Abstract
We have studied the effect of the cholinergic agonist carbachol on the spontaneous release of glutamate in cultured rat hippocampal cells. Spontaneous excitatory postsynaptic currents (sEPSCs) through glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type channels were recorded by means of the patch-clamp technique. Carbachol increased the frequency of sEPSCs in a concentration-dependent manner. The kinetic properties of the sEPSCs and the amplitude distribution histograms were not affected by carbachol, arguing for a presynaptic site of action. This was confirmed by measuring the turnover of the synaptic vesicular pool by means of the fluorescent dye FM 1–43. The carbachol-induced increase in sEPSC frequency was not mimicked by nicotine, but could be blocked by atropine or by pirenzepine, a muscarinic cholinergic receptor subtype M1 antagonist. Intracellular Ca2+ signals recorded with the fluorescent probe Fluo-3 indicated that carbachol transiently increased intracellular Ca2+ concentration. Since, however, carbachol still enhanced the sEPSC frequency in bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetate-loaded cells, this effect could not be attributed to the rise in intracellular Ca2+ concentration. On the other hand, the protein kinase inhibitor staurosporine as well as a down-regulation of protein kinase C by prolonged treatment of the cells with 4β-phorbol 12-myristate 13-acetate inhibited the carbachol effect. This argues for an involvement of protein kinase C in presynaptic regulation of spontaneous glutamate release. Adenosine, which inhibits synaptic transmission, suppressed the carbachol-induced stimulation of sEPSCs by a G protein-dependent mechanism activated by presynaptic A1-receptors.
The occurrence of memory deficits in neurodegenerative disorders, such as Alzheimer disease, or the effects of cholinergic drugs on learning and memory have revealed an important role of the central cholinergic system in human cognitive functions (1–4). The hippocampus, which is known to be involved in memory formation (1, 4), receives cholinergic neurons from the basal forebrain. The cholinergic septohippocampal pathway modulates the activity of the hippocampus. For instance it is involved in the control of the theta rhythmic activity (5).
Hippocampal cells express cholinergic nicotinic and muscarinic receptors with a predominance of the latter (5). Five muscarinic cholinergic receptor subtypes (M1–M5) have been identified (6). M1, M3, and M5 are coupled to phospholipase C activation resulting in the production of the second messengers inositol trisphosphate (IP3) and diacylglycerol, whereas M2 and M4 inhibit adenylate cyclase activity (6). Immunoprecipitation studies, measurements of mRNA levels and of ligand binding sites indicate that the M1-subtype is the most abundant muscarinic receptor in the hippocampus (7). However, the role of M1-receptor activation and the subsequent signaling pathway in neurotransmitter release are only poorly understood. We have studied the effect of the cholinergic agonist carbachol on glutamate release by measuring spontaneous excitatory postsynaptic currents (sEPSCs) by means of the whole-cell patch-clamp technique. In addition, we have measured directly presynaptic vesicle turnover by means of the styryl dye FM 1–43 (8, 9). Our results provide evidence for a muscarinic presynaptic enhancement of spontaneous transmitter release by carbachol. This modulation depends on stimulation of M1-receptors and subsequent activation of protein kinase C (PKC). Adenosine, which is known to inhibit synaptic transmission (10), prevented the carbachol effect by a G protein-dependent mechanism.
MATERIALS AND METHODS
The preparation of the hippocampal cell cultures from 3- to 5-day-old neonatal Sprague–Dawley rats has been described in detail elsewhere (9, 11, 12). The protocol consists in an enzymatic treatment (3.4 mg/ml trypsin type XI + 0.9 mg/ml DNase type IV) followed by a gentle mechanical dissociation in Ca2+-free Hanks’ solution supplemented with 12 mM MgSO4, 0.4 mg/ml DNase, and 3 mg/ml BSA. The cell suspension was centrifuged twice (80 × g) and the hippocampal cells were then plated on poly-l-ornithine-coated glass coverslips inside a small volume (≈160 μl) limited by a cloning cylinder fixed with silicone grease (Dow Corning). The minimal essential medium was supplemented with 29.2 mg/ml glutamax, 6,000 mg/liter glucose, 25 mg/liter insulin, 100 mg/liter transferrin, 5 mg/liter gentamycin, and 10% fetal calf serum. At day 2 cytosine β-d-arabinoside (3 μM) was added to reduce astrocyte growth, 2% B-27 supplement was added, and and the fetal calf serum was reduced to 5%. All experiments were performed 7–16 days after the plating of the cells.
