Abstract
An adverse intrauterine environment can increase the incidence of hypertension and other cardiovascular disease risk factors. However, in clinical and experimental studies the magnitude of the effect is variable. Possibly, the relative influence of the prenatal environment on cardiovascular disease is determined in part by genetic factors that predispose individuals to the development of environmentally induced hypertension. We tested this hypothesis by comparing the effects of prenatal dexamethasone treatment (Dex, 300 μg kg−1i.p. on days 15 and 16 of gestation) in borderline hypertensive rats (BHR) and control Wistar–Kyoto (WKY) rats. Blood pressure, heart rate and plasma corticosterone values were measured at rest during the middle of the day, and during 1 h of restraint stress in the adult offspring using indwelling arterial catheters implanted at least 4 days prior to data collection. Compared with the saline (vehicle) control treatment, prenatal dexamethasone significantly (P < 0.05) increased baseline mean arterial pressure in male (123 ± 2 versus 131 ± 3 mmHg, saline versus Dex) and female (121 ± 2 versus 130 ± 2 mmHg, saline versus Dex) BHR, but not in male (108 ± 3 versus 113 ± 2 mmHg, saline versus Dex) or female (112 ± 2 versus 110 ± 2 mmHg, saline versus Dex) WKY rats. Relative to saline treatment, prenatal Dex also significantly increased baseline heart rate (328 ± 6 versus 356 ± 5 beats min−1, saline versus Dex) and plasma corticosterone (5 ± 2 versus 24 ± 4 μg dl−1, saline versus Dex), and prolonged the corticosterone response to acute stress, selectively in female BHR. However, prenatal Dex significantly enhanced the arterial pressure response to acute stress only in female WKY, while Dex augmented the elevation in heart rate during stress only in male rats. We conclude that prenatal dexamethasone increased baseline arterial pressure selectively in BHR, and plasma corticosterone only in female BHR. In contrast, prenatal Dex enhanced cardiovascular reactivity to stress in both BHR and WKY rats.
Numerous studies have documented that prenatal programming can increase baseline arterial pressure and modulate cardiovascular and endocrine responses to acute stress (Barker, 2000; Doyle et al. 2000; Dodic et al. 2002; Ward et al. 2003; Igosheva et al. 2004; Jones et al. 2007). In humans, prenatal programming has been suggested as an alternative to genetic mechanisms to explain the correlation of racial background with the incidence of hypertension (Forrester, 2004). Other investigators have proposed that prenatal programming might be an epiphenomenon; genetic factors that predispose individuals to cardiovascular disease might also program placental insufficiency (Henriksen & Clausen, 2002). A synthesis of the two hypotheses suggests the importance of genetic background in the susceptibility to prenatal programming in humans (Szitanyi et al. 2003; Fowden et al. 2006). Therefore, we performed the present study to test the hypothesis that the genetic predisposition to environmentally induced hypertension in borderline hypertensive rats (BHR) can exacerbate adverse effects of prenatal programming on arterial pressure and stress responsiveness.
In most epidemiological studies, programming is indexed by low birth weight (Huxley et al. 2000; Szitanyi et al. 2003). Experimental models of programming include several interventions that can result in low birth weight, the most common being prenatal administration of glucocorticoids to the dam or manipulation of her diet during pregnancy (Moritz et al. 2005). We selected the model of glucocorticoid administration because of its clinical relevance and evidence that elevated fetal exposure to glucocorticoids is a primary mediator of programming in several experimental models (Moritz et al. 2005; Alexander, 2006). Additionally, prenatal glucocorticoid administration has specifically been associated with elevated blood pressure in humans (Doyle et al. 2000).
Experiments were performed in BHR and Wistar–Kyoto rats (WKY; control strain). BHR are the first generation offspring of spontaneously hypertensive rats (SHR) and WKY; previously only the male offspring have been studied (Lawler et al. 1988). They exhibit moderate hypertension under baseline conditions and are susceptible to environmentally induced hypertension (Lawler et al. 1988; Sanders & Lawler, 1992). In the present experiments, SHR or WKY (genetic control strain) dams were mated with WKY males to produce BHR and WKY offspring. On days 15 and 16 of gestation pregnant dams were treated with the glucocorticoid dexamethasone (Dex) or saline by subcutaneous injection. Baseline mean arterial pressure and heart rate were determined in conscious freely moving adult male and female offspring instrumented with indwelling arterial catheters. To determine if stress responses were enhanced by prenatal treatment, cardiovascular and endocrine (corticosterone, insulin and glucose) responses were determined in rats prior to and during 1 h of restraint stress.
Methods
Ethical approval
Experimental protocols were approved by the Institutional Animal Care and Use Committee, and were performed with strict adherence to all American Association for Accreditation of Laboratory Animal Care International (AAALAC), National Institutes of Health and National Research Council guidelines.
Animals
BHR and WKY rats were bred in-house from SHR and WKY rats (Charles River Laboratories) and housed in an AAALAC accredited animal care facility on a 12 : 12 h light/dark cycle. Rats were maintained on a normal sodium diet, and food and water were provided ad libitum.
Prenatal treatment
Female WKY (n = 8) and SHR (n = 11) rats were mated with male WKY rats during the estrous phase of their cycle as identified by vaginal smear. Females were housed overnight with males and pregnancy was confirmed by sperm positive vaginal smear the following day (day 0 of pregnancy). Pregnant dams were each housed separately and assigned to either Dex or saline treatment groups. On days 15 and 16 of gestation Dex (300 μg kg−1) or saline (vehicle) was administered by subcutaneous injection. In preliminary experiments higher doses of Dex (400–600 μg kg−1 day−1) resulted in decreased viability in BHR pups, therefore these doses were not studied further. The number of litters per treatment group was as follows: WKY-Saline (3), WKY-Dex (5), BHR-Saline (5) and BHR-Dex (6). Not all pups were used in this study.
