Abstract
The purpose of this study was to determine the effects of 6–8 weeks of chronic spinal cord isolation (SI, removal of descending, ascending and afferent inputs), compared with the same duration of spinal cord transection (ST, removal of descending input only) on hindlimb motoneurone biophysical properties. Adult female Sprague–Dawley rats were placed into three groups: (1) control (no removal of inputs), (2) ST and (3) SI. The electrophysiological properties from sciatic nerve motoneurones were recorded from deeply anaesthetized rats. Motoneurones in SI rats had significantly (P < 0.01) lower rheobase currents and higher spike afterhyperpolarization amplitudes and input resistances compared with motoneurones in control rats. A higher percentage (χ2, P = 0.01) of motoneurones in SI than control rats demonstrated frequency-current (f–I) relationships consistent with activation of persistent inward currents. Motoneurone steady state f–I slopes determined by increasing steps of 500 ms current pulses were significantly lower (P < 0.02) in SI than control rats. Motoneurone spike frequency adaptation measured using 30 s square-wave current injections (1.5–3.0 nA above the estimated rhythmic firing threshold), was similar for control and SI motoneurones. Changes in motoneurone properties following SI did not differ from ST. These findings indicate that the removal of afferent and ascending inputs along with descending inputs has little additional affect on motoneurone properties than removal of descending inputs alone. This study is the first to demonstrate that intact ascending and afferent input does not modify the effects of spinal transection on basic and rhythmic firing properties of rat hindlimb motoneurones.
Spinal cord transection eliminates supraspinal input to motoneurones below the transection site. Although voluntary drive to the motoneurone is eliminated, the afferent connections remain intact. Excitation of these afferents can lead to the activation of intact segmental reflexes (Li & Bennett, 2003), exciting the motoneurones and subsequently generating involuntary muscle contractions (Alaimo et al. 1984; Ishihara et al. 2002). For example, muscle electromyography activity after spinal cord transection (ST) is reduced only by ∼50–75% (Ishihara et al. 2002). These involuntary contractions following ST may reflect the accentuation of voltage-dependent persistent inward currents (PIC) (Bennett et al. 2001b) that enhance motoneurone excitability and, in the absence of inhibition from supraspinal systems, may contribute to periodic prolonged muscle contractions (spasticity) (Bennett et al. 2001a,b; Li & Bennett, 2003; Li et al. 2004). Furthermore, fully developed spasticity after ST results in partial recovery of muscle atrophy and reversion back from a higher percentage of fast-type muscle fibres to the more comparable proportions of slow and fast fibres seen in controls (Harris et al. 2007). Although some biophysical properties of motoneurones below the site of ST may undergo significant changes such as depolarization of resting membrane potential and voltage threshold (Cope et al. 1986; Hochman & McCrea, 1994; Beaumont et al. 2004), decreased afterhyperpolarization (AHP) duration (Czeh et al. 1978; Cope et al. 1986; Hochman & McCrea, 1994) and a rightward shift in the frequency–current (f–I) relationship (Beaumont et al. 2004), a large degree of heterogeneity is maintained. Thus, like muscle, afferent-induced activation of segmental reflexes after ST may help maintain, to a certain degree, the biophysical properties of the affected motoneurones. The question remains, would a lack of afferent and ascending input to motoneurones in addition to ST induce further changes in motoneurone biophysical properties compared with ST alone?
To address this issue, we use the spinal cord isolation (SI) model which combines ST with surgical ablation of afferent and ascending inputs (Grossman et al. 1998). The SI model abolishes segmental reflexes (Pierotti et al. 1991) and the hindlimb muscles are almost completely quiescent such that the integrated electromyography of the soleus muscle of SI rats is < 1% of normal control values (Gomez-Pinilla et al. 2004; Roy et al. 2007b). Unlike chronic ST, spasticity does not develop following chronic SI, myofibres undergo severe atrophy and remain atrophied, and myofibre types are transformed to predominantly fast-type without reverting back to those seen in controls (Harris et al. 2007). Furthermore, SI has a more pronounced effect on muscular force properties and different myosin heavy chain proportions than ST (Grossman et al. 1998; Roy et al. 2002; Talmadge et al. 2002). Despite the known effects of SI on muscle, the effect of SI on motoneurone biophysical properties remains unknown. However, it is known that 2 to 3 weeks after ST along with a bilateral deafferentation, which is very similar to the SI model, motoneurone electrotonic lengths in cats decreased and input resistances (Rin) and excitatory post-synaptic potentials increased, suggesting an overall atrophy of motoneurones (Gustafsson et al. 1982), although recent evidence shows that motoneurone soma size may be unaffected after SI (Chalmers et al. 1992; Roy et al. 2007a). Furthermore, following ST, the decreased slope of the f–I relationship of motoneurones below the lesion can be prevented by 1 month of daily passive cycling exercise (Beaumont et al. 2004). Perhaps this demonstrates the importance of regular afferent input in determining the properties of motoneurones.
Finally, neurotrophins are important for motoneurone survival and maintenance and may modulate the expression of ion channel subunits that render the motoneurone more or less excitable (Lesser et al. 1997; Rose et al. 2004). After SI the ventral horn has lower levels of brain-derived neurotrophic factor and neurotrophin 3 proteins and mRNA (Gomez-Pinilla et al. 2004). A decrease in neurotrophins after spinal cord injury may affect the number, density, composition, activation and/or location of the motoneurone ion channels thereby altering their conductance and subsequently eliciting changes in motoneurone electrophysiological properties. Since neurotrophins are located in both efferent and afferent pathways (Mitsumoto & Tsuzaka, 1999), SI may induce greater change to neurotrophin expression in the lumbar spinal cord and consequently to motoneurone electrophysiological properties compared with ST.
The purpose of the present study was to assess and compare the effects of ST and SI on the biophysical properties of rat hindlimb motoneurones. We hypothesized that, in SI, the ablation of ascending and afferent inputs in addition to the spinal transection would have a more pronounced effect on motoneurones than the lack of descending inputs induced by ST alone. We compared a series of active and basic motoneurone properties and found that ST and SI motoneurones have decreased f–I slopes, increased incidence of bistability but not PIC amplitude, and similar spike frequency adaptation (SFA) patterns compared with control motoneurones. Furthermore, ST and SI motoneurones have lower rheobase currents and higher Rin values and spike afterhyperpolarization (AHP) amplitudes compared with control motoneurones. No differences existed between ST and SI motoneurone properties. These findings suggest that afferent and ascending inputs do not preserve the biophysical properties of motoneurones following ST. Preliminary results have been published elsewhere (Button et al. 2005, 2006a, c).
Methods
Experimental animals
Adult female Sprague–Dawley rats were used in this study. Control animals (275–325 g, n = 33) were from the University of Manitoba (Winnipeg, MB, Canada). The ST (250–350 g, n = 15) and SI (225–300 g, n = 14) animals received surgery at the University of California, Los Angeles (UCLA). Animals were maintained at UCLA for 30 days after surgery and then shipped to the University of Manitoba. All animals were housed individually with 12 : 12 h light–dark cycle and provided water and food ad libitum. The room temperature was maintained at ∼25°C. When electrophysiological recordings were made from the SI and ST animals, rats had been chronically spinal isolated or spinal transected for approximately 6–8 weeks. Control rats were approximately the same age when electrophysiological recordings were made, but did not undergo SI or ST. All procedures were approved by the animal ethics committee of the University of Manitoba and the UCLA Chancellor's Animal Research Committee and were in accordance with the guidelines of the Canadian Council of Animal Care and followed the American Physiological Society Animal Care Guidelines.
ST and SI surgical procedures
These procedures were performed at the University of California, Los Angeles. Animals were acclimated to the laboratory conditions for 1 week prior to the surgical procedures and maintained at the University of California, Los Angeles, for 30 days post-operatively prior to shipment to the University of Manitoba. The rats were deeply anaesthetized with ketamine hydrochloride and xylazine (100 and 5 mg kg−1 body weight, respectively, i.p.). A surgical level of anaesthesia was maintained with supplements of ketamine as needed. All surgical procedures were performed under aseptic conditions.