The experimental procedures for the electrophysiological measurements and analyses have been described (13, 14). Briefly, the sEPSCs were recorded at a holding potential of −50 mV by means of the whole-cell configuration of the patch-clamp technique (15). We prefer the nomenclature “spontaneous” rather than “miniature” EPSC throughout, although most of the external solutions contained tetrodotoxin (0.1–0.6 μM) to block Na+ channels. The currents, filtered at 1–2 kHz, were sampled on-line at 10 kHz (gain: 20 mV/pA, EPC7 amplifier, List Electronics, Darmstadt, Germany) on the hard disk of a 75-MHz Pentium computer running with the ced 6.0 software (Cambridge Electronics Design, Cambridge, U.K.). The external medium contained 135 or 150 mM NaCl, 5 mM KCl, 2 mM MgCl2, 10 mM CaCl2, 10 mM Hepes, 30 mM glucose, 0.01 mM bicuculline, and 0.1 mM CdCl2, pH 7.4 (NaOH). The pipette solution contained 135 mM CsF or Cs gluconate, 2 mM MgCl2, 5–10 mM EGTA, 4 mM Na2ATP, 0.2–0.4 mM GTP, and 10 mM Hepes, pH 7.2 (CsOH). Carbachol- and adenosine-containing solutions were added by gravity to the experimental chamber on the stage of an inverted microscope. The currents were analyzed with a fully automated software written by A. Dityatev (Molecular Neurobiology Center, University of Hamburg, Hamburg, Germany) based on a published algorithm (16). To quantify the frequency change, the cells were always voltage-clamped for at least 5 min prior to the addition of the drugs or their vehicle and the sEPSCs were sampled for two times for 3.1 s per min during that period. The change in sEPSC frequency, 5 min after drug application, was obtained by sampling sEPSCs five to seven times for 3.1 s each. The results were expressed as: freqafter carbachol/freqbefore carbachol.
The effect of carbachol on the cycling of the vesicular pool in presynaptic boutons has been investigated with the fluorescent probe FM 1–43 (8, 9). All experiments were carried out with a confocal Laser Scan microscope (LSM 410, Zeiss) as described (12, 13, 17). For the FM 1–43 loading into presynaptic boutons the cells were superfused with the external medium described above and supplemented with 0.01 mM 6-cyanonitroquinoxaline-2,3-dione (CNQX); 0.01 or 0.1 mM hexamethonium, and 0.1 mM CdCl2. The dye concentration in the loading solution was 15 μM, the exposure time was 2 min. After extensive washout of superficial FM 1–43 from the cells, the dye was unloaded from the labeled boutons by electrical field stimulation (20 Hz for 30 s) (12, 13). Intracellular Ca2+ concentrations ([Ca2+]i) were measured with the same confocal microscope in cells loaded with 10 μM Fluo-3-AM for 30–50 min (13, 17).
All experiments were carried out at room temperature. When possible, the data are presented as mean ± SEM.
RESULTS
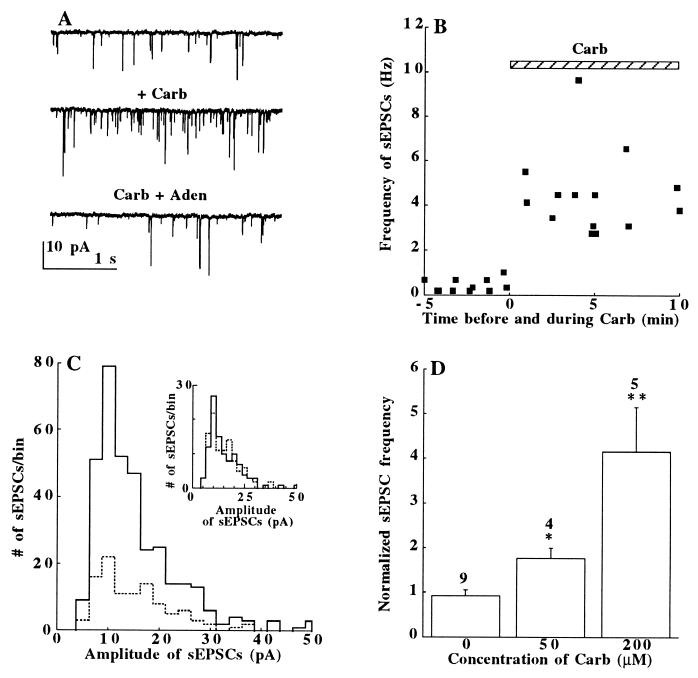
We have used the nonselective cholinergic agonist carbachol to investigate the mechanism of cholinergic stimulation on spontaneous glutamate release by recording sEPSCs in enzymatically dissociated neonatal rat hippocampal cells in culture. Fig. 1A shows whole-cell patch-clamp recordings of sEPSCs through glutamate activated α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptor channels. Superfusion of the cells with carbachol (200 μM), in the presence of the γ-aminobutyric acid type A (GABAA) receptor blocker bicuculline (10 μM), increased the frequency of sEPSCs. This effect developed