Body weight
Since prenatal Dex has been reported to decrease body weight at birth (Ortiz et al. 2003; O'Regan et al. 2004), rat pups were weighed on day 1 of birth. Pups were sexed and weaned at 3 weeks of age. Body weight was also measured at 10 weeks of age to determine if prenatal Dex treatment altered adult body weight. Subsequent experiments were performed in adult male (n = 63) and female (n = 61) BHR and WKY offspring (see Table 1 for ages and numbers of animals in each group).
Table 1.
Age, oestrous cycle day, plasma insulin, thymus weight and numbers of animals per group
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| WKY–saline | WKY–Dex | BHR–saline | BHR–Dex | WKY–saline | WKY–Dex | BHR–saline | BHR–Dex | |
| Age (weeks) | 13.2 ± 0.2 | 13.4 ± 0.5 | 14.1 ± 0.6 | 14.1 ± 0.3 | 13.7 ± 0.5 | 13.1 ± 0.2 | 13.2 ± 0.3 | 13.7 ± 0.4 |
| (n = 10) | (n = 16) | (n = 14) | (n = 21) | (n = 10) | (n = 20) | (n = 14) | (n = 19) | |
| Oestrous cycle day | 1.6 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.2 | N/A | N/A | N/A | N/A |
| Thymus wt/body wt (mg kg−1) | 966 ± 55 | 1023 ± 84 | 1134 ± 67 | 1010 ± 62 | 647 ± 44 | 609 ± 37 | 803 ± 42a | 768 ± 42 |
| Baseline insulin (ng ml−1) | 2.36 ± 0.29 | 1.72 ± 0.17 | 1.62 ± 0.22 | 1.86 ± 0.24 | 2.11 ± 0.37 | 2.47 ± 0.25 | 2.01 ± 0.27 | 2.54 ± 0.48 |
| 10 min insulin (ng ml−1) | 2.70 ± 0.18 | 2.11 ± 0.20 | 2.17 ± 0.30 | 2.17 ± 0.25 | 2.63 ± 0.44 | 3.18 ± 0.19 | 2.94 ± 0.64 | 2.98 ± 0.51 |
| 60 min insulin (ng ml−1) | 1.82 ± 0.19 | 1.81 ± 0.20 | 1.97 ± 0.32 | 1.90 ± 0.23 | 1.74 ± 0.32 | 2.14 ± 0.19 | 2.18 ± 0.38 | 2.46 ± 0.28 |
| No. of animals, baseline values | 10 | 16 | 14 | 21 | 10 | 20 | 14 | |
| No. of animalsl Stress MAP & HR | 9 & 9 | 14 & 13 | 10 & 10 | 16 & 16 | 10 & 10 | 18 & 17 | 11 & 10 | 14 & 13 |
| No. of animals Stress hormones & glucose | 7–8 | 14 | 10 | 15–16 | 6–8 | 15 | 9–10 | 13–14 |
a, P < 0.05 relative to WKY–saline; MAP, mean arterial pressure; HR, heart rate.
Surgical procedures
All surgical procedures were performed using aseptic technique. At least 72 h prior to performing experimental protocols the rats were instrumented with indwelling arterial catheters for measurement of arterial pressure as previously described (Scheuer et al. 2004). Briefly, rats were anaesthetized with inhaled isoflurane (2–4% isoflurane in oxygen at a 1 l min−1 flow rate), with the depth of anaesthesia maintained such that there was no reflex withdrawal to hind paw pinch. A small skin incision was made, then the femoral artery was isolated and a catheter was inserted and advanced into the abdominal aorta. The distal end of the catheter was tunnelled under the skin to exit between the scapulae and sutured in place. The subcutaneous and cutaneous layers of the incision in the leg were individually sutured closed and the arterial catheter was filled with sterile heparin (1000 U ml−1) and plugged. For recovery from surgery rats were placed in warm padded cages and monitored until they could move about and groom normally. Following implantation of the arterial catheter the rats were singly housed for the duration of the experiment.
Experimental protocols
Baseline arterial pressure measurement
Each day the animals were brought to the laboratory in their home cages and remained there throughout the baseline period. The arterial catheter was connected via extension tubing to a pressure transducer (Maxxim Medical), then the rat was free to move normally within the cage until it was placed in the restrainer. The signal was processed using a MacLab system (ADInstruments) connected to a Macintosh computer. Mean arterial pressure and heart rate were calculated on-line. Arterial pressure was recorded continuously for 3 h on two to three separate days to adapt the rats to the procedure, with the last hour of the final day used to determine baseline arterial pressure and heart rate.
Restraint stress
Following the baseline arterial pressure measurement on the final day, most rats were subjected to a 1 h restraint stress by placing each rat in a clear Plexiglas restrainer; restraint stress data were obtained from 53 male and 49 female rats. The number of animals per group for various parameters is provided in Table 1. Blood samples (300 μl) were obtained from most animals from the arterial catheter following the baseline period prior to restraint and at 10 and 60 min during restraint for the measurement of blood glucose (One Touch Ultra glucose meter; LifeScan, Johnson & Johnson) and plasma corticosterone and insulin (see below). Since this blood sample was obtained in the middle of the day, basal morning (nadir) corticosterone concentration was not assessed. In female rats, a vaginal smear was taken on the day of restraint stress to determine the phase of the oestrous cycle. Rats were killed with an overdose of inhaled isoflurane and the adrenal and thymus glands were removed and weighed.
Corticosterone and insulin assays
Corticosterone and insulin concentrations were determined using commercially available radioimmunoassay (RIA) kits. Corticosterone was assayed using the MP Biomedicals rat 125I-RIA kit as previously described (Scheuer et al. 2004). Insulin was assayed with the Linco Research (St Charles, MO, USA) rat insulin RIA kit (catalogue no. RI-13K). The insulin assay sensitivity limit is 0.1 ng ml−1.