The spinal cord of the ST rats was completely transected at a mid-thoracic level as previously described (Talmadge et al. 2002). Briefly, a dorsal midline incision was made, muscles overlying the spinal column from T6–T9 were separated from the spinal column, and a partial laminectomy was performed at T7–T8. Two to three drops of lidocaine (2%) were applied to the exposed spinal cord and the spinal cord was completely transected with microdissection scissors. The cut ends of the spinal cord were lifted gently to verify the completeness of the transection. Gelfoam was packed between the cut ends of the cord. The fascia and muscles surrounding the spinal column were sutured (4-0 chromic gut) over the transection site and the skin incision was closed (4-0 Ethilon sutures).
The SI procedure for rats has been described in detail elsewhere (Grossman et al. 1998). Briefly, rats in the SI group were subjected to complete spinal cord transections at mid-thoracic (∼T7) and upper sacral (∼S1) levels with a complete bilateral dorsal rhizotomy between the two transection sites. A longitudinal midline skin incision was made dorsal to the spinal column from the T6 to the S2 vertebral levels and the muscles overlying the spinal column were separated from the spinal column. A partial laminectomy was performed to remove the spinous processes, a trough (∼2 mm wide) was made in the midline of the column between vertebral levels T7 and S2, and the dura mater was opened longitudinally along the midline. Once the above procedures were successfully completed the dorsal roots from T7 to S2 were cut bilaterally as close to the spinal cord as possible, retracted to their point of exit, and then cut as close to the exit site as possible. Following the dorsal rhizotomy, the spinal cord was completely transected at approximately T7–T8 and S1–S2 and a complete transection was verified as described above. The transection sites were packed with Gelfoam. A strip of gelfilm was placed along the length of exposed spinal cord to minimize adhesions between the spinal cord and the overlying tissues. The paravertebral muscles and fascia surrounding the spinal column and the skin incision were sutured as described above. A schematic illustration of the SI surgery and details of postoperative care have been published previously (Roy et al. 1992; Hyatt et al. 2003).
An antibiotic (Baytril – enrofloxacin) was administered orally (2 ml (125 ml water)−1) for the first 5 days. An analgesic was administered (Buprenex – buprenorphin, 0.05 mg kg−1, s.c.) twice daily for the first 2 days. During recovery, the rats were housed individually in polycarbonate cages (26 cm × 48 cm) in a room maintained at 26 ± 1°C, with a 12 : 12 h light–dark cycle. Post-surgical care involved manual expression of the bladder three times per day for the first 2 weeks and two times per day for the remainder of the study. Following ST and SI surgery, the rats used their forelimbs to move around their cages to access food and water ad libitum. Although we did not measure electromyographic activity of muscles following ST or SI, the hindlimbs of SI animals were completely flaccid and exhibited no reflex activity (no muscle spasms, withdrawal reflexes or toe spread responses) at any point during the 1 month recovery period indicating that the transections and dorsal rhizotomy were complete. The hindlimbs of SI and ST rats were manipulated passively through a full range of movement once per day to maintain joint flexibility. Animal health, based on factors such as body weight, appearance, grooming, and quantity and quality of expressed urine, was assessed daily for both the SI and ST rats and cage bedding was changed to prevent skin infections. Control animals were confined to standard plastic cages for the same time period. The procedures for the care and maintenance of spinal injured animals have been detailed previously (Roy et al. 1992).
Surgery for electrophysiological experiments
Approximately 1 month after the ST or SI surgeries, the rats were shipped to the University of Manitoba for in situ electrophysiology experiments. Animals were anaesthetized with ketamine and xylazine (90 and 10 mg kg−1, respectively, i.p.), and atropine (0.05 mg kg−1 atropine in a 5% dextrose physiological saline vehicle, i.p.) was administered to minimize airway secretions during the subsequent tracheotomy. The surgical procedures included: (1) insertion of a tracheal tube for ventilation (Harvard Apparatus, Canada), (2) catheterization of the femoral artery for continuous monitoring of mean arterial pressure (MAP) and constant infusion of anaesthetic (Pump 11, Harvard Apparatus, Canada), (3) exposure of the left hindlimb sciatic nerve for electrical stimulation, and (4) exposure of the spinal vertebrae and laminectomy from T12 to S1 in a stereotaxic unit.
Physiological saline solution containing ketamine and xylazine (9 and 1 mg h−1, respectively) was infused via the femoral artery to maintain anaesthesia. The depth of anaesthesia was verified continuously via heart rate, MAP, expired CO2 levels, and bilateral toe pinch. Blood pressure was maintained between 80 and 110 mmHg and respiration was kept at a tidal volume of 2.0–2.5 ml and a ventilation rate of 60–80 strokes min−1. Expired CO2 levels were measured via a CAPSTAR 100 CO2 analyser (CWE Inc., USA) and maintained between 3 and 4% by adjusting tidal volume and/or ventilation rate. Rectal temperature was monitored and maintained near 37°C using a feedback homeothermic blanket control unit (Harvard Apparatus, Canada). The head, thoracic and lumbar vertebrae, hips, and left foot were immobilized with clamps, and the open leg and back incisions were used to make an oil bath around the sciatic nerve and spinal cord, respectively. The dura mater covering the spinal cord was incised, and the large dorsal roots comprised of afferents from the left hind-limb in control and ST rats were cut and reflected over the right side of the cord. An opening was made in the pia mater just lateral to the entry zone of these roots into the cord to allow penetration of the glass microelectrode for intracellular recording. Since SI rats had their dorsal roots removed, a greater medial-to-lateral opening in the pia matter was made along the L2–L4 vertabrae approximately where the dorsal roots were found in ST and control animals. Prior to the search for motoneurones, respiratory movement was minimized by performing a left unilateral pneumothorax.
Additional drugs and solutions
To reduce blood pressure and respiration-related movement artifacts and to stabilize the animal for optimal electrophysiological recordings, several additional solutions were administered intravenously. The rat received: (1) a solution of 100 mm NaHCO3 (Fisher scientific) and 5% dextrose (Fisher Scientific) in double-distilled H2O, and (2) pancuronium bromide (0.2 mg kg−1). Pancuronium bromide was injected prior to the start of the electrophysiological recordings and then re-administered as needed to maintain paralysis of the respiratory and hindlimb muscles, a requirement for optimal intracellular recordings.
Measurement of motoneurone basic properties
Glass microelectodes (1.0 mm thin-walled, World Precision Instruments, USA) were pulled with impedances of approximately 10 MΩ (Kopf Vertical Pipette Puller, David Kopf Instruments, USA), and filled with 2 m potassium citrate. The tip of the electrode was positioned over the incision in the pia mater and lowered into the cord with an inchworm microdrive system (Burleigh Instruments Inc., USA) in steps of 5–10 μm. The sciatic nerve was stimulated with a bipolar silver chloride electrode at a frequency of 1 pulse s−1 (0.1–0.2 mA for 0.1 ms) while the microelectrode was advanced through the cord and the field potential was monitored continuously. Evidence of successful impalement of a motoneurone was indicated by: (1) a sudden increase in membrane potential to at least 50 mV; (2) an antidromic action potential spike amplitude greater than 55 mV with a positive overshoot; and (3) a reproducible latency of less than 2.5 ms from the stimulation artifact. During recording, an Axoclamp intracellular amplifier system (Axoclamp 2B, Axon Instruments Inc., USA) was used either in a bridge or a discontinuous current-clamp mode (DCC; 2–10 kHz switching), with capacitance maximally compensated. Basic motoneurone properties recorded from resting membrane potential in bridge mode included: resting membrane potential, spike height, AHP amplitude and half-decay time of orthodromic action potentials evoked by brief (0.5 ms) supramaximal intracellular current injections (averaged from at least 40 spikes). Rheobase current (the minimum amplitude of a 50 ms square-wave current required to elicit an action potential 50% of the time), voltage threshold (membrane voltage at which a spike was triggered 50% of the time), and cell Rin (averaged from 60, 1 nA hyperpolarizing current pulses each lasting 100 ms) were determined in the DCC mode.
