the GABAAR, LICI is very likely mediated through the GABABR.
It is likely that the interneuronal circuits that mediate SICI and SIHI also differ. The argument in support of this notion is again that increasing the test MEP amplitude from ∼0.2 mV to ∼1 mV had opposite effects on SICI and SIHI (Daskalakis et al. 2002). This dissociating effect of test MEP amplitude on the extent of SICI and SIHI, which was no longer present when using larger test MEP amplitudes of 1.0 mV and 1.9 mV (Table 3), is incompatible with the view that SICI and SIHI are being mediated by the same population of inhibitory interneurons. Furthermore, we found differential effects of DZP on SICI and SIHI (see Fig. 3A and C): DZP enhanced SICI, but produced a trend towards a decrease of SIHI.
The question that remains is whether the same or different inhibitory interneuronal circuits mediate LICI and SIHI. Daskalakis et al. (2002) suggested that these circuits are similar but admitted that there are also significant differences between these two forms of inhibition, in particular their duration. More recently, it was suggested that LICI is more closely related to LIHI (tested at interstimulus intervals of ∼40 ms) than SIHI (tested at intervals of ∼10 ms) (Kukaswadia et al. 2005). We found that SICI was suppressed more strongly by LICI than SIHI (Figs 5 and 9). Measuring both interactions, SIHI–SICI and LICI–SICI, in the same experiment allowed us to directly compare the extents of these interactions. The difference between the suppressive effect of SIHI on SICI versus that of LICI on SICI was significant (0.69 ± 0.03 versus 0.97 ± 0.06; P < 0.01; data averaged over all three sessions at Baseline). This finding renders it very unlikely that LICI and SIHI are being mediated by the same interneuronal circuit. Importantly, LICI and SIHI alone were adjusted to ∼50% inhibition (Fig. 1). Therefore, there was no difference in the magnitude of LICI and SIHI that could have accounted for their different suppressive effects on SICI.
In summary, determination of SICI, LICI and SIHI by means of paired-pulse TMS allows non-invasive testing of three distinct inhibitory interneuronal circuits in human motor cortex. This is of great importance given the large body of evidence for morphologically and functionally distinct inhibitory interneurons in the neocortex (Markram et al. 2004), which may be affected differently in neurological or psychiatric diseases (Chen, 2004).
Effects of DZP on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI
Careful readjustment of CS and TS intensities at the Post2 measurement (see Fig. 1) resulted in a close matching of SICI, LICI and SIHI post-drug with baseline values (i.e. ∼50% inhibition, Table 3). This allowed us to assign any change in the interactions between these inhibitory circuits specifically to drug action. DZP partially reinstalled SICI in the presence of LICI (Fig. 9B). This may be interpreted as a non-linear enhancement of postsynaptic GABAAR-mediated neurotransmission by DZP, although this interpretation is necessarily indirect as our investigations are at the systems level. There is evidence from one previous study that the effects of DZP on SICI depend on both the CS and TS intensity (Ilic et al. 2002). At a TS intensity of 130% RMT, which closely matches the mean TS intensity in the DZP session in our study (128 ± 6% RMT pre-DZP; 130 ± 8% RMT post-DZP), Ilic and colleagues found a marked gradient of DZP effects on SICI as a function of CS intensity: DZP increased SICI particularly at low CS intensities that produced weak SICI, while the increase at higher CS intensities that produced close to maximum SICI was clearly less (Ilic et al. 2002; see their Fig. 2G. These findings suggest that the enhancement of SICI by DZP manifests particularly in conditions of low presynaptic GABA release. Suppression of SICI in the presence of LICI is most likely due to a reduction of presynaptic GABA release from SICI-mediating interneurons by presynaptic autoinhibition (Sanger et al. 2001). Thus, the DZP-induced increase in SICI in the presence of LICI (i.e. very low presynaptic GABA release) was most likely more pronounced than the DZP-induced increase in SICI in the absence of LICI (i.e. sufficient presynaptic GABA release to result in ∼50% inhibition). The partial restoration of SICI in the presence of LICI by DZP can thus be interpreted as the ‘additional’ DZP effect on SICI at very low levels of SICI on top of the DZP effect on SICI at medium levels of SICI (which we had corrected for by readjusting SICI to ∼50% inhibition). Conversely, SICI in the presence of SIHI was far less suppressed (see Fig. 5). This reduction of presynaptic GABA release in the presence of SIHI was probably not sufficient to unmask the non-linear enhancing effect of DZP on SICI.