Statistical analysis
All data are expressed as mean ± standard error (s.e.m.). Data analyses were performed separately for males and females because large between-sex differences in some variables could mask the main effects of prenatal treatment and strain. Analyses were performed using 1- or 2-way analysis of variance (ANOVA) for effect of group (WKY–saline, WKY–Dex, BHR–saline and BHR–Dex) and, when applicable, time (repeated measure). When the overall ANOVA detected significant (P = 0.05) between-group differences, post hoc analysis was performed using Duncan's new multiple range and Fisher's protected least significant difference tests; if Duncan's indicated a significant difference Fisher's test was used to calculate the probability value. Effects of prenatal Dex treatment were determined by post hoc comparison of WKY–saline- versus WKY–Dex-treated rats and BHR–saline versus BHR–Dex-treated rats. To determine the effects of strain that were independent of prenatal Dex treatment, we examined post hoc analysis results for saline-treated WKY rats relative to saline-treated BHR. When the ANOVA detected a significant interaction between the effects of group and time, 1-way ANOVA was performed separately for different time points. For the heart rate changes in response to stress, the total integrated increase over the hour of stress was calculated relative to baseline (i.e. area under the curve) and analysed. Adrenal and thymus weights were normalized to body weight for quantification and analysis. The stage of the oestrous cycle on the day of the experiment could affect variables being studied. To determine if there was a significant between-group difference for day of oestrous cycle on the day of the experiment in females, values were assigned as follows: dioestrous day 1 = 1, dioestrous day 2 = 2, pro-oestrous = 3 and oestrous = 4. P values less than 0.10 are specified in the Results section.
Results
The average age at which the rats were studied and the average stage of oestrous cycle in female rats did not differ significantly between groups (Table 1).
Effects of prenatal Dex
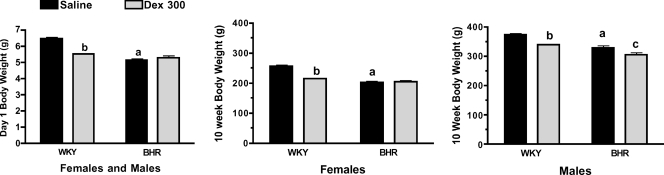
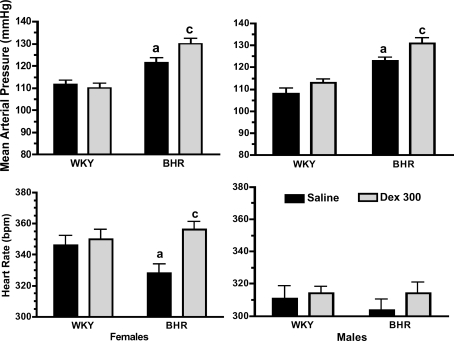
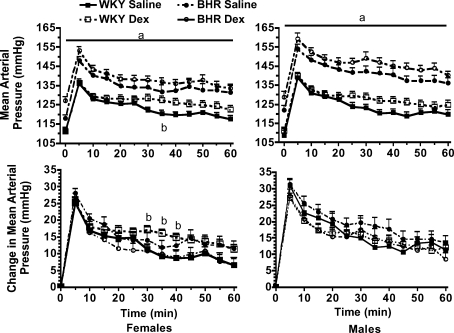
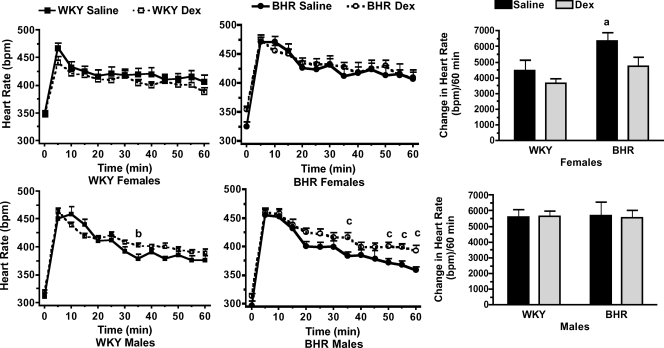
Prenatal Dex treatment significantly reduced body weight in 1-day-old WKY (P < 0.01), but not BHR pups (Fig. 1, left). At the age of 10 weeks, Dex-treated rats weighed less than saline-treated rats (P < 0.01) with the exception of the female BHR (Fig. 1, middle and right). Prenatal Dex increased baseline arterial pressure in male and female BHR (P < 0.01, Fig. 2, top), and significantly increased heart rate only in female BHR (P < 0.01, Fig. 2, bottom). Stress significantly increased both arterial pressure and heart rate (P < 0.01 for time, Figs 3 and 4). In WKY females only, arterial pressure was significantly greater at the 35 min time point (Fig. 3 left, top), and the change in pressure from baseline was significantly greater at several time points in the Dex-treated relative to the saline-treated rats (Fig. 3 left, bottom). Heart rate during stress was significantly higher in prenatal Dex- versus saline-treated BHR males at multiple time points, and in WKY males at the 35 min time point (Fig. 4, bottom left and middle). Prenatal Dex did not alter the integrated change in heart rate during stress (Fig. 4, right panel).
Figure 1. Body weights on day 1 in male and female pups combined (left), and in female (middle) and male rats (right) at 10 weeks of age.
a, P≤ 0.05 for BHR–saline versus WKY–saline; b, P≤ 0.05 for WKY–Dex versus WKY–saline; and c, P≤ 0.05 for BHR–Dex versus BHR–saline.
Figure 2. Baseline mean arterial pressure in mmHg (top) and heart rate in beats min−1 (bpm; bottom) in female (left) and male (right) rats.
a, P≤ 0.05 for WKY–saline versus BHR–saline; and c, P≤ 0.05 for BHR–Dex versus BHR–saline.