rapidly and persisted for many minutes (Fig. 1B). It was poorly reversible after washout of the drug. Plots of amplitude distribution histograms (Fig. 1C) indicate that carbachol increased the sEPSC frequency without affecting the current amplitudes [(14.4 ± 0.4 pA (n = 102) before and 14.4 ± 0.5 pA (n = 342) after carbachol]. Fig. 1C Inset shows that with an equal number of sEPSCs (n = 102), the amplitude distribution histograms under control conditions and in the presence of carbachol are similar. Furthermore, the rise times (10–90%) of the sEPSCs were not changed (0.85 ± 0.04 ms, n = 102, in control vs. 0.83 ± 0.02 ms, n = 342, in carbachol). Taken together, these results strongly argue for a presynaptic site of action of carbachol. The carbachol effect on sEPSC frequencies was concentration-dependent with 50 μM producing about a 2-fold (P < 0.05, n = 4) and 200 μM a 4- to 5-fold (P < 0.01, n = 5) enhancement of the sEPSC frequency (Fig. 1D). A similar effect was also observed with acetylcholine (50 μM, 2.5 ± 0.07-fold increase, P < 0.05, n = 4, not shown). The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type channel blocker 6-cyano-nitroquinoxaline-2,3-dione (10 μM) reversibly suppressed the sEPSCs in control solution and in carbachol-containing solutions (14).
Figure 1.
(A) sEPSCs recorded before carbachol (Top), after carbachol (Carb, 200 μM, Middle), and after adenosine addition in the presence of carbachol (Carb+Aden, 50 μM; Bottom). (B) The time-course of carbachol action on sEPSC. Bar = drug application. (C) Amplitude distribution histograms of sEPSCs sampled for 19 s before (⋅⋅⋅⋅⋅⋅) and after the addition of 200 μM carbachol (——). (Inset) The histograms with an equal number (n = 102) of sEPSCs to demonstrate their same amplitudes; bin size 2.5 pA. (D) Carbachol increased the sEPSC frequency (normalized to the controls = 1) in a concentration-dependent manner. The number of cells tested is above each bar. ∗, P < 0.05; ∗∗, P < 0.01. The data presented in A–C were collected from different cells.
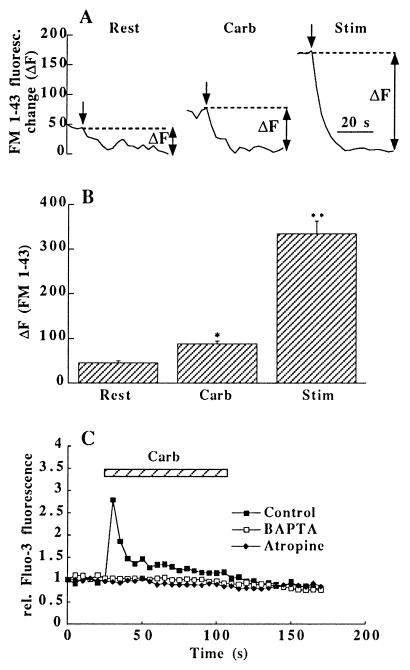
To provide further evidence for presynaptic modulation of exocytosis by carbachol, the styryl dye FM 1–43 was used to study the cycling of synaptic vesicles (9, 18). FM 1–43 (15 μM) was added for 2 min to resting cells before (Rest) and during treatment with carbachol (Carb, 200 μM). After the washout of the superficial dye, the cells were electrically stimulated to unload the endocytosed FM 1–43 from the presynaptic vesicular pool (12, 19). This was accompanied by a reduction of the fluorescence (ΔF, Fig. 2 A and B), which reflects the amount of dye specifically incorporated into the synaptic vesicles during the 2 min exposure. Carbachol doubled the uptake of dye measured in boutons identical to those that had been labeled at rest without the drug. The total size of the releasable pool was estimated by the amount of dye taken up into the same boutons during a third exposure period with 30 s field stimulation (20 Hz) followed by a 90 s period of endocytosis in FM 1–43 containing solution (19). In the presence of carbachol about a quarter of the total releasable pool recycled during the 2 min exposure to carbachol (Fig. 2B). In addition to the 29 boutons that could be identified in two culture dishes after each dye exposure (Fig. 2B), carbachol also increased the number of visible boutons as compared with Rest (not shown).
