Abstract
Pregnancy is associated with hyperphagia, increased fat mass, hyperleptinaemia and hyperprolactinaemia. The neuroendocrine control of bodyweight involves appetite-regulating centres in the hypothalamus, containing both orexigenic and anorexigenic neurons that express leptin receptors (LepR). In the rat, central leptin resistance develops during mid pregnancy, well after hyperphagia becomes apparent, to negate the appetite suppressing effects of leptin. We have investigated the hypothalamic response to leptin during pregnancy and examined the role of pregnancy hormones in inducing these changes. We have shown that there are multiple levels of leptin resistance during pregnancy. Despite elevated serum leptin, neuropeptide Y and agouti related peptide mRNA in the arcuate nucleus are not suppressed and may even be increased during pregnancy. LepR mRNA and leptin-induced pSTAT3 expression, however, are relatively normal in the arcuate nucleus. In contrast, both LepR and leptin-induced pSTAT3 are reduced in the ventromedial hypothalamic nucleus. Injecting α-melanocyte-stimulating hormone (α-MSH) into the brain, to bypass the first-order leptin-responsive neurons in the arcuate nucleus, also fails to suppress food intake during pregnancy, suggesting that pregnancy is also a melanocortin-resistant state. Using a pseudopregnant rat model, we have demonstrated that in addition to the changes in maternal ovarian steroid secretion, placental lactogen production is essential for the induction of leptin resistance in pregnancy. Thus, hormonal changes associated with pregnancy induce adaptive changes in the maternal hypothalamus, stimulating food intake and then allowing elevated food intake to be maintained in the face of elevated leptin levels, resulting in fat deposition to provide energy stores in preparation for the high metabolic demands of late pregnancy and lactation.
Introduction: metabolic adaptation to pregnancy
Pregnancy places significant physiological demands on a female mammal, with a conflict arising between the needs of the developing fetuses and the requirements to maintain the health of the mother. To cope with these novel demands, the hormones of pregnancy induce a coordinated range of adaptations to physiological functions in the mother. These adaptations include changes in the cardiovascular and respiratory systems, changes in immune function, behavioural changes, and extensive changes in neuroendocrine mechanisms governing homeostatic processes (Russell et al. 2001). One of the most profound adaptations is in energy homeostasis, where the mother faces competing needs of meeting her own energy demands, supplying nutrients to the growing fetus, and also establishing a positive energy balance to promote storage of energy resources in preparation for the huge metabolic demands of lactation. There are differences in nutrient partitioning to ensure adequate nutrition to the offspring, and changes in metabolic processing within different tissues. Perhaps the most important adaptation, however, is an increase in appetite and food intake. The process of ‘eating for two’, or ‘eating for many’, in the case of rodents, starts long before the actual metabolic demand affects the mother. Hence, the hormone-induced increase in food intake represents a true adaptive response to prepare the female for motherhood. In this review, we summarize the pregnancy-induced changes in the homeostatic systems that normally regulate food intake, and discuss the neuroendocrine mechanisms underlying these changes.
Neuroendocrine regulation of body weight
Body weight in most individuals is held relatively stable, even in the face of daily fluctuations in energy intake and energy expenditure, by complex interactions between short- and long-term regulators of energy balance. Hormones produced by the stomach (ghrelin) and gut (cholecystokinin and peptide YY) stimulate and suppress food intake, respectively, on a daily basis (Saper et al. 2002). Long-term regulators of bodyweight include the adipocyte-derived hormone, leptin, and to a lesser extent, insulin. Both are produced in the periphery and act centrally to maintain bodyweight homeostasis. Before the discovery of leptin (Zhang et al. 1994), it had been postulated that a secreted molecule circulates in the blood, increases in proportion to total body fat and modulates energy intake and energy expenditure over long periods of time (Weigle, 1994). Leptin fulfilled the criteria of an adiposity signal; circulating levels of leptin in humans and rodents correlate with body adiposity and levels change in accordance with nutritional status (Maffei et al. 1995; Considine et al. 1996). The essential role of leptin in the control of food intake has been confirmed, and is extensively reviewed elsewhere (Elmquist et al. 1998b; Friedman & Halaas, 1998; Schwartz et al. 2000; Gao & Horvath, 2007). Leptin enters the central nervous system (CNS), binds to receptors in hypothalamic nuclei involved in energy intake, and decreases food intake. Unfortunately for dieters, leptin works best as a starvation signal; leptin levels fall during fasting, triggering counter measures to conserve energy and increase appetite (Ahima et al. 1996). The evolutionary role of leptin suggests that rather than acting as an obesity signal, low levels of leptin stimulate appetite and food intake and promote fat storage to return bodyweight to a previous ‘set-point’ (Schwartz et al. 2000).
