Abstract
Agonist-activated Ca2+ entry plays a critical role in Ca2+ signalling in non-excitable cells. One mode of such entry is activated as a consequence of the depletion of intracellular Ca2+ stores. This depletion is sensed by the protein STIM1 in the endoplasmic reticulum, which then translocates to regions close to the plasma membrane where it induces the activation of store-operated conductances. The most thoroughly studied of these conductances are the Ca2+ release-activated Ca2+ (CRAC) channels, and recent studies have identified the protein Orai1 as comprising the essential pore-forming subunit of these channels. Although evidence suggests that Orai1 can assemble as homomultimers, whether this assembly is necessary for the formation of functional CRAC channels and, if so, their relevant stoichiometry is unknown. To examine this, we have used an approach involving the expression of preassembled tandem Orai1 multimers comprising different numbers of subunits into cells stably overexpressing STIM1, followed by the recording of maximally activated CRAC channel currents. In each case, any necessity for recruitment of additional Orai1 units to these preassembled multimers in order to form functional channels was evaluated by coexpression with a dominant-negative Orai1 mutant. In this way we were able to demonstrate, for the first time, that the functional CRAC channel pore is formed by a tetrameric assembly of Orai1 subunits.
CRAC channels (Ca2+ release-activated Ca2+ channels) mediate the store-operated Ca2+-selective conductance in the plasma membrane that plays a critical role in T lymphocyte activation (Hoth & Penner, 1992; Hoth & Penner, 1993; Zweifach & Lewis, 1993). Orai1 was first identified as a key component of these channels as a result of a linkage analysis in a pedigree of two infant patients suffering from a form of hereditary severe combined immunodeficiency (SCID), the primary defect of which was characterized as a specific loss of CRAC channel activity (Feske et al. 2006). In addition, genome-wide RNAi screens for CRAC channel activity using Drosophila S2 cells (Feske et al. 2006; Vig et al. 2006b; Zhang et al. 2006) identified Orai (the Drosophila homologue of Orai1) as the gene product responsible. Finally, studies of mutations of certain conserved acidic residues in the transmembrane domains of Orai1 (and Orai) confirmed that this protein constitutes the pore-forming subunit of the CRAC channel (Prakriya et al. 2006; Yeromin et al. 2006; Vig et al. 2006a). In contrast, whilst expression of either of the other two Orai family members (Orai2 and Orai3) has occasionally been reported to result in increased store-operated currents, particularly when extensively overexpressed (Lis et al. 2007), it should be noted that the biophysical and pharmacological properties of the resulting currents are quite distinct from those associated with the CRAC channels.
For many ion channels, the formation of a functional ion pore requires the multimeric assembly of various numbers of individual subunits. Studies have shown that both Drosophila Orai and mammalian Orai1 can assemble into multimers (Vig et al. 2006a; Gwack et al. 2007). However, neither of these studies has indicated whether such assembly is necessary for the formation of functional channels and, if so, what the exact subunit stoichiometry of the relevant multimer may be. Resolving this is particularly important for the CRAC channels as both biophysical and molecular evidence indicates that they are unlike other channels, including other store-operated channels (e.g. members of the TRPC family), and other Ca2+-selective channels (e.g. voltage-gated Ca2+ channels, and the Ca2+-selective TRPV5/6 channels).
To examine this question, we have used an approach involving the expression of Orai1 in the form of preassembled multimers of various lengths in cells stably expressing a consistent level of STIM1. Any requirement of these various multimers to recruit additional Orai1 subunits in order to form functional CRAC channels was assessed by coexpression of a dominant negative Orai1 mutant.