Effects of BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI
The current model of TMS measures of intracortical inhibitory circuits suggests that SIHI and LICI suppress SICI by presynaptic GABABR-mediated autoinhibition (Sanger et al. 2001; Daskalakis et al. 2002). The negative interaction between LICI and SIHI was suggested to be, at least partly, also due to a GABABR-mediated autoinhibition within the neuronal circuit being responsible for both LICI and SIHI (Daskalakis et al. 2002). In contrast to our findings (see Fig. 7), Daskalakis et al. (2002) showed reduced SIHI in the presence of LICI when matched for test MEP amplitude but not when matched for TS intensity. The authors of this study provided three possible explanations for their finding: (i) SIHI and LICI preferentially target low-threshold cortical neurons; the pulse pair CS100–TS1mVCS100 however, probably activated higher-threshold cortical neurons than the single TS1mV pulse even though matched for test MEP amplitude. Thus, the finding might indicate that high-threshold neurons activated by the pulse pair CS100–TS1mVCS100 are less susceptible to SIHI as compared to low-threshold neurones activated by the single TS1mV pulse. This is unlikely given our results that SIHI is reduced in the presence of LICI when matched for both test MEP amplitude and TS intensity; (ii) a saturation effect within a common circuit mediating both SIHI and LICI. This argument can be discarded by our finding that SIHI and LICI are mediated by different neuronal circuits (see above); (iii) LICI inhibits SIHI via presynaptic GABAB receptor-mediated autoinhibition. This explanation is best compatible with our findings and therefore the most likely one. Hence, BAC was expected to increase all three negative interactions SIHI–SICI, LICI–SIHI and LICI–SICI.
However, we did not find any effect of BAC on any of these interactions. To interpret this finding it is important to note that we readjusted SICI, LICI and SIHI alone at the Post2 measurement to match baseline values (i.e. ∼50% inhibition, see Fig. 1 and Table 3). If, according to the current model, the negative interactions between SIHI–SICI, LICI–SIHI and LICI–SICI are really due to GABABR-mediated presynaptic autoinhibition, then the readjustment at the Post2 measurement, which aimed to correct for BAC effects on the postsynaptic GABABR that mediates LICI, at the same time inadvertently also readjusted for GABABR-mediated neurotransmission at presynaptic sites that we believe are responsible for the negative interactions between all three forms of inhibition. Thus, the lack of any effect of BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI cannot be taken as specific evidence but is compatible with the idea that these interactions are GABABR mediated.
This reasoning would not hold true however, if the presynaptic GABABR at inhibitory interneurons mediating SICI and SIHI showed a different sensitivity to BAC than the postsynaptic GABABR mediating LICI. In this case, correction for BAC effects on LICI at the Post2 measurement would not have equally adjusted for BAC effects on presumed presynaptic GABABRs mediating the interactions SIHI–SICI, LICI–SIHI and LICI–SICI. Two different subtypes of GABABRs can be distinguished in the brain, the molecular diversity of which most likely derives from splice variants GABAB1a and GABAB1b of the heteromeric GABAB(1,2)R (Kaupmann et al. 1997, 1998). Although the GABABR subunit isoforms 1a and 1b are differentially localisd at pre- and postsynaptic sites and show functional diversity (Bettler et al. 1998; Perez-Garci et al. 2006; Shaban et al. 2006; Vigot et al. 2006; Chu et al. 2007), their pharmacology of their binding to receptor agonists as well as antagonists is similar, which suggests that only those genetic sequences common to both GABAB1a and GABAB1b isoforms are directly involved in ligand binding (Kaupmann et al. 1997). Specifically, no difference in the binding affinity of GABAB1a and GABAB1b isoforms to BAC was observed (Kaupmann et al. 1997). However, if there were any differences between the pre- and postsynaptic effects of BAC these are very likely to be small, needing a large sample size to be demonstrated.
It may still be argued that, with our experimental design, we were not able to detect a stronger negative interaction between LICI and SICI post-BAC intake, because SICI in the presence of LICI was already completely suppressed at baseline (saturation effect). However, this would have posed a problem only if BAC exhibited higher affinity to pre- versus postsynaptic GABABRs, for which there is no evidence (Kaupmann et al. 1997).