Figure 3. Mean arterial pressure during 60 min of restraint stress in female (left) and male (right) rats.
Absolute values are shown in the top panels, changes from baseline are below. a, P≤ 0.05 for BHR–saline versus WKY–saline; and b, P≤= 0.05 for WKY–Dex versus WKY–saline. Stress significantly (P < 0.01) increased arterial pressure above baseline; for clarity this is not noted within the figure.
Figure 4. Heart rate in beats min−1 (bpm) during 60 min of restraint stress in female (top) and male (bottom) rats.
Absolute values for WKY (left panel) and BHR (middle panel) are shown separately for clarity. Right panel shows the integrated change in heart rate over 60 min of restraint. a, P≤ 0.05 for BHR–saline versus WKY–saline; b, P≤ 0.05 for WKY–Dex versus WKY–saline; and c, P≤ 0.05 for BHR–Dex versus BHR–saline. Stress significantly (P < 0.01) increased heart rate above baseline; for clarity this is not noted within the figure.
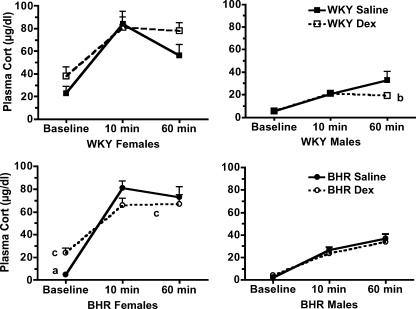
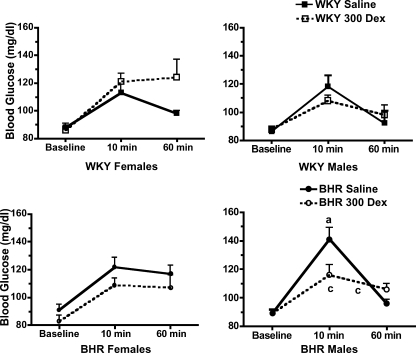
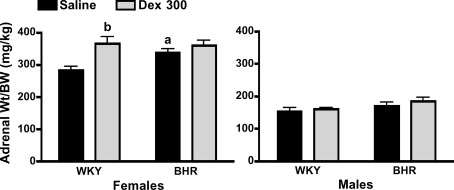
Stress significantly increased blood glucose and plasma corticosterone concentrations (P < 0.01 for time, Figs 5 and 6). In female BHR, prenatal Dex significantly (P < 0.01) increased midday baseline plasma corticosterone, but did not alter the absolute values for corticosterone at either 10 or 60 min of restraint (Fig. 5, bottom left). Prenatal Dex prolonged the corticosterone response to stress in female BHR as evidenced by the significant attenuation of the reduction in plasma corticosterone between 10 and 60 min of restraint (P = 0.01). In contrast, in males prenatal Dex reduced plasma corticosterone at 60 min only in WKY rats (P = 0.04, Fig. 5, right panel). Plasma glucose was unaffected by prenatal Dex in female rats (Fig. 6, left panel). In male BHR, Dex treatment significantly attenuated the glucose response to stress at 10 min (P = 0.01, Fig. 6, bottom right), and attenuated the subsequent fall in glucose between 10 and 60 min of stress (P < 0.01). Overall, stress increased plasma insulin concentration (P < 0.01 for time, Table 1). However, prenatal Dex did not significantly alter insulin levels during stress (Table 1). Adrenal weight normalized to body weight was increased by prenatal Dex only in female WKY rats (P < 0.01, Fig. 7), while thymus weight was unaffected by prenatal Dex (Table 1).
Figure 5. Plasma corticosterone (Cort) concentration during the baseline period and after 10 and 60 min of restraint stress in WKY (top) and BHRs (bottom).
a, P≤ 0.05 for BHR–saline versus WKY–saline; b, P≤ 0.05 for WKY–Dex versus WKY–saline; and c, P≤ 0.05 for BHR–Dex versus BHR–saline. Stress significantly (P < 0.01) increased plasma corticosterone concentration; for clarity this is not noted within the figure.
Figure 6. Blood glucose concentration during the baseline period and after 10 and 60 min of restraint stress in WKY (left) and BHRs (right).
a, P≤ 0.05 for BHR–saline versus WKY–saline; and c, P≤ 0.05 for BHR–Dex versus BHR–saline. Stress significantly (P < 0.01) increased blood glucose concentration; for clarity this is not noted within the figure.
Figure 7. Adrenal weight normalized to body weight in female (left) and male (right) rats.
a, P≤ 0.05 for BHR–saline versus WKY–saline; and b, P≤ 0.05 for WKY–Dex versus WKY–saline.
Differences between WKY and BHR
Data from WKY and BHR saline-treated rats were compared. Body weights at day 1 and 10 weeks of age were significantly lower in BHR relative to WKY rats (P < 0.01, Fig. 1). Baseline arterial pressure was significantly greater in both male and female BHRs relative to WKY rats (P < 0.01, Fig. 2), while heart rate was reduced in BHR females only (P < 0.01, Fig. 2). The change in arterial pressure during stress was not significantly different between rat strains (Fig. 3). The integrated heart rate response to stress was greater (P = 0.02) in female BHR relative to WKY rats, but was unaffected by strain in male rats (Fig. 4). Unexpectedly, baseline plasma corticosterone was lower in female BHRs relative to WKY rats (P = 0.05, Fig. 5). There were no other between-strain differences in plasma corticosterone. Plasma glucose at 10 min of stress was greater in BHR males relative to WKY males (P < 0.01, Fig. 5), but there were no effects of rat strain on plasma insulin concentration (Table 1). Adrenal weight normalized to body weight was significantly increased in BHR females relative to WKY females (P < 0.01, Fig. 7), while thymus weight was significantly increased in BHR males only (P = 0.04, Table 1).