Measurement of motoneurone frequency–current (f–I) relationship
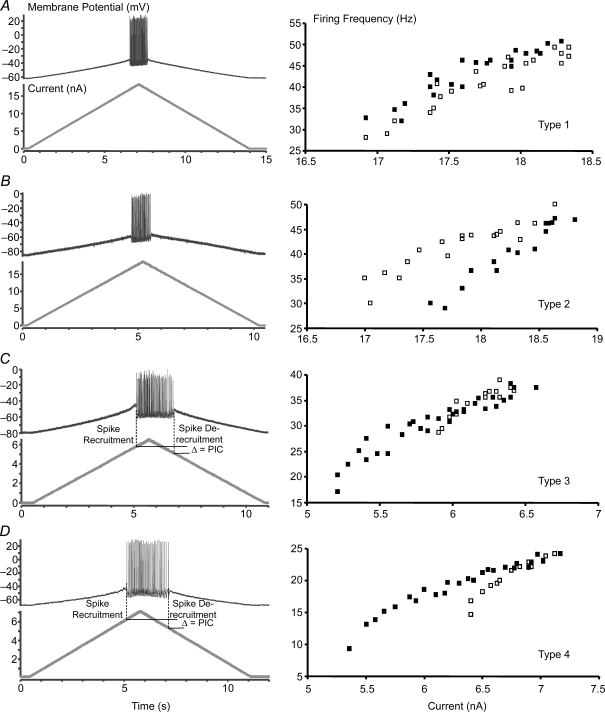
After measuring the basic properties, the motoneurone f–I relationship was measured in two ways. First, cells were challenged with slow current ramps (range: 0.5–6.0 nA s−1), and the voltage response was measured in DCC mode (see Button et al. (2006b) for a complete description of this measurement). Peak amplitude of the ramp depended on the rhythmic threshold of the motoneurone and an attempt was made to evoke trains of impulses containing 10–75 spikes over a 0.5–2.5 s duration. The ramps were used to determine the motoneurone f–I relationship, to evoke voltage-dependent plateaus, and to estimate the underlying PIC as previously described (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001b) (Fig. 1). Since PICs are activated at membrane potentials depolarized greater than −60 mV (Li & Bennett, 2003), only those cells with a resting membrane potential of −60 mV were used to determine f–I relationships. During the current ramps, PIC was estimated from the difference in injected current at spike recruitment compared with spike de-recruitment (Fig. 1C and D, left). Second, cells were challenged with an incremental series of 500 ms square-wave pulse current injections and the voltage response was measured in DCC mode. Current was gradually increased and decreased by 1–5 nA steps from minimum to maximum steady-state firing until blocking occurred before the end of the 500 ms period, after which current steps of decreasing intensity were administered (see Cormery et al. 2005). Five hundred millisecond square-wave pulse current injections were used to determine minimum and maximum steady-state firing frequencies (SSFF) (Fig. 2A). The f–I slope for each motoneurone was determined by plotting the relationship between the minimum current and mean firing frequency of the last three interspike intervals during the 500 ms square-wave pulse current injection and the maximum current and the mean firing frequency of the last three interspike intervals and the slope of the line was calculated (Fig. 2B).
Figure 1. F–I relationships for control, ST and SI motoneurones were determined by using 5 s up–down ramp injections.
The left panels illustrate rhythmic discharge of the motoneurone in response to a ramp current (bottom trace). In C and D the dashed lines indicate the current at spike recruitment and spike de-recruitment. Estimated PIC was calculated by subtracting the current at spike de-recruitment from the current at spike recruitment. The right panels are the plotted f–I relationships which show a plot of the instantaneous firing frequency and current from traces on the left. Open and filled squares represent up and down phases of the ramp, respectively. A, type 1 motoneurone f–I relationship demonstrated a firing frequency slope that overlaps. B, type 2 motoneurone f–I relationship demonstrated a clockwise hysteresis. C, type 3 motoneurone f–I relationship demonstrated a linear regression line with some self-sustained firing. D, type 4 (bottom) motoneurone f–I relationship demonstrated a counter-clockwise hysteresis. Ramp data shown here were recorded from 4 different control motoneurones.
Figure 2. Motoneurones from control, ST and SI groups were capable of discharging rhythmically when injected with sustained 500 ms current pulses.
A, for each motoneurone the intensity of the 500 ms current pulses was gradually increased until steady-state firing occurred, and was increased further in approximately 1–5 nA steps until blocking began to take place. At that time, current was decreased by approximately the same current steps until the cell stopped firing. Not all 500 ms current pulses are shown. B, the f–I relationship plotted from the 500 ms current pulses in A was used to determine the minimum and maximum SSFFs (mean of last 3 intervals during 500 ms of current injection), current required to evoke SSFFs and slope. Each data point represents the SSFF and current intensity for each 500 ms current pulse injected into the motoneurone.
Measurement of motoneurone spike frequency adaptation (SFA)
To evoke rhythmic firing, cells were injected with 30 s square-wave current pulses at an amplitude exceeding rhythmic firing threshold (the minimum current at which the motoneurone would fire for at least 10 s). From these recordings we determined the change in spike frequency over time. A 30 s current pulse was chosen on the basis that very little motoneurone SFA is seen after this time point (Sawczuk et al. 1995). Once the threshold current for rhythmic firing was determined, a square-wave pulse of current was injected into the motoneurone for 30 s (Button et al. 2007) and the membrane voltage was measured in DCC and bridge mode (Fig. 3A). Time was allotted after each 30 s current injection for the motoneurone to repolarize back to the resting membrane potential prior to the next attempt. For each cell we used one to two current amplitudes that ranged from 1.5 to 3 nA greater than threshold current for rhythmic firing and analysed motoneurone SFA by the following process: (1) we counted the number of discharges in 1 s bins beginning at the onset of firing (Fig. 3B); (2) we normalized the number of spikes in each 1 s bin such that the final bin in the 30 s period of firing always contained 5 spikes; (3) these 1 s bins were pooled into six 5 s bins; and (4) [1 −(bin5/bin1)] ratio was used as an index of SFA. The rationale for using this measure of SFA and a more detailed description of the methods have been published (Button et al. 2007).
Figure 3. Thirty seconds of a sustained supra-threshold current injection results in continuous firing that gradually decreases (SFA).
Control, ST and SI motoneurones were subjected to a 30 s sustained supra-threshold current injection to determine their SFA pattern. A shows current injected and spikes (voltage measured in DCC mode). B shows the instantaneous firing frequency rate (black line, right axis) and the normalized number of spikes discharged per second (left axis). The 1 s bins were normalized such that the final bin in the 30 s period of spike discharge always contained 5 spikes.
After recording all basic and active properties of the motoneurone, the microelectrode was backed out of the motoneurone in 5 μm steps, and the extracellular voltage was recorded. Typically, experiments yielded two to four motoneurones with complete and acceptable complements of data. At the end of the experiment, the rat was killed by an overdose of KCl and a bilateral pneumothorax.
Statistics
Only motoneurones that passed all electrophysiological requirements were used for the statistical analyses (see Methods). We performed a one-way ANOVA to determine if motoneurone basic and active properties differed between the control, ST and SI groups. A Tukey post hoc analysis was used where significant main effects were present. Pearson product moment correlation was used to determine relationships between several properties. χ2 analysis was used to determine whether significant differences were present in the motoneurone f–I relationship type determined by ramp current injections between control, ST and SI motoneurones. Data are expressed as either mean ± s.d. or as a distribution.
Results
Overall, we recorded the basic and active properties of 96 motoneurones from 33 control rats, 71 motoneurones from 15 ST rats, and 57 motoneurones from 14 SI rats. Typically, each rat yielded 1–5 motoneurones with acceptable data (see Methods). Some of the control motoneurone data reported here have been published elsewhere (Button et al. 2006b; Button et al. 2007). In the present study, the elimination of supraspinal input via spinal transection altered the basic properties and rhythmic firing behaviour of rat hindlimb motoneurones. Surprisingly, the elimination of afferent input in addition to spinal transection (spinal isolation) had no further effect on the biophysical properties of the motoneurone.