Methods
Cell lines and constructs
The cell line stably expressing STIM1 was generated using the Flp-In™-293 system (Invitrogen) following the manufacturer's instructions. In this system, the gene of interest is incorporated into the genome of the cell at a single engineered Flp recombination target site via an Flp recombinase-mediated DNA recombination, followed by selection with hygromycin B. This cell line is therefore isogenic for STIM1. Stable expression of the relevant Orai1 constructs in this cell line was achieved by transfection with the appropriate construct, and selection using 5 μg ml−1 puromycin. For transient expression, cells were transfected with 0.75 μg DNA of the selected construct, together with 0.25 μg of an EYFP construct using an Amaxa Nucleofector II following the manufacturer's guidelines. The basic Orai1 monomer construct and the E106Q mutant, both of which were FLAG-tagged at the C-terminal, were as described by Gwack et al. (2007). Multimeric constructs were prepared by subcloning the protein coding region of Orai1 MO70, in which the stop codon was deleted, into the pBluescript SK+ vector. A linker was prepared that would provide a six amino acid sequence (QLNQLE) between each subunit at a site just prior to the Orai1 start codon in the final construct. The subunit plus linker was then excised and ligated into the cut Orai1 MO70 backbone using restriction sites containing non-recleavable compatible cohesive ends. This allowed addition of further linked subunits as required. Orientation and number of the subunits in the final constructs were confirmed by restriction analysis.
Immunocytochemistry
Cells were plated onto poly l-lysine-coated coverslips 24 h before being fixed with 2% ultra-pure formaldehyde in phosphate-buffered saline (PBS), permeabilized with TBS containing 0.2% Triton X-100, washed, and incubated with blocking buffer (2% IgG-free BSA, 0.05% Tween in TBS). Cells were incubated with anti-FLAG M2 antibody (Cell Signaling Technology, Inc., Danvers, MA, USA), followed by Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA) secondary antibodies. Immunofluorescence was analysed by confocal imaging using a Nikon D-Eclipse C1 laser-scanning confocal system. Alexa Fluor images were obtained with 488 nm excitation using an argon ion laser (Spectra Physics, Mountain View, CA, USA), and HcRed images with 543 nm excitation using a HeNe laser (Melles Griot, Carlsbad, CA, USA). Images were acquired and analysed using EZ-C1 software (Nikon).
Electrophysiology
Cells were patch-clamped 42–50 h after transfection using EYFP fluorescence as a marker for transfected cells. Whole cell recordings of macroscopic CRAC channel current were obtained essentially as previously described (Mignen et al. 2007), using sequential 250 ms voltage pulses to −40 mV and +60 mV delivered every 2 s from a holding potential of 0 mV. Current–voltage relationships were recorded using 150 ms voltage ramps from −100 mV to +60 mV. The extracellular (bath) solution contained (mm): NaCl 140, MgCl2 1.2, CaCl2 10, CsCl 5, d-glucose 30, Hepes 10 (pH 7.4). The internal (pipette) solution contained (mm): caesium acetate 140, NaCl 10, MgCl2 3.72, EGTA 10, Hepes 10 (pH 7.2). Adenophostin A (2 μm) was added to this solution to deplete intracellular stores and maximally activate the CRAC channels. The calculated free [Mg2+] in this solution (3 mm) was designed to inhibit the activation of MIC/MagNum currents, which was also confirmed by checking for any significant changes in the outward current at +60 mV. Experiments were discarded if any such significant changes were observed. Currents were sampled at 20 kHz during the voltage steps and at 5.5 kHz during the voltage ramps, and digitally filtered off-line at 1 kHz. Initial current–voltage relationships obtained before activation of the CRAC channel currents, were averaged and used for leak subtraction of subsequent current recordings. In addition, to confirm that the adenophostin A had not begun to activate the CRAC channels immediately on achieving the whole-cell condition, La3+ (100 μm) was added at the end of the experiment to inhibit the currents through the activated CRAC channels, and the resulting values compared with those recorded initially on achieving the whole-cell condition. All experiments were carried out at room temperature (20–22°C).
Data analysis
All data are presented as means ± s.e.m. Statistical significance was determined using Student's t test, or by one-way ANOVA as appropriate, with a value of P < 0.05 taken as significant.
