Abstract
Transcranial magnetic stimulation (TMS) was initially used to evaluate the integrity of the corticospinal tract in humans non-invasively. Since these early studies, the development of paired-pulse and repetitive TMS protocols allowed investigators to explore inhibitory and excitatory interactions of various motor and non-motor cortical regions within and across cerebral hemispheres. These applications have provided insight into the intracortical physiological processes underlying the functional role of different brain regions in various cognitive processes, motor control in health and disease and neuroplastic changes during recovery of function after brain lesions. Used in combination with neuroimaging tools, TMS provides valuable information on functional connectivity between different brain regions, and on the relationship between physiological processes and the anatomical configuration of specific brain areas and connected pathways. More recently, there has been increasing interest in the extent to which these physiological processes are modulated depending on the behavioural setting. The purpose of this paper is (a) to present an up-to-date review of the available electrophysiological data and the impact on our understanding of human motor behaviour and (b) to discuss some of the gaps in our present knowledge as well as future directions of research in a format accessible to new students and/or investigators. Finally, areas of uncertainty and limitations in the interpretation of TMS studies are discussed in some detail.
Introduction
Recent work on electrophysiology of non-invasive brain stimulation has contributed to identifying physiologically active interactions between different cortical regions in awake, healthy human subjects. The cortical motor output has been studied in particular detail, as the output of the primary motor cortex (M1) can be objectively measured in the form of a motor evoked potential (MEP). Using surface electromyographic (EMG) recording electrodes, most commonly positioned on the skin overlying the hand muscles, a compound MEP can be elicited in response to a single suprathreshold transcranial magnetic stimulation (TMS) pulse delivered to M1. The MEP amplitude elicited by stimulation of M1 can be modulated by a preceding conditioning pulse delivered either to the same cortical area or elsewhere, allowing the exploration of intra- and inter–regional physiological interactions in real time.
Detailed study of the corticofugal discharge in response to a motor cortical stimulus by Amassian et al. (1989) revealed multiple components of the MEP. These can be observed either by epidural recordings or by measuring single motor unit recordings with needle electrodes, and consist of a short latency direct wave (D-wave) followed by several longer latency indirect waves (I-waves). The D-wave is thought to result from direct depolarisation of the initial axon segment of the corticospinal neuron and is most effectively activated in human subjects by transcranial electrical stimulation or high intensity TMS. The I-waves following the d-wave occur sequentially with a periodicity of approximately 1.5 ms, reflecting the delay required for synaptic discharge. Thus, the first I-wave (I1) is thought to be generated through the depolarisation of an axon synapsing directly onto a corticospinal neuron (i.e. monosynaptically), while following I-waves (I2 and later) may require local polysynaptic circuits. I-waves can be elicited using relatively low TMS intensities in humans and are thus readily amenable to study.
The last few years have seen a flurry of investigations into inter-regional physiological interactions linking M1 with other ipsilateral and contralateral motor regions, parietal cortex, cerebellum and sensory afferents. A new picture of M1 is gradually emerging in which its role is considerably greater than that of the passive servant of higher order motor regions – rather, M1 may be seen as performing a complex integration of multiregional influences that result in purposeful motor behaviour. Some of the mechanisms involved in this process are now better understood, but many questions remain unanswered.
The first aim of this review is to update the reader on this rapidly changing subject, highlighting in the process the gaps in current knowledge. The second aim is to assist investigators by illustrating the electrophysiological interactions tested with TMS that may influence goal specific motor behaviour in humans.
Because of the very extensive literature on TMS, we chose to review in this paper electrophysiological interactions tested with TMS in particular detail, rather than extensively reviewing work on the use of TMS to induce a ‘virtual lesion’, only partially discussed, or on the interactions of TMS with other techniques like EEG, MEG and MRI.
Resting and activity-dependent interactions between M1 and other regions or afferents are grouped into intrahemispheric (within M1), interhemispheric (M1 to M1) and interregional (e.g. premotor cortex or cerebellum to M1). For the sake of simplicity, these interactions are separated into inhibitory and excitatory, but it should be kept in mind that they are likely to overlap to some extent, such that what is measured represents a net effect. Separating such influences often requires subtle manipulations of stimulus parameters. A summary of the net interregional influences to be considered is provided in Fig. 1.
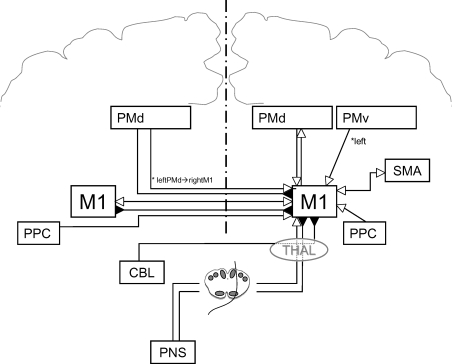
Figure 1. Summary of inter-regional influences on the primary motor cortex.
The currently described influences of other brain areas on the output of the primary motor cortex (M1) are shown. Open arrows denote facilitation, while filled arrows denote inhibition. In many cases the influence shown represents a net effect of several specific interactions, whose details are discussed in the relevant section of the text and are shown in subsequent figures. These influences include projections from motor areas in the ipsi- and contralateral hemispheres and the effects of afferent sensory input. PMd = dorsal premotor cortex; PMv = ventral premotor cortex; SMA = supplementary motor area; PPC = posterior parietal cortex; CBL = cerebellum; THAL = thalamus; PNS = peripheral nervous system.
Intrahemispheric interactions within M1
The interactions within M1 that are currently known to modulate its output are illustrated in Fig. 2.
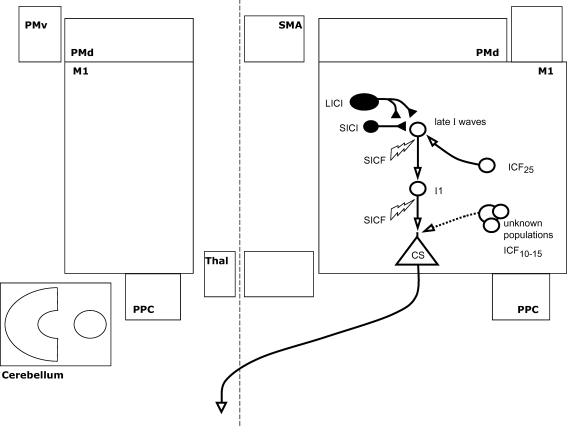
Figure 2. Interactions within the primary motor cortex.
Intracortical interactions believed to modulate the output of the primary motor cortex (M1) are shown. Each element represents a separate neuronal population within M1. Facilitatory and inhibitory populations are shown as open and filled elements, respectively. This layout forms the ‘common basis’ onto which interregional influences (in following figures) are superimposed. I1 and ‘late I-waves’ represent the populations responsible for generating the earliest and later I-waves (respectively) in response to transcranial magnetic stimulation. These are shown here in series, reflecting the temporal sequence following stimulation, but this does not necessarily reflect their anatomy. Short and long interval intracortical inhibition (SICI and LICI) and intracortical facilitation (ICF) at an interstimulus interval of 25 ms are believed to modulate the later I-waves. Short interval intracortical facilitation (SICF) enhances both early and later components of the I-wave. ICF at 10–15 ms is shown as a dotted line, as there is uncertainty regarding relative cortical and spinal contributions.
Facilitation within M1
Facilitatory interactions occurring locally within M1 can be studied by delivering two TMS pulses through the same coil (or two overlapping coils targeting the same cortical area), referred to generically as paired-pulse TMS. This approach has revealed two categories of local facilitation.
Intracortical facilitation (ICF) of a test MEP can be elicited at interstimulus intervals (ISIs) of 6–25 ms, using a subthreshold conditioning stimulus (CS) to influence the response to a subsequent suprathreshold test stimulus (TS). This effect was first described by Kujirai et al. (1993) in a now classic paper reporting facilitation of the test MEP at intervals of 10–15 ms. Facilitation becomes stronger with increasing CS intensity (Kujirai et al. 1993), but tends to be weaker with increasing TS intensity (Daskalakis et al. 2004). The question arises as to whether ICF may be merely a ‘rebound’ phenomenon from the robust inhibition described by these authors at shorter interstimulus intervals (see below) or whether it represents a separate phenomenon. Ziemann et al. (1996c) demonstrated that short interval intracortical inhibition (SICI) occurs at lower CS intensities than ICF, becoming stronger with increasing intensities. Furthermore the two phenomena behave differently depending on the current direction of conditioning and test pulses: while inhibition can be elicited regardless of the direction of current flow, reliable ICF requires a conditioning stimulus to be induced in a postero-anterior (PA) direction. The authors concluded that separate neuronal populations were likely to mediate intracortical inhibition and facilitation (Ziemann et al. 1996c). A cortical (rather than spinal) site of action of the CS in this context was supported by the findings that the CS intensity required to elicit this effect was below the threshold for producing an MEP and that spinal H-reflexes were unaffected. This was investigated further by studying the effect of the CS on descending volleys recorded in cervical epidural electrodes. This approach demonstrated facilitation of the late I-waves at an interstimulus interval of 25 ms, suggesting a synaptic interaction within M1 (Nakamura et al. 1997). However, a recent study by Di Lazzaro et al. (2006) examined cervical descending volleys in more detail: while facilitation of late I-waves was again seen at 25 ms, no changes in the amplitudes or number of I-waves were seen at 10 and 15 ms, despite facilitation of the compound MEP. This raises the possibility that facilitation at these shorter intervals may be mediated by subtle changes in spinal excitability. However, in the same study, test MEPs generated by delivering an electrical TS directly to cervical epidural electrodes were not facilitated by a magnetic cortical CS, making such a spinal interaction unlikely. An alternative and more likely possibility is that any additional corticospinal discharge produced in the presence of a CS is temporally dispersed, and thus not apparent in the mean I-wave traces. Thus, while ICF at 25 ms appears likely to have a cortical origin, the site of facilitation at 10–25 ms is less clear.
Excitatory glutamatergic interneurons within M1 and N-methyl-d-aspartate (NMDA) receptors appear to influence ICF (Ziemann, 2003). NMDA antagonists have been shown in two separate studies to abolish (dextromethorphan) or even reverse (memantine) ICF measured at 10 or 15 ms (Ziemann et al. 1998a; Schwenkreis et al. 1999). This issue has been clouded somewhat by the demonstration that ICF is unaffected by the non-competitive NMDA antagonist ketamine, given at a subanaesthetic dose (Di Lazzaro et al. 2003). However, while ketamine is thought to reduce transmission at NMDA receptors, it is also believed to increase glutamate release and transmission at AMPA synapses. Furthermore, the increase in unconditioned test MEP amplitude after ketamine makes the lack of effect on ICF difficult to interpret. ICF is also thought to be modulated by GABAA activity, since it is reduced by the GABAA agonist lorazepam and abolished by ethanol, which potentiates GABA-mediated currents (Ziemann et al. 1995, 1996b; Ziemann, 2004). This is consistent with the idea that the inhibition of I3 waves that is responsible for short interval inhibition (SICI – see below) may persist as late as 20 ms after the CS (Hanajima et al. 1998). Thus the phenomenon of ICF is likely to be influenced by glutamatergic facilitation tempered by persisting GABAergic inhibition.
The interactions between ICF and other physiological processes have not been explored extensively. Ziemann et al. (1996c) demonstrated in a triple pulse TMS protocol that SICI and ICF can be shown to interact in an approximately linear relation, e.g. strong SICI might abolish ICF, further supporting a different origin for these two processes. In another triple pulse TMS protocol, ICF tested in M1 in the setting of cerebello-M1 inhibition (described below) appears to be enhanced (Daskalakis et al. 2004). However, a within-group correlation analysis suggested that this is likely to be due to a reduction in the SICI component rather than an increase in the excitatory component (tested at 10 ms), making a direct interaction with the excitatory population unlikely.
A different kind of facilitatory interaction can be demonstrated within M1 over shorter interstimulus intervals. This short interval intracortical facilitation (SICF, also known as I-wave facilitation) occurs when a suprathreshold stimulus (S1, in this case considered as the test stimulus, TS) is followed by a subthreshold stimulus (S2, in this case considered as the conditioning stimulus, CS) (Ziemann et al. 1998c), or alternatively when two stimuli near motor threshold are given consecutively (Tokimura et al. 1996). Using this approach, facilitation can be demonstrated at three distinct ISIs after the first stimulus: 1.1–1.5, 2.3–2.9 and 4.1–4.4 ms. If S2 is fixed at 90% of resting motor threshold (RMT) and the intensity of S1 is gradually increased, the first facilitatory peak is observed with an S1 of 70% RMT: further increasing the intensity of S1 produces second and third peaks at approximately 90% and 100%, respectively, with latencies that shorten with increasing S1 intensity. This effect is absent if S1 precedes a transcranial electric (instead of magnetic) S2, implying a cortical site of such facilitation, and it was proposed that the three facilitatory peaks observed reflect the generation of subsequent I-waves by S1 (Tokimura et al. 1996; Ziemann et al. 1998c). This was demonstrated conclusively for the earliest such peak by showing similar effects in the descending volleys generated by such stimuli in cervical epidural electrodes (DiLazzaro et al. 1999). A study of the precise timings of these interactions and their relation to stimulus intensity shed light on the contrast between this phenomenon and that mediating inhibition at similar intervals. If S1 < S2 (with S1 subthreshold and S2 suprathreshold) inhibition occurs mainly in the I3-wave. By contrast, if S1 = S2 or S1 > S2 (with S1 suprathreshold), facilitation occurs, but this is primarily in the I2 (or even I1) wave latency range. Thus the facilitation appears to take place one I-wave cycle earlier than the inhibition. Ilic and colleagues have proposed that this is because after a suprathreshold S1 the excitatory interneurons mediating the later I-waves are still hyperexcitable at the time of the earlier I-waves resulting from S2 (Ilic et al. 2002). Thus, while SICI and ICF are mediated via a trans-synaptic action on excitatory interneurons, SICF may instead involve a direct action on the initial axon segment of these excitatory interneurons (Ilic et al. 2002).