Figure 2.
Carbachol increases vesicle recycling and [Ca2+]i. (A) FM 1–43 was loaded into vesicles of individual boutons at rest without (Rest) and with addition of carbachol (Carb), or during electrical field stimulation (Stim); unloading of the dye (arrows) was achieved by 30 s periods of field stimulation (20 Hz); ΔF in the three traces indicates the dye uptake into a releasable vesicular pool of the same bouton. (B) Plot of ΔF for 29 identical boutons in two culture dishes under conditions described in A; the differences between the columns are highly significant (∗, P < 0.001; ∗∗, P < 0.0001). (C) Changes in [Ca2+]i measured after incubation of the cells in Fluo-3-AM (15 μM, >30 min); carbachol (200 μM) produced a transient Ca2+ rise in boutons identified by subsequent FM 1–43 loading; preincubation of the cells with BAPTA-AM (10 μM, >45 min) or atropine (10 μM, 2 min) inhibited this effect completely; average of 21 synaptic regions in 2 cultures.
The increased release of glutamate by carbachol could be due to either a nicotinic (20) or a muscarinic effect. Since nicotinic cholinergic ion channels are highly Ca2+-permeable (21, 22) this could account for the enhanced neurotransmitter release. We tested this hypothesis by measuring changes in the [Ca2+]i by means of the Ca2+-indicator Fluo-3-AM in synaptic regions after exposure to nicotine (40 or 80 μM) or carbachol (100 or 200 μM). While nicotine failed to have any effect on [Ca2+]i (not shown), carbachol produced a pronounced transient rise in [Ca2+]i (Fig. 2C), presumably due to Ca2+ release from intracellular stores (23, 24). This effect could be prevented by atropine (10 μM) but not by the nicotinic receptor blocker hexamethonium (100 μM; data not shown). Preincubation of the cells with the Ca2+-chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester (BAPTA-AM; 10 μM, >30 min), or with thapsigargin (2 μM, >1 h; data not shown) completely inhibited the rise in [Ca2+]i by carbachol.
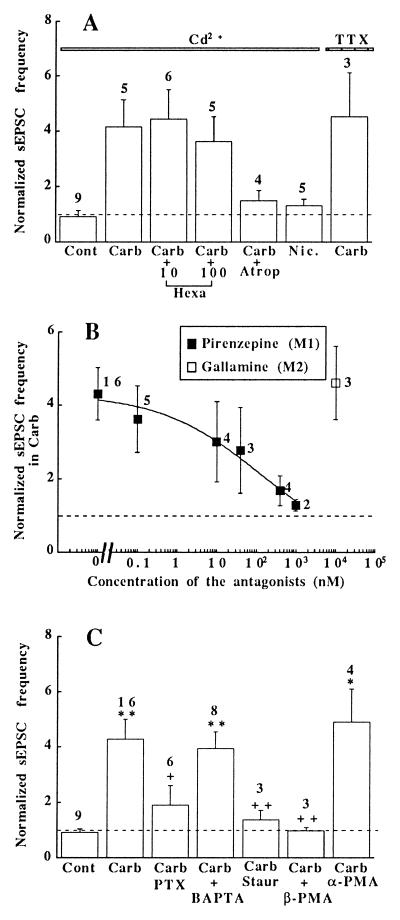
Fig. 3A shows the normalized (controls = 1) sEPSC frequencies under different experimental conditions. The nicotinic antagonist hexamethonium (10 or 100 μM) did not suppress the effect of carbachol, whereas atropine (10 μM) blocked it almost completely. Correspondingly, nicotine (40 μM) had no effect on the sEPSC frequency. Blockade of Na+ channels by tetrodotoxin and of Ca2+ channels by Cd2+ did not prevent the carbachol effect, excluding a possible involvement of increased excitation and enhanced Ca2+ influx. The carbachol-induced change in sEPSC frequency, however, was antagonized in a dose-dependent manner by pirenzepine, a M1-muscarinic receptor antagonist (6), whereas gallamine, which is more potent on the M2-receptor subtype (6, 25), had no effect (Fig. 3B).
Figure 3.
