Abstract
The time around birth is accompanied by behavioural and physiological adaptations of the maternal brain, which ensure reproductive functions, maternal care and the survival of the offspring. In addition, profound neuroendocrine and neurobiological adaptations have been described with respect to behavioural and neuroendocrine stress responsiveness in rodents and human mothers. Thus, the hormonal response of the hypothalamo-pituitary-adrenal (HPA) axis and the response of the sympathetic nervous system to emotional and physical stressors are severely attenuated. Moreover, anxiety-related behaviour and emotional responsiveness to stressful stimuli are reduced with the result of general calmness. These complex adaptations of the maternal brain are likely to be a consequence of an increased activity of brain systems with inhibitory effects on the HPA axis (such as the oxytocin and prolactin systems) and of a reduced activity of excitatory pathways (noradrenaline (norepinephrine), corticotrophin-releasing factor and opioids). Experimental manipulation of these systems using complementary approaches indeed demonstrates their importance in these maternal brain adaptations. Maternal stress adaptations are not only important for the healthy prenatal development of the offspring by preventing excessive glucocorticoid responses and in the promotion of postnatal maternal behaviour, but are also vital for the well-being of the mother and her mental health.
Introduction
Across all mammalian species, both physiological and behavioural changes occur throughout pregnancy in order to prepare the mother for the birth. These changes include the onset of maternal behaviours (i.e. maternal aggression, milk production and let-down and nursing of offspring) (Rosenblatt et al. 1994; Neumann et al. 2001) to ensure the development and survival of the offspring. Moreover, profound alterations have been shown in pregnancy and lactation with respect to maternal stress-coping style with severely attenuated activity of the hypothalamo-pituitary-adrenal (HPA) axis (Stern et al. 1973; Neumann et al. 1998b; Russell et al. 1999; Lightman et al. 2001; Kammerer et al. 2002; de Weerth & Buitelaar, 2005). Such changes seem to be essential for the healthy development of the offspring; for example, to prevent excessive circulating stress hormone levels. Moreover, there is a growing body of evidence to suggest that these altered stress responses are also important for maternal mental health.
In the following review, we focus on the physiological, behavioural and molecular adaptations underlying the stress hypo-responsiveness, with particular emphasis on the brain oxytocin (OXT) and prolactin (PRL) systems.
Animal research
The end of pregnancy and the onset of lactation is accompanied by the activation of neurobiological systems that are directly related to reproductive functions (i.e. maternal behaviour and nurturing of the newborn). Specifically, the neuropeptide OXT is increasingly synthesized in hypothalamic supraoptic nucleus/paraventricular nucleus (SON/PVN) neurons and secreted into the blood to promote labour during parturition and to release milk (e.g. in response to suckling). Another neuropeptide, PRL is not only up-regulated in lactotrophs to ensure lactogenesis, but also in the hypothalamus and is similarly involved in the regulation of maternal behaviour. In addition, during lactation a chronic elevation in plasma corticosterone has been described under basal conditions (see Table 1) (Stern et al. 1973; Walker et al. 1995; Windle et al. 1997b) accompanied by alterations of the diurnal pattern of activity (for review see Lightman et al. 2001). Increased vasopressin expression in parvocellular PVN neurons (Walker et al. 2001), accompanied by an enhanced sensitivity of the PVN to vasopressin (Toufexis et al. 1999b), may contribute to the altered basal activity of the HPA axis during lactation. Of importance, despite the elevated basal activity of the HPA axis, it has been shown in various mammalian species that the responsiveness of the HPA axis to a broad variety of psychological and physiological stressors is severely attenuated from mid-pregnancy through to the end of lactation (Stern et al. 1973; Walker et al. 1995; Windle et al. 1997b; Neumann et al. 1998b; Shanks et al. 1999; Johnstone et al. 2000; Lightman et al. 2001; Neumann, 2001; Brunton & Russell, 2003). In agreement, the expression of corticotrophin-releasing factor (CRF) within the PVN is reduced both in pregnancy, which might be related to elevated glucocorticoid levels and negative feedback effects (Douglas & Russell, 1994; Johnstone et al. 2000; da Costa et al. 2001), and during lactation (Lightman et al. 2001; Walker et al. 2001). Moreover, the pituitary sensitivity to CRF is reduced due to reduced CRF receptor binding at pituitary corticotrophs (Neumann et al. 1998a). The lactation-associated reduction in CRF expression has also been described in the central nucleus of the amygdala, a region important not only for HPA axis regulation, but also for emotionality (Davis & Whalen, 2001). A potential mechanism underlying the attenuated CRF system is via the immediate-early gene nur77 (Nerve Growth Factor-induced B (NGFI-B)), which controls CRF gene expression and is up-regulated after emotional stress (Kirschbaum et al. 1999), but to a lesser extent in the hypothalamus of pregnant mice (Douglas et al. 2003). As the brain CRF system is the main stimulator of the HPA axis, lowered activity of the system may contribute to the attenuated corticotrophin (ACTH) and corticosterone responses observed during pregnancy and lactation. Thus, reduced (re)activity of the brain CRF system may also be related to behavioural changes of the dam including reduced anxiety (Hard & Hansen, 1985; Windle et al. 1997b; Toufexis et al. 1998; Neumann, 2003), but also enhanced maternal behaviour (Pedersen et al. 1991) and maternal aggression when protecting the offspring (Gammie et al. 2004).