The effects of SICF are suppressed in the period following a peripheral sensory stimulus, suggesting an inhibitory interaction between afferent inputs and the interneuron populations responsible for I-wave generation. There is also a suppression of ICF in this context, but this occurs at lower CS intensities than for SICF (Zittel et al. 2006), reinforcing the hypothesis of different mechanisms for these two phenomena.
Inhibition within M1
Two principal types of local intracortical inhibition can be studied using paired pulse TMS. Short interval intracortical inhibition (SICI) was first described by Kujirai et al. (1993) and can be elicited by a subthreshold CS followed by supra threshold TS. At interstimulus intervals (ISIs) of 1–6 ms the test motor response is inhibited by the conditioning shock. Two main phases of inhibition have been described, at ISIs of 1 ms and 2.5 ms (Fisher et al. 2002; Roshan et al. 2003). Based on indirect evidence such as the lack of change in spinal reflexes, Kujirai et al. (1993) originally suggested that SICI was the result of synaptic interactions occurring within M1. A later study used direct recordings of descending spinal cord volleys to confirm that the initial I1-wave was suppressed by the CS, indicating that SICI seems to be mediated at the cortical level (Nakamura et al. 1997). An important limitation of this study was that the intensity of the CS was relatively large, raising the possibility that the CS alone could depolarize the axon, causing subsequent refractoriness during TS delivery. Therefore, it was not until 1998 that Di Lazzaro and collaborators provided the first direct evidence that SICI originated at the cortical level (DiLazzaro et al. 1998). In their study, a subthreshold CS suppressed the size of both the descending spinal cord volleys and the MEP evoked by the suprathreshold TS. Inhibition of the descending spinal volleys was most pronounced at an ISI of 1 ms and disappeared by 5 ms. This inhibition was evident for all later I-waves but not the I1-wave. Pharmacological studies have continued to provide more detailed information about the mechanisms of SICI. It has been shown that GABAA agonists enhance SICI (Ziemann et al. 1996a; Ilic et al. 2002). However, a single dose of the GABAA antagonist flumazenil did not alter SICI, suggesting that there might be no tonic activity at the benzodiazepine binding site of the GABAA receptor in the normal human M1 (Jung et al. 2004). It has also become apparent that inhibition at the short ISI of 1 ms does not depend on GABAA, while ‘true’ SICI at an ISI of 2.5 ms is likely to be mediated by GABAergic inhibition at the intracortical level (Fisher et al. 2002; Roshan et al. 2003), supporting the point of view that they are mediated by different mechanisms. Previously, Fisher et al. (2002) proposed that SICI at an ISI of 1 ms may be due to refractoriness or changes in axonal excitability of excitatory interneurons. In this scenario the subthreshold CS would convey excitatory interneurons into the refractory state, leading to less impact of the TS reflected as inhibition. Thus, with increasing TS intensity less inhibition would be expected, as the TS would activate more non-refractory interneurons. However, the fact that SICI at 1 ms increases with TS intensity at rest and decreases with voluntary muscle contraction (Roshan et al. 2003) argues against simple axonal refractoriness. In addition, a recent study showed that SICI at an ISI of 1 and 2.5 ms decreased to a similar extent during the cortical silent period (CSP) (Ni et al. 2007). Since the CSP is unlikely to affect axonal refractoriness, a synaptic mechanism is very likely responsible for SICI at 1 ms ISI.
While SICI can be considered as a well-characterised ‘standard’ TMS parameter, much less is known about the inhibitory phenomenon occurring at longer interstimulus intervals. Long interval intracortical inhibition (LICI) is elicited by a suprathreshold CS and TS applied at ISIs of approximately 50–200 ms (Valls-Sole et al. 1992; Wassermann et al. 1996) – thus two MEPs are elicited, of which the second is smaller in amplitude. Previous evidence has suggested that LICI at ISIs longer than 50 ms is mediated within M1 rather than subcortical structures (Nakamura et al. 1997). Although this evidence supports the view that LICI is related to reduced corticofugal excitability, it still remains unclear whether the same population of neurons mediates LICI and SICI. Pharmacological studies suggest that LICI is mediated by GABAB receptors (Werhahn et al. 1999; McDonnell et al. 2006) while SICI is primarily mediated by GABAA receptors (Ziemann, 2003). Nevertheless, the involvement of different receptor subtypes does not in itself exclude the possibility of a shared neuronal population mediating these two inhibitory phenomena.
Recent studies have shown that SICI and LICI interact with each other. SICI increases with higher test MEP amplitudes, while LICI decreases with higher test MEP amplitudes (Chen & Curra, 2004). These findings suggest that motor cortical neurons recruited at low TS intensities are more susceptible to LICI than to SICI, while those recruited at higher intensities appear to be more susceptible to SICI than LICI. On this basis, it is likely that different populations of inhibitory interneurons mediate LICI and SICI. In addition, previous evidence has shown that SICI is reduced in the presence of LICI at matched size of test MEP amplitude and test stimulus intensity, suggesting an inhibitory effect of LICI on SICI (Chen & Curra, 2004). While most studies of SICI and ICF have been implemented using distal hand muscles, it has been shown that relatively similar phenomena occur also in more proximal arm representations as well (Chen et al. 1998).
Interhemispheric interactions (M1–M1)
The interhemispheric interactions between homologous M1s currently described, and their relationships to intracortical processes, are illustrated in Fig. 3.
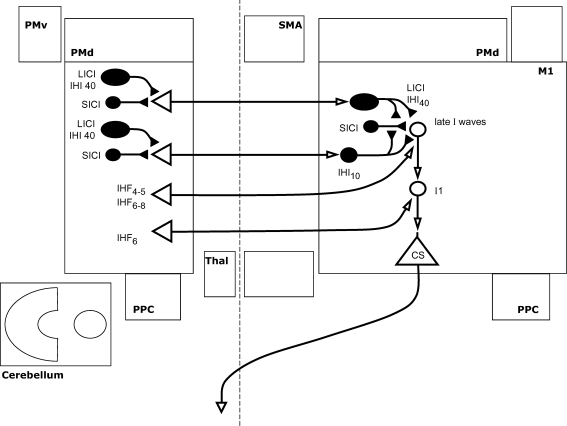
Figure 3. Interhemispheric interactions between primary motor cortices.
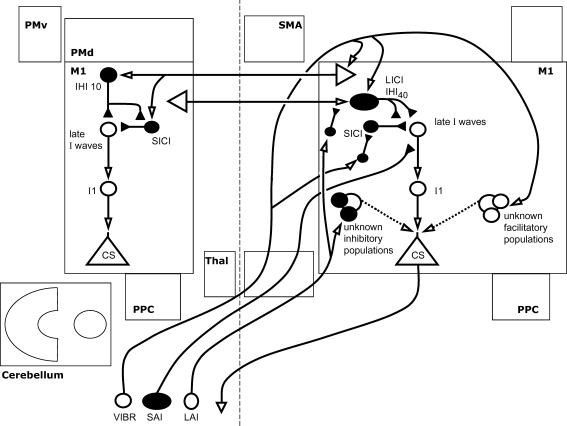
Interhemispheric inhibition and facilitation (IHI and IHF) at the interstimulus intervals shown are illustrated, along with their interactions with local intracortical circuits where known. Open arrows denote facilitation, while filled arrows denote inhibition. Thus IHI is shown as mediated by a facilitatory transcallosal population synapsing onto a local inhibitory population. IHI10 (shown here as the second interhemispheric interaction from the top) can be conditioned by short or long interval intracortical inhibition (SICI and LICI) in the conditioning hemisphere, and itself suppresses SICI in the target hemisphere. IHI40 (shown as the top-most interaction) may share a common inhibitory effector population with LICI. Of the interactions described, only IHF at 6 ms is thought to modulate the early I-waves, while the others affect later I-waves. Of the facilitatory interhemispheric interactions shown, IHI6 requires a test stimulus with current flow in an anterior direction, while IHI6–8 requires a posterior current.
Interhemispheric facilitation between primary motor cortices
Transcallosal projections between the two M1 hand areas are known to exist in monkeys (Jenny, 1979). That such projections can convey information between the hemispheres is suggested by the detection of evoked potentials over M1 following electrical or magnetic stimulation of the contralateral M1, both in animal models and in humans (Hanajima et al. 2001; Chowdhury & Matsunami, 2002). Using a paired pulse TMS technique with one coil over each M1 hand area, Ferbert et al. (1992) investigated interactions between the two M1s. While inhibition was their most striking finding (see below), they also described a facilitation occurring in some subjects, at shorter ISIs, which was ‘capricious’ and poorly reproducible. This phenomenon was further investigated by Hanajima et al. (2001), who found that such interhemispheric facilitation (IHF) is reliably obtainable under particular conditions. Small test MEPs were used (approximately 0.3 mV) with slight tonic voluntary contraction of the right FDI (ipsilateral to the site of the conditioning stimulus) maintained throughout, and facilitation occurred only with a TS delivered such that the induced current is in an antero-posterior (AP) direction, suggesting that it primarily affects the I3-wave generators. The ISI required for facilitation was 4–5 ms, but allowing for the time taken to generate I3 waves in the target hemisphere, this implies a facilitatory interaction approximately 10 ms after the CS. Facilitation only occurred following a CS of relatively low intensity (5–10% above active motor threshold (AMT)) given with the induced current in a medial direction. A similar effect could also be produced using an electrical CS (inducing a contralateral d-wave) and it was originally suggested that corticospinal discharge may be necessary for IHF to occur, whether mediated by axon collaterals of pyramidal cells or by a separate neural population. However, further investigation of IHF at low CS intensities makes this conclusion unlikely. Baumer et al. (2006) demonstrated reliable IHF at rest following a conditioning stimulus to M1 at two very subthreshold intensities. At 60% of active motor threshold, IHF occurred at an interval of 6 ms, with the TS current in a postero-anterior (PA) direction (unlike Hanajima et al.). At 80% of AMT, IHF occurred at 6–8 ms ISI, with the TS current in an antero-posterior (AP) direction. The I-wave components of the test pulse affected in these two conditions are likely to be predominantly I1 and I3, respectively. The authors suggested that the longer ISIs could be explained by the activation of slower-conducting fibres at these lower CS intensities, and that at higher intensities such facilitation may have been overwhelmed by concomitant inhibition. In cats the cortical area of the distal forelimb has an excitatory transcallosal connection to the homologous motor cortex, but this is surrounded by a larger area of inhibition (Sanuma & Okuda, 1962). It may be that the relatively poor spatial resolution of TMS means that this robust surround inhibition predominates in most circumstances. The role of this form of interhemispheric facilitation in motor control of bilateral arm movements remains to be determined (Swinnen et al. 1993; Whitall et al. 2000; Schambra et al. 2003; Luft et al. 2004; Duque et al. 2005).
Interhemispheric inhibition between primary motor cortices
In contrast to interhemispheric facilitation, interhemispheric inhibition (IHI) is more robust and occurs over a wide range of ISIs (6–50 ms) (Ferbert et al. 1992; Daskalakis et al. 2002). This form of inhibition is lacking in patients with ischaemic lesions affecting transcallosal populations, supporting the idea that this phenomenon is mediated via the corpus callosum (Boroojerdi et al. 1996). Emerging evidence suggests that IHI elicited at relatively short ISIs (e.g. 8–10 ms) is mediated by different mechanisms than that elicited at longer intervals (e.g. 40 ms). Therefore short (IHI10) and long (IHI40) interval IHI will be discussed separately. Other than the ISI, the stimulation parameters required to elicit IHI10 and IHI40 are similar. Both require a suprathreshold CS and TS intensity adequate to elicit an MEP of 0.5–1.5 mV in amplitude (Kukaswadia et al. 2005). Both are also believed to be dependent on GABAB-mediated neurotransmission in the target hemisphere (IHI10: Daskalakis et al. 2002; Kukaswadia et al. 2005; IHI40: Kukaswadia et al. 2005). This was confirmed for long latency IHI by a recent study of pharmacological modulation by GABA agonists: IHI at ISIs of up to 200 ms was strengthened after application of the GABAB agonist baclofen, suggesting that long interval IHI is most likely mediated by postsynaptic GABAB receptors (Irlbacher et al. 2007).