Basic motoneurone properties after SI and ST
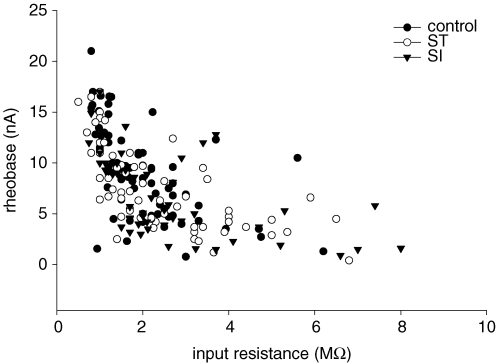
Basic and active motoneurone properties are summarized in Table 1. Control and SI motoneurones had similar resting membrane potentials and voltage thresholds. On the other hand, motoneurones in SI rats had 27% lower rheobase current and 42% higher Rin than motoneurones in control rats (Fig. 4A and B, respectively). SI had no effect on mean spike height and AHP half-decay time, but increased AHP amplitude by 56% compared with control motoneurones (Fig. 4C) (see Table 1, Basic properties). The distributions for rheobase current, Rin, spike AHP amplitude and AHP duration are shown in Fig. 5. Control and SI motoneurones had similar maximum and minimum values for each of the three basic properties (Fig. 5A–C), but different distributions between these values for rheobase current (Fig. 5A), Rin (Fig. 5B), and AHP amplitude (Fig. 5C). However, motoneurone AHP duration distributions were very similar (Fig. 5D). The relationship between rheobase current and Rin (Fig. 6) was maintained following control (slope =−0.14, r =−0.56), ST (slope =−0.24, r =−0.64), and SI (slope =−0.23, r =−0.52). In all cases, the correlation for each group was significant (P < 0.0001, data not shown). There were no significant differences between SI and ST motoneurones for any basic property (Table 1; Figs 4, 5 and 6).
Table 1.
A summary of basic (numbers 1–7) and active (numbers 8–14) motoneurone properties for control, ST and SI groups
| Motoneurone property | Control | Spinal cord transected | Spinal cord isolated | P value | |
|---|---|---|---|---|---|
| Basic properties | |||||
| 1 | Resting membrane potential (mV) | −66.4 ± 9.3 (60) | −66.9 ± 8.1 (47) | −68.0 ± 7.3 (43) | 0.61 |
| 2 | Voltage threshold (mV) | −48.0 ± 8.5 (60) | −49.7 ± 8.3 (47) | −50.6 ± 9.0 (44) | 0.31 |
| 3 | Rheobase current (nA) | 9.6 ± 4.4 (59) | 7.3 ± 4.0 (47) | 7.0 ± 4.3 (44) | *< 0.01 |
| *< 0.02 | |||||
| 4 | Input resistance (MΩ) | 1.9 ± 1.1 (56) | 2.3 ± 1.4 (47) | 2.7 ± 1.9 (43) | *< 0.05 |
| 0.32 | |||||
| 5 | AHP amplitude (mV) | 1.6 ± 1.5 (60) | 2.6 ± 1.5 (47) | 2.5 ± 1.7 (44) | *< 0.05 |
| **< 0.01 | |||||
| 6 | AHP 1/2 decay time (ms) | 14.8 ± 5.4 (60) | 14.4 ± 3.9 (47) | 14.9 ± 5.0 (44) | 0.88 |
| 7 | Spike height (mV) | 73.2 ± 12.7 (53) | 74.5 ± 17.1 (41) | 74.6 ± 15.3 (40) | 0.88 |
| f–I relationship | |||||
| 8 | Estimated PIC (nA) | 0.49 ± 0.4 (44) | 0.53 ± 0.4 (40) | 0.55 ± 0.3 (30) | 0.83 |
| 9 | SSFF Min current (nA) | 11.3 ± 3.8 (37) | 9.8 ± 5.2 (26) | 9.3 ± 4.4 (31) | 0.13 |
| 10 | SSFF Max current (nA) | 20.6 ± 5.9 (37) | 24.4 ± 10.7 (26) | 20.9 ± 10.7 (31) | 0.3 |
| 11 | SSFF (Min) (Hz) | 34.8 ± 11.1 (37) | 25.7 ± 6.3 (26) | 28.1 ± 10.4 (31) | *< 0.001 |
| **< 0.001 | |||||
| 12 | SSFF (Max) (Hz) | 104.3 ± 35.0 (37) | 84.2 ± 31.2 (26) | 78.8 ± 40.3 (31) | *< 0.01 |
| 0.21 | |||||
| 13 | SSFF f–I slope (Hz nA−1) | 6.5 ± 2.4 (37) | 5.0 ± 1.8 (26) | 5.1 ± 2.5 (31) | *< 0.02 |
| **< 0.02 | |||||
| Spike frequency adaptation | |||||
| 14 | SFA 5 s bincount ratio [1 − (bin5/bin 1)] | 0.69 ± 0.11 (21) | 0.63 ± 0.14 (14) | 0.68 ± 0.09 (14) | 0.5 |
Throughout the table, values are presented as means ± s.d. with the number of motoneurones (N) recorded from. Abbreviations: afterhyperpolarization (AHP), persistent inward current (PIC), steady state firing frequency (SSFF), firing frequency (FF) and spike frequency adaptation (SFA). Number 8 only includes PICs of f–I relationship types 3 and 4.
* and ** denote a significant difference between control versus SI and control versus ST, respectively. A Tukey post hoc analysis was used where a significant main effect (P < 0.05) was present. There were no significant differences between ST versus SI for any measure.
Figure 4. Following SI, motoneurone basic properties become like those typically seen in slow motoneurones.
Motoneurone basic properties from control, ST and SI groups are displayed as means +s.d. Overall, SI motoneurones have significantly lower rheobase currents (A) and significantly higher Rin values (B) and AHP amplitudes (C) compared with control. ST motoneurones have significantly lower rheobase currents, a trend to have higher mean Rins than control and have significantly higher motoneurone AHP amplitudes than control. There were no significant differences between ST and SI for any of the three motoneurone properties. * denotes a significant difference from control.
Figure 5. Although mean values for SI motoneurone basic properties become like those typically seen in slow motoneurones they still retain a range of values which indicate that heterogeneity is maintained (Fig. 1).
Motoneurone basic properties from control, ST and SI groups are displayed as distributions and represent data of individual motoneurones from all experiments, whereas statistical comparisons in the Results were derived from means calculated for each group of motoneurones (Table 1). Shown is the range of values recorded from control, ST and SI motoneurones for rheobase current (A), Rin (B), AHP amplitude (C) and AHP duration (D). Following ST and SI, motoneurone rheobase current, Rin and AHP amplitude were similar to control motoneurones at the low and high values but the values between these were shifted to the left for rheobase and shifted to the right for Rin and AHP amplitudes.
Figure 6. When motoneurone rheobase current is plotted against Rin, control, ST and SI motoneurone group values overlap considerably.
Motoneurone values are paired as rheobase current and Rin from individual motoneurones for control, ST and SI groups. Correlation coefficients for the control, ST and SI groups were all significant and very similar (see Results).
Active motoneurone properties after SI and ST
f–I relationship and PIC amplitude determined by ramp current injections
To determine motoneurone PIC amplitude, we plotted the motoneurone f–I relationships of ramp current injections from 44 control, 40 ST and 30 SI motoneurones. This procedure has been used in our (Button et al. 2006b) and other (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001b) laboratories to estimate motoneurone PIC amplitude. Only those motoneurones with a minimum resting membrane potential of −60 mV were used in the PIC analysis. Motoneurone f–I relationships plotted from ramp current injections could be categorized into four distinct types based on spike recruitment and de-recruitment thresholds from the ascending and descending portions of the ramp currents (Fig. 1A–D). These f–I types have been described in detail previously (Button et al. 2006b), and include: (a) type 1 overlapping, (b) type 2 adapting, (c) type 3 linear + sustained, and (d) type 4 acceleration. All four f–I relationship types were observed in motoneurones from all groups of rats (Figs 1 and 7).