Results
STIM1 has been shown to be an essential, and potentially rate-limiting, component in the activation of the CRAC channels (Roos et al. 2005; Spassova et al. 2006; Peinelt et al. 2006; Soboloff et al. 2006; Mercer et al. 2006). Therefore, to reduce the possibility that variations or limitations in STIM1 expression might influence the magnitude of recorded CRAC channel currents, we used a HEK293 cell line engineered to stably overexpress a consistent level of STIM1 (STIM1-stable cells) throughout this study. To confirm that expression of Orai1 in these cells results in the generation of significant macroscopic CRAC channel currents, we stably expressed Orai1 monomers in the STIM1-stable cells. Depletion of the intracellular Ca2+ stores using a buffered Ca2+-free pipette solution containing the potent InsP3 receptor agonist adenophostin A (2 μm) (Mignen et al. 2007) resulted in the development of significant inward currents at negative potentials, reaching a maximal value, after approximately 75–80 s, of 3.74 ± 0.51 pA pF−1 at −40 mV (n = 15) (Fig. 1A). This is equivalent to a greater than 8-fold increase over the corresponding currents in untransfected STIM1-stable cells (0.44 ± 0.03 pA pF−1, n = 13). The recorded currents were completely inhibited by La3+ (100 μm), and showed a characteristic inwardly rectifying current–voltage relationship, with reversal potentials greater than +60 mV (Fig. 1B). Along with their specific activation by store depletion, these features are consistent with currents reflecting the activity of CRAC channels.
Figure 1. The E106Q mutant Orai1 inhibits CRAC channel currents in STIM1- stable cells stably expressing wild-type Orai1.
A, inward CRAC channel currents measured at −40 mV in STIM1-stable cells stably expressing the wild-type Orai1 either alone (black bar) or after transfection with the E106Q mutant Orai1 (red bar). Data are presented as means ± s.e.m.; n = 15 and 10, respectively. B, representative current–voltage relationships for CRAC channel currents measured in STIM1-stable cells stably expressing wild-type Orai1 monomers either alone (black trace), or after transfection of the E106Q mutant Orai1 (red trace). C, upper panels, images showing the absence of significant background fluorescence in untransfected STIM1-stable cells. Shown is a DIC image of STIM1-stable cells (left panel), and the same field in confocal mode following immunocytochemistry with the anti-FLAG antibody (right panel). Lower panel, confocal image showing that transfection with the E106Q mutant Orai1 in STIM1-stable cells stably expressing the Orai1 monomer does not affect the plasma membrane localization of Orai1. Orai1 is detected in the confocal image by an antibody targeting the C-terminal FLAG tag and shown in green. Those cells transfected with the E106Q mutant are distinguished by cotransfection of a nuclear-targeted HcRed plasmid (pHcRed1-N1, Clontech, Palo Alto, CA, USA).
To determine whether functional CRAC channels required the assembly of the stably expressed Orai1 monomers into multimeric complexes, we utilized an Orai1 construct in which the glutamate at position 106 was mutated to a glutamine. This charge-neutralizing mutation (or the equivalent E178Q mutant in Drosophila Orai) has been reported to profoundly inhibit CRAC channel currents (Yeromin et al. 2006; Vig et al. 2006a; Lis et al. 2007) and/or store-operated Ca2+ entry (Vig et al. 2006a; Gwack et al. 2007), indicating that it functions as a dominant-negative construct. Consistent with these previous reports, transfection of the E106Q construct into the STIM1-stable cells stably expressing the Orai1 monomers resulted in a profound inhibition of recorded inward CRAC channel currents to a value of only 0.55 ± 0.16 pA pF−1 (n = 10) at −40 mV (Fig. 1A). Importantly, this effect of expression of the E106Q mutant cannot be ascribed to any obvious inhibition of the expression of Orai1, or its trafficking to the membrane (Fig. 1C). Instead, this effect of the E106Q mutant on the CRAC channel currents indicates that monomeric Orai1 alone does not constitute functional CRAC channels, and that the formation of such channels requires the multimeric assembly of the Orai1 monomers. Moreover it demonstrates that, by coexpressing the E106Q mutant construct, its incorporation into such multimeric assemblies results in the profound inhibition of the resulting currents.
This pronounced dominant-negative effect of the E106Q mutant suggests that the coexpression of this construct with various preassembled multimers of Orai1 might provide a relatively simple means to determine the subunit composition of the CRAC channels. In this way, coexpression of the E106Q mutant with a multimer that contains fewer subunits than the functional channels would be expected to reduce the relevant measured current as the dominant-negative effect of the E106Q mutant would become apparent on its incorporation into the extended multimer. In contrast, coexpression of the E106Q mutant with a multimer that is, itself, capable of forming a functional channel would have essentially no effect on the measured currents, as the E106Q mutant would be excluded from such a functional multimer. Multimers containing fewer subunits than required to form a functional channel, but which can combine with themselves to form such a channel, might be expected to display an intermediate current magnitude in the presence of the E106Q mutant, reflecting the relative ability of the mutant to compete with such a combination of identical multimeric subunits.