Interactions within the target hemisphere
Evidence for differing mechanisms of IHI at these two ISIs comes primarily from studies of their interactions with other inhibitory phenomena. A number of studies have examined the interactions of such phenomena with IHI within the ‘target’ hemisphere (i.e. that receiving the inhibition), and it has been suggested that LICI and IHI40 may be mediated by an overlapping population of inhibitory neurons. As nicely reviewed by Kukaswadia et al. (2005) the evidence for this is three-fold: (1) both parameters preferentially affect lower threshold M1 interneurons (Gerloff et al. 1998; Daskalakis et al. 2002); (2) both require a suprathreshold CS (Kujirai et al. 1993; Daskalakis et al. 2002; Chen et al. 2003); and (3) both inhibit SICI in the receiving hemisphere (Sanger et al. 2001; Chen, 2004). However, a third phenomenon, long afferent inhibition (LAI – discussed below), helps to shed more light on the relationship between IHI and LICI. It is known that LAI directly inhibits LICI (Sailer et al. 2002; Chen, 2004). Kukaswadia et al. (2005) found that LAI also directly inhibits IHI40. They therefore concluded that LICI is probably more closely related to IHI40 than to IHI10. This idea is consistent with the finding that IHI8 decreases with voluntary muscle activation (Chen et al. 2003), while IHI40 (Chen et al. 2003) and LICI (Valls-Sole et al. 1992; Wassermann et al. 1996) both show little change. Thus far, the differential effects of LICI on IHI40versus IHI10 in the target hemisphere have not yet to our knowledge been tested directly. The relationships between SICI and IHI40versus IHI10 have likewise not been directly compared (in fact, little is known regarding the relationship between SICI and IHI40). However, it is known that IHI10 inhibits SICI in the target hemisphere and is decreased in the presence of LAI (Kukaswadia et al. 2005). This occurs only under certain conditions and, more importantly, the amount of decrease in IHI10 is not related to the strength of LAI or IHI10. Thus, LAI probably does not inhibit IHI10 directly, but both may act on a similar neuronal population (Gilio et al. 2003). In contrast, LAI strongly inhibits IHI40, in most cases changing inhibition to facilitation, and the decrease in IHI40 is directly related to the strength of LAI (Kukaswadia et al. 2005). Therefore, LAI and IHI40 seem to show a direct inhibitory interaction, with LAI inhibiting IHI40.
Interactions within the conditioning hemisphere
The above studies describe intracortical interactions with IHI within the target hemisphere. A recent study has examined the effects of such intracortical interactions within the conditioning hemisphere on IHI targeting the contralateral hemisphere (Lee et al. 2007). IHI10 and IHI40 were elicited with the CS in the presence or absence of SICI, LICI or ICF (with stimulus intensities adjusted to maintain MEP amplitudes). Both forms of IHI were suppressed in the presence of SICI or LICI but were unaffected by ICF. This last result could be interpreted as suggesting that the corticospinal output and the transcallosal projections mediating IHI arise from different neuronal populations. However, this conclusion should be guarded in view of the recent data casting doubt on the cortical site of ICF's action (Di Lazzaro et al. 2006). Moreover, in a separate study the effect of SICF within the conditioning hemisphere on IHI40 (but not IHI10) was examined (Avanzino et al. 2007). IHI was enhanced by SICF at identical latencies to the facilitation of the contralateral MEP (1.5 ms and 3.0 ms, coinciding with the I1 and I2 waves, respectively), suggesting that circuits modulating transcallosal and corticospinal projections have at least similar properties. The fact that IHI10 and IHI40 are affected similarly by SICI, LICI and ICF (Lee et al. 2007) raises the possibility that a shared population may convey each form of inhibition across the corpus callosum, with different target populations influenced at different latencies. However, the impact of SICI on IHI might depend on the intensity of the CS for SICI (Kujirai et al. 1993), which was kept constant in the study.
Summary of interhemispheric M1–M1 interactions
As described above, the approach of paired pulse TMS between the two M1 hand areas has revealed at least three facilitatory and two inhibitory distinct interactions, depending on the parameters used (ISI, coil orientation and intensities of CS and TS). Facilitation or inhibition can even be produced at overlapping ISIs, depending on the nature of the CS and TS, suggesting that such interactions are likely to occur in parallel. With regard to the cell populations involved, it is likely that even in the case of IHI the transcallosal projections are excitatory, synapsing onto local inhibitory circuits within the target hemisphere. From such manipulation of physiological parameters as described above it is not possible to infer whether the various phenomena are mediated by distinct transcallosal populations or whether common projections are used with, for example, different coding characteristics. Consistent with these findings, it was reported that down-regulation of excitability of one motor cortex modulates cortical excitability in the opposite M1 and motor function in the ipsilateral hand in health (Schambra et al. 2003; Kobayashi et al. 2004; Johansen-Berg et al. 2007) and disease (Ward & Cohen, 2004; Talelli et al. 2006; Fregni & Pascual-Leone, 2006).
Inter-regional interactions
The inter-regional cortico–cortical interactions currently described that are known to modulate the output of M1 (excluding M1-to-M1) are illustrated in Fig. 4.
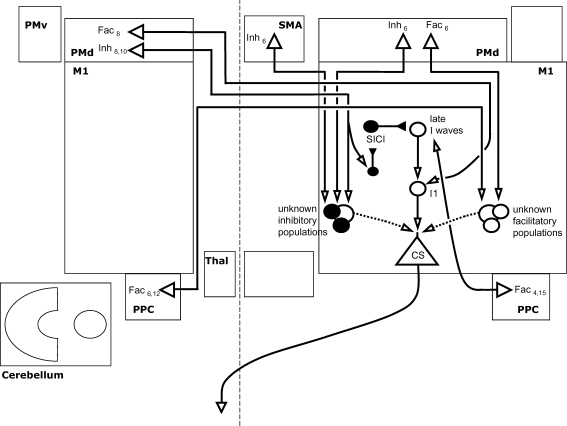
Figure 4. Model of Inter-regional interactions of nonprimary cortical areas with M1.
Interactions with M1 are shown of both ispi- and contralateral dorsal premotor cortices (PMd) and posterior parietal cortices (PPC), and of ipsilateral supplementary motor area (SMA). Open arrows denote facilitation, while filled arrows denote inhibition. Interactions with local intracortical circuits are shown where known, but for most only a facilitatory or inhibitory influence has been demonstrated. Inter-regional projections are shown as facilitatory, synapsing onto facilitatory or inhibitory local circuits, but this arrangement is not certain. The influence of the PMd on either side is facilitatory or inhibitory depending crucially on the conditioning stimulus intensity used.
Interactions between non-primary and primary motor areas
M1–PMd interactions. Non-primary motor areas are also capable of influencing motor cortical output including the ventral and dorsal premotor cortex, supplementary motor area and cingulate cortex (Chouinard & Paus, 2006). The dorsal premotor cortex (PMd) has attracted particular attention in this regard because of its recognised role in movement selection (Cisek & Kalaska, 2005) and its dense anatomical connection to M1 in monkeys (Ghosh & Porter, 1988). Two main approaches have been taken to investigating PMd's influence on its ipsilateral M1. The first involves applying repetitive TMS (rTMS) to PMd, using a protocol known to up- or down-regulate cortical excitability, and afterwards assessing motor cortical excitability in M1 with single pulse TMS. An rTMS protocol used to produce a transient reduction in excitability, applying subthreshold stimuli at 1 Hz, was applied to PMd and resulted in a reduction of MEP amplitudes elicited from M1 (Gerschlager et al. 2001) but increased paired pulse excitability at a 7 ms ISI and shortened the cortical silent period (Munchau et al. 2002). Conversely, applying an rTMS protocol that increases cortical activity (5 Hz at 90% AMT) to PMd had the opposite effects: MEP amplitudes were increased and paired pulse excitability at 7 ms ISI was reduced (Rizzo et al. 2004). Together, these rTMS studies show that manipulations of PMd excitability modulate M1 corticospinal excitability in a similar direction, suggesting at first glance a facilitatory influence of PMd on M1.
The second approach has employed two coils in a paired pulse protocol. Civardi et al. (2001) showed that a subthreshold CS (defined by the M1 MEP threshold) over PMd reduces the excitability of the ipsilateral M1, with a maximum effect at an ISI of 6 ms – this ipsilateral PMd–M1 inhibition requires a CS given at 90% of AMT with antero-posterior current flow. The authors argued that this interaction did indeed involve conditioning PMd rather than acting via current spread to M1, on the basis of spatial separation (conditioning at an intermediate point produces no inhibition), temporal separation (a time course that is distinct from SICI) and the effect of coil orientation (Civardi et al. 2001). However, in addition to this inhibitory interaction, facilitation could also be elicited if a higher conditioning intensity was used (120% AMT).
Mochizuki et al. (2004a) applied a similar paired pulse approach to investigate the interhemispheric interaction between PMd and the contralateral M1. At ISIs between 4 and 20 ms (with a CS to the right PMd and a TS to the left M1), they found significant inhibition of the test MEP using a CS intensity of either 90% RMT (with an ISI of 8 ms) or 110% RMT (ISI of 8–10 ms). This interhemispheric PMd–M1 inhibition is spatially specific for PMd (as not detected when stimulating 2 cm anterior, lateral or medial to the target area) but not for the hemisphere (Baumer et al. 2006). Stimulation of the left PMd and right M1 revealed the same results (Baumer et al. 2006; Koch et al. 2006). This interaction can be distinguished from M1-to-M1 IHI at the 90% CS intensity (on the basis of a lower threshold and differing effects of voluntary contraction) but this distinction is less clear at the suprathreshold intensity. Interhemispheric PMd–M1 inhibition has also been described at the longer interstimulus interval of 150 ms, using a latero-medial CS at 110% of AMT (Mochizuki et al. 2004b), but at such a long interval the effect cannot be assumed to be transmitted transcallosally. The effect of PMd stimulation on the contralateral M1 seems to depend on the stimulation intensities used, as demonstrated recently by Baumer et al. (2006). Conditioning the left PMd with low stimulus intensity (80% of AMT) and targeting the right M1 (with small test MEPs), interhemispheric PMd–M1 facilitation was described at an ISI of 8 ms (Baumer et al. 2006). This facilitation was dependent on a postero-anterior current flow for the TS, providing indirect evidence that this form of facilitation preferentially affects I1 waves in the target hemisphere.
A mechanism proposed for these interregional effects is the activation of long distance projections from the PMd to ipsi- or contralateral M1, consistent with anatomical studies showing dense connections between those areas, which are known to be both inhibitory and facilitatory (Ghosh & Porter, 1988; Tokuno & Nambu, 2000). Details of how these long-range projections interact with the intracortical circuits described above are not well known. The only study to directly address this question has been Mochizuki et al. (2004a), who showed that interhemispheric PMd–M1 inhibition was associated with a reduction in intracortical inhibition in the target hemisphere (SICI at ISI of 2 ms).
M1–SMA interactions. The supplementary motor area (SMA) is by contrast a more difficult area to target than the lateral premotor cortex (PMd and PMv), as it is located in the interhemispheric fissure, relatively unexposed on the surface of the hemisphere. TMS can reliably elicit MEPs from leg muscles by stimulating the leg area of M1 adjacent to SMA (Gerloff et al. 1997; Perez et al. 2004). Stimulation of the SMA is therefore possible if cortical elements targeted by TMS have a threshold comparable in the lower limb area of M1 and in the SMA. However, there are few electrophysiological studies of SMA stimulation in healthy subjects. Civardi et al. (2001) found that a conditioning stimulus applied over the SMA, defined as a cortical area 3 cm anterior to the M1 leg area (1–4 cm anterior to Cz), reduced the excitability of the ipsilateral M1 at an ISI of 6 ms, indicating that SMA stimulation is likely to lead to changes in activity in anatomically connected regions in a way similar to that seen after stimulation of PMd (Civardi et al. 2001). Matsunaga et al. (2005) used 5 Hz suprathreshold rTMS (110% AMT) over the SMA to investigate the effects on ipsilateral M1 excitability. Stimulation increased the MEP amplitudes (as for PMd), but in this case SICI/SICF was unchanged as were cortical silent period and H-reflexes. Thus an inhibitory interaction is suggested by the paired pulse approach (Civardi et al. 2001), whereas excitatory rTMS appears to cause facilitation (Matsunaga et al. 2005).
Posterior parietal cortex (PPC)
The two coil paired-pulse approach has recently also revealed a facilitatory interaction between the posterior parietal cortex (PPC, defined as the P4 position on the 10–20 EEG system) and both the ipsilateral and contralateral M1 (Koch et al. 2007). Significant facilitation of an MEP elicited from the ipsilateral M1 was observed at ISIs of 4 ms and 15 ms when a CS of 90% RMT was used (postero-anterior current direction). This PPC–M1 facilitation was not seen at higher or lower CS intensities, or with the opposite conditioning coil orientation. Single motor unit recordings suggested that PPC stimulation enhances the I3-wave component of the test MEP, a finding that may explain the surprisingly short ISI of 4 ms (as late I-waves may take as long as 7 ms to leave the motor cortex –Day et al. 1989). In addition to the ipsilateral effect, facilitation of MEPs elicited from the contralateral M1 was also observed. This was seen at a CS intensity of 90% RMT and ISIs of 6 ms and 12 ms. The precise anatomical pathways mediating these effects are not known, and it may be that such facilitation involves parieto-premotor projections (which are known to be more numerous than direct parieto-motor projections). An interesting feature of this effect is that, unlike premotor-M1 or M1–M1 interactions inhibition was not observed at any of the CS intensities tested.