Figure 7. Control, ST and SI motoneurones have very similar PIC amplitudes, but their f–I relationship type distribution differ in that ST and SI groups have an increased number of motoneurones that demonstrate PIC.
Four distinct types of f–I relationships are present in control, ST and SI: (1) type 1 overlapping, (2) type 2 adapting, (3) type 3 linear plus sustained, and (4) type 4 acceleration (see Fig. 1). A greater percentage of motoneurones from ST and SI than control rats are categorized as f–I relationship types 3 and 4 (activation of PIC) and a smaller percentage as type 2 (no activation of PIC). χ2 analysis, χ2= 16.6, revealed a significant difference (P < 0.01) in the distribution of the f–I relationships among the motoneurone groups. The inset shows the distributions of motoneurone PIC amplitudes for each group that were calculated from only f–I relationship types 3 and 4. Note that the range of values and the mean (Table 1) were very similar for each group.
The PIC can be estimated from f–I relationship types 3 and 4 by subtracting the current at spike de-recruitment from the current at spike recruitment (Fig. 1C and D). The average PIC amplitudes (see Table 1, f–I relationship) of control and SI motoneurones were similar. Furthermore, the frequency distributions of types 3 and 4 f–I relationships (which indicate the presence of an PIC) of control and SI motoneurones overlapped (Fig. 7, inset). However, χ2 analysis revealed that there was a significant difference in the distribution of f–I relationship types between control and SI motoneurones; more SI motoneurones demonstrated f–I relationships that indicated the presence of PIC (types 3 and 4), compared with controls (Fig. 7). The average motoneurone PIC amplitude and frequency distribution of motoneurones exhibiting PIC in ST rats were similar to those of SI rats (Fig. 7, inset). Similar to the case for SI rats, however, there was a greater percentage of motoneurones demonstrating f–I relationships that indicate the presence of PIC (types 3 and 4) (Fig. 1C and D) in ST compared with control rats (Fig. 7). There was no correlation found between motoneurone PIC amplitude and resting membrane potential in each group (control, ST and SI groups, data not shown). Other ramp properties (current and firing frequency at spike recruitment and spike de-recruitment) were similar in control, ST and SI rats. The similarity in the effects of ST and SI suggest that a loss of descending input plays a larger role in determining motoneurone bistability than the loss of afferent and ascending inputs.
The effect of SI or ST on motoneurone f–I relationship properties determined by 500 ms current injections
We determined the motoneurone f–I relationship by injecting 500 ms square-wave pulses into 37 control, 26 ST and 31 SI motoneurones. This allowed us to determine the minimum and maximum SSFFs (SSFF Min and Max, respectively), the minimum current required evoking these SSFFs, and ultimately the f–I slope for each motoneurone (Fig. 2). These 500 ms current injections have been employed previously to describe changes in motoneurone f–I relationships following 2–4 weeks of ST (Beaumont et al. 2004) and 2 weeks of hindlimb unloading (Beaumont et al. 2004; Cormery et al. 2005). Motoneurone SSFF Min was 19% lower and SSFF Max was 24% lower in SI than control rats (see Table 1, f–I relationship, Fig. 8A), as might be expected based on the higher spike AHP amplitudes (measured by short 0.5 ms suprathreshold current injections at resting membrane potential) for the motoneurones from SI than control rats and the increased incidence of the number of motoneurones demonstrating PIC (see Discussion for details). Motoneurone f–I slopes also were ∼20% lower in SI than control rats (Fig. 8B). In addition, the distribution of each of the aforementioned three motoneurone properties was shifted significantly to the left in SI compared with control rats (Fig. 9).
Figure 8. Motoneurone 500 ms steady state firing frequencies, currents and f–I slopes are decreased after ST and SI, indicating decreased motoneurone input–output gain.
A, motoneurones from ST and SI rats require less current to induce lower SSFF Min and have lower firing frequencies at similar SSFF Max currents than motoneurones from control rats. Data are presented as means ± s.d. (see Table 1). * denotes a significant difference for motoneurone SSFF Min between control and ST and SI groups. ** denotes a significant difference for motoneurone SSFF Max between control and SI groups. B, compared with the control group, there is a reduction or a shift to the right in the f–I slopes of motoneurones in the ST and SI groups. The slope values in text are the mean of the slopes derived using linear regression from all points on the f–I curve as shown in Fig. 2B (not just the minimum and maximum values plotted above). Slope values are presented as means (see Table 1). *** denotes a significant difference for motoneurone f–I slope between control and SI and ST groups.
Figure 9. Following ST and SI, the range of values for motoneurone 500 ms f–I relationship properties are substantially decreased compared with control.
Motoneurone 500 ms f–I relationship properties from control, ST, and SI groups are displayed as distributions and represent data of individual motoneurones from all experiments, whereas statistical comparisons in the Results were derived from means calculated for each group of motoneurones (Fig. 5, Table 1). Shown is the range of values recorded from control, ST, and SI motoneurones for SSFF Min (A), SSFF Max (B) and SSFF slopes (C). Following ST and SI, motoneurone SSFF Min, SSFF Max and SSFF slopes were similar to control motoneurones at the low values but the values above these were shifted to the left. However, ST and SI motoneurones still retain a range of values which indicate that heterogeneity is maintained.
In general, motoneurones in ST and SI rats had similar f–I relationship properties recorded from 500 ms current injections. Motoneurone SSFF Min was 26% and f–I slopes ∼20% lower in ST than control rats (Fig. 8B). Unlike in SI rats, however, the motoneurone SSFF Max was unaffected in ST rats. All mean values (Fig. 8) and distributions (Fig. 9) of the 500 ms f–I relationship properties were similar for the SI and ST groups. Once again, the similarity in the effects of ST and SI suggests that a loss of descending input plays a larger role in determining the gain of motoneurone f–I relationship than the loss of afferent and ascending inputs.
The effect of SI or ST on motoneurone spike frequency adaptation
To determine whether motoneurone spike frequency adaptation is influenced by ST or SI, we injected 30-s square-wave current pulses into 21 control, 14 ST, and 14 SI motoneurones. The average number of spikes discharged in 1-s bins was plotted over 30 s for each group of motoneurones (Fig. 10). These spike counts are normalized to a frequency of 5 spikes s−1 in the final 1 s bin to more clearly visualize differences in SFA patterns between groups. When 1 s bins are pooled into six 5 s bins, the decline in the number of spikes discharged from the first to the second to last 5 s of firing (1 −[bin5/bin1]) provides a sensitive index of SFA (Button et al. 2007). One-way ANOVA showed no difference in the mean SFA index among the three groups. In addition, the distributions were similar among the groups (Fig. 10, inset). Thus, neither ST nor SI significantly influenced motoneurone SFA patterns.
Figure 10. The pattern of motoneurone spike frequency adaptation is similar for control, ST and SI motoneurones.
Motoneurones from control, ST and SI rats have a similar rate of decrease in the number of spikes (placed in 1 s bins) throughout 30 s of rhythmic discharge (see Fig. 3). Data are presented as means ± s.d. The inset shows the distributions of the SFA index (1 − bin5/bin1) that we developed and previously published as a simple and reliable way to measure motoneurone SFA (see Methods). The range of values was very similar for each group. Furthermore, the mean SFA indexes were similar among control, ST and SI motoneurones (see Table 1).