Supplemental analysis
As in many other studies on prenatal programming (Levitt et al. 1996; McDonald et al. 2003; Igosheva et al. 2007; Singh et al. 2007), we studied more than one pup from each litter. However, to ensure that this did not affect the validity of our data, we performed an additional analysis on the data for baseline arterial pressure averaging data from all rats from a single litter and using the average number as one observation. As with the previous analysis, prenatal Dex had no significant effect on mean arterial pressure in WKY rats (111 ± 2 and 109 ± 1 mmHg for saline and Dex, respectively, in females; 109 ± 3 and 113 ± 2 mmHg, in males). In BHR, prenatal Dex significantly increased mean arterial pressure in both females (122 ± 2 versus 130 ± 2 mmHg, saline versus Dex, P < 0.01) and males (122 ± 3 and 132 ± 3 mmHg, saline versus Dex, P = 0.02). This supplemental analysis verifies the results reported above.
Discussion
This study demonstrates that BHR, who have a genetic predisposition to environmentally induced hypertension, are more susceptible to the hypertensive effects of dexamethasone-mediated prenatal programming compared with WKY rats. In female offspring, the hypertension was associated with elevated midday baseline heart rate and plasma corticosterone, in addition to a prolonged corticosterone response to acute stress. In male rats, the hypertension was associated with elevated heart rate during acute stress, although the integrated change in heart rate was not affected. However, prenatal Dex programmed enhanced cardiovascular responses to stress preferentially in WKY rats. Further experiments using additional strains of rats are needed to determine if the results reported here are generalisable to other models and strains of rats, or if the influence of genetic background on the effects of the intrauterine environment is specific to the BHR.
The BHR model
Essential hypertension results from a complex, poorly understood, interaction of multiple genetic and environmental factors (Izzo & Black, 2003). Analogously, BHR express a genetically based moderate elevation in baseline blood pressure and are sensitive to environmental factors known to elevate blood pressure in humans, although previous studies have been performed only in male BHR (Sanders & Lawler, 1992). Environmental factors known to increase blood pressure in BHR include psychological stress, a high salt diet, a high fructose diet or exposure to cold (Sanders & Lawler, 1992; Han et al. 1998; Masai et al. 2001). Susceptibility of BHR to the prenatal environment was suggested by a study demonstrating that a maternal high salt diet from conception through weaning increased arterial pressure in adult male BHR fed a 1% sodium chloride diet (Hunt & Tucker, 1993). The present study provides novel evidence that female BHR also have moderately elevated baseline arterial pressure, and that prenatal glucocorticoid treatment programs a further elevation in baseline arterial pressure in BHR but not WKY rats. The increases in baseline arterial pressure programmed by prenatal Dex treatment, when added to the moderate genetic hypertension of the BHR, results in baseline mean arterial pressures above 130 mmHg. Previous reports provide no consensus regarding increased stress reactivity of blood pressure, heart rate and the hypothalamic–pituitary–adrenal (HPA) axis in male BHR relative to WKY rats (Woodworth et al. 1990; Sanders & Lawler, 1992; Mansi & Drolet, 1997; Fuchs et al. 1998; Mansi et al. 1998). We did not observe any increase in cardiovascular or HPA axis reactivity in saline-treated male BHR relative to WKY rats, although the glucose response to stress was enhanced in the male BHR. Our observation of an enhanced heart rate response to stress in female BHR has not been reported previously. Collectively, our data suggest that BHR can be used as a unique model to study the interactive influences of genetic predisposition, prenatal environment and the adult environment on the development of hypertension.
Prenatal programming
Hypertension is one of the most commonly reported consequences of prenatal exposure to excess glucocorticoids (Benediktsson et al. 1993; Levitt et al. 1996; Celsi et al. 1998; Langdown et al. 2001; Sugden et al. 2001; Ortiz et al. 2003; O'Regan et al. 2004; Alexander, 2006; Woods, 2006; Singh et al. 2007). Surprisingly, prenatal Dex treatment did not increase baseline arterial pressure in male or female WKY rats in the current study. Several factors could explain this discrepancy including the Dex dose, timing of treatment, rat strain used and method of blood pressure measurement. Dex was administered at the optimal time in gestation to elicit an increase in baseline arterial pressure (Ortiz et al. 2001), at a dose within the range that glucocorticoids are used clinically to promote lung maturation prior to anticipated premature birth (Hardman et al. 2006). Since prenatal Dex-induced hypertension has been reported in rats as early as 3 weeks (Ortiz et al. 2003) any elevation in baseline arterial pressure should have been evident when we measured arterial pressure in adult rats. BHR are the F1 generation of an SHR–WKY cross, therefore WKY are the most appropriate control animals for comparison of Dex programming effects. However, nearly all previous studies have utilized the Sprague–Dawley or Wistar rat strains (Benediktsson et al. 1993; Levitt et al. 1996; Celsi et al. 1998; Langdown et al. 2001; Martins et al. 2003; Ortiz et al. 2003; O'Regan et al. 2004; Dagan et al. 2006; Woods, 2006; Igosheva et al. 2007; Singh et al. 2007). Although we would expect similar effects of Dex in WKY as in Wistar and Sprague–Dawley rats, we cannot rule out the possibility that differences in sensitivity to the programming effects of Dex may exist even among normotensive strains.