Afferent input and somatosensory cortex
Although peripheral nerve stimulation provides a strong and temporally precise afferent input, it stimulates a mixed population of nerve fibres, including muscle afferents, cutaneous afferents, joint afferents and motor efferents. Hence, it is not surprising that studies of the effects of peripheral nerve stimulation on cortical excitability provide a set of heterogeneous results whose underlying mechanisms and origin of the interactions are difficult to interpret. Two different approaches are most frequently used: (1) a paired-pulse protocol combining a peripheral and a cortical stimulus to study sensorimotor interactions, and (2) repetitive peripheral nerve stimulation as a tool to elicit changes in cortical excitability (with or without implementing the paired-pulse technique).
Activity in afferent pathways can condition motor cortical excitability in a paired-pulse protocol (summarised in Fig. 5). For example, a conditionings electrical stimulus applied to a mixed nerve (most often the median or digital nerve at the wrist) has an inhibitory effect on motor cortex excitability. These effects, more evident at ISIs of 20 ms and 200 ms, are described as short- (SAI) and long-latency afferent inhibition (LAI), respectively (Tokimura et al. 2000).
Figure 5. Influence of somatosensory afferent input on M1 excitability.
The effects of long and short afferent inhibition (LAI and SAI) and of muscle vibration (VIBR) on M1 excitability and on intracortical circuits are shown. Open arrows denote facilitation, while filled arrows denote inhibition. LAI and SAI suppress late I-waves in the contralateral M1. Vibration increases M1 excitability but the effect on the I-wave profile is not known. The effect of IHI from the opposite M1 is reduced in the presence of LAI. Muscle vibration reduces SICI but increases LICI in the contralateral M1, while increasing IHI targeting the ipsilateral M1 (with increased SICI and reduced M1 excitability in that hemisphere).
Many experiments have confirmed that the M1 hand area receives short latency input from peripheral receptors (Friedman & Jones, 1981; Darian-Smith & Darian-Smith, 1993). The most direct evidence that somatosensory input modulates the motor output at a cortical level in humans comes from recordings of corticospinal volleys in patients with implanted electrodes in the cervical epidural space (Tokimura et al. 2000). These showed that I2 and I3 waves were reduced at an interval appropriate for SAI, whereas the I1 wave remained unchanged at any ISI. Similar findings were observed for mixed nerve stimulation and digit nerve stimulation of separate fingers. Based on these findings, it seems likely that reduced corticofugal output is the cause of the reduced MEPs. However, whether the afferent input travels directly to M1 or proceeds via the primary somatosensory cortex (S1) is still under investigation. Pharmacological investigations have revealed roles for both the cholinergic and GABAergic systems in SAI. The anticholinergic drug scopolamine reduces SAI (Di Lazzaro et al. 2000), which is also impaired in Alzheimer's disease (Di Lazzaro et al. 2004). SAI shows dissociated responses to positive allosteric modulators of the GABA receptor, lorazepam and diazepam, becoming weaker with the former and stronger with the latter (Di Lazzaro et al. 2005). Given that SAI may be seen as a marker of cholinergic function, it is interesting to consider that these two benzodiazepines are also dissociated with respect to their effects on memory function (profoundly impaired by lorazepam but not diazepam). Thus it may be that lorazepam reduces SAI via an effect on cholinergic function.
Indirect evidence for a cortical site of action of LAI originates from the finding that F-wave amplitudes remain unchanged at an ISI of 200 ms (Chen et al. 1999). As for SAI, it remains to be determined if this effect is mediated through direct somatosensory projections to M1 or indirectly through the primary somatosensory cortex. As mentioned above, in the presence of LAI, both LICI and IHI40 are reduced (Sailer et al. 2002; Kukaswadia et al. 2005).
Similar results have been shown for cutaneous stimulation of digital nerves. MEPs were inhibited when a TMS pulse was delivered 25–50 ms after homotopic stimulation of a digital nerve (Classen et al. 2000; Tamburin et al. 2005). However, when the TMS pulse preceded the cutaneous stimulus (ISI 16–22 ms) or if the ISI was longer than 50 ms (up to 200 ms) MEPs were facilitated. In a muscle heterotopic to the site of the cutaneous stimulus a reversed pattern of MEP size was found. In the presence of a cutaneous stimulus applied ∼35 ms before a conditioning TMS pulse, a reduction in SICI was described (Ridding & Rothwell, 1999), suggesting that the afferent input provoked by the digital stimulus had a direct effect on circuits involved in intracortical inhibition, most likely as an interference with later I-waves weakening the efficacy of the cortical conditioning stimulus.
Introduced by Stefan et al. (2000) another approach combines peripheral and low frequency cortical stimulation in a repetitive, timing-specific pattern. Resembling mechanisms of associative plasticity in animal slice preparations by pairing pre- and postsynaptic action potentials (Wigstrom et al. 1986; Markram et al. 1997), this paired associative stimulation (PAS) protocol consists of a peripheral stimulus (most often electrical stimulation of the median nerve at the wrist) that is followed by a suprathreshold TMS stimulus to the contralateral M1 targeting a muscle innervated by the stimulated nerve.
The ISI is determined by the time lag in evoking an MEP from M1 via activation of the primary somatosensory cortex (S1). The shorter the ISI for facilitatory PAS (shorter than 25 ms), the more likely later inputs to corticospinal neurons are targeted by PAS, as the afferent input would arrive after firing of the initial input (I1 input) produced by the TMS pulse. Thus, if the peripheral stimulus is given approximately at the N20 latency of a somatosensory evoked potential (SEP) plus ∼1–4 ms for the S1 to M1 transit time (ISI of approximately 20–25 ms) an increase in the conditioned MEP can be found, if the PAS procedure is repeated for a period of ∼30 min (Stefan et al. 2000). Conversely, at even shorter ISIs, most often 10 ms, a decrease of cortical excitability as measured by a reduced MEP size is found after repeated stimulation (Wolters et al. 2003).
In a relaxed muscle, the intensity of the peripheral stimulus and the TMS stimulus used for PAS need to be suprathreshold to induce long-lasting changes in cortical excitability (Stefan et al. 2000; Wolters et al. 2003). The reason for this is still unclear, but it is thought that associative plasticity requires either a certain amount of synaptic activity or the neuronal population that is targeted by PAS might have high thresholds for activation. Interestingly, weak voluntary muscle contraction further enhances the after-effect of PAS compared to a resting condition (Kujirai et al. 2006). Also, the direction of the current flow in the brain following a subthreshold TMS pulse significantly alters the effectiveness of this procedure: tested during voluntary contraction, PAS using subthreshold TMS with AP current flow and 25 ms ISI is superior to PAS using subthreshold TMS with PA current flow (Kujirai et al. 2006) in eliciting excitability changes, presumably reflecting the later arrival of inputs preferentially activated by AP pulses (I3 input; Di Lazzaro et al. 2001).
The after-effects of PAS are relatively long lasting (duration up to 90 min) and have topographical specificity (Stefan et al. 2000). Furthermore they can be abolished by the application of the N-methyl-d-aspartate (NMDA) receptor antagonist dextromethorphan (Stefan et al. 2002; Wolters et al. 2003). Recently it was described that motor learning prior to PAS can also prevent induction of the LTP-like plasticity in M1 for several hours (Stefan et al. 2006) suggesting the occlusion of further plastic changes after maximized LTP following training. Conversely, Ziemann et al. (2004) found even greater reduction in cortical excitability following a PAS protocol that usually elicits LTD-like plasticity when it was preceded by motor learning, supporting the idea that PAS exerts its action via LTP/LTD-like mechanisms.
A modulation of cortical excitability can also be elicited by repetitive mixed peripheral nerve stimulation (PNS). Trains of five slightly suprathreshold pulses of 1 ms duration delivered at 10 Hz for at least 1.5 h resulted in a somatotopically specific increase of MEPs only in muscles innervated by the stimulated nerve (Ridding et al. 2000a; Ridding & Taylor, 2001; Kaelin-Lang et al. 2002), outlasting the end of the stimulation by ∼20 min (Kaelin-Lang et al. 2002). The somatotopy and the fact that MEPs and maximal peripheral M-waves were not altered in response to electrical brainstem stimulation suggest a cortical site of action (Kaelin-Lang et al. 2002), consistent with the lack of alteration of F-waves (Ridding et al. 2000a). No changes in motor thresholds (RMT and AMT), SICI and ICF have been found after repetitive mixed PNS, but pharmacological blockage of the effect of PNS was seen after administration of lorazepam, a positive allosteric modulator of the GABAA receptor (Kaelin-Lang et al. 2002). PNS has also been shown to increase the beneficial effects of motor training when applied for a period of 1–2 h immediately preceding the motor training period (Kaelin-Lang et al. 2005; Sawaki et al. 2006). Interestingly, proprioceptive input originating in the training motions does not appear to be sufficient to elicit substantial changes in cortical plasticity (Kaelin-Lang et al. 2005; Lotze et al. 2003).
Another form of somatosensory input is provided by low amplitude muscle vibration, which stimulates predominantly large Ia fibres and can mimic joint proprioception (Burke et al. 1976), clearly influencing excitability in somatosensory pathways (Cohen & Starr, 1985). If M1 corticospinal excitability is tested using a TMS pulse after 1 s of hand muscle vibration, MEP amplitudes are found to increase in the vibrated muscle while decreasing in adjacent non-vibrated muscles (Rosenkranz & Rothwell, 2003). Although muscle vibration certainly alters spinal excitability (Claus et al. 1988), there are associated changes in paired pulse TMS parameters which strongly suggest an effect at the level of M1: SICI targeting the vibrated muscle is reduced, while LICI is enhanced (the converse changes are seen in surrounding muscles, Rosenkranz & Rothwell, 2003). This muscle-specific surround inhibition suggests that the effects of proprioceptive input on M1 are more spatially specific at rest than those of cutaneous inputs, which give rise to less exquisitely somatotopic changes (Classen et al. 2000; Tamburin et al. 2001). Such a cortical change in response to muscle vibration is consistent with the observation in baboons that proprioceptive afferent input, unlike cutaneous input, projects directly to the motor cortex (Hore et al. 1976). Using a two-coil paired pulse approach, it has been shown that vibration of a hand muscle is also associated with stronger IHI targeting the motor cortical representation of the contralateral homologous muscle, with increased SICI and reduced MEP amplitudes in that muscle (Swayne et al. 2006).
Cutaneous anaesthesia of one hand increases MEP amplitudes in muscles immediately proximal to the deafferented hand (Ziemann et al. 1998b; Brazil-Neto et al. 1993) and in hand muscles in the unanaesthesized hand (Werhahn et al. 2002b) in the absence of excitability changes in other body part representations. This effect was blocked by a positive modulator of the GABAA receptor, lorazepam. MEPs resulting from brainstem electrical stimulation remained unchanged suggesting that the effect is probably of cortical origin. Additionally, IHI targeting the unanaesthesized hand muscles decreased during the anaesthetic procedure (Werhahn et al. 2002b). These results were interpreted as indicative that acute hand deafferentation can elicit a focal increase in cortical excitability in the hand motor representation contralateral to the deafferented cortex that is influenced by transcallosal interactions and GABAergic neurotransmission. Interestingly, these effects appeared to rebalance in the setting of chronic deafferentation following amputations. In the somatosensory domain, cutaneous anaesthesia of one hand results in focal rapid improvements in tactile spatial acuity in the opposite hand that are accompanied by increased cortical SEP amplitudes elicited by stimulation of the unanaesthesised hand (Werhahn et al. 2002b). These results are consistent with the idea that deafferentation of a cortical representation influences the homotopic representation in the opposite hemisphere; perhaps supporting the unanaesthesised hand's need to tackle enhanced environmental requirements, and is consistent with interhemispheric competition models of sensory processing. It is of relevance that these principles appear to operate also after cortical lesions like stroke, in which cutaneous anaesthesia of a healthy hand exerts beneficial effects on motor function of a paretic hand after stroke in both motor and somatosensory domains (Floel et al. 2004; Voller et al. 2005).