Table 1.
Examples of neuroendocrine and behavioural alterations observed in pregnancy and lactation
| Behavioural alterations | References |
|---|---|
| HPA axis alterations | |
| Chronic basal hypercorticalism and altered diurnal pattern | (Stern et al. 1973; Walker et al. 1995; Windle et al. 1997b; Lightman et al. 2001) |
| Decreased responsiveness of HPA axis (ACTH, corticosterone) to psychological and physiological stressors | (Stern et al. 1973; Windle et al. 1997b; Neumann et al. 1998a; Shanks et al. 1999; Lightman et al. 2001; Neumann et al. 2001; Walker et al. 2001; Brunton & Russell, 2003) |
| Decreased stressor perception and stress-induced expression of c-fos in limbic brain regions | (da Costa et al. 1996) |
| Alterations in excitatory pathways | |
| Decreased noradrenergic excitatory tone in the PVN | (Toufexis et al. 1998; Douglas, 2005) |
| Reduced excitatory opioid tone on CRF neurons | (Douglas et al. 1998) |
| Attenuated pituitary sensitivity to CRF | (Neumann et al. 1998a; Toufexis et al. 1999a) |
| Decreased sympathetic responsiveness to stressors | (Douglas et al. 2005) |
| Alterations in inhibitory pathways | |
| Elevated OXT system activity | (Insel, 1990; Douglas & Russell, 1994) |
| Increased prolactin synthesis and binding | (Pi & Grattan, 1999; Torner et al. 2002) |
| Decreased CRF mRNA expression in the PVN | (Johnstone et al. 2000; Lightman et al. 2001; Walker et al. 2001) |
| Alterations in behaviour | |
| Increased maternal behaviour including aggressive behaviour | (Rosenblatt et al. 1994; Neumann et al. 2001) |
| Increased calmness, reduced anxiety and reduced emotional responsiveness to stressors | (Carter et al. 2001; Heinrichs et al. 2001; Glynn et al. 2004) |
There is also a loss of excitatory inputs of the HPA axis in pregnancy, including a reduced noradrenergic excitatory tone within the hypothalamic PVN (Toufexis et al. 1998; Douglas et al. 2005), which contributes to its attenuated responsiveness during lactation. Further, there is a lower expression of noradrenergic α1A-adrenoceptors in the parvo- and magnocellular PVN of pregnant rats (Douglas et al. 2005). These alterations could contribute to the loss of hypothalamic noradrenergic excitation and the low level of CRF gene expression within the hypothalamus found under resting conditions. Another excitatory input to the HPA axis that is attenuated in pregnancy is that of endogenous opioids (Douglas et al. 1998; Kammerer et al. 2002; Kofman, 2002). The effects of endogenous opioids are reversed during parturition, when opioids appear to inhibit, rather than stimulate, HPA axis activity (Wigger et al. 1999). Further, endogenous opioid actions on OXT neurons within the hypothalamic PVN differ between virgin and pregnant rats (see Fig. 1) (Douglas et al. 1995; Wigger & Neumann, 2002). As brain OXT is an important regulator of the activity of the HPA axis (Windle et al. 1997a; Neumann et al. 2000a; Neumann, 2003), the inhibitory effects of endogenous opioids on intra-PVN release of OXT in virgins and the excitatory effects of endogenous opioids in pregnant rats are of interest in the context of mechanisms regulating stress adaptations peripartum (Wigger & Neumann, 2002).