A more likely explanation for the differences between this study and those of previous investigators is that the blood pressure effects reported following prenatal glucocorticoid treatment depend on the method used to measure blood pressure. Most previous studies have relied on the tail cuff plethysmography to estimate baseline arterial pressure, with many of these studies reporting elevations in blood pressure at similar or lower doses of Dex than were used in the current study (Celsi et al. 1998; Langdown et al. 2001; Ortiz et al. 2001, 2003; Sugden et al. 2001; O'Regan et al. 2004). However, the tail cuff method requires restraining and heating animals, usually measures only systolic blood pressure at only one or a limited number of time points and assumes that there are no differences in cardiovascular responses to restraint and/or heat stress between treatment groups (Kurtz et al. 2005; Nathanielsz, 2006). This assumption is challenged by recent studies in other models of prenatal programming known to be dependent on elevated glucocorticoids that have reported enhanced cardiovascular responses to acute stress but no elevation in baseline blood pressure when blood pressure is assessed by radiotelemetry (Tonkiss et al. 1998; Igosheva et al. 2004, 2007). Relatively few investigators have directly measured blood pressure with indwelling arterial catheters, and they have often measured pressure for only brief (minutes) or unspecified time periods (Benediktsson et al. 1993; Levitt et al. 1996; Martins et al. 2003; McDonald et al. 2003). McDonald et al. (2003) reported no effect of prenatal glucocorticoid treatment on blood pressure under true baseline conditions in freely moving rats in which blood pressure was measured over 24 h. Martins et al. (2003) also failed to detect an elevation in baseline arterial pressure with prenatal betamethasone treatment. Conversely, Levitt et al. (1996) observed elevated arterial pressure in Dex-treated rats, although they used brief (10 min) recordings with indwelling arterial catheters, and Benediktsson et al. (1993) reported elevated blood pressure when assessed with three measurements of unspecified length. The values for arterial pressure reported here were obtained using indwelling arterial catheters in rats that were freely moving within their home cages at least 4 days following survival surgery. To obtain an accurate resting baseline, blood pressure was measured in the animal's home cage continuously for 3 h on two to three consecutive days, with the final hour of recording on the final day used to determine the reported baseline values. This recording duration was selected based on preliminary experiments in our laboratory demonstrating that blood pressure continued to stabilize during the first 2 h of recording. Even in the absence of an effect on baseline blood pressure, this and previous studies report other programming effects such as reduced body weight at birth and enhanced stress reactivity with prenatal glucocorticoid treatment (Martins et al. 2003; McDonald et al. 2003). Thus, it seems likely that variations in methods of blood pressure measurement techniques account for differences in reported effects of prenatal glucocorticoids on baseline arterial pressure between studies (Benediktsson et al. 1993; Ortiz et al. 2001, 2003; Armitage et al. 2004; O'Regan et al. 2004; Nathanielsz, 2006). The validity of the present results is supported by a recent statement from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research that recommends that baseline blood pressure be determined by direct measurement of blood pressure by indwelling catheters or by radiotelemetry whenever feasible (Kurtz et al. 2005).
Enhanced cardiovascular responses to acute stress, even in the absence of elevated arterial pressure, are associated with the development of cardiovascular disease later in life (Izzo & Black, 2003) and may be programmed prenatally in humans (Ward et al. 2003). In rats, prenatal stress (Igosheva et al. 2004) or dietary protein restriction (Tonkiss et al. 1998) increased arterial pressure during or following acute stress. Here we reported that prenatal Dex increased heart rate in male rats and arterial pressure in female WKY rats during acute restraint; however, we did not monitor blood pressure and heart rate during the post-stress recovery period. Furthermore, there might have been greater effects of prenatal Dex on the cardiovascular response to a stressor that elicits smaller elevations in arterial pressure and heart rate than does restraint. It is also possible that prenatal Dex could differentially modulate the stress response in females during different phases of the oestrous cycle; however, this cannot be addressed by the present study for two reasons. First, oestrous cycle phase was determined on only one day, so it is not clear that the female rats were cycling normally. Second, there were not sufficient numbers of rats to subdivide the females into groups according to phase of the cycle. Therefore, additional experiments would be needed to address this issue.
Elevated HPA axis function in adulthood is proposed as one mechanism mediating effects of an adverse prenatal environment to increase risk for cardiovascular and other diseases in adulthood (Clark, 1998; Phillips et al. 1998; Owen et al. 2005). In the present study, prenatal Dex treatment induced an increase in adrenal weight in female WKY, and an increase in midday baseline corticosterone and an attenuated recovery from peak corticosterone concentration in female BHR, suggesting that HPA feedback inhibition was impaired. In humans, birth weight has been linked to elevated cortisol levels in adulthood (Clark, 1998; Phillips et al. 1998; Kapoor et al. 2006). In animal studies, others have reported increased HPA axis function resulting from prenatal programming (Kapoor et al. 2006). The gender specificity of the HPA axis programming is variable, with the vulnerability of the HPA axis to programming depending on the timing and duration of the exposure to an adverse intrauterine environment in addition to the age at which the adult animal is studied (Owen et al. 2005; Kapoor et al. 2006). While we found evidence for elevated HPA axis function only in female rats, we did not measure sensitivity to the effects of glucocorticoids, which can also be affected by prenatal programming (Bertram & Hanson, 2001; Seckl & Meaney, 2004; Kapoor et al. 2006). Furthermore, we measured baseline corticosterone values in the middle of the day, and at only one time point at rest and two time points during stress. Although BHR females in general had increased adrenal weights, prenatal Dex did not cause a significant further increase in adrenal weight in these rats, suggesting that the 24 h average adrenocorticotropic hormone concentration was not elevated by prenatal Dex. Clearly, additional studies focusing on the assessment of the HPA axis are required to fully evaluate HPA axis function in this model.
Insulin resistance, altered glucose handling and increased risk for diabetes are reported metabolic effects of prenatal programming (Phillips et al. 1998; Barker, 2003; Armitage et al. 2004; Fagerberg et al. 2004; Sloboda et al. 2005; Stocker et al. 2005), and are risk factors for cardiovascular disease (Izzo & Black, 2003). In the present study, Dex-treated BHR males had a reduced peak glucose response to stress, followed by an attenuated recovery, and there were no effects of Dex on insulin in either male or female rats. However, we did not measure fasting glucose and insulin or glucose tolerance, which may have been altered by the prenatal Dex treatment.