Leptin action in the CNS
Much work on leptin action in the hypothalamus has focused on two populations of neurons in the arcuate nucleus that have opposing effects on food intake. LepR are found in the mediobasal hypothalamus, in nuclei that have been implicated in feeding, thermogenesis and hormone regulation, and also in a variety of non-hypothalamic sites and the brainstem (Mercer et al. 1996b; Schwartz et al. 1996b; Cheung et al. 1997; Fei et al. 1997; Elmquist et al. 1998a). Within the arcuate nucleus, LepR have been localized on a population of orexigenic neurons (Mercer et al. 1996a) that coexpress neuropeptide Y (NPY) and agouti-related peptide (AGRP) (Broberger et al. 1998). Leptin acts to inhibit these neurons and regulates the level of NPY mRNA (Stephens et al. 1995; Ahima et al. 1996; Schwartz et al. 1996b). In fasted rodents, and ob/ob and db/db mice, NPY gene expression is increased, and this increase is blunted by leptin treatment (Stephens et al. 1995; Ahima et al. 1996; Schwartz et al. 1996b). Anorexigenic proopiomelanocortin (POMC) neurons in the arcuate nucleus also contain LepR mRNA (Cheung et al. 1997) and leptin increases the level of POMC mRNA (Schwartz et al. 1997). In fasted rodents or ob/ob mice, POMC mRNA is reduced, and this decrease is prevented by leptin administration (Schwartz et al. 1997). POMC neurons produce the anorectic peptide α-melanocyte-stimulating hormone (α-MSH), and mutations in the POMC gene which prevent α-MSH production result in obesity in mice and humans (Spiegelman & Flier, 2001). Electrophysiological studies on transgenic mice have shown that leptin activates POMC neurons directly and decreases the firing rate of NPY/AGRP neurons that normally exhibit an inhibitory GABAergic tone onto POMC neurons (Cowley et al. 2001).
Mechanisms of leptin resistance
Despite the clear role of leptin to suppress appetite, most obese individuals have high plasma leptin concentrations, proportional to their increased body fat (Maffei et al. 1995; Considine et al. 1996) and leptin administration has weight-reducing effects in only a subset of obese people (Heymsfield et al. 1999). More commonly, obese humans are resistant to elevated endogenous leptin and to exogenously administered leptin. Thus, mechanisms of leptin resistance are of key interest in the efforts to understand rising levels of obesity. Resistance to leptin is evident in mice (db/db) and rats (fa/fa) with mutant leptin receptors, and in mice that develop obesity due to other reasons such as diet-induced obesity, age-related obesity, New Zealand Obese (NZO) mice and mice that lack melanocortin-4 receptors (MC4-R) (Halaas et al. 1997; Zhang & Scarpace, 2006). Various mechanisms have been identified in these animal models of obesity and leptin resistance. Diet-induced obese and NZO mice have peripheral leptin resistance (Halaas et al. 1997) due to impaired leptin transport into the brain, and this can be bypassed by central leptin injections (El-Haschimi et al. 2000; Hileman et al. 2002). Peripheral leptin resistance is also seen in obese Otsuka Long–Evans fatty rats but again, they still respond to central leptin (Niimi et al. 1999). In humans there is a correlation between plasma and CSF leptin levels, and the ratio of these two factors is lower in obese individuals (Schwartz et al. 1996a), implicating reduced leptin transport into CSF as a possible cause of leptin resistance. Leptin transport into the brain is thought to be via a saturable transport mechanism, possibly involving short-form LepR at the blood–brain barrier (Hileman et al. 2002). Therefore, human leptin resistance may be caused by a lack of leptin transport into the brain (Caro et al. 1996; Banks, 2004). More recently, however, it has been shown that functional uptake of leptin can occur directly in regions of the brain outside the blood brain barrier, via arcuate nucleus neurons extending processes down into the median eminence and having direct contact with the peripheral circulation (Faouzi et al. 2007). Thus, the arcuate nucleus displays a more rapid response and increased sensitivity to circulating leptin than other deeper hypothalamic sites. In DIO mice, the arcuate nucleus remains unresponsive to central leptin injection (Munzberg et al. 2004) (whereas other hypothalamic nuclei were responsive), which suggests that in some leptin resistant models, resistance also occurs centrally. In fact, age-related obesity is associated with central leptin resistance involving decreased LepR mRNA and protein (Fernandez-Galaz et al. 2001) and impaired leptin signal transduction (Scarpace et al. 2000; Zhang & Scarpace, 2006). Rats on a high-fat diet become obese and unresponsive to central leptin gene therapy due to decreased LepR expression (Wilsey et al. 2003), which is reversed if animals are calorie-restricted (Wilsey & Scarpace, 2004). Agouti (Ay) mice are hyperleptinaemic due to antagonism of the melanocortin system responsible for down-stream regulation of food intake (Fan et al. 1997), and are hence, obese. While they do not respond to leptin with a decrease in food intake, they are not technically leptin resistant as they show normal intracellular responses to leptin. This demonstrates that leptin resistance may involve defects in multiple mechanisms at the hypothalamic level.
Physiological regulation of leptin responses
Pregnancy is an example of transient leptin resistance. This is beneficial to allow build up of long-term energy stores required for successful reproduction. Pregnancy and lactation are both states of physiological hyperphagia, a desirable adaptation that supports the growing conceptus and provides adequate energy in preparation for lactation. Similar changes can occur in response to other physiological demands, such as seasonal breeding (Tups et al. 2004). Our studies have focused on pregnancy in the rat as a model to better understand homeostatic regulation of body weight and to contribute to understanding leptin resistance, which has implications for obesity. We have investigated the mechanisms triggering leptin resistance (Augustine & Grattan, 2007), and the mechanisms that limit the actions of leptin during the pregnancy-induced state of hyperleptinaemia (Ladyman & Grattan, 2004, 2005). During pregnancy there are peripheral actions of leptin involving placental/fetal interactions that have been reviewed in great depth (Henson & Castracane, 2000, 2006). Therefore, this review will focus on neuroendocrine adaptations that occur in the maternal brain during this physiologically demanding time.