Discussion
It is reasonable to expect that the change in the biophysical properties of motoneurones seen after ST would be more pronounced after SI. Motoneurone adaptations would be expected to occur in association with the dramatic decrease in muscle fibre size and shift towards ‘faster’ myosin heavy chain phenotypes reported in the hindlimb muscles of SI rats (Grossman et al. 1998; Roy et al. 2002) and because SI has a greater effect on muscle tension-related properties than ST (Roy et al. 2002; Talmadge et al. 2002). However, the population of hindlimb motoneurones in SI rats maintained a degree of heterogeneity for both basic and active properties as illustrated by a range of values and measures of variation that were comparable to those observed in motoneurones of control and ST rats. Thus, there is an unusual dissociation between the effects of spinal isolation on muscle fibres and motoneurone properties. This illustrates that despite the more pronounced changes in muscle properties following SI compared with ST, SI does not appear to have a greater influence on motoneurone properties. Furthermore, the aforementioned results clearly show that a lack of descending input (ST) alone affects active and basic properties of motoneurones caudal to the site of the transection. Removing the afferent and ascending inputs to the motoneurones in addition to the descending input (SI) had no further effect on their basic or active properties.
SI and ST motoneurones have lower rheobase currents and higher Rin values
Rheobase current and Rin are indices of motoneurone size and excitability. Specifically, motoneurones with a low rheobase and high Rin are easily excited, relatively small and are more likely to innervate slow-twitch muscle fibres (Zengel et al. 1985), and are therefore classified as slow motoneurones. Conversely, motoneurones with a high rheobase and low Rin are less easily excited, relatively large and usually innervate fast-twitch muscle fibres, and are classified as fast motoneurones. After ST and SI, rheobase and Rin shifted towards values typically seen in slower and more excitable cells. There are several possible explanations for this. The most obvious is motoneurones become smaller after ST or SI. Morphologically, motoneurone soma sizes show either no change after ST and SI (Chalmers et al. 1992) or become reduced after ST (Kitzman, 2005). There is a loss, however, in the number of primary, secondary and tertiary dendrites following ST (Gazula et al. 2004; Kitzman, 2005) and spinal cord contusion (Bose et al. 2005); however, effects of SI on dendritic structure remain unknown. Due to a trimming of the dendritic tree after spinal cord injury, the overall motoneurone size may become smaller whether or not the soma size is affected. The reported reductions in rat motoneurone cell capacitance following ST (Beaumont et al. 2004) and decrease in electrophysiologically calculated motoneurone diameter of cat motoneurones following ST with a partial dorsal rhizotomy surgery very similar to SI (Gustafsson et al. 1982), further suggest that chronic inactivity may diminish overall motoneurone size.
An alteration in ion channel expression could have caused motoneurone rheobase and Rin to change in the 6–8 week period following ST and SI. Neurotrophins are important for motoneurone survival and maintenance and may modulate the expression of ion channel subunits that render the motoneurone more or less excitable (Lesser et al. 1997; Rose et al. 2004). Neurotrophins are transported from the muscle to the motoneurone (retrograde) and from the motoneurone to the muscle (anterograde) (Mitsumoto & Tsuzaka, 1999). After ST or SI (or spinal cord injury) the ventral horn has lower levels of brain-derived neurotrophic factor and neurotrophin 3 proteins and mRNA (Gomez-Pinilla et al. 2004). Furthermore, motoneurone properties in ST rats are maintained near control values when fetal tissue, which contains and may continue to secrete neurotrophins, is transplanted into the spinal cord immediately after the transection (Beaumont et al. 2004). Thus, a decrease in neurotrophin activity in the spinal cord after ST or SI may affect the number, density, composition, activation and/or location of the motoneurone ion channels thereby altering the conductances that underly rheobase and Rin. However, it remains unknown whether or not neurotrophin levels are differentially altered following SI compared with ST. It is likely that a combination of these factors contributed to the changes in motoneurone rheobase and Rin after ST or SI.
Descending but not afferent and ascending inputs have greater influence on motoneurone properties
Muscle spasms observed after ST may be due to a number of factors including afferent input, enhanced interneuronal excitability and persistent inward currents (PIC) that are sufficient to activate motoneurones in the absence of descending inhibition. Since EMG activity is nearly eliminated and spasms are not observed after SI (Gomez-Pinilla et al. 2004; Roy et al. 2007b), it may be assumed that increased excitability of interneurones is insufficient to activate motoneurons in the absence of sensory feedback. Therefore, we expected motoneurons deprived of sensory input for 6–8 weeks (chronic SI) to have different properties than motoneurones with intact sensory input (chronic ST) for 6–8 weeks. This, however, was not the case suggesting that motoneurone properties are primarily dependent upon descending inputs after ST. Monoaminergic inputs increase motoneurone excitability (Hounsgaard et al. 1988; Heckman et al. 2003; Perrier et al. 2003; Gilmore & Fedirchuk, 2004). This excitability is especially apparent from the work of Harvey et al. (2006a, b, c) illustrating the effects of monoamines on motoneurone basic properties, PICs, and firing frequency rates after acute and chronic ST. Thus, the lack of descending monoaminergic input to the motoneurones following chronic ST and SI and the chronic adaptation to the loss of these inputs seems to be a major contributor to the change in motoneurone properties in the current study.
Similar to ST, a higher percentage of SI motoneurones demonstrate the presence of PIC
An increase in the presence of PIC is another indication that motoneurone ion channel profiles and other receptors may have changed after ST or SI. PICs are depolarizing currents generated by voltage-gated Na+ and Ca2+ channels that slowly inactivate (Lee & Heckman, 1999; Li & Bennett, 2003) when the membrane potential is depolarized above activation threshold. These currents can mediate plateau potentials that allow self-sustained rhythmic firing and bistability (Kiehn & Eken, 1998). Bistable motoneurones tend to have relatively lower rheobase currents, suggesting that small motoneurones are influenced by PIC differently than large motoneurones (Lee & Heckman, 1998b). The shift towards lower rheobase currents in ST and SI motoneurones may contribute to the increased number of motoneurones exhibiting PICs. Alternatively, ST and SI motoneurones may be more sensitive to serotonin (5-HT) and noradrenaline (NA). Serotonergic and NA input to motoneurones via projections from the raphe nucleus and locus coerulus, respectively, of the brainstem (Hounsgaard et al. 1988; Heckman et al. 2003), enhances PIC and renders the motoneurone more excitable (Heckman et al. 2003; Gilmore & Fedirchuk, 2004). After acute ST, descending input from the brainstem is eliminated and facilitation of the PIC by 5-HT and NA is compromised. Four weeks later, ST motoneurones become supersensitive to 5-HT and NA such that small amounts of exogenous 5-HT and NA are sufficient to activate motoneuronal 5-HT receptors (Harvey et al. 2006a; Rank et al. 2007). There is also an increase in motoneurone sensitivity to residual endogenous 5-HT in the spinal cord (Harvey et al. 2006b) which enhances PIC and increases motoneurone bistability. Similar effects should be expected after SI. The increase in motoneurone PIC and subsequently bistability plays a role in the development of spasticity following ST (Bennett et al. 2001a, b; Li & Bennett, 2003; Li et al. 2004).
There is no difference between control, ST and SI motoneurone PIC amplitudes
Acute and chronic ST motoneurones have distinct differences in their ability to activate PIC channels and PIC size. Very little PIC is seen after acute ST compared with chronic ST (Bennett et al. 2001b; Harvey et al. 2006c; Li et al. 2007). However, a direct comparison of PIC amplitude between normal control (no transection) motoneurones and chronic ST motoneurones has not been previously reported. As we found no difference between control, ST and SI motoneurone PIC amplitudes, there may be an increase in 5-HT and NA receptor sensitivity to residual endogenous monoamines in the 6–8 week period following chronic ST and SI (Harvey et al. 2006a, b), which is enough to maintain motoneurone PICs to match PIC amplitudes seen in those motoneurones where the monoaminergic systems are left intact (control motoneurones).