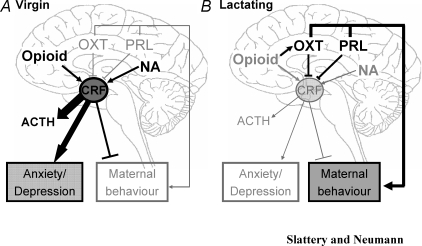
Figure 1. Schematic representation of some of the neuroendocrine alterations that occur between virgin and lactating dams.
Virgins are characterized by having higher levels of opioid and noradrenergic (NA) inputs to the PVN than in lactating animals but lower expression of oxytocin (OXT) and prolactin (PRL). Whereas opioids have no apparent influence on OXT in virgin animals, they have been shown to increase OXT in the PVN during lactation. Furthermore, the effect of endogenous opioids on CRF release is attenuated during lactation. The combined effect of these alterations is an enhanced corticotrophin-releasing factor (CRF) release in response to stress in virgins, which leads to the release of corticotrophin (ACTH) and increased anxiety- and depression-related behaviour in comparison to lactating animals. By contrast, the low OXT and PRL levels, as well as elevated the CRF level, leads to lower levels of maternal behaviour in virgins compared with lactating animals. Thus disruption of these factors may contribute to postpartum depression, which is associated with increased anxiety and depression, as well as reduced maternal care. Black and thick arrows represent the predominant pathways in virgins and lactating animals while the grey and thin arrows show the systems that are at low levels (i.e. OXT and PRL in virgins) or attenuated (i.e. opioid and NA in lactation). Blocked lines represent factors which are inhibitory; factors with thick and black lines again represent the predominant systems in virgins or lactating animals compared with one another.
Involvement of brain OXT and PRL in decreased stress responsiveness
Both OXT and PRL have important reproductive functions including promotion of labour and milk ejection and lactogenesis, respectively, when released from the neurohypophysis (OXT) or adenohypophysis (PRL) into the blood. Furthermore, as potential neuromodulators/neurotransmitters, they are synthesized and released within the brain, and significant effects on maternal behaviour (among others) have been established (Pedersen & Prange, 1979; Neumann & Landgraf, 1989; Neumann et al. 1993b, 1994a,b; Bridges et al. 2001; Torner et al. 2002).
OXT
Despite a general activation of the OXT system around birth in direct relation to reproductive functions (Pedersen & Prange, 1979; Insel, 1986; van Leengoed et al. 1987; Neumann & Landgraf, 1989; Landgraf et al. 1991; Neumann et al. 1993a, b; Insel et al. 1997), responses of the OXT system to stimuli not directly related to parturition or lactation were found to be lower both in pregnancy and lactation (Lightman & Young, 1989; Walker et al. 1995; Neumann et al. 1995, 1998a, 2001; Douglas et al. 1998). OXT neurons generally respond to emotional, physical or pharmacological stressors with elevated neurohypophysial release into the blood (Neumann et al. 1993a, 1995, 1998a; Douglas et al. 1998; Wotjak et al. 1998; Neumann, 2002; Landgraf & Neumann, 2004) and release within the hypothalamus (Wigger & Neumann, 2002; Bosch et al. 2004), and the amygdala (Bosch et al. 2004, 2005) in male and female rats (Nishioka et al. 1998; Wotjak et al. 1998; Neumann, 2002; Landgraf & Neumann, 2004; Ebner et al. 2005). Recently, we have extended previous findings (Windle et al. 1997a) and demonstrated that endogenous brain OXT regulates the activity of the HPA axis both in virgin female and male rats. Thus, central infusion of a selective OXT receptor antagonist dis-inhibited the release of ACTH and corticosterone under basal and stress-induced conditions (Neumann et al. 2000b,c). Effects of OXT were found to depend on the brain region (PVN, amygdala or medio-lateral septum) and experimental conditions (basal versus stress) studied (Neumann et al. 2000a; Neumann, 2001). How can these effects of brain OXT on HPA axis responses be related to the peripartum period? Chronic infusion of OXT into the brains of virgin rats attenuated neuronal and neuroendocrine responses to stress (Windle et al. 2004) making an involvement of endogenous OXT on HPA axis responses during the peripartum period probable. However, our own experimental evidence indicates that blockade of brain OXT receptor actions in pregnant or lactating rats does not result in a dis-inhibition of the HPA axis in response to various stressors (novel environment, swimming or maternal defence) as found in virgins (Neumann et al. 2000b,c). Similarly, during parturition, when the brain OXT system is highly activated (Neumann et al. 1993b), the non-responsiveness of the HPA axis to repeated air puff applied in the home cage in rats or to novel environment in mice (Douglas et al. 2003) is not due to a significant inhibition by OXT at this time. Therefore, it is likely that other additional inhibitory factors, among them endogenous opioids and PRL, act in concert with OXT to attenuate HPA axis hormonal responses in the peripartum period (see Fig. 1).