To illustrate this, the anticipated effects of coexpression of the E106Q mutant with each of the multimeric constructs are summarized in Fig. 2. The analysis presented is undoubtedly simplistic. We have, for example, ignored any potential complications arising from differences in the levels of expression of the various constructs, or in their relative ability to assemble either with each other or with endogenous monomers. However, at least at a qualitative level, it is clear that the different models examined predict very distinct patterns of current magnitude within each group under the defined experimental conditions. Thus, if CRAC were a dimer or a trimer then, in the presence of the E106Q mutant, currents greater than those seen in untransfected STIM1 cells should only be observed when the specific relevant construct (i.e. the dimer or trimer) is transfected. However, if CRAC is a tetramer, increased currents in the presence of the mutant would be expected on transfection of either a dimer or a tetramer, but not with a trimer.
Figure 2. Predicted composition of expressed channel stoichiometries in cells coexpressing the E106Q mutant Orai1 and each of the tandem Orai1 multimers.
Wild-type Orai1 subunits (blue circles) and E106Q Orai1 (black circles) are shown for each transfected multimer (dimer, trimer or tetramer), together with the predicted possible combinations of the transfected subunits based on the stoichiometry of functional CRAC channels being either a dimer, a trimer, or a tetramer. Those assemblies predicted to result in functional channels are shown with a central ‘pore’ in red, and predicted non-functional channels are represented with a pore in white. The consequent predicted overall effect on CRAC channel currents in the presence of the E106Q mutant is indicated. See text for details.
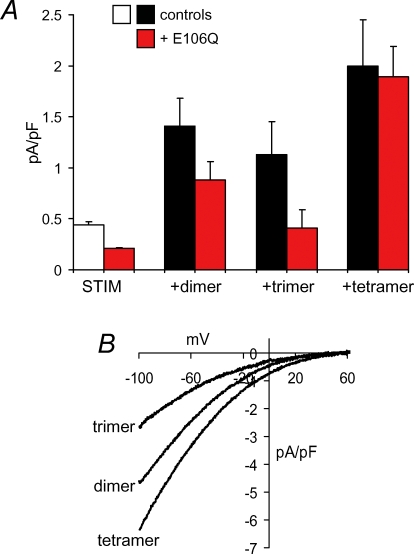
To examine this, we generated a series of cDNA constructs in which the individual wild-type Orai1 coding sequences were linked by insertion of six amino acids after deletion of the stop codon. These constructs were transfected into the STIM1-stable cells either alone, or together with the E106Q mutant Orai1, and the resulting macroscopic CRAC channel currents recorded and compared to the corresponding currents in untransfected STIM1-stable cells, and in the same cells expressing the E106Q mutant. The data obtained are shown in Fig. 3. In each case, transfection of the multimeric constructs alone resulted in currents that were significantly greater (2.5- to 4.5-fold) than those recorded in the untransfected STIM1-stable cells, but were not significantly different from each other (P = 0.22, one-way ANOVA) (Fig. 3A). The recorded currents displayed features characteristic of CRAC channels, including marked inward rectification, and reversal potentials of approximately +60 mV (Fig. 3B). Moreover, Western blot analysis of the FLAG-tagged constructs indicated that they were successfully expressed as intact proteins of the approximate appropriate size based on their predicted molecular masses (see online Supplemental material, Supplementary Fig. 1). These data indicate that each of these multimeric proteins is expressed intact, is able to traffic correctly to the membrane, and forms functional channels – either by itself or by association with endogenous Orai1 subunits. However, when coexpressed with the monomeric E106Q mutant, it is clear that the increased currents observed on expression of the trimer construct are completely lost, resulting in values that are not significantly different from those seen in the STIM1-stable cells transfected with the E106Q mutant (P = 0.14) (Fig. 3A). Such a pronounced inhibitory effect indicates that the increased currents seen on expression of this construct alone must reflect a requirement for some event that has the potential to be impacted by the incorporation of the mutant Orai1. The precise nature of any such event remains unclear and may involve, for example, the linking of trimeric constructs – either as complete units or parts of such units – to form a higher order assembly. However, whatever their origin, the profound effect of the E106Q mutant on the resulting currents clearly indicates that the CRAC channel pore cannot be composed of an individual trimeric assembly of Orai1 subunits. In contrast, significant increases in currents are still observed with the dimeric and tetrameric Oria1 constructs in the presence of the E106Q mutant (P = 0.002 and 0.0001, respectively, when compared to the STIM1-stable cells transfected with the same mutant). This indicates that, unlike the trimeric construct, both the dimeric and tetrameric Orai1 constructs have at least the potential to form functional CRAC channels.