Cerebello-thalamo-cortical interactions
The most distant brain area over which TMS has been shown to modulate the motor cortical output is the cerebellum (summarised in Fig. 6). Cerebello-cortical (CbC) interactions in humans were originally described by Ugawa et al. (1995). They investigated how a CS over the cerebellum influences the amplitude of an MEP elicited by a subsequent TS over the contralateral M1. Either an electrical (Ugawa et al. 1991) or a magnetic cerebellar CS (Ugawa et al. 1995) resulted in a net suppression of corticomotor excitability at ISIs of 5–7 ms, as reflected in the decreased amplitude of MEPs elicited by a magnetic TS over M1 (Ugawa et al. 1995). In contrast, CbC inhibition was not observed when an electrical TS was applied over M1, suggesting an interaction upstream of the corticospinal neurons. Inhibition of TMS-induced MEP in M1 by a magnetic cerebellar CS has been consistently replicated (Pinto & Chen, 2001; Daskalakis et al. 2004). CbC inhibition can be best obtained with a double-cone coil positioned 3–5 cm lateral to the inion, with the induced current flowing upward in the cerebellar cortex (Meyer et al. 1994; Werhahn et al. 1996). The intensity of the cerebellar CS is usually set at 5–10% below AMT for direct recruitment of the corticospinal tract at the level of the foramen magnum when the double-cone coil is placed over the inion (Werhahn et al. 1996).
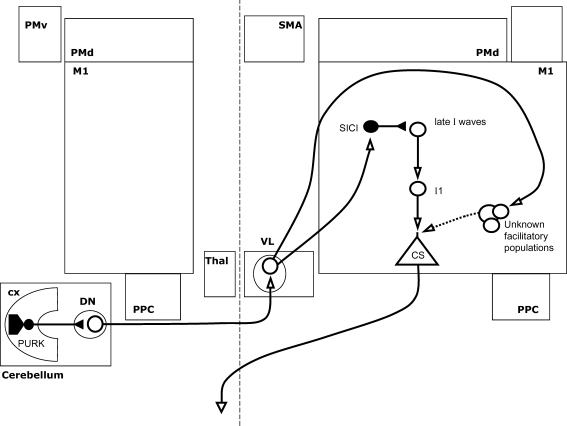
Figure 6. Cerebello-thalamo-cortical interactions modulating M1 excitability.
A magnetic stimulus over the cerebellar cortex (CX) suppresses excitability of the contralateral M1 in response to a second stimulus. This interaction is shown here: open arrows denote facilitation, while filled arrows denote inhibition. Stimulation is thought to activate inhibitory projections from the purkinje cells of the cortex (PURK) to the dentate nucleus (DN), suppressing an excitatory projection to the ventrolateral thalamus (VL), and in turn suppressing thalamocortical projections. Although M1 excitability is suppressed, short interval intracortical inhibition (SICI) is decreased in this context. While intracortical facilitation (ICF) also appears to be increased, this is thought to result from the reduced SICI rather than a change in facilitatory circuits.
A truly cerebellar origin of the suppression of M1 evoked by a magnetic CS applied over the base of the skull has been challenged. Indeed, with a flat figure-of-eight coil, a significant amount of such inhibition elicited at ISIs of 7–9 ms results from the simultaneous activation of afferent peripheral nerve fibres in the brachial plexus (Ugawa et al. 1995; Werhahn et al. 1996). This is supported by the fact that 1 Hz rTMS over the right cerebellum results in a reduction of MEPs elicited from the contralateral M1 that is comparable to the MEP reduction after 1 Hz rTMS over the posterior neck area (Gerschlager et al. 2002). However, when a double-cone coil is positioned over the base of the skull for the CS, the suppression of MEP amplitudes in a paired-pulse protocol (CbC-M1) starts at latencies similar to that of an electrical cerebellar CS, i.e. 5 ms (Liepert et al. 2004; Battaglia et al. 2006). Moreover, this MEP suppression is absent in patients with lesion of the cerebellar cortex or efferent cerebello-thalamo-cortical pathway, or when an electrical TS is applied over M1 (Ugawa et al. 1995). Therefore, the net suppression of M1 elicited at ISI 5 ms by a double-cone coil over the base of the skull has been attributed to genuine CbC inhibition.
As recently suggested by studies in patients with a variety of strokes, the key cerebellar structures involved in CbC interactions elicited by a magnetic cerebellar CS are the superior cerebellum and the dentate nucleus (Liepert et al. 2004; Battaglia et al. 2006). The dentate nucleus exerts a background tonic facilitatory drive onto the contralateral M1 through synaptic relay in the ventral lateral thalamus. This dentato-thalamo-cortical pathway is one of the many cerebello-cortical loops that specifically link cerebellar and cortical areas through dedicated channels (Middleton & Strick, 2000; Dum et al. 2002; Ramnani, 2006). The activity of the dentate nucleus is under the inhibitory control of the Purkinje cells, whose axons are the exclusive output of the cerebellar cortex. It has been proposed that a magnetic cerebellar CS activates the Purkinje cells; this results in an inhibition of the dentate nucleus that leads in turn to a disfacilitation of the contralateral M1, due to a reduction in dentato-thalamo-cortical facilitatory drive (Pinto & Chen, 2001; Daskalakis et al. 2004). However, this is still under debate as the short ISIs of ∼5 ms to elicit inhibition of the dentate nuclei would necessitate an extremely fast inhibitory system. Moreover, data from rTMS studies over the cerebellum inconclusively showed a reduction (Fierro et al. 2007) or an increase (Oliveri et al. 2005) of ICF in M1, but no changes in SICI. However, as reviewed above, the origin of ICF at shorter interstimulus intervals may be mediated by subtle changes in spinal excitability, further supporting the possibility of peripheral effects of magnetic stimulation of the cerebellum. One should remark that the use of a flat figure-of-eight coil in these studies would promote simultaneous activation of afferent peripheral nerve fibres in the brachial plexus (Ugawa et al. 1995; Werhahn et al. 1996) and therefore might lead to this result. Thus, a specific inhibitory effect of rTMS on the dentate-thalamo-cortical pathway has yet to be proven.
CbC inhibition is more pronounced for small test MEPs (0.5 mV) elicited by slightly suprathreshold TS than for large test MEPs (2 mV) (Ugawa et al. 1995; Pinto & Chen, 2001). This may reflect either a preferential inhibition of the neuronal elements generating the I1 wave (which have a lower threshold than the D-wave and later I-waves) or the fact that the dentato-thalamo-cortical pathway projects predominantly to the core of cortical muscle representations, where the motor threshold may be lower (Pinto & Chen, 2001). The cell populations within M1 targeted by these projections are likely to include both pyramidal cells and inhibitory interneurons (Shinoda et al. 1993; Daskalakis et al. 2004).
CbC interactions with the intracortical populations mediating SICI, ICF and LICI have been tested by Daskalakis et al. (2004), who used a triple-pulse TMS protocol with small adjustments of the test MEP amplitudes. A magnetic cerebellar CS reduces SICI in the opposite M1, most likely through reduced facilitatory dentato-thalamo-cortical drive to intracortical inhibitory interneurones. This reduction in SICI may shift the intracortical balance of excitability toward excitation, leading to the observed increase in ICF. Finally, in the presence of LICI, CbC inhibition is decreased. The mechanism of this interaction is unclear and could result either from a saturation effect if LICI and CbC inhibition converge onto the same population of cortical inhibitory interneurons, or alternatively from changes in subcortical excitability.
Further evidence for the presence of a tonic facilitatory drive from the dentate-thalamo-cortical pathway onto M1 in healthy humans is provided by studies in patients with cerebellar stroke or degeneration. These have consistently demonstrated an increased RMT in the contralateral M1 (as well as increased SICI and decreased ICF) (Liepert et al. 2004; Battaglia et al. 2006).
Interrupting tonic contraction: silent periods
Contralateral silent period
In a voluntarily contracted muscle, the MEP elicited by a single suprathreshold TMS pulse is followed by a period of EMG inhibition called the contralateral silent period (CSP) (Fuhr et al. 1991). While there is evidence that the early part of the CSP is mediated by spinal mechanisms, the later part is thought to result from suppression of neural output by interneurons at the cortical level (Fuhr et al. 1991; Tergau et al. 1999). Cracco et al. (1989) have shown that cortical stimulation excites inhibitory interneurons (probably Golgi-II cells with long axons) connected to the pyramidal cells, thus decreasing corticospinal neuron firing (Cracco et al. 1989). The CSP duration is greater following an antero-posterior and biphasic stimulus than a postero-anterior stimulus, and correlates strongly with the amplitude of the evoked MEP, raising the possibility that it may depend on activity in recurrent collaterals from discharging pyramidal tract neurons (Orth & Rothwell, 2004). The CSP has been reported to be prolonged following administration of either oral tiagabine (a GABA re-uptake inhibitor) or intrathecal baclofen (a GABAB agonist), suggesting that the CSP, like LICI, may be mediated by GABAB populations (Siebner et al. 1998; Werhahn et al. 1999). However, this effect of baclofen was not replicated in studies using oral (McDonnell et al. 2006) or intravenous administration (Inghilleri et al. 1996).
The study by Werhahn et al. (1999) also provided evidence of a reciprocal relationship between CSP and SICI, in that tiagabine increased CSP duration while weakening SICI. Daskalakis et al. (2006) used rTMS in order to evaluate the effects of several different stimulation frequencies (1, 10 and 20Hz) on SICI and CSP (Daskalakis et al. 2006). They showed that the rTMS-induced change in SP was associated with a change in SICI and this inverse relationship was greatest in the highest stimulation condition (i.e. 20 Hz). Recently, this interaction was explored in a human study by Ni et al. (2007). The authors used a triple-pulse protocol, investigating SICI and ICF during different time points of the CSP. While SICI was decreased (80–140 ms following the stimulus that induced the CSP) ICF was increased, followed by normalization of both parameters after termination of the CSP. Since the lack of SICI was already present at low CS intensities (∼60% aMT) and the threshold of inhibitory interneurons is known to be lower than that of facilitatory interneurons (Chen et al. 1998), the decrease of SICI during the CSP is likely to be due to a decreased inhibition rather than increased facilitation. This relationship may be seen as analogous with the suppression of SICI in the presence of LICI, another GABAB-mediated phenomenon (Sanger et al. 2001), and is consistent with previous lines of evidence from both animal and human studies demonstrating that activation of presynaptic GABAB receptors inhibits further release of GABA (Deisz, 1999). Interestingly, the inhibition of SICI by LICI was also observed during the CSP (Ni et al. 2007), further supporting this hypothesis.
Ipsilateral silent period
Application of a single suprathreshold TMS pulse to the M1 ipsilateral to a tonic voluntary contraction can cause an interruption of the ongoing voluntary EMG activity known as the ipsilateral silent period (iSP), even in the absence of an ipsilateral MEP (Ferbert et al. 1992; Meyer et al. 1995). Several lines of evidence suggest that the iSP is mediated by fibres passing through the corpus callosum: iSPs were absent or delayed in patients with agenesis or surgical lesions of the corpus callosum (Meyer et al. 1995), but were preserved in patients with subcortical cerebrovascular lesions that interrupted the corticospinal tract but spared the corpus callosum (Boroojerdi et al. 1996). In addition, in young children the iSP is significantly shorter than in adults; the protracted development and myelination of the corpus callosum are paralleled by the appearance and strengthening of the iSP (Heinen et al. 1998).
Several studies have investigated the iSP's relationship to IHI (Ferbert et al. 1992; Chen et al. 2003). Chen et al. (2003) have examined the effects of different stimulus intensities and current directions on the two forms of interhemispheric inhibition. They showed that paired-pulse IHI measured with a 40 ms ISI, both at rest and during muscle activation, significantly correlated with iSP duration for some of the stimulus intensities and current directions tested, while IHI at an ISI of 8 ms and iSP did not correlate under any of the experimental conditions. These results suggest that while common neuronal populations may mediate IHI40 and iSP, the same is not true of IHI8. Thus iSP and IHI8 are separate phenomena, mediated perhaps through different sets of transcallosal fibres or, alternatively, different sets of effector neurons in the contralateral ‘target’ M1. The duration of the iSP can be modulated by a CS delivered to the stimulated hemisphere in a paired pulse protocol: the iSP can be suppressed by a subthreshold CS delivered 3 ms before the suprathreshold TS (Trompetto et al. 2004) or enhanced by a CS at motor threshold intensity delivered 1.5 or 3 ms after the TS (Avanzino et al. 2007). These are the same protocols used to elicit SICI and SICF, respectively, implying that the iSP is conditioned in a similar manner to the contralateral corticospinal output – the cell population giving rise to the transcallosal projection mediating the iSP seems therefore to be subject to similar modulation to the pyramidal output, although they do not necessarily have to be the same population.
State-dependent intra- and interhemispheric interactions
We have so far described different physiological interactions which modulate the output of M1 while the system is at rest, defined as muscle relaxation (except for the silent periods). It may be expected that the behaviour of these interactions should change depending on the behavioural state. If these parameters play a functional role in motor control then one may expect changes when subjects engage in preparation or performance of a motor task. Such movement-related changes have indeed been described for a number of these interactions, but there are still plenty of unknowns.