Prenatal programming by glucocorticoids and birth weight
Most clinical studies use birth weight as a biomarker for prenatal programming (Huxley et al. 2000). Correspondingly, a reduction in birth weight is commonly observed in animal models of programming, including prenatal glucocorticoid exposure (Gardner et al. 1998; Louey & Thornburg, 2005). Some studies report subsequent catch-up growth postnatally (Cleasby et al. 2003; O'Regan et al. 2004). In both humans (Huxley et al. 2000) and rats (O'Regan et al. 2004) high rates of catch-up growth are associated with higher blood pressure. In the current study Dex decreased birth weight in the WKY, but not BHR rats, although even without prenatal Dex exposure BHR pups were smaller than WKY saline-treated controls. Dex-treated male and female WKY remained smaller at 10 weeks compared with the saline controls, indicating they did not undergo complete catch-up growth. This may be one additional factor that could account for the lack of effect of prenatal Dex on baseline blood pressure in WKY rats. Nonetheless, prenatal Dex treatment altered cardiovascular and endocrine function in BHR though mechanisms independent of a further reduction in birth weight. This supports the hypothesis that low birth weight is simply an indicator of adverse intrauterine environment rather than the mechanism of intrauterine programming (Louey & Thornburg, 2005; Nathanielsz, 2006).
Perspectives
An adverse prenatal environment can raise the risk of cardiovascular disease later in life by multiple mechanisms that include increasing the risk for hypertension and enhancing stress reactivity (Barker, 2000; Doyle et al. 2000; Eriksson et al. 2000; Huxley et al. 2000; Ward et al. 2003; Fagerberg et al. 2004; Jones et al. 2007). However, the magnitude of the effect is variable. For example, prenatal glucocorticoid exposure increased adolescent systolic arterial pressure by 4.1 mmHg (Doyle et al. 2000), and a review of the literature reported that the relationship between increased blood pressure and decreased birth weight averages about 2 mmHg kg−1, with a wide range of between-study values (Huxley et al. 2000). Furthermore, the incidence of cardiovascular disease mortality is only partially accounted for by birth weight (Barker, 2000). The present study contributes two important findings. First, in BHR, who have a genetic predisposition to environmentally induced hypertension, there is an exacerbation of the hypertensive effects of prenatal programming. Second, relatively moderate effects of both genetic background and prenatal programming on baseline blood pressure are additive in BHR, and thus combine to exacerbate cardiovascular disease risk in both males and females. In addition, this study shows the female BHR have elevated baseline arterial pressure and adrenal weights, and enhanced changes in heart rate in response to stress compared with female WKY rats. Future basic and clinical studies should examine the role of genetic factors on the deleterious effects of an adverse prenatal environment.
Acknowledgments
This study was supported in part by grant R01 HL 076807 from the National Heart, Lung and Blood Institute.
References
- Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Comp Physiol. 2006;290:1–10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–357. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Coronary heart disease: a disorder of growth. Horm Res. 2003;59:35–41. doi: 10.1159/000067843. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CRW. Glucocorticoid exposure in utero: a new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA. Animal models and programming of metabolic syndrome. Br Med Bull. 2001;60:103–121. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- Celsi G, Kistner A, Aizman R, Eklof A-C, Ceccatelli S, Santiago AD, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- Clark PM. Programming of the hypothalamo-pituitary-adrenal axis and the fetal origins of adult disease hypothesis. Eur J Pediatr. 1998;157:S7–S10. doi: 10.1007/pl00014289. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Kelly PAT, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- Dagan A, Gattineni J, Cook VI, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Comp Physiol. 2006;292:R1230–R1235. doi: 10.1152/ajpregu.00669.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodic M, Moritz K, Koukoulas I, Wintour EM. Programmed hypertension: kidney, brain or both? Trends Endocrinol Metab. 2002;13:403–408. doi: 10.1016/s1043-2760(02)00693-8. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci. 2000;98:137–142. [PubMed] [Google Scholar]
- Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Bondjers L, Nilsson P. Low birth weight in combination with catch-up growth predicts the occurrence of metabolic syndrome in men at late middle age: the atherosclerosis and insulin resistance study. J Intern Med. 2004;256:254–259. doi: 10.1111/j.1365-2796.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- Forrester T. A critical evaluation of the fetal origins hypothesis and its implications for developing countries. J Nutr. 2004;134:211–216. doi: 10.1093/jn/134.1.191. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- Fuchs LC, Hoque AM, Clarke NL. Vascular and hemodynamic effects of behavioral stress in borderline hypertensive and Wistar-Kyoto rats. Am J Physiol Regul Comp Physiol. 1998;274:375–382. doi: 10.1152/ajpregu.1998.274.2.R375. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Jackson AA, Langley-Evans SC. The effect of prenatal diet and glucocorticoids on growth and systolic blood pressure in the rat. Proc Nutr Soc. 1998;57:235–240. doi: 10.1079/pns19980037. [DOI] [PubMed] [Google Scholar]
- Han S, Chen X, Cox B, Yang C-L, Wu Y-M, Naes L, Westfall T. Role of neuropeptide Y in cold stress-induced hypertension. Peptides. 1998;19:351–358. doi: 10.1016/s0196-9781(97)00297-0. [DOI] [PubMed] [Google Scholar]