Food intake during pregnancy
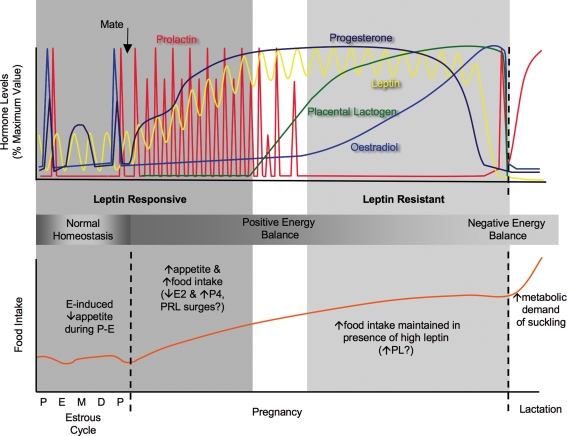
During pregnancy and lactation, adequate nutrition of the mother is crucial for survival of the young. Hence, leptin resistance, which can be considered as a state in which a starvation-like signal is maintained despite sufficient food intake, is a physiological adaptation that is necessary to maintain increased food intake and fat deposition in the face of rising levels of leptin. In rats, food intake increases by up to 50% during pregnancy, decreases the day prior to parturition, then increases by up to 300% during lactation compared to non-pregnant rats (Cripps & Williams, 1975; Shirley, 1984; Ladyman & Grattan, 2004) (see Fig. 1). Importantly, increased food intake during pregnancy precedes the metabolic demand, establishing a positive energy balance, resulting in an increase in maternal body fat (Shirley, 1984). These stores are later depleted due to suckling-induced mobilization of fat stores (Naismith et al. 1982). The initial increase in appetite is likely to be due to the absence of the anorectic effect of oestradiol, plus high circulating concentrations of progesterone. Oestradiol specifically reduces meal size (Eckel & Geary, 2001; Asarian & Geary, 2002) and increases energy utilization (Wade & Gray, 1979). Ovariectomised (OVX) rats and mice have increased food intake and weight gain (Tarttelin & Gorski, 1971; Clegg et al. 2007), which is reversed if treated with oestrogen. Progesterone is orexigenic and administration to either ovariectomised or intact rats and mice increases food intake (Hervey & Hervey, 1967). Plasma leptin concentrations increase as pregnancy advances, reaching peak levels on day 19 followed by a rapid decline prior to parturition (Kawai et al. 1997; Amico et al. 1998; Ladyman & Grattan, 2004). The source of increased leptin secretion during pregnancy is species dependent. In humans, placental production of leptin significantly contributes to leptin levels in the maternal circulation (Masuzaki et al. 1997). In the rat, the placenta is not a major source of hyperleptinaemia during pregnancy, and high serum leptin is more likely to be due to increased leptin mRNA expression in maternal fat (Kawai et al. 1997). Also, in rats and mice, increased levels of circulating binding proteins of placental origin are thought to contribute to hyperleptinaemia during pregnancy (Gavrilova et al. 1997; Seeber et al. 2002). Despite elevated plasma leptin concentrations during pregnancy, increased food consumption is maintained, a paradox considering leptin's pivotal role in suppressing food intake. The presence of leptin-binding proteins in the plasma during pregnancy (Gavrilova et al. 1997; Seeber et al. 2002) may impair leptin action by restricting leptin access to the brain. Our group and others, however, have shown that leptin resistance occurs at the hypothalamic level in pregnant rats, with a region-specific loss of hypothalamic LepR mRNA (Garcia et al. 2000; Ladyman & Grattan, 2005) and suppression of leptin signal transduction (Garcia et al. 2000; Ladyman & Grattan, 2004, 2005). Pregnant females do not show suppression of food intake in response to exogenous leptin (Mounzih et al. 1998; Johnstone & Higuchi, 2001; Ladyman & Grattan, 2004; Lecklin et al. 2005).
Figure 1. Schematic representation of the changing hormonal milieu and food intake during the rat four-day oestrous cycle, followed by pregnancy and lactation (see text for sources of data).
Levels are expressed as a percentage of maximum levels. During the oestrous cycle surges of oestradiol, progesterone and prolactin occur on pro-oestrus (P). Food intake on oestrous (E) displays the oestrogen-induced decrease in appetite, while food intake increases on metoestrus (M) and dioestrus (D), coincident with increased progesterone levels at this time. After mating, prolactin surges are initiated, which provide luteotrophic support for corpus luteum production of progesterone to be maintained throughout pregnancy. The placenta forms around day 7–8 of pregnancy and secretes placental lactogen at relatively constant levels, resulting in inhibition of endogenous prolactin secretion via negative feedback. Oestradiol levels gradually rise during pregnancy, and just prior to parturition the changing ratio between increasing oestradiol and decreasing progesterone causes the late-parturition surge of prolactin. Prolactin levels are then constantly elevated during lactation due to the suckling stimulus. Leptin levels show a circadian fluctuation that persists into pregnancy, when overall levels rise to be significantly elevated by day 7 of pregnancy and remain elevated until the day prior to parturition. Even in the face of elevated leptin, appetite and food intake are increased during pregnancy (significantly by day 4), creating a state of positive energy balance. Non-pregnant and early pregnant rats are responsive to the satiety actions of leptin. However by day 14 of pregnancy, rats are leptin resistant. Coincident with the timing of pregnancy-induced leptin resistance is the changing pattern of lactogen hormone secretion, from phasic prolactin surges to chronic placental lactogen secretion. During lactation the rat is in a state of negative energy balance, as food intake is increased by up to 300% to meet the high metabolic demands of the suckling young.