Figure 3. Effect of expression of tandem Orai1 multimers with and without the E106Q mutant Orai1.
A, inward CRAC channel currents measured at −40 mV in untransfected STIM1-stable cells (white bar) and after transfection of the wild-type Orai1 multimers either alone (black bars) or along with 0.75 μg DNA of the E106Q mutant Orai1 (red bars). Data are presented as means ± s.e.m.; n = 9–13. B, representative current–voltage relationships for CRAC channel currents measured in STIM1-stable cells after transfection of the respective wild-type Orai1 multimers.
A problem in determining which of these two constructs, the dimers or the tetramers, actually constitutes the functional pore of the CRAC channel is that that they have the potential to be converted into each other – the dimers could coassemble to form tetramers, or the tetramers may constitute functional channels in the form of two parallel dimers. To resolve this, we chose to replace one of the wild-type subunits in the tetramer construct with one bearing the E106Q mutation, thereby forming a WT-WT-WT-E106Q tetramer. The prediction was that, if the functional channels were a tetramer, the inclusion of the mutant subunit would result in a profoundly reduced current. However, if the tetramer were forming functional dimeric channels, then a proportion of the resultant dimers (i.e. those consisting of two wild-type Orai1 subunits) would be available to generate increased current. Measurement of CRAC channel currents in cells transfected with this construct revealed inward currents at −40 mV of 0.35 ± 0.07 pA pF−1 (n = 11), a value not significantly different from that recorded in the untransfected STIM1-stable cells (P = 0.11). This demonstrates that the currents seen with the tetramer construct do not result from the formation of functional dimeric channels, and that functional CRAC channels are formed specifically from a tetramer of Orai1 subunits. Significantly, the above data also demonstrate that the incorporation of a single mutant subunit in a tetrameric Orai1 construct is sufficient to profoundly inhibit the resulting currents. This is, perhaps, the most direct confirmation of the dominant-negative effect of the E106Q mutation.
To finally confirm that a tetrameric assembly of Orai1 subunits represents the functional CRAC channel pore, we generated a STIM1-stable cell line that was stably expressing the Orai1 tetramer construct. Maximally activated inward store-operated currents in the Orai1-tetramer stable cell line were 5.55 ± 0.69 pA pF−1 at −40 mV (n = 12) (Fig. 4A). As shown for the similar cell line stably expressing the Orai1 monomer, these large currents displayed all the features of genuine CRAC channel currents, including complete inhibition by La3+ (100 μm), pronounced inward rectification, and very positive reversal potentials (> +60 mV) (Fig. 4B). In addition, of course, their activation was entirely dependent on store depletion. As predicted, transfection of the E106Q mutant in these cells stably expressing the Orai1 tetramer had no effect on the recorded CRAC channel currents (Fig. 4A; mean value of inward current at −40 mV = 5.36 ± 0.90 pA pF−1, n = 7; P = 0.43 compared to untransfected stable Orai1-tetramer cells). This stands in marked contrast to the effect of the mutant in cells stably expressing the monomeric Orai1, which produced an 85% inhibition of the recorded CRAC currents (see Fig. 1, above). Two important facts arise from these data. First, they demonstrate that it is unlikely that the functional CRAC channel pore is made up of any higher order Orai1 multimer (e.g. a pentamer, hexamer, etc.), as any functionality of such a multimer would be expected to be inhibited by coexpression with the E106Q mutant. Secondly, the data also show that any inhibitory effect of the E106Q mutant on currents induced by transfection of the various tandem constructs (see Fig. 3) cannot reflect an effect caused by expression of excess Orai1 relative to available STIM1. Thus, the data show that sufficient STIM1 is present to support CRAC channel currents of almost 6 pA pF−1, or some 3-fold greater than the largest currents recorded on transient transfection of the various tandem constructs. Moreover, the fact that expression of additional Orai1 (albeit in a mutant form) failed to reduce these currents, shows that even this additional non-functional Orai is not able to effectively titrate STIM1 away from the functional channels. As we discussed above, because STIM1 has been shown to be a potentially rate-limiting component in the activation of the CRAC channels (Roos et al. 2005; Spassova et al. 2006; Peinelt et al. 2006; Soboloff et al. 2006; Mercer et al. 2006), we deliberately used a cell line engineered to stably overexpress a consistent level of STIM1 (STIM1-stable cells) throughout this study. Clearly, the above data demonstrate that levels of STIM1 in these STIM1-stable cells cannot be rate-limiting under the present experimental conditions.