- The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2006. [Google Scholar]
- Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–114. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- Hunt RA, Tucker DC. Developmental sensitivity to high dietary sodium chloride in borderline hypertensive rats. Hypertension. 1993;22:524–550. doi: 10.1161/01.hyp.22.4.542. [DOI] [PubMed] [Google Scholar]
- Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Klimova O, Anishchenko T, Glover V. Prenatal stress alters cardiovascular responses in adult rats. J Physiol. 2004;557:273–285. doi: 10.1113/jphysiol.2003.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igosheva N, Taylor PD, Poston L, Glover V. Prenatal stress in the rat results in increased blood pressure responsiveness to stress and enhanced arterial reactivity to neuropeptide Y in adulthood. J Physiol. 2007;582:665–674. doi: 10.1113/jphysiol.2007.130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo JI, Black HR. Hypertension Primer. Philadelphia: Lippincott, Williams & Wilkens; 2003. [Google Scholar]
- Jones A, Beda A, Ward AMV, Osmond C, Phillips DIW, Moore VM, Simpson DM. Size at birth and autonomic function during psychological stress. Hypertension. 2007;49:548–555. doi: 10.1161/01.HYP.0000257196.13485.9b. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of the hypothalamo–pituitary–adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals: Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council On High Blood Pressure Research. Arterioscler Thromb Vasc Biol. 2005;25:e22–e33. doi: 10.1161/01.ATV.0000158419.98675.d7. [DOI] [PubMed] [Google Scholar]
- Langdown ML, Smith ND, Sugden MC, Holness MJ. Excessive glucocorticoid exposure during late intrauterine development modulates expression of cardiac uncouplng proteins in adult hypertensive male offspring. Pflugers Arch. 2001;442:248–255. doi: 10.1007/s004240100519. [DOI] [PubMed] [Google Scholar]
- Lawler JE, Cox RH, Sanders BJ, Mitchell VP. The Borderline Hypertensive Rat: a model for studying the mechanisms of environmentally induced hypertension. Health Psychol. 1988;7:137–147. doi: 10.1037//0278-6133.7.2.137. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev. 2005;81:745–751. doi: 10.1016/j.earlhumdev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Franco KL, Brown JM, Jenkins SL, Nathanielsz PW, Nijland MJ. Betamethasone in the last week of pregnancy causes fetal growth retardation but not adult hypertension in rats. J Soc Gynecol Invest. 2003;10:469–473. doi: 10.1016/s1071-5576(03)00151-5. [DOI] [PubMed] [Google Scholar]
- Mansi JA, Drolet G. Chronic stress induces sensitization in sympathoadrenal response to stress in borderline hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R813–R820. doi: 10.1152/ajpregu.1997.272.3.R813. [DOI] [PubMed] [Google Scholar]
- Mansi JA, Rivest S, Drolet G. Effect of immobilization stress on transcriptional activity of inducible immediate-early genes, corticotropin-releasing factor, its type 1 receptor, and enkephalin in the hypothalamus of borderline hypertensive rats. J Neurochem. 1998;70:1556–1566. doi: 10.1046/j.1471-4159.1998.70041556.x. [DOI] [PubMed] [Google Scholar]
- Martins JP, Monteiro JC, Paixao AD. Renal function in adult rats subjected to prenatal dexamethasone. Clin Exper Pharm Physiol. 2003;30:32–37. doi: 10.1046/j.1440-1681.2003.03787.x. [DOI] [PubMed] [Google Scholar]
- Masai M, Fujioka Y, Fujiwara M, Morimoto S, Miyoshi A, Suzuki H, Iwasaki T. Activation of Na+/H+ exchanger is associated with hyperinsulinemia in borderline hypertensive rats. Eur J Clin Invest. 2001;31:193–200. [PubMed] [Google Scholar]
- Moritz KM, Boon WM, Wintour EM. Glucocorticoid programming of adult disease. Cell Tissue Res. 2005;322:81–88. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J. 2006;47:74–82. doi: 10.1093/ilar.47.1.73. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Quan A, Zarzar F, Weinburg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Barker DJ, Fall CHD, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endorinol Metab. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci Biobehav Rev. 1992;16:207–217. doi: 10.1016/s0149-7634(05)80181-2. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol. 2004;286:H458–H467. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, Moritz KM. Prenatal glucocorticoid exposure results in altered AT1/AT2, nephron deficit and hypertension in rat offspring. J Physiol. 2007;579:503–513. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda DM, Challis JRG, Moss TJM, Newnham JP. Synthetic glucocorticoids: antenatal administration and long-term implications. Curr Pharm Des. 2005;11:1459–1472. doi: 10.2174/1381612053507873. [DOI] [PubMed] [Google Scholar]
- Stocker CJ, Arch JRS, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc. 2005;64:143–145. doi: 10.1079/pns2005417. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- Szitanyi P, Janda J, Poledne R. Intrauterine undernutrition and programming as a new risk of cardiovascular disease in later life. Physiol Res. 2003;52:389–395. [PubMed] [Google Scholar]
- Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VLM. Prenatal malnutrition-induced changes in blood pressure; dissociation of stress and nonstress responses using radiotelemetry. Hypertension. 1998;32:108–114. doi: 10.1161/01.hyp.32.1.108. [DOI] [PubMed] [Google Scholar]
- Ward AMV, Moore VM, Steptoe A, Cockington RA, Robinson JS, Phillips DIW. Size at birth and cardiovascular responses to psychological stressors: evidence for prenatal programming in women. J Hypertens. 2003;22:2295–2301. doi: 10.1097/00004872-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Comp Physiol. 2006;291:R1069–R1075. doi: 10.1152/ajpregu.00753.2005. [DOI] [PubMed] [Google Scholar]
- Woodworth CH, Knardahl S, Sanders BJ, Kirby RF, Johnson AK. Dam strain affects cardiovascular reactivity to acute stress in BHR. Physiol Behav. 1990;47:139–144. doi: 10.1016/0031-9384(90)90052-6. [DOI] [PubMed] [Google Scholar]