Changes in leptin action during pregnancy
Despite high leptin, NPY mRNA is either unchanged (Rocha et al. 2003) or even increased during pregnancy (Garcia et al. 2003), while levels of POMC remain unchanged (Garcia et al. 2003; Rocha et al. 2003). An increase in NPY mRNA would be expected to occur during a fast when leptin levels are low, or in the absence of leptin (Schwartz et al. 1996b). Thus, these results suggest that the maternal brain is receiving a starvation-like signal from the periphery and therefore promotes hyperphagia, in the face of high leptin concentrations, by increasing orexigenic signalling in the arcuate nucleus. The mechanism of this leptin resistance is not known. Leptin signalling in the CNS has been extensively reviewed, and multiple pathways are involved (Bjorbaek & Kahn, 2004; Fruhbeck, 2006). The most important pathway activated is thought to be the janus kinase-signal transducer and activator of transcription (JAK/STAT3) pathway. Neuron specific deletion of STAT3 leads to hyperphagia and obesity (Gao et al. 2004). We have shown that LepR mRNA in the arcuate is normal during pregnancy, and hence used the phosphorylation of STAT3 as a functional marker to assess leptin-induced signalling in arcuate neurons. We observed reduced overall levels of pSTAT3 activation in the arcuate nucleus after leptin administration in pregnant rats (Ladyman & Grattan, 2004), although the number of cells showing leptin-induced pSTAT3 was not different from non-pregnant animals (Ladyman & Grattan, 2005). Despite the fact that the number of cells showing leptin-induced pSTAT3 in the arcuate nucleus during pregnancy is normal, the NPY and POMC neurons are not responding appropriately to leptin, suggesting that some other signalling pathway may be altered.
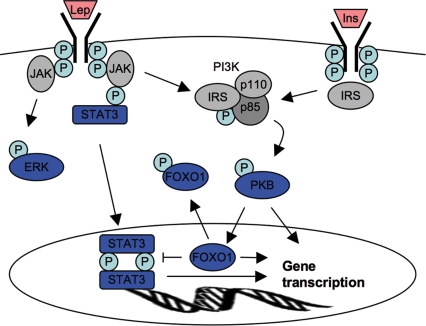
Recent data show that leptin action is also dependent on a range of additional signal transduction pathways, including the phosphoinositol-3 kinase/protein kinase B (PI3K/PKB) and mitogen-activated protein kinase/extracellular regulated protein kinase (MAPK/ERK) pathways. Leptin action on NPY and AgRP may be independent of STAT3 (Bates et al. 2003), and appears to be mediated by PI3K/PKB (Spanswick et al. 1997). The tyrosine residue Tyr1138, located on the intracellular tail of the leptin receptor, is critical for leptin receptor activation via the JAK/STAT3 pathway. Mice that have had Tyr1138 replaced with a serine residue (s/s knock-in mouse), are unable to activate STAT3 and are obese and hyperphagic and have decreased energy expenditure, but have normal reproductive function and increased linear growth (Bates et al. 2003). NPY mRNA levels are near normal in the s/s mice, while POMC mRNA in the hypothalamus is decreased, suggesting that the STAT3-signalling pathway is important for POMC/α-MSH signalling, whereas STAT3-independent signalling may be important for leptin action on NPY gene expression. The acute regulation of orexigenic arcuate neurons by leptin, as measured by c-fos immunoreactivity and electrophysiological changes, does not require STAT3-mediated transcription (Munzberg et al. 2007). Other LepR-mediated signals may therefore contribute to leptin's inhibition on orexigenic neurons. Thus, it is possible that leptin signalling through the PI3K/PKB or MAPK pathway may be altered during pregnancy. One potential mechanism whereby this pathway might be compromised would be the activation of the forkhead transcription factor (FOXO1), an important mediator in insulin signalling in the hypothalamus. Activated FOXO1 blocks the action of both leptin- and insulin-induced decreases in food intake (Kim et al. 2006; Kitamura et al. 2006), directly stimulating NPY/AgRP expression and suppressing POMC by antagonizing transcriptional actions of activated STAT3 (Kim et al. 2006; Kitamura et al. 2006). Leptin and insulin suppress appetite, at least in part, by inactivating FOXO1 through a PKB-mediated phosphorylation of FOXO1. It is possible that increased FOXO1 in the hypothalamus during pregnancy might cause loss of leptin responses, even in the presence of normal pSTAT3. These signalling pathways are illustrated in Fig. 2.
Figure 2. Diagrammatic representation of interactions between leptin (Lep) and insulin (Ins) signal transduction pathways.
P = phosphorylation events. The three pathways discussed (MAPK/ERK, STAT3, PI3K/PKB) are highlighted in blue. The Jak/STAT3 pathway is the best characterized; LepR mediates signalling via the receptor-associated Jak2 tyrosine kinase, which phosphorylates the tyrosine residue Tyr1138 on the intracellular tail of the LepR. Phosphorylation of Tyr1138 recruits the cytoplasmic transcription factor STAT3, which is phosphorylated by Jak2. Phosphorylated STAT3 molecules form dimers, and translocate into the nucleus to activate gene transcription. Note, leptin can activate all three pathways, and recent work suggests that the PKB pathway is required for STAT-mediated signalling, possibly by phosphorylating and thereby inactivating FOXO1, which can otherwise act to inhibit STAT-mediated gene transcription.