PRL
There is an increase in plasma PRL levels seen at the end of pregnancy (Grattan, 2001), as well as an up-regulation of PRL and PRL receptor expression within the hypothalamus in the peripartum period (Pi & Grattan, 1999). Furthermore, our studies have revealed an increased neuronal PRL synthesis within the hypothalamus in late pregnancy and lactation (Torner et al. 2004). The relevance of this centrally synthesized PRL is provided by the fact that PRL is locally released from neurons within selected hypothalamic regions, including the PVN, in lactating rats in response to suckling (Torner et al. 2004). The detailed function of such centrally released PRL in the context of suckling remains to be elucidated. However, in the context of altered stress responsiveness peripartum, it is of interest to note that acute or chronic intracerebroventricular (i.c.v.) administration of PRL to virgin female rats results in attenuated stress-induced secretion of ACTH and corticosterone accompanied by an elevated basal plasma level of ACTH (Torner et al. 2001; Donner et al. 2007). Furthermore, chronic PRL administration has also been demonstrated to attenuate neuronal (hypothalamic fos mRNA and CRF mRNA) responses to restraint stress (Donner et al. 2007). Thus, the lactation-like neuroendocrine state induced by i.c.v. application of PRL indicates a strong involvement of high PRL expression within the maternal brain in altered stress-coping style and stress attenuation. In support of this, use of antisense oligodeoxynucleotides directed against the long form of PRL receptors in the brain resulted in a significant dis-inhibition of stress-induced ACTH secretion in lactating rats, demonstrating that brain PRL is a central factor inhibiting the HPA axis response in lactation (Torner et al. 2001).
Alteration of behavioural stress responses peripartum: involvement of OXT and PRL
The reduced emotional responsiveness observed during lactation can be explained, at least in part, by an enhanced activity of the brain OXT and PRL systems as both have anxiolytic properties, especially peripartum (Neumann et al. 2000b; Torner et al. 2002). The anxiolytic effect of OXT could be localized within the central amygdala via local infusion (Bale et al. 2001) as also shown by reverse microdialysis studies (Neumann, 2001); however, further regions, including the PVN, may be involved. Further, OXT released within the PVN and the central amygdala is correlated with the level of maternal aggressive behaviour displayed by the lactating dam, and such locally released OXT promotes maternal aggression (Bosch et al. 2005). It is interesting that in virgin female and male rats and male mice (Ring et al. 2006), administration of synthetic OXT directly into the hypothalamic PVN (i.c.v. in male mice) results in reduced anxiety levels, both on the elevated plus-maze and the light–dark box, an effect which we could block with an inhibitor of the extra cellular regulated kinase (ERK) signalling cascade (Neumann et al. 2007). Furthermore, a chronic elevation of brain OXT concentrations in virgin rats was directly related to an attenuation of the emotional response to an acute noise stress (Windle et al. 1997a, 2004). Therefore, in addition to their main roles in promoting maternal behaviour (Pedersen & Prange, 1979; Insel, 1990; Neumann et al. 1994a,b; Torner et al. 2004), the emerging evidence clearly demonstrates that the high levels of brain OXT and PRL (Insel, 1990; Douglas & Russell, 1994; Mann & Bridges, 2002; Torner et al. 2004) also play a crucial role in the dampened emotional responsiveness observed in the peripartum period.