Changes affecting M1 during movement preparation
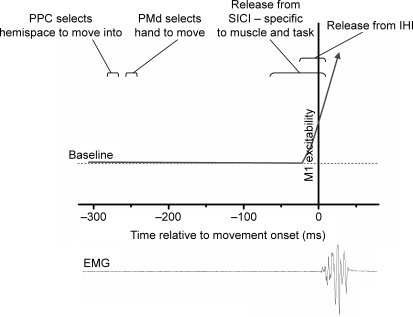
Corticospinal and intracortical parameters can be assessed in the context of reaction time protocols, providing a picture of changing physiological interactions leading up to movement execution. Using single pulse TMS applied at a number of time points after a ‘Go’ cue (in a simple reaction time protocol), three studies have described a gradual increase in corticospinal excitability starting 80–120 ms prior to movement onset (Rossini et al. 1988; Leocani et al. 2000; Nikolova et al. 2006). In the study of Leocani et al., this finding was accompanied by a suppression of MEPs in the contralateral resting hand (if the dominant right hand was being moved). The role of excitability changes in the α-motorneuron pool, which was not studied in detail in these early investigations, was evaluated more recently. It appears that the true ‘lead time’ between premovement excitability increases in the motor cortex and the spinal cord may be of the order of 10–15 ms: shorter than previously thought (MacKinnon & Rothwell, 2004; Schneider et al. 2004). This faster build-up of motor cortex excitability is perhaps not surprising when one considers that healthy volunteers may have a total reaction time of around 100 ms. Recent work suggests that motor cortical excitability is also modulated by the expectancy of the need to make a movement. In an elegant version of the simple reaction time task (SRTT), van Elswijk and colleagues manipulated the interval between a preparatory stimulus and a response stimulus in order to create four time intervals at which subjects had various expectancies of the likelihood of a cue to move. Not only were reaction times shorter with high cue expectancy (relative to intervals with a low expectancy), but MEP amplitudes to a single TMS pulse were also increased (van Elswijk et al. 2007). Thus it would seem that premovement modulation of M1 excitability is exquisitely sensitive to the precise nature of the upcoming task, and is modulated in advance of expected movements.
Paired pulse TMS can be used in a similar manner to investigate intracortical excitability changes in relation to movement. Reynolds & Ashby (1999) demonstrated that SICI begins to decrease approximately 95 ms prior to the onset of a phasic movement, and that this change is seen in the agonist but not antagonist muscle groups. As a local intracortical phenomenon, SICI would be well placed to modulate the relationship between adjacent intracortical representations via changes in horizontal connections. It was thus speculated that the reduction in SICI could contribute to the focal increase in corticospinal excitability affecting the target muscle (Reynolds & Ashby, 1999). Conversely, SICI targeting a neighbouring uninvolved hand muscle may become stronger in some subjects when tested in relation to phasic finger movements (Stinear & Byblow, 2003a). SICI also increases after a no-go signal in a go/no-go reaction task protocol (Sohn et al. 2002). These results are consistent with a role of SICI in actively suppressing execution of prepared movements. A comparison of synchronized versus syncopated externally paced finger movements has also suggested that movement-related SICI changes may be task dependent (Byblow & Stinear, 2006).
The precise timing of SICI changes has been recently investigated in a simple reaction time protocol, revealing that inhibition is in fact stronger more than 70 ms before movement onset, but is then progressively abolished relative to rest (Nikolova et al. 2006). A trend was also observed for ICF to become weaker from 150 ms before movement. This reduction in inhibition is likely in fact to occur closer to the onset of movement than described here, as this study did not test for early subtle increases in spinal excitability. With this in mind, it may be the case that the reduction in SICI occurs alongside (or later than) the increase in MEP amplitudes. If so, this would suggest that SICI modulation is unlikely to drive the corticospinal excitability increase, but may serve to focus it appropriately to the task. There is a further inherent difficulty in this kind of experiment in that premovement MEP facilitation may distort the degree to which apparent SICI in fact reflects activity in the inhibitory population. While the phasic movement experiments of Stinear & Byblow (2003b) make efforts to correct for this, it would be technically very challenging to do so across a range of time points – it is possible that such a consideration may affect the changes reported by Nikolova and colleagues. In the case of phasic movements the question arises as to whether afferent feedback, known to focally reduce SICI (Rosenkranz & Rothwell, 2003), may be responsible for the observed changes. However, reduced SICI has been observed during imagined thumb abduction movements, suggesting that afferent feedback is not necessary to produce these changes (Stinear & Byblow, 2003b). Thus it seems likely that both motor drive and afferent feedback may contribute to movement-related modulation of SICI.
Using a two coil approach it is also possible to test the activity of inter-regional interactions during movement preparation. If tested in a simple reaction time protocol, IHI targeting the moving hand is reversed to become IHF in the period immediately before movement onset (Murase et al. 2004). This effect is more prominent when tested for IHI targeting the dominant hand (Duque et al. 2007). It was suggested that this reversal of tonic inhibition may allow for accuracy of movement when the hands need to be used separately. In view of the importance of bimanual control in primate evolution it could be speculated that the role of interhemispheric interactions between the hand areas may differ between unimanual and bimanual tasks, but this has not been directly tested yet. Indirect evidence that this may be the case is provided by studying changes in MEP amplitudes (in response to a single TMS pulse) in a hand muscle before a bimanual movement. MEPs increase or decrease during this period depending on both the agonist–antagonist and kinematic relationships between the two moving fingers (Duque et al. 2005). This suggests that information describing such relationships is coded at the level of M1, but does not directly support a role for IHI/IHF in this process.
A similar approach has been employed to investigate activity in the inhibitory and facilitatory interhemispheric PMd–M1 interactions described above during a choice reaction time task (Koch et al. 2006). During movement preparation these investigators found a crucial timing dependence: the facilitatory effect on MEP amplitude was evident 75 ms after the cue if the target hand was being moved (but not the contralateral hand), while the inhibitory effect was evident 100 ms after the cue if the contralateral hand was being moved (but not the target hand). Interestingly, both the inhibitory and facilitatory influences of PMd on M1 were absent at all other time intervals before movement. The authors speculated that the reaction cue may initially cause both left and right hand movements to be specified, with the incorrect movement being eliminated at a later stage. The expectation of the need to move may thus cause the interhemispheric interactions to be suppressed, only for the relevant interaction to become active during the appropriate premovement time window. Thus, the left PMd exerts a brief facilitatory or inhibitory influence on the right M1 depending on which hand is to be selected to move, supporting a role for the left PMd in movement selection. This dependence of the interhemispheric PMd–M1 interaction on the motor state is in keeping with recent work which used the effect of a TMS input on haemodynamics in remote areas (during fMRI imaging) to assess functional connectivity. This approach also demonstrated that the PMd–M1 interaction is inhibitory at rest but facilitatory during movement preparation (Bestmann et al. 2007). A recent paper by Davare et al. (2006) supports this point of view, demonstrating that 1 Hz rTMS over the left PMd impairs movement preparation as tested in a pinch-lift task.
The brief periods of activity in the PMd–M1 interactions (Koch et al. 2006) occur considerably earlier than the excitability changes within M1 described above (although simple and choice reaction time protocols are being compared). This is consistent with the relative timings of the two regions as revealed by attempting to prolong reaction times with a short train of repetitive TMS. This delayed responses if given early in the reaction time to PMd or if given later to M1 (Schluter et al. 1998). However, it should be noted that this result refers to the PMd ipsilateral to the active M1, whereas the PMd–M1 interaction tested by Koch et al. (2006) conditioned the contralateral PMd. The time course of the ipsilateral PMd–M1 interaction described by Civardi et al. (2001) would be interesting in this regard, but has not been investigated.
A time-dependent facilitation has also been described between the right posterior parietal cortex (PPC) and the ipsilateral M1 in a choice reaction time task (Koch et al. 2007). Significant facilitation, equivalent to that seen at rest, was seen 50 ms after the ‘Go’ cue but not at other premovement time intervals. This occurred if the subject was preparing to move towards the left hemispace but not if movement was towards the right, suggesting that the PPC may play a role in directional planning early on in movement.
There is indirect evidence that the cerebellum exerts an influence on M1 during movement preparation: the premovement facilitation normally observed in response to M1 stimulation is reduced in patients with spinocerebellar degeneration (Nomura et al. 2001). Furthermore, abnormally diffuse movement-related cortical potentials can be demonstrated prior to movement in patients with stroke affecting the contralateral cerebellum, a finding that resolves with clinical improvement (Gerloff et al. 1996). It has been suggested that cerebello-thalamo-cortical projections to M1 intracortical inhibitory interneurons may dynamically focus the motor output through regulation of surround cortical inhibition (Pinto & Chen, 2001). However, CbC interactions have not been studied during movement preparation, so further evidence is necessary before such conclusions can be drawn.
From the work described above, a picture begins to emerge of the changes in interactions targeting M1 that lead up to the execution of a movement – this is illustrated in Fig. 7. The timings shown are likely to be approximate, as subtle changes in spinal excitability were not tested in several of the studies cited. It should also be emphasised that the interactions involving PPC and PMd are based on choice reaction time experiments, whereas the other changes shown were in the context of a simple reaction time task. While the PPC and PMd should make more decisive contributions in the choice reaction time protocol, the sequence of events is likely to be similar: a directional selection (PPC), then a hand selection (PMd), leading to increasing excitability in the relevant M1, which is then refined by focused task-specific changes in SICI (and IHI), followed finally by movement onset. Changes in these interactions may be seen as preparatory tuning of the motor output before release: this is likely to occur at a variety of intervals depending on the advance information available about the movement being planned. This model describes movement preparation only in terms of the interactions for which such experiments have been performed. Changes may occur in many or all of the other M1 interactions described, and this is a major gap in current knowledge. Perhaps more importantly, it should be kept in mind that the validity of this model is likely to vary widely depending on the particular behavioural setting.
Figure 7. Changes affecting M1 excitability during movement preparation.
The increase in excitability of M1 in response to a magnetic stimulation before movement onset is shown by the blue line, increasing from approximately 100 ms prior to the onset of muscle activity. Above, an approximate sequence of facilitatory inputs from the posterior parietal cortex (PPC) and dorsal premotor cortex (PMd) are shown, followed by a reduction of short interval intracortical inhibition (SICI) and abolition of interhemispheric inhibition (IHI) from the contralateral M1. It should be noted that while the experiments represented all involved physiological measurements during a reaction time, the timings shown relate to a variety of types of cue and task and therefore may not be accurate in relation to each other (see text). The inputs from the PPC and PMd may select the hemispace to be moved into and the hand to move, respectively. It may also be speculated that the relatively late reduction in SICI, which is muscle group specific, allows the increasing excitability to be focused appropriately before the onset of activity.
Changes during muscle contraction
Corticospinal excitability to single pulse TMS is increased in the agonist muscle during established tonic contraction (Hess et al. 1986). This is felt to result from a combination of increases at the cortical and spinal levels. Furthermore, changes in the response of the contralateral homologous muscle have been documented, with the direction of the effect (increase or decrease) depending on the level of voluntary contraction as well as the type of muscle contractions. While low force levels produced by one hand lead to a decrease in MEP amplitude in the homologous muscle (Liepert et al. 2001), high force levels (25–50% MVC) lead to increased responses (Hess et al. 1986; Muellbacher et al. 2000). Increased responses are seen even in patients with agenesis of the corpus callosum (Meyer et al. 1995) suggesting increased excitability at the spinal level. However, the simple reaction time study of Leocani et al. (2000) reported suppression of responses in the contralateral hand soon after the onset of movement. This was observed only in the non-dominant hand during movements of the dominant hand. IHI is likewise stronger from the dominant to the non-dominant hemisphere than the other way around (Netz et al. 1995), so it may be that there is initial transcallosal suppression of contralateral excitability followed by a later phase of spinal facilitation, at least for the tasks in which these parameters have been evaluated. Both SICI and ICF are significantly weaker during tonic contraction of a hand muscle than at rest – this effect is relatively focal in that while it is also observed in neighbouring hand muscles it does not apply to more proximal arm muscles (Ridding et al. 1995).
During slight tonic contraction, IHI targeting the active hand (tested using a suprathreshold CS to the contralateral M1) is unchanged, whereas that targeting the contralateral resting hand becomes slightly stronger (Ferbert et al. 1992). The effect of preactivation of the target muscle has also been investigated using stimulation parameters that produce IHF at rest (CS of 80% AMT, small target MEP amplitudes). Under these conditions, facilitation is still seen at an ISI of 8 ms but is absent at 6 ms (Baumer et al. 2006).
The intrahemispheric PMd–M1 inhibition observed by Civardi et al. (2001) was significantly weaker if tested with slight tonic preactivation of the target muscle. Likewise, under conditions that produce interhemispheric PMd–M1 facilitation at rest, this effect is abolished or masked by preactivation of the target muscle (Baumer et al. 2006). However, in both of these studies lower TS intensities were used in the active state (in order not to produce larger baseline MEPs), making it difficult to rule out altered I-wave profiles in the test MEP as a possible cause of the observed reduced effect of the CS. Under conditions that at rest produce inhibition in the interhemispheric PMd–M1 interaction, Mochizuki et al. (2004a) investigated instead the effect of preactivating the homonymous muscle contralateral to the target muscle (i.e. contralateral to the CS) – unlike IHI between the motor cortices, this manoeuvre did not affect the PMd–M1 interaction. So for these interhemispheric interactions, facilitation has been tested targeting the active hand, whereas inhibition has been tested targeting the resting hand – the effects of preactivating the conditioning and target hemispheres remain to be determined.