Dependence of the carbachol effect on activation of M1-receptors and PKC. (A) Fold increase in sEPSC frequency above control values (Cont = 1) under the following conditions (solutions contained 100 μM Cd2+ and 0.2 μM tetrodotoxin): Carb, 200 μM carbachol; Hexa, 10 or 100 μM hexamethonium + carbachol (200 μM); Atrop, 10 μM atropine + carbachol (200 μM); Nic., 40 μM nicotine. (B) The effect of carbachol (200 μM) on the sEPSC frequency was inhibited in a dose-dependent manner by the M1-antagonist pirenzepine (▪) but not by gallamine, a preferential M2-antagonist (□). (C) The carbachol effect depended on activation of G proteins and PKC, but not on elevation of [Ca2+]i: untreated cells (Cont); cells treated with carbachol alone (Carb, 200 μM), or after pretreatment with one of the following substances: PTX (150 ng/ml for 14–19 h); BAPTA (2–10 μM BAPTA-AM for >30 min); staurosporine (Staur, 800 nM for 70–100 min); β-PMA or α-PMA (100 nM 4β- or 4α-PMA for 14–19 h). ∗, P < 0.05 and ∗∗, P < 0.01 (vs. Cont); ++, P < 0.01 (vs. Carb). The number of cells tested is indicated above each bar.
G proteins (Gi and/or Go) seem to participate in the regulation of the sEPSC frequency by carbachol, since pretreatment of the hippocampal cells with pertussis toxin (PTX; 150 ng/ml) prevented the effect (Fig. 3C). Activation of M1-muscarinic receptors leads to the production of IP3 and diacylglycerol (6) which, in turn, may either cause a release of Ca2+ from IP3-sensitive stores and/or activation of PKC (26). If the elevation of Ca2+ (Fig. 2C) is responsible for the increased sEPSC frequency, treatment of the cells with BAPTA-AM should prevent the carbachol effect. However, after the BAPTA loading, carbachol still increased the sEPSC frequency. Also depletion of [Ca2+]i stores with thapsigargin (0.5–1 μM, n = 4, data not shown) failed to affect the change in sEPSC frequency (27). These results show that carbachol does not enhance the sEPSC frequency by the increase in [Ca2+]i. On the other hand, inhibition of the effect by staurosporine points to a possible role of a protein kinase in mediating the carbachol-induced modulation of synaptic transmission. This was further tested by treating the cells with the phorbol ester 4β-phorbol 12-myristate 13-acetate (4β-PMA; 100 nM) for 14–19 h to produce a down-regulation of PKC (26, 28). After this treatment, carbachol no longer enhanced the sEPSC frequency. However, the modulatory effect was still observed after treating the cells with the inactive analogue 4α-PMA. It is worth mentioning that carbachol (200 μM) and acute applications of PMA (100 nM) increased the sEPSC frequency to the same extent (14), but these effects were not additive, suggesting a common site of action (not shown). In contrast, 4β-PMA and activation of adenylate cyclase by forskolin did act additively (not shown) (29).
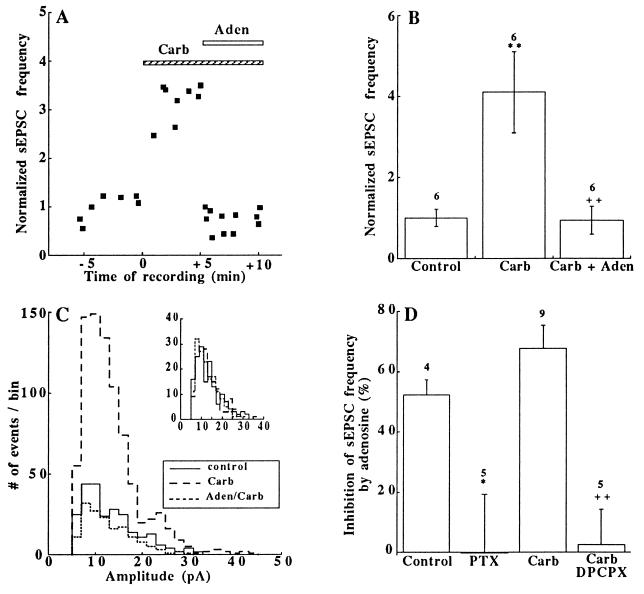
Adenosine that inhibits synaptic transmission (30, 31) also rapidly inhibited the carbachol response (Figs. 1A and 4 A and B). The amplitudes of sEPSCs were unaffected by adenosine (50 μM) (Fig. 4C), which argues for a presynaptic site of action of the drug (30, 31). Adenosine inhibited the sEPSC frequency without or with carbachol (Fig. 4D). However, because of the higher sEPSC frequency in the presence of carbachol, the absolute inhibition of the frequency by adenosine was larger than in the controls (Fig. 4B). Pretreatment of the cells with PTX (150 ng/ml) or with the A1-adenosine receptor blocker 8-cylcopentyl-1,3-dipropylxanthine (DPCPX) (5 μM) abolished this inhibition (Fig. 4D). Adenosine seems to inhibit the carbachol action by a G protein-dependent mechanism down-stream to PKC activation, since in the presence of 4β-PMA (100 nM) it reduced the sEPSC frequency by 42 ± 4% in five out of eight cells tested.