The melanocortin system
The melanocortin system is one downstream pathway activated in response to leptin, triggered through leptin action on POMC neurons. α-MSH is a derivative of the POMC precursor and acts on MC3 and MC4 receptors which have been localized using in situ hybridization in the paraventricular, dorsomedial hypothalamic, ventromedial hypothalamic and lateral hypothalamic areas (Mountjoy et al. 1994; Harrold et al. 1999). MC3-R and MC4-R knockout mice are obese and a small percentage of severely obese humans appear to be linked to MC4R mutations (reviewed by Spiegelman & Flier, 2001). Intracerebroventricular (i.c.v.) injection of melanotan II, a melanocortin agonist, inhibits feeding in hyperphagic animal models, while administration of SHU9119, a specific melanocortin antagonist, completely blocks this inhibition in feeding (Fan et al. 1997). To bypass any leptin resistance in first-order arcuate neurons in pregnant rats, and determine whether other parts of the leptin response pathway were altered, we injected α-MSH i.c.v. Such a treatment effectively reduces food intake in mice made leptin resistant with DIO (Hansen et al. 2001). Pregnant rats did not respond to central α-MSH administration with a decrease in food intake, as was seen in non-pregnant rats, suggesting that pregnancy is also an α-MSH-resistant state (S. R. Ladyman, unpublished data). Other animal models of hyperphagia and obesity respond to α-MSH or the melanocortin agonist, melanotan-II (Scarpace et al. 2003; Zhang et al. 2004), suggesting that this phenomenon might be unique to pregnancy. The mechanism for this is unknown. However, AGRP is an endogenous antagonist of melanocortin receptors, and therefore we hypothesized that there might be an increase in AGRP during pregnancy (as reported previously by Rocha et al. (2003). AGRP and NPY are coexpressed in a high percentage of arcuate neurons (Broberger et al. 1998), and over-expression of AGRP results in obesity similar to that seen in the Agouti and MC4-R knockout mice. Using in situ hybridization, we found AGRP mRNA did not change during pregnancy (S. R. Ladyman, unpublished data), although other groups have shown an increase during pregnancy (Rocha et al. 2003). Reasons for this discrepancy are unclear at this time but may be due to different time points or methodologies used. Despite this discrepancy, either no change or elevated AGRP in the face of elevated leptin during pregnancy is further evidence for leptin resistance at the level of the arcuate nucleus.
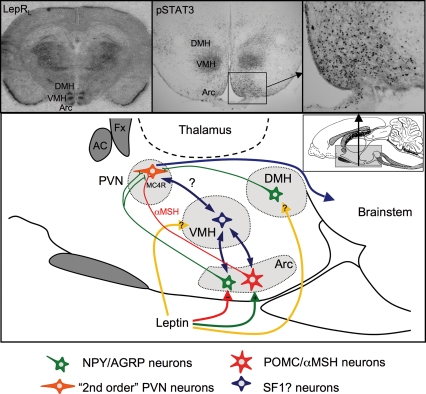
The arcuate nucleus is thought to be central in the control of appetite and body weight regulation because it contains anorexigenic and orexigenic neurons that release neuropeptides that control appetite. LepR are colocalized on these neurons and animals lacking leptin or its receptor have altered gene expression of these neuropeptides. However, unlike the obesity seen in db/db mice lacking functional LepR in all tissue, conditional gene-knockout of LepR just from POMC neurons (Balthasar et al. 2004) only results in a mildly obese phenotype. Similarly, specific deletion of the NPY gene in ob/ob mice only partially restores body weight (Erickson et al. 1996). This suggests that additional neuronal populations, elsewhere in the hypothalamus, must also be involved in mediating the anorectic actions of leptin. The ventromedial hypothalamic nucleus (VMH) has been known for some time to be involved in suppressing food intake. Early studies showed that lesions of the VMH resulted in hyperphagia, morbid obesity and impaired thermoregulation, while lesions in the lateral hypothalamus resulted in death from starvation, thereby declaring these areas as key sites in the control of energy balance (reviewed by Elmquist et al. 1999). Recently, interest in the VMH has regained strength, after retrograde tracing studies show inputs from arcuate nucleus neurons into the VMH and LHA (Sternson et al. 2005). Leptin receptors are expressed in the VMH (Elmquist et al. 1998a) and conditional knockout of LepR on specific neurons in the VMH leads to obesity of a similar magnitude to that observed in POMC-specific LepR knockouts (Dhillon et al. 2006). We have shown that in leptin-resistant pregnant rats, there is a decrease in the long form of LepR in the VMH and decreased leptin-induced pSTAT3 levels, indicative of reduced leptin signalling in this area (Ladyman & Grattan, 2005). This evidence, and the role of leptin-responsive neurons in the VMH, suggests that the VMH is also a first-order leptin responsive area and is an important site for the control of food intake, especially during pregnancy (see Fig. 3).
Figure 3. The leptin responsive network in the hypothalamus.