Human research
In humans, progressively increasing levels of placental CRF and gradually decreasing levels of CRF-binding protein (Magiakou et al. 1996a,b) may contribute to the elevated basal activity of the HPA axis at the end of pregnancy. Additionally, attenuation of the responsiveness of the HPA axis, and other relevant systems, to physiological or psychological stressors has been found both in pregnant and lactating women (Nisell et al. 1985; Schulte et al. 1990; Altemus et al. 1995; Kammerer et al. 2002; for review see de Weerth & Buitelaar, 2005). For example, the pituitary responses to an i.v. bolus of CRF were found to be attenuated in lactating women (Magiakou et al. 1996a). Further, and in line with animal studies, in late human pregnancy and lactation, increased calmness, a more positive mood state and a reduced emotional response to stressful life events have been described (Carter et al. 2001; Heinrichs et al. 2001; Glynn et al. 2004). These changes are likely to be due to a reduced activity of the brain CRF system, among others. Of importance, suckling-related factors, possibly including the activation of the brain OXT and PRL systems, were recently shown to contribute to the reduced HPA axis response to stress and to the positive mood state (Heinrichs et al. 2001). Thus, the experimental evidence accumulated to date in humans is largely in agreement with the findings from animal research. Thus, brain CRF, OXT and PRL are likely to play a significant role in altered emotionality in humans in the peripartum period. Therefore, disruption of these systems may be a risk factor for the development of postpartum mood disorders.
Significance of the adaptations of the maternal stress responsiveness peripartum
The maternal adaptations described above, with the significant attenuation of hormonal stress responses in pregnancy and lactation, are important for the healthy prenatal development of the offspring by preventing excessive levels of circulating glucocorticoids (Altemus et al. 1995; McCormick et al. 1995; Vallee et al. 1997; Weinstock, 2001). For example, it has recently been demonstrated that lactating dams whose mothers were exposed to stressful stimuli during pregnancy do not display the normal adaptations observed in pregnancy (Bosch et al. 2007). In more detail, these dams had elevated CRF and vasopressin mRNA expression within the PVN suggesting a dysregulation of the stress circuitries, which in turn leads to the elevated HPA axis reactivity found in these rats (Bosch et al. 2007).
Therefore, we hypothesize that the attenuation of the stress responsiveness around birth is also vital for the well-being of the mother and her mental health. Thus, in humans, the postpartum period is a time of increased vulnerability to mood disorders (O'Hara & Swain, 1996; Llewellyn et al. 1997; Pedersen, 1999; Mastorakos & Ilias, 2000), which can last up to a year and significantly affect the development of the newborn and the family unit. Prior history of depressive disorders, lack of social support and stressful life events, particularly during pregnancy, increase the risk of perinatal mood disorders (O'Hara & Swain, 1996; Pedersen, 1999). However, to date, the underlying mechanisms of postpartum mood disorders are largely unknown, but it is likely that disruption of the mechanisms leading to reduced stress-hyporesponsiveness during lactation plays a significant role.
In general, it is well accepted that CRF is a relevant neuropeptide involved in the pathogenesis of psychopathologies, including depression- and anxiety-related disorders, as a hyperactive state of the HPA axis has been found in patients with major depression (Nemeroff, 1996; Mitchell, 1998; Arborelius et al. 1999; Wong & Licinio, 2001; Bakshi et al. 2002; Heinrichs & Koob, 2004). Accordingly, the reduced activity of the brain CRF system may represent a protective mechanism of the maternal brain to cope with the dramatic alteration in various hormonal systems, in particular gonadal steroids, around birth. Because gonadal steroids are important regulators of the HPA axis and the CRF system (Kirschbaum et al. 1999; Young et al. 2001), the large shifts in hormone levels that occur peripartum may particularly contribute to the relapse of major depression in women with a history of psychopathologies. Thus, general suppression of the activity of the HPA axis during lactation was hypothesized to prevent the development of depression in vulnerable women (Carter et al. 2001). In support of our hypothesis of dampened CRF acitivity being important for the well-being of the mother, elevated CSF concentrations of CRF were found in rhesus macaque females that abused their infants. These females also rated higher in their level of anxiety and aggression (Maestripieri et al. 2005). According to another hypothesis, the central suppression of hypothalamic CRF causes the increased vulnerability to affective disorders postpartum (Magiakou et al. 1996a; Mastorakos & Ilias, 2000) as a more pronounced attenuation of the ACTH response to a CRF bolus was found in women with postpartum blues compared with euthymic women.