Figure 4.
Adenosine suppressed the carbachol-induced increase in sEPSC frequency. (A) Time-course of adenosine (Aden) action on sEPSC frequency after carbachol (Carb) application. (B) Normalized sEPSC frequencies in control (n = 1), 5 min after the addition of carbachol (Carb, 200 μM) and 5 min after the addition of adenosine (50 μM) in a carbachol-containing medium (Carb + Aden). ∗∗, P < 0.01 (vs. control); ++, P < 0.01 (vs. Carb). The number of cells tested is indicated above each bar. (C) Amplitude distribution histograms of sEPSCs sampled for 22.2 s before (——), after the addition of 200 μM carbachol (– – –), and in the presence of carbachol + adenosine (50 μM) (⋅⋅⋅⋅⋅⋅). (Inset) Histograms with an equal number (n = 156) of sEPSCs in each case; bin size 2.5 pA. (D) Inhibition (%) by adenosine (50 μM) of the sEPSC frequency; control: adenosine alone; PTX: after PTX treatment (150 ng/ml for 14–23 h); carbachol: after carbachol (200 μM) treatment; Carb, 8-cylcopentyl-1,3-dipropylxanthine: after treatment with carbachol (200 μM) and the A1-adenosine blocker 8-cylcopentyl-1,3-dipropylxanthine (5 μM). ∗, P < 0.05 (vs. control); ++, P < 0.01 (vs. Carb). The number of cells tested is indicated above each bar.
DISCUSSION
Most studies reported in the literature have dealt with inhibitory effects of muscarinic receptor stimulation on synaptic transmission in hippocampal slices (32, 33). We now show that muscarinic cholinergic stimulation of cultured hippocampal cells can increase the sEPSC frequency. A similar observation has been reported in hippocampal slices cocultured with septal explants, after stimulation of septal cholinergic neurons (34). Although that effect could be inhibited by atropine, the detailed mechanism of action has not been further analyzed. Carbachol modulates the spontaneous glutamate release probably by activating presynaptic M1-muscarinic receptors. Since the spontaneous glutamate release can be enhanced by an elevation of [Ca2+]i (13, 35), the transient Ca2+ elevation produced by carbachol could have increased the sEPSC frequency. However, BAPTA-loaded cells still responded to carbachol. Furthermore, thapsigargin not only failed to change [Ca2+]i in presynaptic boutons (12) but also the frequency of sEPSCs (27) indicating that Ca2+ ions released from the IP3-sensitive stores are not involved in this modulatory process. It is worth noting that previous immunohistochemical experiments revealed a nonuniform distribution of the IP3-receptors in hippocampal cells. They are mainly located in the dendritic shaft (but not in spines) and are rarely found in axons or nerve terminals (24, 36). Our results seem to differ from those showing that in CA1 hippocampal neurons BAPTA loading prevented the potentiating effect of acetylcholine on N-methyl-d-aspartate responses (37). This muscarinic effect was due to inhibition of IP3-dependent Ca2+ release, while PKC inhibition had no effect. These results, however, were probably due to a postsynaptic effect of acetylcholine in hippocampal slices that we have not studied.