Inset (top, left) shows a representative in situ hybridization for the long form of the leptin receptor (LepR). Note strong signal in the arcuate (Arc), ventromedial (VMH) and dorsomedial (DMH) hypothalamic nuclei. Insets (top, middle and top, right) show leptin-induced activation of pSTAT3, as a functional marker for leptin responsive neurons. Note distribution in the same hypothalamic nuclei. The main panel shows a diagram of a sagittal section through the hypothalamus, showing the known and postulated (?) interactions between key hypothalamic nuclei. Leptin stimulates anorexigenic POMC neurons and inhibits orexigenic NPY/AGRP neurons in the arcuate nucleus. These neurons project to other hypothalamic nuclei, notably the paraventricular nucleus (PVN), stimulating and antagonizing melanocortin receptors (MC4R) in this nucleus, and thus regulating further downstream pathways. Leptin also acts directly in the VMH and DMH, to influence the appetite regulatory pathways in as yet poorly defined ways. Leptin actions in the VMH appear to be particularly important in the adaptive response to pregnancy.
Hormonal mechanisms inducing leptin resistance during pregnancy
Leptin resistance during pregnancy is likely to be driven by the hormonal changes characteristic of this state, such as elevated progesterone, leptin, prolactin and placental lactogen and loss of the cyclical elevations in serum oestradiol (see Fig. 1). Leptin levels in blood are elevated during pregnancy (Kawai et al. 1997; Amico et al. 1998; Ladyman & Grattan, 2004), and chronic high leptin has been shown to induce leptin resistance (Sahu, 2002). However, it is unlikely that leptin resistance during pregnancy is caused by elevated leptin, as levels of leptin rise slowly during pregnancy, and are only elevated two- to threefold for a few days prior to development of leptin resistance (Ladyman & Grattan, 2004). To induce leptin resistance with leptin, prolonged infusions (> 14 days) of high levels of leptin are required (Sahu, 2002; Zhang & Scarpace, 2006). Pregnant ob/ob mice treated with leptin throughout pregnancy become leptin resistant at mid-pregnancy, but it is unlikely to be a leptin-induced effect, as non-pregnant ob/ob mice can be identically treated with leptin for up to 6 months with no observed resistance to leptin action (Mounzih et al. 1998). Gonadal steroid hormone concentrations are altered dramatically during pregnancy, and are known to influence feeding behaviour. Oestradiol has an inhibitory effect on food intake, specifically reducing meal size (Eckel & Geary, 2001; Asarian & Geary, 2002) and increasing energy utilization (Wade & Gray, 1979). Oestradiol acts within the brain to increase leptin sensitivity (Clegg et al. 2006), and thus, low oestradiol during pregnancy might reduce leptin responsiveness. In contrast, administration of progesterone or progesterone metabolites to either OVX or intact rats increases food intake (Hervey & Hervey, 1967; Wade, 1975; Chen et al. 1996). Progesterone-treated rats have an increased body weight and food intake but maintain normal plasma and CSF leptin levels (Grueso et al. 2001), suggesting progesterone may inhibit CNS action of leptin. Elevated levels of prolactin and the closely related hormone, placental lactogen, are also characteristic of pregnancy and lactation (Grattan, 2001) and may contribute to the hyperphagia at these times. In virgin female rats, prolactin infusion induces hyperphagia in a dose-dependent manner (Gerardo-Gettens et al. 1989; Sauve & Woodside, 1996), and this is a central effect, involving direct actions of prolactin in the hypothalamus (Sauve & Woodside, 2000).
To investigate the hormonal mechanisms involved in inducing leptin resistance during pregnancy, we have examined leptin responses in pseudopregnant rats, which have identical hormone profiles to early pregnancy. Like pregnancy, pseudopregnancy is associated with high progesterone, hyperprolactinaemia and hyperphagia (Tarttelin & Gorski, 1971; Smith et al. 1975; Augustine & Grattan, 2007). Thus, pseudopregnancy in the rat provided an ideal model to investigate the effect of maternal hormones on appetite regulation, without the confounding influence of placental hormones. Central leptin resistance does not develop until midpregnancy in the rat, whereas hyperphagia develops almost immediately (Ladyman & Grattan, 2004). Thus, pregnancy-induced hyperphagia is not primarily caused by leptin resistance, but would be facilitated by the subsequent development of leptin resistance, which probably plays a role in the rapid increase in food intake later in pregnancy (Johnstone & Higuchi, 2001; Rocha et al. 2003). Leptin resistance develops between day 7 and 14 of pregnancy (Ladyman & Grattan, 2004), coincident with the development of the placenta and onset of secretion of placental lactogen (see Fig. 1). Thus, we hypothesized that placental lactogen may be a critical factor influencing appetite regulatory centres in the brain. Early pregnant, and pseudopregnant rats are characterized by pulsatile prolactin secretion and hyperphagia, but continue to have a relatively normal response to leptin (Ladyman & Grattan, 2004; Augustine & Grattan, 2007). In contrast, during the second half of pregnancy, when placental lactogen is chronically elevated, animals show a loss of response to leptin (Ladyman & Grattan, 2004). Therefore, it is possible that the pattern of prolactin secretion, whether phasic or continually high, might exert different physiological responses in regards to appetite regulation. Twice-daily injections of prolactin into the paraventricular nucleus of virgin rats induces a feeding response of similar magnitude to that observed during the first week of pregnancy (Sauve & Woodside, 2000), but chronically elevated prolactin, such as seen in lactating rats, increases food intake to a greater extent (Woodside, 2007). It seems likely that leptin resistance might contribute to this effect of chronic prolactin, as leptin infusion results in only a transient decrease in food intake during lactation (Woodside et al. 2000). Hence, we investigated if the pattern of prolactin secretion caused changes in feeding and hypothalamic responses to leptin, by giving pseudopregnant rats continuous exposure to high concentrations of prolactin. Under these conditions, the leptin-induced decrease in food intake was completely suppressed (Augustine & Grattan, 2007). Thus, by giving chronic i.c.v. prolactin infusions to mimic the pattern of placental lactogen secretion during the second half of pregnancy, we were able to induce leptin resistance in pseudopregnant female rats in the absence of any metabolic load induced by pregnancy or fetal development (Augustine & Grattan, 2007). The exact neuroendocrine changes that are occurring in the hypothalamus in response to chronic lactogenic infusion are currently unknown but may involve a loss in functional leptin receptors in key hypothalamic nuclei, or a loss in leptin signalling via intracellular signalling cascades.