Other factors possibly involved in these mood disorders include brain vasopressin and OXT, which exert opposite effects on anxiety-like behaviour and contribute to the regulation of HPA axis responses (Landgraf et al. 1995; Purba et al. 1996; for review see (Landgraf & Neumann, 2004). Also, relationships between PRL and mood state, including depression and anxiety, have been found with high circulating PRL levels in breastfeeding women associated with hypo-anxiety (Asher et al. 1995) and lower levels with the occurrence of depression (Abou-Saleh et al. 1998). Furthermore, according to the monoamine hypothesis, depression results from underactivity of noradrenaline and serotonin systems, and accepted treatment is generally with their respective reuptake inhibitors (Ressler & Nemeroff, 1999; Wong & Licinio, 2001). However, whether these factors are also dysregulated in postpartum mood disorders is presently unknown.
Conclusions
The findings from animal and human studies in late pregnancy and lactation, reveal profound physiological adaptations of neuroendocrine and behavioural stress responses occurring at various brain levels. The adaptations ensure the healthy development of the offspring by preventing excess prenatal glucocorticoid exposure and appropriate maternal care in the postpartum period. These adaptations include activation of brain OXT and PRL systems, which act to attenuate the HPA axis activity and emotional responsiveness in the peripartum period, coupled with a dampening of opioid and noradrenergic systems, which are the main excitatory inputs of the HPA axis. If these important adaptations are prevented, for example by chronic life stress especially during pregnancy, hormonal or other physiological events around birth may alter the activity of the brain CRF, OXT, vasopressin and PRL systems thus increasing the risk for mood disturbances and post-partum depression.
References
- Abou-Saleh MT, Ghubash R, Karim L, Krymski M, Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23:465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80:2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Asher I, Kaplan B, Modai I, Neri A, Valevski A, Weizman A. Mood and hormonal changes during late pregnancy and puerperium. Clin Exp Obstet Gynecol. 1995;22:321–325. [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Musch W, Bredewold R, Slattery DA, Neumann ID. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: implications for postpartum mood disorder. Psychoneuroendocrinology. 2007;32:267–278. doi: 10.1016/j.psyneuen.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Bridges R, Rigero B, Byrnes E, Yang L, Walker A. Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology. 2001;142:730–739. doi: 10.1210/endo.142.2.7931. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Hypothalamic-pituitary-adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J Neuroendocrinol. 2003;15:633–637. doi: 10.1046/j.1365-2826.2003.01045.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Ma X, Ingram CD, Lightman SL, Aguilera G. Hypothalamic and amygdaloid corticotropin-releasing hormone (CRH) and CRH receptor-1 mRNA expression in the stress-hyporesponsive late pregnant and early lactating rat. Brain Res Mol Brain Res. 2001;91:119–130. doi: 10.1016/s0169-328x(01)00137-1. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 1996;742:177–184. doi: 10.1016/s0006-8993(96)00962-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy – a review. Neurosci Biobehav Rev. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Donner N, Bredewold R, Maloumby R, Neumann ID. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci. 2007;25:1804–1814. doi: 10.1111/j.1460-9568.2007.05416.x. [DOI] [PubMed] [Google Scholar]
- Douglas AJ. Central noradrenergic mechanisms underlying acute stress responses of the hypothalamo-pituitary-adrenal axis: adaptations through pregnancy and lactation. Stress. 2005;8:5–18. doi: 10.1080/10253890500044380. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology. 2003;144:5268–5276. doi: 10.1210/en.2003-0461. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone HA, Wigger A, Landgraf R, Russell JA, Neumann ID. The role of endogenous opioids in neurohypophysial and hypothalamo-pituitary-adrenal axis hormone secretory responses to stress in pregnant rats. J Endocrinol. 1998;158:285–293. doi: 10.1677/joe.0.1580285. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Meddle SL, Toschi N, Bosch OJ, Neumann ID. Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. J Neuroendocrinol. 2005;17:40–48. doi: 10.1111/j.1365-2826.2005.01272.x. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Neumann I, Meeren HK, Leng G, Johnstone LE, Munro G, Russell JA. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci. 1995;15:5049–5057. doi: 10.1523/JNEUROSCI.15-07-05049.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AJ, Russell JA. Corticotrophin-releasing hormone, proenkephalin A and oxytocin mRNA's in the paraventricular nucleus during pregnancy and parturition in the rat. Gene Ther. 1994;1(Suppl. 1):S85. [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res. 2001;133:153–171. doi: 10.1016/s0079-6123(01)33012-1. [DOI] [PubMed] [Google Scholar]