In our experiments, carbachol increased the sEPSC frequency most likely by activating PKC. Since Ca2+ channels were blocked with Cd2+, the carbachol action was independent of the entry of Ca2+ through presynaptic voltage-gated Ca2+ channels. This is in agreement with previous reports showing that phorbol esters increase excitatory and inhibitory synaptic transmission in the presence of Cd2+ in hippocampal cells (14, 29). However, the exact molecular mechanism of action of PKC activators on neurotransmission is unknown. Phorbol esters enhance the exocytosis from bovine adrenal chromaffin cells by increasing the size of the readily releasable vesicular pool (38). In these cells, PKC activation appears to modulate a late step in secretion, but the Ca2+ sensitivity remains unaffected. A similar phenomenon seems to occur in our experiments with cultured hippocampal cells. Although we have no information that PKC substrates are involved in the increase in sEPSC frequency, several of them have been characterized in nerve cells. Thus, carbachol stimulates the phosphorylation of GAP-43 (39), a known PKC substrate that is thought to play a major role in neurotransmitter release and long-term potentiation (40). In addition, Munc-18, myristoylated alanine-rich C kinase substrate (MARCKS), soluble NSF attachment proteins (SNAP-25), and α-SNAP can be phosphorylated by PKC (41–44). Interestingly, protein kinase A activation can also lead to facilitation of transmitter release in hippocampal cells (29, 45) by directly increasing the probability of exocytosis (46). Again, this presynaptic effect is independent of changes in [Ca2+]i (46). Our results provide additional evidence that PKC activation could be involved in the regulation of synaptic plasticity (47, 48) by a muscarinic pathway. In agreement with this, muscarinic activation has been shown to facilitate the induction of long-term potentiation (49).
Adenosine is released from nerve cells during periods of ischemia but it can also accumulate as a result of breakdown of ATP that is released from presynaptic vesicles together with noradrenaline, serotonine, and acetylcholine (50). Adenosine inhibits synaptic transmission by activating presynaptic receptors by a G protein-dependent pathway (30, 31). The receptors are mainly expressed in axons of hippocampal neurons (51). A recent report showed that in hippocampal slices adenosine blocks acetylcholine-activated excitatory responses through an A1-receptor dependent mechanism (52). Our results agree with a G protein-dependent inhibition of spontaneous glutamate release by adenosine in untreated cells as well as after potentiation of the release by PKC activation. Since this inhibition occurred not only after carbachol treatment but also after direct activation of PKC by 4β-PMA, we conclude that the site of action on exocytosis must be downstream of the PKC activation. Again, it cannot be due do inhibition of Ca2+ influx in the nerve terminals since the Ca2+ channels had been blocked with Cd2+.
In conclusion, our results suggest an important PKC-dependent regulatory pathway for exocytosis. Stimulation of M1-receptors by carbachol can increase the sEPSC frequency through this pathway in a Ca2+-independent manner. Furthermore, adenosine reverses the PKC-induced stimulation of the spontaneous glutamate release. However, further studies are needed to clarify the exact site of action of the PKC isoform(s) activated by M1-muscarinic receptors, as well as the inhibitory action of adenosine on this effect.
Acknowledgments
We are most grateful to Charlotte Becker for preparing the cell cultures and to Beat H. Gähwiler (Zurich) for comments on the manuscript. This work was supported by Swiss National Science Foundation Grant 31-45099.95 to H.R.
ABBREVIATIONS
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester
- 4α- or 4β-PMA
4α- or 4β-phorbol 12-myristate 13-acetate
- M1–M5
muscarinic cholinergic receptor subtypes
- IP3
inositol trisphosphate
- PKC
protein kinase C
- PTX
pertussis toxin
- sEPSCs
spontaneous excitatory postsynaptic currents
- [Ca2+]i
intracellular Ca2+ concentration
References
- 1.Everitt B J, Robbins T W. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Growdon J H. Life Sci. 1997;60:993–998. doi: 10.1016/s0024-3205(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 3.Iversen S D. Life Sci. 1997;60:1145–1152. doi: 10.1016/s0024-3205(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 4.Zola-Morgan S, Squire L R. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- 5.Dutar P, Bassant M H, Senut M C, Lamour Y. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- 6.Hulme E C, Birdsall N J M, Buckley N J. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 7.Levey A I. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 8.Betz W J, Mao F, Bewick G S. J Neurosci. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan T A, Reuter H, Wendland B, Schweizer F E, Tsien R W, Smith S J. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- 10.Lupica C R, Dunwiddie T V. In: Presynaptic Receptors in the Mammalian Brain. Dunwiddie T V, Lovinger D M, editors. Boston: Birkhauser; 1993. pp. 104–126. [Google Scholar]