In the present study, PIC amplitude following ST was smaller than previously reported by others who also studied this lesion (Bennett et al. 2001b). One of the major differences in our measurement of motoneurone PIC was the use of anaesthetics (ketamine and xylazine) and the time period following ST at which motoneurone recordings were made. It has been suggested (Hultborn & Kiehn, 1992; Hultborn, 1999) and found (Guertin & Hounsgaard, 1999; Button et al. 2006b) that PICs are substantially decreased when animals are anaesthetized with barbiturates. We previously reported (Button et al. 2006b) that PICs recorded from motoneurones of unaesthetized decerebrated rats did not differ from PICs recorded from motoneurones of rats anaesthetized with a mixture of ketamine (an N-methyl-d-aspartate receptor antagonist) and xylazine (an α2-adrenoceptor agonist). Thus, the discrepancy between PIC amplitude reported here and elsewhere is probably not due to the use of our anaesthetic. In the present study, PIC amplitudes were recorded from motoneurones following 6–8 weeks of ST, whereas in Bennett et al. (2001b) PIC amplitudes were recorded from motoneurones following 1.5–7 months of ST. A period of 6 to 8 weeks following ST may not be long enough to allow for full development of spasticity, which may explain the smaller PIC amplitudes seen here. Perhaps PICs in rat lumbar motoneurones are actually somewhat smaller than those of sacral–caudal motoneurones. The functions of the rat hindlimb (Button et al. 2006b) versus tail (Bennett et al. 2001b) are quite different, and may be accompanied by differences in motoneurone PIC amplitude. Finally, although changes in neurotrophin levels occur following ST and SI (Gomez-Pinilla et al. 2004), which can significantly alter motoneurone properties (Gonzalez & Collins, 1997), their involvement in the mechanisms underlying PIC is probably limited.
Motoneurone f–I slopes are decreased similarly after SI and ST
We found a reduction in f–I slopes following ST or SI that indicates a decreased input–output gain of the motoneurone, suggestive of a change of ion channel profiles. As a decrease in AHP amplitude increases the f–I slope (Hounsgaard et al. 1988; Hounsgaard & Kiehn, 1989; Hultborn et al. 2004), the most logical explanation for the smaller motoneurone f–I slope following ST and SI was an increase in AHP amplitude, which has been reported for motoneurones of ST rats previously (Beaumont et al. 2004; Petruska et al. 2007). These properties, however, reverted towards control values after fetal tissue transplants and/or passive cycling exercise (Beaumont et al. 2004), or step training (Petruska et al. 2007), probably because of increased neurotrophin levels below the transection (Gomez-Pinilla et al. 2001; Beaumont et al. 2004). SI results in decreased neurotrophin levels in the spinal cord (Gomez-Pinilla et al. 2004) which may increase motoneurone AHP amplitudes generated by large (BK) and small (SK2) Ca2+-activated K+ channel conductances (Powers & Binder, 2001), and subsequently diminish the motoneurone gain. In addition, motoneurones with high Rin values tend to have large AHP amplitudes (Gardiner, 1993; Cormery et al. 2005).
The decrease firing frequencies after ST and SI may be due to a greater percentage of motoneurones demonstrating the presence of PIC. It has been demonstrated by Li et al. (2004) that both an increase in membrane conductance as a result of the activation of Na+ and Ca2+ PIC channels and a subthreshold oscillation of the Na+ PIC leads to slow motoneurone firing rates in ST motoneurones. Furthermore, when Na+ PIC is increased in ST motoneurones by increased 5-HT2 receptor activation firing frequency is significantly reduced (Harvey et al. 2006a).
SI and ST do not affect motoneurone SFA
Spike frequency adaptation is a time-dependent decrease in motoneurone firing frequency that can be further broken down into initial, early and late adaptation phases. Until now, the effects of SI or ST on motoneurone SFA were unknown. Previously, our laboratory reported that the firing rate declines to a lesser extent during a 30 s current injection (less SFA) in motoneurones that exhibit a high PIC and low rheobase (Button et al. 2007). Given previous evidence of the tendency for smaller, more excitable motoneurones to exhibit less SFA in control animals (Kernell & Monster, 1982; Spielmann et al. 1993), one might expect the more easily excitable motoneurones from SI and ST rats to exhibit less SFA. Instead, SFA was unaffected by SI or ST despite the apparent increase in motoneurone excitability. SFA, however, was measured using currents injected into the motoneurone that were just above that required for rhythmic discharge. At higher currents motoneurone SFA patterns start to differ (Button et al. 2007), and perhaps after ST and SI, SFA patterns could have been distinguished from those in control rats at higher currents. Nonetheless, like control, ST and SI motoneurones were able to fire for prolonged periods (≥ 30 s) without any measurable change in SFA pattern.
On a continuum from slow- to fast-type motoneurones, the rate of SFA covaries with the rate of fatigue of the innervated muscle fibres. For example, fast motor units demonstrate much greater rates of SFA and fatigue, compared with slow motor units (Kernell & Monster, 1982). Following 6 months of ST and 60 days of SI soleus muscle fibres become faster and there is approximately a 40% reduction in soleus fatigue resistance compared with control (Roy et al. 2002; Talmadge et al. 2002). Even after prolonged ST where there is reversion back from a higher percentage of fast-type muscle fibres to the more comparable proportions of slow and fast fibres seen in controls, muscle fatigue resistance remains suppressed (Harris et al. 2006). Since the SFA patterns reported here showed no difference between control, ST and SI motoneurones, the matching of SFA and muscle fatigue within motor units may be lost following ST and SI.
There is dissociation between the effect of SI on motoneurones and muscle fibre type
Following SI, muscle fibre types are severely atrophied and converted to mainly express type 2 myosin heavy chains (Grossman et al. 1998; Harris et al. 2007). It has been reported previously that muscle type can influence motoneurone type. For example cross-reinnervation of the medial gastrocnemius (MG) nerve onto the soleus muscle for 9–11 months leads to motoneurone biophysical property changes such that MG motoneurones behave more like soleus motoneurones (Foehring et al. 1987). Furthermore, chronic electrical stimulation of cat MG nerve for 2–3 months converts all muscles fibres to slow type, with some of the innervating motoneurones changing their properties to those of motoneurones normally innervating soleus (Munson et al. 1997). In the present study, motoneurone properties following SI maintained heterogeneity and did not match the changes that are known to take place in muscle fibre type (motoneurones did not change to become more ‘fast’-like in properties). Unfortunately, the motoneurone properties reported here were after only 6–8 weeks of SI, which may not be enough time for changes in motoneurones to be significantly influenced by their target muscle – indeed, this time factor may explain the rather limited change in motoneurone properties that occurs following chronic muscle stimulation (Munson et al. 1997). Efferent innervation of muscle is left intact after SI enabling uptake of trophic substances by the motoneurone, which may be enough to maintain motoneurone properties. Furthermore, with the exception of motoneurone size, dorsal rhizotomy only has minimal effects on motoneurone properties (Kuno et al. 1974; Gustafsson et al. 1982). Therefore, the current results do not support a significant effect of muscle-derived substances on motoneurone properties following spinal cord transection.
In conclusion, we show that following 6–8 weeks of chronic ST or chronic SI, motoneurone Rin, rheobase current, spike AHP amplitude and bistability tend to become like those observed in more excitable motoneurones. ST and SI motoneurones also have decreased f–I slopes and minimum firing frequencies but retain ability to discharge for short and long durations in response to an electrical input. Even though SI and ST motoneurones have a greater tendency to be bistable, overall PIC amplitude is not greater than control motoneurones, maybe owing to increased monoaminergic receptor sensitivity. Although the SI procedure is one of the most ‘severe’ experimental models of spinal cord injury, the range of values for basic and active motoneurone properties indicate that the heterogeneity among motoneurones is relatively maintained. Finally, unlike muscle, it appears that the development of spasticity is not required (for at least up to 6–8 weeks) to maintain motoneurone biophysical properties.
Acknowledgments
This research was supported by grants from NSERC, CIHR, the Canada Research Chairs program, and NIH (NS 16333). Financial support for D.C.B., J.M.K. and T.M. was provided by NSERC PGSB and Manitoba Health Research Council (MHRC), NSERC PDF, and MHRC PDF, respectively. The authors would like to thank Farrell Cahill, Gilles Detillieux and Matt Ellis at University of Manitoba for technical assistance.