When considering the changes observed in M1 output during muscle contraction it must be considered whether sensory afferent input may make a contribution. In the context of a muscle contraction it is difficult to separate the effects of motor drive from those of the resulting proprioceptive feedback. The physiological changes described above that can be induced by muscle vibration may, however, provide information about the proprioceptive component originating from muscle spindles. In fact, the effect of vibration on the contralateral M1 is strikingly similar to that of tonic contraction – a focal increase in corticospinal excitability and decrease in SICI, with surround inhibition (Rosenkranz & Rothwell, 2003). Like tonic contraction, vibration also increases IHI targeting the contralateral homologous muscle (Swayne et al. 2006). While this may contribute to the IHI change seen with contraction, motor drive is likely to be important as well, as corticospinal excitability in the contralateral M1 is reduced with vibration (whereas it is increased in tonic contraction). LAI and SAI resulting from a peripheral nerve stimulus can be tested in the context of a movement, such that the test TMS stimulus is given soon after the onset of a voluntary movement. At rest LAI obtained from cutaneous stimulation acts not only on muscles affecting the stimulated finger (homotopic LAI) but also on neighbouring muscles (heterotopic LAI): during movement both forms of LAI affecting the resting digit are enhanced, while those affecting the moving digit are abolished (Voller et al. 2005). In a similar protocol, homotopic SAI is enhanced during movement in the resting digit and absent in the moving digit, while heterotopic SAI is absent altogether during movement (Voller et al. 2006). Thus the changes in afferent inhibition in the context of movement also appear to demonstrate properties of surround inhibition, focusing the inhibitory effect onto neighbouring resting muscles while disinhibiting the moving muscle. This could be explained in theory either by afferent inhibition causing surround inhibition or alternatively being shaped by it. It is clear from the case of muscle vibration that sensory afferent input on its own can drive surround inhibition, so it seems reasonable that the same might apply to SAI/LAI. This has not been investigated in detail for SAI/LAI induced by mixed nerve stimulation during movement.
Cerebello-cortical inhibition, demonstrated in the FDI at rest, is absent during voluntary isometric contraction of the target muscle. This effect may result from a reduction in cerebello-thalamo-cortical pathway excitability or alternatively from a decreased susceptibility of M1 to CbC inhibition during contraction of the target muscle (Pinto & Chen, 2001). Whereas CbC targeting the right FDI is unaffected by proximal voluntary contraction of the left arm, it is abolished by isometric contraction of the right arm. This effect can be attributed either to a decreased excitability of the Purkinje cells or to reduced activity of the dentato-thalamo-cortical pathway during proximal muscle contraction.
Physiological changes resulting from training
Repetitive training in a simple motor task results in changes in M1 excitability that are well documented. Focally increased MEP amplitudes (specific to the trained muscle) can be induced in as little as 30 min of training (Classen et al. 1998; Butefisch et al. 2000; Muellbacher et al. 2001). The direction of a thumb movement in response to a single TMS pulse can also be altered by training over a similar period, suggesting that motor practice can influence directional coding at the level of M1 (Classen et al. 1998). Because changes identified with TMS were not present with transcranial electrical stimulation indicating an intracortical substrate (Classen et al. 1998) and because GABAergic agents dampened this form of plasticity (Butefisch et al. 2000), modulation of excitability in horizontal connections such as those reported following motor training in rats (Rioult-Pedotti et al. 1998) may represent a contributory mechanism. Conversely, low frequency 1 Hz TMS applied to M1 immediately after practice resulted in disruption of improvement in motor performance in a pinch-grip task. The disruptive effect was specific for M1 and was not found when stimulating the visual cortex indicating that M1 is involved in the early phase of motor consolidation (Muellbacher et al. 2002).
ICF is increased following training of a task involving simple repetitive wrist movements (Lotze et al. 2003) but no change in ICF is detected after a complex sensorimotor task (McDonnell & Ridding, 2006). Clear changes in SICI can also be induced by motor training. Liepert et al. (1998) observed that performance of repetitive thumb movements induced a global reduction in SICI targeting hand muscles (Liepert et al. 1998). If subjects were also instructed to keep the fourth dorsal interosseus muscle relaxed throughout, then SICI targeting this muscle increased, while still decreasing in the thumb (APB). Thus, changes in SICI can be muscle and task specific. A similar SICI reduction has been observed in leg muscles following skilled (but not un-skilled) training in an ankle movement task (Perez et al. 2004). It is tempting to conclude from these studies that during acquisition of a new motor skill a reduction in GABAergic inhibition may somehow facilitate an increase in the strength of horizontal cortico-cortical connections, as has been observed in rat motor cortex (Rioult-Pedotti et al. 1998), but a role for SICI in this process is yet to be directly demonstrated.
Long-term changes of intracortical excitability after repeated training (months to years) have also been reported: both ICF and SICI are less strong at baseline in musicians, who have undergone extensive training in complex finger movements than in non-musicians (Nordstrom & Butler, 2002), and ICF can be increased in pianists following training in an unfamiliar piece of music (D'Ausilio et al. 2006). Reduced baseline ICF in this context may arguably be seen as allowing greater scope for increase in response to training, while weak SICI may facilitate such changes. It is interesting in this regard that musicians' dystonia, a condition regarded as a form of maladaptive plasticity, is associated with weak SICI (Rosenkranz et al. 2005). A more recent study with musicians examined SICI across a range of CS intensities and found that musicians had stronger SICI than non-musicians at higher intensities (Rosenkranz et al. 2007). The apparent discrepancy between this result and that of Nordstrom & Butler (2002) may be due to the fact that the earlier study tested SICI at only one conditioning stimulus (CS) intensity, which was fixed relative to the active motor threshold. Comparison of the two studies reveals that they recorded markedly different motor thresholds in the musician groups: with only one CS it is impossible to know whether the study of Nordstrom & Butler was testing SICI in a different part of the CS curve from the later study. The study of Rosenkranz et al. (2007) also found steeper MEP recruitment curves and greater changes in excitability following a paired associative stimulation protocol. The authors argue that the long-term effects of intensive motor practice may be to increase the capacity for training-related synaptic modification. However, it should be kept in mind that these results may represent epiphenomena of differences in anatomical structures between musicians and non-musicians (Gaser & Schlaug, 2003). Furthermore, differences in baseline skills at the time of the testing, genetic predispositions or simply endophenotypic traits may also contribute to interindividual differences.
In view of these intracortical training effects, it may be expected that interhemispheric interactions between the motor cortices would be modulated by training that relies on accurate interhemispheric coordination. Indirect evidence that this might be the case is provided by the experiments of Shim et al. (2005), in which subjects were asked to practise an unfamiliar bimanual task (Shim et al. 2005). Prior to practice, slight tonic contraction (max 15% MVC) of the training hand produced inhibition of MEP amplitudes (to a single pulse) in the non-training hand, whereas after practice this inhibition was selectively reduced in fingers involved in the task. A further hint that IHI as tested with the paired pulse technique may be involved in bimanual training is provided by the observation that it is significantly weaker in musicians than in non-musicians (Ridding et al. 2000b). However, a direct demonstration of a change in IHI with bimanual training is so far lacking.
One fundamental skill that human and non-human primates have is the ability to execute with one hand a task that was learned with the opposite hand, referred to as intermanual transfer (Halsband & Lange, 2006). Recent evidence points to a role for IHI in the intermanual transfer of procedural motor learning (Perez et al. 2007). In this study, subjects were trained in a unimanual serial reaction time task, resulting in performance improvements in not only the trained hand but also the untrained hand. Training was associated with reductions in SICI in either hemisphere, but intriguingly decreased IHI targeting the untrained hemisphere correlated with performance improvements in the untrained hand, that is, with the magnitude of the intermanual transfer function (Perez et al. 2007).
Discussion
It may be appreciated from the previous sections that the amount of information available and our understanding of the interactions shaping the characteristics of motor cortical output have advanced considerably in recent years. Despite these advances, much more work is required to fully understand the cortical mechanisms underlying human motor control. While the review component of this paper focuses to a larger extent on what is known in the field of TMS, the discussion will focus on what is not known, possible pitfalls in interpretations of TMS data, and interesting research directions that emerge from the described work.
Caveats in the interpretation of these studies
First, it is important to point out that direct evidence proving the anatomical substrates of the interactions modulating the output of M1, as shown for example in Fig. 1, is largely missing. Wiring diagrams of the sort presented here, and seen frequently in published material, are useful in that they describe the relationships between the several phenomena that TMS is able to probe, and they further provide experimental models against which new hypotheses can be more easily formulated and tested. Also, these models provide new investigators with a comprehensive description of other interactions to be controlled for in future experiments. While some of the connections depicted in the figures are based on evidence sound enough to make educated guesses as to the neural structures involved, others are speculative and require specific testing. The reader is warned then to interpret these diagrams as works in progress, useful in summarising data already available and posing future questions. The overlapping phenomena of SICI and SICF, for example, are ascribed to the differential actions of low threshold inhibitory or high threshold excitatory interneuron populations, depending on the conditioning stimulus intensity used. However, although anatomical candidates for these populations are present in the motor cortex, there is insufficient evidence to confidently assign these phenomena to particular neuron types. Interneurons may be dedicated to individual kinds of interaction, as the wiring diagrams suggest, or alternatively may be able to exert more than one kind of influence on the pyramidal cell depending on the input received. Likewise, the interhemispheric interactions discussed above are described as consisting of excitatory transcallosal projections onto inhibitory or excitatory local circuits within the target M1. This fits with currently available neuroanatomical data (largely based on animal work) and would explain observed findings, but direct evidence is still lacking. It should also be remembered in this regard that apparent changes in excitability as measured by TMS do not necessarily reflect actual changes in neural activity within the target populations (Similar limitations apply for BOLD fMRI or blood flow studies). A neuron which is partially depolarised but has not reached firing threshold may appear to have increased in activity in response to a TMS stimulus while not actually being physiologically active. Thus while excitability to the artificial input of TMS is likely to be highly correlated with neuronal activity, the two are not necessarily equivalent. A recent study by Allen et al. (2007) addresses this issue in more detail.
Secondly, evidence from recent studies clearly points to fundamental differences in the way these intracortical interactions operate in the setting of different behavioural or cognitive tasks or motor states. In other words, the review of the literature is consistent with the view that each behavioural state may condition fundamentally different cortical interactions (Allen et al. 2007). Therefore, care should be taken to avoid generalising conclusions on the role of specific inhibitory or excitatory processes to tasks or behaviours beyond those specifically studied. Clearly, more work is needed to understand the extent to which task-specificity influences the direction and magnitude of changes in the various TMS measures described above.
Finally, the specific relationship between physiological changes and motor behaviour remains elusive. Most studies have focused on describing the association between specific neurophysiological changes and behavioural modifications. When changes in physiology and behaviour correlate such findings are often interpreted as suggestive of a beyond association link. However, it should be kept in mind that these findings do not prove a cause-and-effect connection between the physiological change and the motor behaviour. This is a gap that has not yet been crossed in most studies, except when using TMS as a tool to elicit ‘virtual lesions’ (O'Shea et al. 2007; Cohen et al. 1997). Still more work is needed to prove that the associations between specific functional interactions and behaviour represent more than mere epiphenomena of the specific behaviour. The design of these types of experiments represents one of the crucial challenges ahead of us.
Necessary controls
The studies reviewed have over time shown an increasing degree of sophistication, attention to detail and effort to control for confounding variables. This is quite important to keep in mind. For example, from a technical point of view, for all the interactions that may be illustrated by paired pulse TMS protocols the intensities used for the conditioning and test stimuli are crucial to the outcome. This is true of intracortical interactions: if two TMS pulses are applied to M1 separated by 2.5 ms, for example, their relative intensities will determine whether SICF or SICI is elicited (Fisher et al. 2002; Roshan et al. 2003). This consideration is also crucial in inter-regional interactions, where careful changes in CS and TS intensities can change IHI into IHF (Baumer et al. 2006). This serves to underline the important fact that paired pulse TMS studies by no means provide a clean measure of an isolated neural population – rather they describe the outcome of an interaction representing the net effect of several overlapping influences. It is also worth bearing in mind that the relative ease with which physiological interactions may be elicited in a TMS experiment does not necessarily reflect the relative importance of their roles in vivo: while IHI can be more readily elicited than IHF, for example, this may reflect the way these parameters are tested rather than aspects of their function. This consideration of overlapping interactions has important implications for investigators planning to measure the changes in a particular interaction with regard to a physiological or behavioural manipulation. In order to extract the true behaviour of the neural population of interest it may not be enough to use one set of CS and TS intensities. The safest approach to this potentially confounding problem is to study a range of intensities, providing a recruitment curve for the activity of that interaction. Examples of studies that included this approach are a study of SICI after stroke (Butefisch et al. 2003) and another study of intermanual transfer of procedural knowledge in healthy subjects (Perez et al. 2007).