- 11.Malgaroli A, Tsien R W. Nature (London) 1992;357:134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- 12.Reuter H. Neuron. 1995;14:773–779. doi: 10.1016/0896-6273(95)90221-x. [DOI] [PubMed] [Google Scholar]
- 13.Bouron A, Reuter H. Neuron. 1996;17:969–978. doi: 10.1016/s0896-6273(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 14.Bouron A. FEBS Lett. 1997;404:221–226. doi: 10.1016/s0014-5793(97)00135-x. [DOI] [PubMed] [Google Scholar]
- 15.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Ankri N, Legendre P, Faber D S, Korn H. J Neurosci Methods. 1994;52:87–100. doi: 10.1016/0165-0270(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 17.Reuter H, Porzig H. Neuron. 1995;15:1077–1084. doi: 10.1016/0896-6273(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 18.Betz W J, Mao F, Smith C B. Curr Opin Neurobiol. 1996;6:365–371. doi: 10.1016/s0959-4388(96)80121-8. [DOI] [PubMed] [Google Scholar]
- 19.Ryan T A, Smith S J, Reuter H. Proc Natl Acad Sci USA. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray R, Rajan A S, Radcliffe K A, Yakehiro M, Dani J A. Nature (London) 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 21.Vidal C, Changeux J P. News Physiol Sci. 1996;11:202–208. [Google Scholar]
- 22.Wonnacott S. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 23.Kudo Y, Ogura A, Iijima T. Neurosci Lett. 1988;85:354–350. doi: 10.1016/0304-3940(88)90590-3. [DOI] [PubMed] [Google Scholar]
- 24.Seymour-Laurent K J, Barish M E. J Neurosci. 1995;15:2592–2608. doi: 10.1523/JNEUROSCI.15-04-02592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel A D, Delmendo R E, Lopez M, Whiting R L. Eur J Pharmacol. 1990;182:335–345. doi: 10.1016/0014-2999(90)90292-e. [DOI] [PubMed] [Google Scholar]
- 26.Nishizuka Y. Nature (London) 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 27.Trudeau L E, Doyle R T, Emery D G, Haydon P G. J Neurosci. 1996;16:46–54. doi: 10.1523/JNEUROSCI.16-01-00046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthies H J G, Palfrey H C, Hirning L D, Miller R J. J Neurosci. 1987;7:1198–1206. doi: 10.1523/JNEUROSCI.07-04-01198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capogna M, Gähwiler B H, Thompson S M. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanziani M, Capogna M, Gähwiler B H, Thompson S M. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 31.Scholz K P, Miller R J. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- 32.Nicoll R A. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 33.Williams S H, Johnston D. In: Presynaptic Receptors in the Mammalian Brain. Dunwiddie T V, Lovinger D M, editors. Boston: Birkhauser; 1993. pp. 27–41. [Google Scholar]
- 34.Gahwiler B H, Brown D A. Nature (London) 1985;313:577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- 35.Zucker R S. J Physiol (Paris) 1993;87:25–36. doi: 10.1016/0928-4257(93)90021-k. [DOI] [PubMed] [Google Scholar]
- 36.Sharp A H, McPherson P S, Dawson T M, Aoki C, Campbell K P, Snyder S H. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markram H, Segal M. J Physiol (London) 1992;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillis K D, Mössner R, Neher E. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 39.Van Hooff C O M, De Graan P N E, Oestreicher A B, Gispen W H. J Neurosci. 1989;9:3753–3759. doi: 10.1523/JNEUROSCI.09-11-03753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benowitz L I, Routtenberg A. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 41.Fujita Y, Sasaki T, Fukui K, Kotani H, Kimura T, Hata Y, Südhof T C, Scheller R H, Takai Y. J Biol Chem. 1996;271:7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- 42.Robinson P J. Mol Neurobiol. 1991;5:87–130. doi: 10.1007/BF02935541. [DOI] [PubMed] [Google Scholar]
- 43.Shimazaki Y, Nishiki T I, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- 44.Whiteheart S W, Griff I C, Brunner M, Clary D O, Mayer T, Buhrow S A, Rothman J E. Nature (London) 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- 45.Chavez-Noriega L E, Stevens C F. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trudeau L E, Emery D G, Haydon P G. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 47.Walaas S I, Greengard P. Pharmacol Rev. 1991;43:299–349. [PubMed] [Google Scholar]
- 48.Tanaka C, Nishizuka Y. Annu Rev Neurosci. 1995;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 49.Burgard E C, Sarvey J M. Neurosci Lett. 1990;116:34–39. doi: 10.1016/0304-3940(90)90382-j. [DOI] [PubMed] [Google Scholar]
- 50.Nicholls D G. Proteins, Transmitters and Synapses. Oxford: Blackwell; 1995. [Google Scholar]
- 51.Swanson T H, Drazba J A, Rivkees S A. J Comp Neurol. 1996;363:517–531. doi: 10.1002/cne.903630402. [DOI] [PubMed] [Google Scholar]
- 52.Morton R A, Davies C H. J Physiol (London) 1997;502:75–90. doi: 10.1111/j.1469-7793.1997.075bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]