References
- Alaimo MA, Smith JL, Roy RR, Edgerton VR. EMG activity of slow and fast ankle extensors following spinal cord transection. J Appl Physiol. 1984;56:1608–1613. doi: 10.1152/jappl.1984.56.6.1608. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Houle JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol. 2001a;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001b;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Bose P, Parmer R, Reier PJ, Thompson FJ. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp Neurol. 2005;191:13–23. doi: 10.1016/j.expneurol.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Button DC, Gardiner KR, Cahill F, Marqueste T, Zhong H, Roy RR, Egerton VR, Gardiner PF. The effects of spinal cord isolation (SI) on rat hindlimb α-motoneurone (α-Mns) electrophysiological properties. FASEB J. 2006a;20:A14 15-C. [Google Scholar]
- Button DC, Gardiner K, Marqueste T, Gardiner PF. Frequency–current relationships of rat hindlimb α-motoneurones. J Physiol. 2006b;573:663–677. doi: 10.1113/jphysiol.2006.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button DC, Gardiner K, Zhong H, Roy RR, Egerton VR, Gardiner PF. In situ frequency-current (f-I) relationships of hindlimb α-motoneurons (α-Mns) in spinal cord transected (ST) and spinal cord isolated (SI) rats. Can J Appl Physiol. 2005;30:S15. [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Cahill F, Gardiner P. Spike frequency adaptation of rat hindlimb motoneurones. J Appl Physiol. 2007;102:1041–1050. doi: 10.1152/japplphysiol.01148.2006. [DOI] [PubMed] [Google Scholar]
- Button DC, Kalmar KM, Gardiner K, Cahill F, Zhong H, Roy RR, Egerton VR, Gardiner PF. Do spinal cord isolation and spinal cord transection differentially influence rat hindlimb α-motoneurone properties? Appl Physiol Nutr Metab. 2006c;31:S15. [Google Scholar]
- Chalmers GR, Roy RR, Edgerton VR. Adaptability of the oxidative capacity of motoneurons. Brain Res. 1992;570:1–10. doi: 10.1016/0006-8993(92)90556-o. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- Cormery B, Beaumont E, Csukly K, Gardiner P. Hindlimb unweighting for 2 weeks alters physiological properties of rat hindlimb motoneurones. J Physiol. 2005;568:841–850. doi: 10.1113/jphysiol.2005.091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh G, Gallego R, Kudo N, Kuno M. Evidence for the maintenance of motoneurone properties by muscle activity. J Physiol. 1978;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Motor-unit properties following cross-reinnervation of cat lateral gastrocnemius and soleus muscles with medial gastrocnemius nerve. I. Influence of motoneurons on muscle. J Neurophysiol. 1987;57:1210–1226. doi: 10.1152/jn.1987.57.4.1210. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J Neurophysiol. 1993;69:1160–1170. doi: 10.1152/jn.1993.69.4.1160. [DOI] [PubMed] [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- Gilmore J, Fedirchuk B. The excitability of lumbar motoneurones in the neonatal rat is increased by a hyperpolarization of their voltage threshold for activation by descending serotonergic fibres. J Physiol. 2004;558:213–224. doi: 10.1113/jphysiol.2004.064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Hodgson J, Edgerton VR. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J Neurophysiol. 2004;92:3423–3432. doi: 10.1152/jn.00432.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Collins WF., 3rd Modulation of motoneuron excitability by brain-derived neurotrophic factor. J Neurophysiol. 1997;77:502–506. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Roy RR, Talmadge RJ, Zhong H, Edgerton VR. Effects of inactivity on myosin heavy chain composition and size of rat soleus fibers. Muscle Nerve. 1998;21:375–389. doi: 10.1002/(sici)1097-4598(199803)21:3<375::aid-mus12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. Non-volatile general anaesthetics reduce spinal activity by suppressing plateau potentials. Neuroscience. 1999;88:353–358. doi: 10.1016/s0306-4522(98)00371-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Katz R, Malmsten J. Effects of chronic partial deafferentiation on the electrical properties of lumbar α-motoneurones in the cat. Brain Res. 1982;246:23–33. doi: 10.1016/0006-8993(82)90138-x. [DOI] [PubMed] [Google Scholar]
- Harris RL, Bobet J, Sanelli L, Bennett DJ. Tail muscles become slow but fatigable in chronic sacral spinal rats with spasticity. J Neurophysiol. 2006;95:1124–1133. doi: 10.1152/jn.00456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RL, Putman CT, Rank M, Sanelli L, Bennett DJ. Spastic tail muscles recover from myofiber atrophy and myosin heavy chain transformations in chronic spinal rats. J Neurophysiol. 2007;97:1040–1051. doi: 10.1152/jn.00622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006a;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006b;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol. 2006c;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. II. Motoneuron electrical properties. J Neurophysiol. 1994;71:1468–1479. doi: 10.1152/jn.1994.71.4.1468. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Curr Opin Neurobiol. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Hyatt JP, Roy RR, Baldwin KM, Edgerton VR. Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am J Physiol Cell Physiol. 2003;285:C1161–C1173. doi: 10.1152/ajpcell.00128.2003. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Roy RR, Ohira Y, Edgerton VR. Motoneuron and sensory neuron plasticity to varying neuromuscular activity levels. Exerc Sport Sci Rev. 2002;30:152–158. doi: 10.1097/00003677-200210000-00003. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue. An intracellular study of gastrocnemius motoneurones of the cat. Exp Brain Res. 1982;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Kitzman P. Alteration in axial motoneuronal morphology in the spinal cord injured spastic rat. Exp Neurol. 2005;192:100–108. doi: 10.1016/j.expneurol.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Kuno M, Miyata Y, Munoz-Martinez EJ. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974;242:273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998a;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998b;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol. 1999;82:2518–2527. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- Lesser SS, Sherwood NT, Lo DC. Neurotrophins differentially regulate voltage-gated ion channels. Mol Cell Neurosci. 1997;10:173–183. doi: 10.1006/mcne.1997.0656. [DOI] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2007;97:1236–1246. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Tsuzaka K. Neurotrophic factors and neuro-muscular disease. II. GDNF, other neurotrophic factors, and future directions. Muscle Nerve. 1999;22:1000–1021. doi: 10.1002/(sici)1097-4598(199908)22:8<1000::aid-mus2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Munson JB, Foehring RC, Mendell LM, Gordon T. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. II. Motoneuron properties. J Neurophysiol. 1997;77:2605–2615. doi: 10.1152/jn.1997.77.5.2605. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol. 2003;548:485–492. doi: 10.1113/jphysiol.2002.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy R, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti DJ, Roy RR, Bodine-Fowler SC, Hodgson JA, Edgerton VR. Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J Physiol. 1991;444:175–192. doi: 10.1113/jphysiol.1991.sp018872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol. 2007;97:3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hodgson JA, Lauretz SD, Pierotti DJ, Gayek RJ, Edgerton VR. Chronic spinal cord-injured cats: surgical procedures and management. Lab Anim Sci. 1992;42:335–343. [PubMed] [Google Scholar]
- Roy RR, Matsumoto A, Zhong H, Ishihara A, Edgerton VR. Rat alpha- and gamma-motoneuron soma size and succinate dehydrogenase activity are independent of neuromuscular activity level. Muscle Nerve. 2007a;36:234–241. doi: 10.1002/mus.20810. [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Khalili N, Kim SJ, Higuchi N, Monti RJ, Grossman E, Hodgson JA, Edgerton VR. Is spinal cord isolation a good model of muscle disuse? Muscle Nerve. 2007b;35:312–321. doi: 10.1002/mus.20706. [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Monti RJ, Vallance KA, Edgerton VR. Mechanical properties of the electrically silent adult rat soleus muscle. Muscle Nerve. 2002;26:404–412. doi: 10.1002/mus.10219. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Caiozzo VJ, Edgerton VR. Mechanical properties of rat soleus after long-term spinal cord transection. J Appl Physiol. 2002;93:1487–1497. doi: 10.1152/japplphysiol.00053.2002. [DOI] [PubMed] [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]