Conclusion
Pregnancy is characterized by hyperphagia and fat deposition, with the subsequent development of leptin resistance allowing the hyperphagia to be maintained even in the presence of elevated leptin secretion from the growing fat deposits. Both the initial increase in food intake and the subsequent development of leptin resistance are driven by the unique pattern of hormonal changes associated with pregnancy. Our work suggests that the establishment of the placenta and the associated pattern of chronically high levels of placental lactogen secretion are essential causes of the leptin resistance seen during the latter parts of pregnancy. Indeed, leptin resistance can be induced in the absence of pregnancy, merely by mimicking the pattern of placental lactogen secretion. Thus, the developing conceptus provides a key neuroendocrine signal to the mother, inducing a resetting of maternal homeostatic mechanisms to facilitate a change in behaviour that will eventually help both mother and offspring cope with the metabolic demands of their new life together.
Acknowledgments
The authors would like to thank Dr Alexander Tups for his help with leptin receptor in situ hybridization and Dr Derik Steyn for his help with the figures. This work was supported by a grant from the Royal Society of New Zealand Marsden Fund.
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Amico JA, Thomas A, Crowley RS, Burmeister LA. Concentrations of leptin in the serum of pregnant, lactating, and cycling rats and of leptin messenger ribonucleic acid in rat placental tissue. Life Sci. 1998;63:1387–1395. doi: 10.1016/s0024-3205(98)00405-6. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Hormones Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Augustine RA, Grattan DR. Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology. 2007 doi: 10.1210/en.2007-1018. in press. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Banks WA. The many lives of leptin. Peptides. 2004;25:331–338. doi: 10.1016/j.peptides.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance [see comment] Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- Chen SW, Rodriguez L, Davies MF, Loew GH. The hyperphagic effect of 3α-hydroxylated pregnane steroids in male rats. Pharmacol Biochem Behav. 1996;53:777–782. doi: 10.1016/0091-3057(95)02142-6. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans [see comment] N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cripps AW, Williams VJ. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br J Nutrition. 1975;33:17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R738–R746. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998a;395:535–547. [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998b;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Faouzi M, Leshan R, Bjornholm M, Thomas H, Jones J, Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Galaz C, Fernandez-Agullo T, Campoy F, Arribas C, Gallardo N, Andres A, Ros M, Carrascosa JM. Decreased leptin uptake in hypothalamic nuclei with ageing in Wistar rats. J Endocrinol. 2001;171:23–32. doi: 10.1677/joe.0.1710023. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MD, Casanueva FF, Dieguez C, Senaris RM. Gestational profile of leptin messenger ribonucleic acid (mRNA) content in the placenta and adipose tissue in the rat, and regulation of the mRNA levels of the leptin receptor subtypes in the hypothalamus during pregnancy and lactation. Biol Reprod. 2000;62:698–703. doi: 10.1095/biolreprod62.3.698. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Lopez M, Gualillo O, Seoane LM, Dieguez C, Senaris RM. Hypothalamic levels of NPY, MCH, and prepro-orexin mRNA during pregnancy and lactation in the rat: role of prolactin. FASEB J. 2003;17:1392–1400. doi: 10.1096/fj.02-0933com. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Barr V, Marcus-Samuels B, Reitman M. Hyperleptinemia of pregnancy associated with the appearance of a circulating form of the leptin receptor. J Biol Chem. 1997;272:30546–30551. doi: 10.1074/jbc.272.48.30546. [DOI] [PubMed] [Google Scholar]
- Gerardo-Gettens T, Moore BJ, Stern JS, Horwitz BA. Prolactin stimulates food intake in a dose-dependent manner. Am J Physiol Regul Integr Comp Physiol. 1989;256:R276–R280. doi: 10.1152/ajpregu.1989.256.1.R276. [DOI] [PubMed] [Google Scholar]
- Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res. 2001;133:153–171. doi: 10.1016/s0079-6123(01)33012-1. [DOI] [PubMed] [Google Scholar]
- Grueso E, Rocha M, Puerta M. Plasma and cerebrospinal fluid leptin levels are maintained despite enhanced food intake in progesterone-treated rats. Eur J Endocrinol. 2001;144:659–665. doi: 10.1530/eje.0.1440659. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MJ, Ball MJ, Morris MJ. Enhanced inhibitory feeding response to α-melanocyte stimulating hormone in the diet-induced obese rat. Brain Res. 2001;892:130–137. doi: 10.1016/s0006-8993(00)03246-7. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Widdowson PS, Williams G. Altered energy balance causes selective changes in melanocortin-4 (MC4-R), but not melanocortin-3 (MC3-R), receptors in specific hypothalamic regions: further evidence that activation of MC4-R is a physiological inhibitor of feeding. Diabetes. 1999;48:267–271. doi: 10.2337/diabetes.48.2.267. [DOI] [PubMed] [Google Scholar]