- Hard E, Hansen S. Reduced fearfulness in the lactating rat. Physiol Behav. 1985;35:641–643. doi: 10.1016/0031-9384(85)90155-6. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Insel TR. Postpartum increases in brain oxytocin binding. Neuroendocrinology. 1986;44:515–518. doi: 10.1159/000124694. [DOI] [PubMed] [Google Scholar]
- Insel TR. Regional changes in brain oxytocin receptors post-partum: time-course and relationship to maternal behaviour. J Neuroendocrinol. 1990;2:539–545. doi: 10.1111/j.1365-2826.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reprod. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, Russell JA. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol. 2000;12:811–822. doi: 10.1046/j.1365-2826.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Adams D, Castelberg B, Glover V. Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth. 2002;2:8. doi: 10.1186/1471-2393-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann I. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–383. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotropin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology. 1989;124:2358–2364. doi: 10.1210/endo-124-5-2358. [DOI] [PubMed] [Google Scholar]
- Llewellyn AM, Stowe ZN, Nemeroff CB. Depression during pregnancy and the puerperium. J Clin Psychiatry. 1997;58(Suppl. 15):26–32. [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci Biobehav Rev. 2005;29:51–57. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996a;81:1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Margioris AN, Dubbert B, Calogero AE, Tsigos C, Munson PJ, Chrousos GP. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin Endocrinol (Oxf) 1996b;44:419–428. doi: 10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Prolactin receptor gene expression in the forebrain of pregnant and lactating rats. Brain Res Mol Brain Res. 2002;105:136–145. doi: 10.1016/s0169-328x(02)00401-1. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period. Postpartum-related disorders. Ann N Y Acad Sci. 2000;900:95–106. doi: 10.1111/j.1749-6632.2000.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. The role of corticotropin releasing factor in depressive illness: a critical review. Neurosci Biobehav Rev. 1998;22:635–651. doi: 10.1016/s0149-7634(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Neumann I. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res. 2001;133:143–152. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- Neumann I. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann I. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- Neumann I, Bosch OJ, Torner L, Miklos S, Waldherr M, Blume A. Society for Neuroscience 37th Annual Meeting. San Diego: Anxiolytic effects of oxytocin within the rat hypothalamic paraventricular nucleus: involvement of ERK1/2 activation; p. 84.13. [Google Scholar]
- Neumann I, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998a;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Koehler E, Landgraf R, Summy-Long J. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994a;134:141–148. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- Neumann I, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re) activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000a;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Neumann I, Landgraf R. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J Neuroendocrinol. 1989;1:305–308. doi: 10.1111/j.1365-2826.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Neumann I, Landgraf R, Bauce L, Pittman QJ. Osmotic responsiveness and cross talk involving oxytocin, but not vasopressin or amino acids, between the supraoptic nuclei in virgin and lactating rats. J Neurosci. 1995;15:3408–3417. doi: 10.1523/JNEUROSCI.15-05-03408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993a;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Neumann I, Porter DW, Landgraf R, Pittman QJ. Rapid effect on suckling of an oxytocin antisense oligonucleotide administered into rat supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol. 1994b;267:R852–R858. doi: 10.1152/ajpregu.1994.267.3.R852. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993b;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Neumann I, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000b;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Neumann I, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Neumann I, Wigger A, Liebsch G, Holsboer F, Landgraf R. Increased basal activity of the hypothalamo-pituitary-adrenal axis during pregnancy in rats bred for high anxiety-related behaviour. Psychoneuroendocrinology. 