An alternative explanation to account for the negative results of BAC on the interactions SIHI–SICI, LICI–SIHI and LICI–SICI is a postsynaptic interference between the inhibitory circuits by shunting. That is, the conditioning inhibitory circuit leads to an increase in membrane conductance in any of the neuronal elements necessary for MEP generation (e.g. the I-wave-generating neurons), which suppresses the efficacy of another inhibitory circuit to hyperpolarise exactly these neuronal elements. Such a mechanism could explain negative interactions between any two inhibitory circuits as well as the lack of any effect of BAC on these interactions, as BAC per se is not expected to have an impact on membrane conductance (Deisz et al. 1997). However, shunting can be largely ruled out for two reasons. First, SICI was clearly differently suppressed by SIHI than by LICI (SIHI–SICI: 0.69 ± 0.03 versus LICI–SICI: 0.97 ± 0.06; P < 0.01; data averaged over all three sessions at baseline). Assuming comparable strength of SIHI and LICI in the triple pulse stimulation (which is reasonable to assume as both SIHI and LICI were matched to ∼50% inhibition), there should be a comparable amount of negative interaction between SIHI–SICI and LICI–SICI if shunting played a major role. Second, intracortical facilitation (ICF) was not suppressed by SIHI (Daskalakis et al. 2002). If SIHI led to suppression of SICI by shunting, then a similar suppression of ICF in the presence of SIHI versus ICF alone, very much like in the interaction SIHI–SICI, had to be expected. Thus, we conclude that shunting does not explain the negative interactions between SIHI–SICI, LICI–SIHI and LICI–SICI. Rather our data further support the currently prevailing model that SIHI and LICI suppress SICI by means of presynaptic GABABR-mediated inhibition (Sanger et al. 2001; Daskalakis et al. 2002). Similarly, the negative interaction between LICI and SIHI can most parsimoniously also be explained by GABABR-mediated presynaptic autoinhibition, in this instance of SIHI mediating interneurons by LICI mediating interneurons.
Conclusion
We conclude that SICI, LICI and SIHI represent three distinct inhibitory circuits in the human motor cortex which can be tested non-invasively by means of paired-pulse TMS. Our data further support the notion that the negative interactions between SIHI and SICI, LICI and SIHI, and LICI and SICI which can be tested by triple-pulse TMS are most likely due to presynaptic GABABR-mediated autoinhibition. The partial restoration of SICI in the presence of LICI but not in the presence of SIHI by DZP reveals a non-linear enhancing effect of DZP on SICI.
References
- Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Chu J, Gunraj C, Chen R. Possible differences between the time courses of presynaptic and postsynaptic GABA(B) mediated inhibition in the human motor cortex. Exp Brain Res. 2007. in the press. [DOI] [PubMed]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA, Billard JM, Zieglgansberger W. Presynaptic and postsynaptic GABAB receptors of neocortical neurons of the rat in vitro: differences in pharmacology and ionic mechanisms. Synapse. 1997;25:62–72. doi: 10.1002/(SICI)1098-2396(199701)25:1<62::AID-SYN8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002;113:1673–1679. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct recordings of descending volleys after transcranial magnetic and electric motor cortex stimulation in conscious humans. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:120–126. [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, Ugawa Y. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151:427–434. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Jung P, Ziemann U. Subtle hemispheric asymmetry of motor cortical inhibitory tone. Clin Neurophysiol. 2004;115:330–340. doi: 10.1016/j.clinph.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol. 2007;118:308–316. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, Kazis A, Mills KR. Lorazepam-induced effects on silent period and corticomotor excitability. Exp Brain Res. 2006;173:603–611. doi: 10.1007/s00221-006-0402-1. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol. 2005;563:915–924. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell RA, Shapiro BE, Chiappa KH, Helmers SL, Cros D, Day BJ, Shahani BT. Hemispheric threshold differences for motor evoked potentials produced by magnetic coil stimulation. Neurology. 1991;41:1441–1444. doi: 10.1212/wnl.41.9.1441. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nature Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABA(B) receptor agonist baclofen. Exp Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Sander T, Samochowiec J, Ladehoff M, Smolka M, Peters C, Riess O, Rommelspacher H, Schmidt LG. Association analysis of exonic variants of the gene encoding the GABAB receptor and alcohol dependence. Psychiatr Genet. 1999;9:69–73. doi: 10.1097/00041444-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Shader RI, Pary RJ, Harmatz JS, Allison S, Locniskar A, Greenblatt DJ. Plasma concentrations and clinical effects after single oral doses of prazepam, clorazepate, and diazepam. J Clin Psychiatry. 1984;45:411–413. [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs WJ, Calvanio R, Macdonell RA, Cros D, Chiappa KH. Physiological motor asymmetry in human handedness: evidence from transcranial magnetic stimulation. Brain Res. 1994;636:270–276. doi: 10.1016/0006-8993(94)91026-x. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]