Figure 4. Expression of the E106Q mutant has no effect on CRAC channel currents in cells stably expressing the Orai1 tetramer.
A, inward CRAC channel currents measured at −40 mV in STIM1- stable cells stably expressing the wild-type Orai1 tetramer either alone (black bar) or after transfection with the E106Q mutant Orai1 (red bar). Data are presented as means ± s.e.m.; n = 12 and 7, respectively. B, representative current–voltage relationships for CRAC channel currents measured in STIM1-stable cells stably expressing wild-type Orai1 tetramer either alone (black trace), or after transfection of the E106Q mutant Orai1 (red trace).
Discussion
In conclusion, we have used a relatively simple approach to examine the multimeric structure of functional CRAC channels involving the linking of the coding sequences of multiple single Orai1 subunits. In this, we were able to show that such constructs can result in the formation of functional CRAC channels. The approach employed is somewhat analogous to those originally used to determine the tetrameric construction of voltage-gated K+ channels, which used mutants that affected the sensitivity of the channels for inhibition by TEA ions (Isacoff et al. 1990; Liman et al. 1992). In contrast to those studies, the approach used here is greatly simplified by the pronounced dominant-negative effect of the E106Q mutant Orai1. Because of this, as we have shown, the association of even a single mutant subunit with any of the wild-type constructs could be readily detected by the profound inhibition of the resulting currents. Of course, we cannot definitively exclude the possibility that a particularly high affinity association between expressed constructs might prevent the incorporation of the mutant, raising the possibility that the channel pore is really a much larger assembly – e.g. an octomeric pore structure formed by the assembly of two tetrameric constructs. The evidence shows that any such linking of the trimeric constructs failed to preclude incorporation of the E106Q mutant, but the development of significant E106Q-resistant currents with the dimer construct suggests that such preferred linkage can occur in this case. Perhaps this is simply a reflection of a strongly favoured configuration of the channel pore as a tetramer. Finally, our findings do not eliminate the possibility of other proteins of, as yet, unknown identity being part of the channel as a whole, and perhaps playing important regulatory functions.
In summary, using the approach described, we have demonstrated for the first time that the functional pore of such channels is specifically formed from a tetrameric assembly of these subunits. Such an arrangement would be consistent with an assembly in which the charged residues from the four individual subunits identified as being critical for Ca2+ selectivity could be arranged in a circle to form a tetrameric ion pore structure (Vig et al. 2006a; Cai, 2007).
Finally, the ability to express functional channels from tetrameric constructs with specifically designed and predetermined stoichiometries will undoubtedly greatly facilitate subsequent investigations of the key functional and structural features of the channel and, for example, may help resolve some of the current inconsistencies in reported findings of the effects of certain mutations influencing the ion selectivity of these channels (Prakriya et al. 2006; Vig et al. 2006a).
Acknowledgments
We thank Drs Anjana Rao and Patrick Hogan (CBR Institute, Harvard) for generously providing essential constructs, and Pauline Leakey, for excellent technical assistance. This work was supported by National Institutes of Health Grants GM040457 to T.J.S. In addition, O.M. was supported in part by funds from the Alfred and Eleanor Wedd Endowment.
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2007.147249/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.147249
References
- Cai X. Molecular evolution and structural analysis of the Ca2+ release-activated Ca2+ channel subunit, Orai. J Mol Biol. 2007;368:1284–1291. doi: 10.1016/j.jmb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-Hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff EY, Jan YN, Jan LY. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990;345:530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 Are Store-Operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2007.147249/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.147249