From a behavioural point of view, when considering the activity of a given physiological measure, it is essential to take the particular motor or behavioural state into consideration. Recent work suggests that monitoring for muscle activity at the time of measurement, while useful, may not be enough. For example, motor activity is absent when the subject is fully at rest, but also in the milliseconds preceding a voluntary movement. Despite the comparable absence of EMG activity, the cortical excitability in the two conditions is fundamentally different. The magnitude and sign of the physiological interactions described changes well in advance of movement onset and may vary with respect to the task being performed (Duque et al. 2005), the hemispace involved (Koch et al. 2007) and even the emotional context (Oliveri et al. 2003). An investigator studying changes in physiological interactions with regard to movement must thus ensure that such aspects of the motor state have been taken into account and adequately controlled for.
Concluding remarks
The last few years have seen an increasing sophistication and detail in the characterisation of the role of different intracortical interactions in motor control. This review, performed by fellows in two active laboratories in the field, has focused on the description of these novel findings and, perhaps more importantly, on warning upcoming students and investigators (even ourselves) of potential pitfalls in the interpretation of these data and some exciting trends for future investigations. In other words, as in other areas of science, it is very important to know what we ‘cannot conclude’ from an otherwise technically flawless data set. Altogether, the available literature points to a view of M1 that is far from the passive servant of higher motor structures. To the contrary, it appears to perform a complex integration of multiple influences, originating in both cerebral hemispheres, in a role as an ultimate gate-keeper that is carefully and differentially tuned to generate well defined motor behaviours.
Acknowledgments
This work was supported by the NIH/NINDS intramural program (JR, MHL, MP, MC, MAD, PR, YV and LGC). OBS was supported by the Patrick Berthoud Charitable Trust, JR was supported by the Alexander von Humboldt-Foundation Germany, YV was supported by a grant from the FSR (Fond Spécial de Recherche) of the Université catholique de Louvain (UCL, Brussels), PR was supported by the German research Foundation (Ra 1391/1-1).
References
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ. Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:401–416. doi: 10.1016/0168-5597(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Quartarone A, Ghilardi MF, Dattola R, Bagnato S, Rizzo V, Morgante L, Girlanda P. Unilateral cerebellar stroke disrupts movement preparation and motor imagery. Clin Neurophysiol. 2006;117:1009–1016. doi: 10.1016/j.clinph.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Baumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, Munchau A. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572:857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsolateral premotor cortex exerts task-dependent causal influences on activity in contralateral primary and secondary motor cortex. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm159. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Byblow WD, Stinear CM. Modulation of short-latency intracortical inhibition in human primary motor cortex during synchronised versus syncopated finger movements. Exp Brain Res. 2006;168:287–293. doi: 10.1007/s00221-005-0205-9. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chen R, Curra A. Measures of cortical inhibition in health and disease. Clin Neurophysiol. 2004;57(Suppl.):691–701. doi: 10.1016/s1567-424x(09)70409-x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Matsunami KI. GABA-B-related activity in processing of transcallosal response in cat motor cortex. J Neurosci Res. 2002;68:489–495. doi: 10.1002/jnr.10223. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Claus D, Mills KR, Murray NM. The influence of vibration on the excitability of alpha motoneurones. Electroencephalogr Clin Neurophysiol. 1988;69:431–436. doi: 10.1016/0013-4694(88)90065-x. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Starr A. Vibration and muscle contraction affect somatosensory evoked potentials. Neurology. 1985;35:691–698. doi: 10.1212/wnl.35.5.691. [DOI] [PubMed] [Google Scholar]
- Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417–424. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- D'Ausilio A, Altenmuller E, Olivetti BM, Lotze M. Cross-modal plasticity of the motor cortex while listening to a rehearsed musical piece. Eur J Neurosci. 2006;24:955–958. doi: 10.1111/j.1460-9568.2006.04960.x. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Darian-Smith I. Thalamic projections to areas 3a, 3b, and 4 in the sensorimotor cortex of the mature and infant macaque monkey. J Comp Neurol. 1993;335:173–199. doi: 10.1002/cne.903350204. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA. GABAB receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/s0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:555–559. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol. 2005;569:315–323. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- DiLazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- DiLazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–447. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Floel A, Nagorsen U, Werhahn KJ, Ravindran S, Birbaumer N, Knecht S, Cohen LG. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Jones EG. Thalamic input to areas 3a and 2 in monkeys. J Neurophysiol. 1981;45:59–85. doi: 10.1152/jn.1981.45.1.59. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Altenmuller E, Dichgans J. Disintegration and reorganization of cortical motor processing in two patients with cerebellar stroke. Electroencephalogr Clin Neurophysiol. 1996;98:59–68. doi: 10.1016/0013-4694(95)00204-9. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Christensen LO, Bestmann S, Rothwell JC. rTMS over the cerebellum can increase corticospinal excitability through a spinal mechanism involving activation of peripheral nerve fibres. Clin Neurophysiol. 2002;113:1435–1440. doi: 10.1016/s1388-2457(02)00156-6. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Porter R. Corticocortical synaptic influences on morphologically identified pyramidal neurones in the motor cortex of the monkey. J Physiol. 1988;400:617–629. doi: 10.1113/jphysiol.1988.sp017139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol (Paris) 2006;99:414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen F, Glocker FX, Fietzek U, Meyer BU, Lucking CH, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Ann Neurol. 1998;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett. 1986;71:235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- Hore J, Preston JB, Cheney PD. Responses of cortical neurons (areas 3a and 4) to ramp stretch of hindlimb muscles in the baboon. J Neurophysiol. 1976;39:484–500. doi: 10.1152/jn.1976.39.3.484. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA (A) and GABA (B) agonists on interhemispheric inhibition in man. Clin Neuro Physiol. 2007;118:308–316. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979;188:137–145. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36(Suppl. 2):T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Sohn YH, Mason A, Considine E, Hallett M. Flumazenil does not affect intracortical motor excitability in humans: a transcranial magnetic stimulation study. Clin Neurophysiol. 2004;115:325–329. doi: 10.1016/s1388-2457(03)00335-3. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin-Lang A, Sawaki L, Cohen LG. Role of voluntary drive in encoding an elementary motor memory. J Neurophysiol. 2005;93:1099–1103. doi: 10.1152/jn.00143.2004. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Koch G, Del Olmo MF, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez SM, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai K, Kujirai T, Sinkjaer T, Rothwell JC. Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol. 2006;96:1337–1346. doi: 10.1152/jn.01140.2005. [DOI] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol. 2005;563:915–924. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Liepert J, Kucinski T, Tuscher O, Pawlas F, Baumer T, Weiller C. Motor cortex excitability after cerebellar infarction. Stroke. 2004;35:2484–2488. doi: 10.1161/01.STR.0000143152.45801.ca. [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Ridding MC. Transient motor evoked potential suppression following a complex sensorimotor task. Clin Neurophysiol. 2006;117:1266–1272. doi: 10.1016/j.clinph.2006.02.008. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol. 2004;528:633–645. doi: 10.1111/j.1469-7793.2000.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Maruyama A, Fujiwara T, Nakanishi R, Tsuji S, Rothwell JC. Increased corticospinal excitability after 5 Hz rTMS over the human supplementary motor area. J Physiol. 2005;562:295–306. doi: 10.1113/jphysiol.2004.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, von Grafin EH, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Machetanz J. Reduction of corticospinal excitability by magnetic stimulation over the cerebellum in patients with large defects of one cerebellar hemisphere. Electroencephalogr Clin Neurophysiol. 1994;93:372–379. doi: 10.1016/0168-5597(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004a;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Terao Y, Okabe S, Furubayashi T, Arai N, Iwata NK, Hanajima R, Kamakura K, Motoyoshi K, Ugawa Y. Effects of motor cortical stimulation on the excitability of contralateral motor and sensory cortices. Exp Brain Res. 2004b;158:519–526. doi: 10.1007/s00221-004-1923-0. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M, Pondev N, Christova L, Wolf W, Kossev AR. Motor cortex excitability changes preceding voluntary muscle activity in simple reaction time task. Eur J Appl Physiol. 2006;98:212–219. doi: 10.1007/s00421-006-0265-y. [DOI] [PubMed] [Google Scholar]
- Nomura T, Takeshima T, Nakashima K. Reduced pre-movement facilitation of motor evoked potentials in spinocerebellar degeneration. J Neurol Sci. 2001;187:41–47. doi: 10.1016/s0022-510x(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Butler SL. Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Exp Brain Res. 2002;144:336–342. doi: 10.1007/s00221-002-1051-7. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, Traversa R, Palmieri MG, Rossini PM. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res. 2003;149:214–221. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–193. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci. 2007;27:1045–1053. doi: 10.1523/JNEUROSCI.4128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res. 2001;140:505–510. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000a;131:135–143. doi: 10.1007/s002219900269. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Exp Brain Res. 2000b;133:249–253. doi: 10.1007/s002210000428. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999;126:536–544. doi: 10.1007/s002210050762. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 2001;537:623–631. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Munchau A, Gerschlager W, Webb RM, Rothwell JC. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Rothwell JC. Motorcortical exitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27:5200–5206. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Butler K, Cordivari C, Lees AJ, Rothwell JC. Pathophysiological differences between musician's dystonia and writer's cramp. Brain. 2005;128:918–931. doi: 10.1093/brain/awh402. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 1988;458:20–30. doi: 10.1016/0006-8993(88)90491-x. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanuma H, Okuda O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol. 1962;25:198–208. doi: 10.1152/jn.1962.25.2.198. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006;37:246–247. doi: 10.1161/01.STR.0000195130.16843.ac. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Schneider C, Lavoie BA, Barbeau H, Capaday C. Timing of cortical excitability changes during the reaction time of movements superimposed on tonic motor activity. J Appl Physiol. 2004;97:2220–2227. doi: 10.1152/japplphysiol.00542.2004. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett. 1999;270:137–140. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- Shim JK, Kim SW, Oh SJ, Kang N, Zatsiorsky VM, Latash ML. Plastic changes in interhemispheric inhibition with practice of a two-hand force production task: a transcranial magnetic stimulation study. Neurosci Lett. 2005;374:104–108. doi: 10.1016/j.neulet.2004.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Kakei S, Futami T, Wannier T. Thalamocortical organization in the cerebello-thalamo-cortical system. Cereb Cortex. 1993;3:421–429. doi: 10.1093/cercor/3.5.421. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88:333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex. 2006;16:376–385. doi: 10.1093/cercor/bhi116. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol. 2003a;89:2014–2020. doi: 10.1152/jn.00925.2002. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Motor imagery of phasic thumb abduction temporally and spatially modulates corticospinal excitability. Clin Neurophysiol. 2003b;114:909–914. doi: 10.1016/s1388-2457(02)00373-5. [DOI] [PubMed] [Google Scholar]
- Swayne O, Rothwell J, Rosenkranz K. Transcallosal sensorimotor integration: effects of sensory input on cortical projections to the contralateral hand. Clin Neurophysiol. 2006;117:855–863. doi: 10.1016/j.clinph.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Walter CB, Lee TD, Serrien DJ. Acquiring bimanual skills: contrasting forms of information feedback for interlimb decoupling. J Exp Psychol Learn Mem Cogn. 1993;19:1328–1344. doi: 10.1037//0278-7393.19.6.1328. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Fiaschi A, Andreoli A, Marani S, Zanette G. Sensorimotor integration to cutaneous afferents in humans: the effect of the size of the receptive field. Exp Brain Res. 2005;167:362–369. doi: 10.1007/s00221-005-0041-y. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Manganotti P, Zanette G, Fiaschi A. Cutaneomotor integration in human hand motor areas: somatotopic effect and interaction of afferents. Exp Brain Res. 2001;141:232–241. doi: 10.1007/s002210100859. [DOI] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124:447–454. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: An electrophysiological study in the macaque monkey. Cereb Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res. 2004;158:133–140. doi: 10.1007/s00221-004-1881-6. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosc. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Voller B, St Clair GA, Dambrosia J, Pirio RS, Lomarev M, Dang N, Hallett M. Short-latency afferent inhibition during selective finger movement. Exp Brain Res. 2006;169:226–231. doi: 10.1007/s00221-005-0140-9. [DOI] [PubMed] [Google Scholar]
- Voller B, St Clair GA, Lomarev M, Kanchana S, Dambrosia J, Dang N, Hallett M. Long-latency afferent inhibition during selective finger movement. J Neurophysiol. 2005;94:1115–1119. doi: 10.1152/jn.00333.2005. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002b;125:1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Taylor J, Ridding M, Meyer BU, Rothwell JC. Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:58–66. doi: 10.1016/0013-4694(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Ziemann U. Pharmacology of TMS. Clin Neurophysiol. 2003;56(Suppl.):226–231. [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998a;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998b;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998c;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996b;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel S, Baumer T, Liepert J. Modulation of intracortical facilitatory circuits of the human primary motor cortex by digital nerve stimulation. Exp Brain Res. 2006;176:425–431. doi: 10.1007/s00221-006-0624-2. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Marinelli L, Buccolieri A, Trompetto C, Abbruzzese G. Creutzfeldt-Jakob disease presenting as corticobasal degeneration: a neurophysiological study. Neurol Sci. 2006;27:118–121. doi: 10.1007/s10072-006-0611-1. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002a;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]