- Henson MC, Castracane VD. Leptin in pregnancy. Biol Reprod. 2000;63:1219–1228. doi: 10.1095/biolreprod63.5.1219. [DOI] [PubMed] [Google Scholar]
- Henson MC, Castracane VD. Leptin in pregnancy: an update. Biol Reprod. 2006;74:218–229. doi: 10.1095/biolreprod.105.045120. [DOI] [PubMed] [Google Scholar]
- Hervey E, Hervey GR. The effects of progesterone on body weight and composition in the rat. J Endocrinol. 1967;37:361–381. doi: 10.1677/joe.0.0370361. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Higuchi T. Food intake and leptin during pregnancy and lactation. Prog Brain Res. 2001;133:215–227. doi: 10.1016/s0079-6123(01)33016-9. [DOI] [PubMed] [Google Scholar]
- Kawai M, Yamaguchi M, Murakami T, Shima K, Murata Y, Kishi K. The placenta is not the main source of leptin production in pregnant rat: gestational profile of leptin in plasma and adipose tissues. Biochem Biophys Res Commun. 1997;240:798–802. doi: 10.1006/bbrc.1997.7750. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145:3704–3711. doi: 10.1210/en.2004-0338. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology. 2005;146:3868–3874. doi: 10.1210/en.2005-0194. [DOI] [PubMed] [Google Scholar]
- Lecklin A, Dube MG, Torto RN, Kalra PS, Kalra SP. Perigestational suppression of weight gain with central leptin gene therapy results in lower weight F1 generation. Peptides. 2005;26:1176–1187. doi: 10.1016/j.peptides.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nature Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol. 1996a;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996b;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Qiu J, Ewart-Toland A, Chehab FF. Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology. 1998;139:5259–5262. doi: 10.1210/endo.139.12.6523. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith DJ, Richardson DP, Pritchard AE. The utilization of protein and energy during lactation in the rat, with particular regard to the use of fat accumulated in pregnancy. Br J Nutr. 1982;48:433–441. doi: 10.1079/bjn19820125. [DOI] [PubMed] [Google Scholar]
- Niimi M, Sato M, Yokote R, Tada S, Takahara J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol. 1999;11:605–611. doi: 10.1046/j.1365-2826.1999.00368.x. [DOI] [PubMed] [Google Scholar]
- Rocha M, Bing C, Williams G, Puerta M. Pregnancy-induced hyperphagia is associated with increased gene expression of hypothalamic agouti-related peptide in rats. Regul Pept. 2003;114:159–165. doi: 10.1016/s0167-0115(03)00119-8. [DOI] [PubMed] [Google Scholar]
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity – adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sauve D, Woodside B. The effect of central administration of prolactin on food intake in virgin female rats is dose-dependent, occurs in the absence of ovarian hormones and the latency to onset varies with feeding regimen. Brain Res. 1996;729:75–81. [PubMed] [Google Scholar]
- Sauve D, Woodside B. Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats. Brain Res. 2000;868:306–314. doi: 10.1016/s0006-8993(00)02344-1. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology. 2000;39:1872–1879. doi: 10.1016/s0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zolotukhin S, Tumer N, Zhang Y. Leptin-induced leptin resistant rats exhibit enhanced responses to the melanocortin agonist MT II. Neuropharmacology. 2003;45:211–219. doi: 10.1016/s0028-3908(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996a;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996b;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seeber RM, Smith JT, Waddell BJ. Plasma leptin-binding activity and hypothalamic leptin receptor expression during pregnancy and lactation in the rat. Biol Reprod. 2002;66:1762–1767. doi: 10.1095/biolreprod66.6.1762. [DOI] [PubMed] [Google Scholar]
- Shirley B. The food intake of rats during pregnancy and lactation. Lab Anim Sci. 1984;34:169–172. [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol Behav. 1971;7:847–852. doi: 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, Klingenspor M. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology. 2004;145:1185–1193. doi: 10.1210/en.2003-1382. [DOI] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Weigle DS. Appetite and the regulation of body composition. FASEB J. 1994;8:302–310. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol. 2004;181:297–306. doi: 10.1677/joe.0.1810297. [DOI] [PubMed] [Google Scholar]
- Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1011–R1020. doi: 10.1152/ajpregu.00193.2003. [DOI] [PubMed] [Google Scholar]
- Woodside B. Prolactin and the hyperphagia of lactation. Physiol Behav. 2007;91:375–382. doi: 10.1016/j.physbeh.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Woodside B, Abizaid A, Walker C. Changes in leptin levels during lactation: implications for lactational hyperphagia and anovulation. Hormones Behav. 2000;37:353–365. doi: 10.1006/hbeh.2000.1598. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Matheny M, Tumer N, Scarpace PJ. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol Aging. 2004;25:1349–1360. doi: 10.1016/j.neurobiolaging.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue [erratum appears in Nature 374, 479] Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Scarpace PJ. Circumventing central leptin resistance: lessons from central leptin and POMC gene delivery. Peptides. 2006;27:350–364. doi: 10.1016/j.peptides.2005.01.024. [DOI] [PubMed] [Google Scholar]