1998b;23:449–463. doi: 10.1016/s0306-4530(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Neumann I, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000c;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nisell H, Hjemdahl P, Linde B, Lunell NO. Sympatho-adrenal and cardiovascular reactivity in pregnancy-induced hypertension. I. Responses to isometric exercise and a cold pressor test. Br J Obstet Gynaecol. 1985;92:722–731. doi: 10.1111/j.1471-0528.1985.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:56–60. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- O'Hara M, Swain A. Rates and risks of postpartum depression – a-meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- Pedersen CA. Postpartum mood and anxiety disorders: a guide for the nonpsychiatric clinician with an aside on thyroid associations with postpartum mood. Thyroid. 1999;9:691–697. doi: 10.1089/thy.1999.9.691. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, McGuire M, Evans DL. Corticotropin-releasing hormone inhibits maternal behavior and induces pup-killing. Life Sci. 1991;48:1537–1546. doi: 10.1016/0024-3205(91)90278-j. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi XJ, Grattan DR. Increased prolactin receptor immunoreactivity in the hypothalamus of lactating rats. J Neuroendocrinol. 1999;11:693–705. doi: 10.1046/j.1365-2826.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–1233. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Factor EM, Mayer AD. Relationship between maternal aggression and maternal care in the rat. Aggress Behav. 1994;20:243–255. [Google Scholar]
- Russell JA, Johnstone H, Douglas AJ, Landgraf R, Wigger A, Shipston M, Seckl JR, Neumann ID. Neuroendocrine stress mechanisms regulating ACTH and oxytocin in pregnancy. In: Yamashita H, editor. Control Mechanisms of Stress and Emotions: Neuroendocrine-based Studies. New York: Elsevier; 1999. pp. 33–51. [Google Scholar]
- Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf) 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J Neuroendocrinol. 1999;11:857–865. doi: 10.1046/j.1365-2826.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Stern JM, Goldman L, Levine S. Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12:179–191. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- Torner L, Maloumby R, Nava G, Aranda J, Clapp C, Neumann ID. In vivo release and gene upregulation of brain prolactin in response to physiological stimuli. Eur J Neurosci. 2004;19:1601–1608. doi: 10.1111/j.1460-9568.2004.03264.x. [DOI] [PubMed] [Google Scholar]
- Torner L, Toschi N, Nava G, Clapp C, Neumann ID. Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur J Neurosci. 2002;15:1381–1389. doi: 10.1046/j.1460-9568.2002.01965.x. [DOI] [PubMed] [Google Scholar]
- Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Rochford J, Walker CD. Lactation-induced reduction in rats' acoustic startle is associated with changes in noradrenergic neurotransmission. Behav Neurosci. 1999a;113:176–184. doi: 10.1037//0735-7044.113.1.176. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Tesolin S, Huang N, Walker C. Altered pituitary sensitivity to corticotropin-releasing factor and arginine vasopressin participates in the stress hyporesponsiveness of lactation in the rat. J Neuroendocrinol. 1999b;11:757–764. doi: 10.1046/j.1365-2826.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N, Walker CD. Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. J Neuroendocrinol. 1998;10:417–427. doi: 10.1046/j.1365-2826.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leengoed E, Kerker E, Swanson HH. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Walker CD, Toufexis DJ, Burlet A. Hypothalamic and limbic expression of CRF and vasopressin during lactation: implications for the control of ACTH secretion and stress hyporesponsiveness. Prog Brain Res. 2001;133:99–110. doi: 10.1016/s0079-6123(01)33008-x. [DOI] [PubMed] [Google Scholar]
- Walker CD, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J Neuroendocrinol. 1995;7:615–622. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Wigger A, Lorscher P, Oehler I, Keck ME, Naruo T, Neumann ID. Nonresponsiveness of the rat hypothalamo-pituitary-adrenocortical axis to parturition-related events: inhibitory action of endogenous opioids. Endocrinology. 1999;140:2843–2849. doi: 10.1210/endo.140.6.6784. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Endogenous opioid regulation of stress-induced oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: a microdialysis study. Neuroscience. 2002;112:121–129. doi: 10.1016/s0306-4522(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997a;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa AP, Ingram CD, Lightman SL. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol. 1997b;9:407–414. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]