Abstract
Natriuretic peptides (NPs), including atrial, brain and C-type natriuretic peptides (ANP, BNP and CNP), bind two classes of cell surface receptors: the guanylyl cyclase-linked A and B receptors (NPR-A and NPR-B) and the C receptor (NPR-C). The biological effects of NPs have been mainly attributed to changes in intracellular cGMP following their binding to NPR-A and NPR-B. NPR-C does not include a guanylyl cyclase domain. It has been denoted as a clearance receptor and is thought to bind and internalize NPs for ultimate degradation. However, a substantial body of biochemical work has demonstrated the ability of NPR-C to couple to inhibitory G proteins (Gi) and cause inhibition of adenylyl cyclase and activation of phospholipase-C. Recently, novel physiological effects of NPs, mediated specifically by NPR-C, have been discovered in the heart and vasculature. We have described the ability of CNP, acting via NPR-C, to selectively inhibit L-type calcium currents in atrial and ventricular myocytes, as well as in pacemaker cells (sinoatrial node myocytes). In contrast, our studies of the electrophysiological effects of CNP on cardiac fibroblasts demonstrated an NPR-C–Gi–phospholipase-C-dependent activation of a non-selective cation current mediated by transient receptor potential (TRP) channels. It is also known that CNP and BNP have important anti-proliferative effects in cardiac fibroblasts that appear to involve NPR-C. In the mammalian resistance vessels, including mesenteric and coronary arteries, CNP has been found to function as an NPR-C-dependent endothelium-derived hyperpolarizing factor that regulates local blood flow and systemic blood pressure by hyperpolarizing smooth muscle cells. In this review we highlight the role of NPR-C in mediating these NP effects in myocytes and fibroblasts from the heart as well as in vascular smooth muscle cells.
The hallmark discovery of atrial natriuretic peptide (ANP) by de Bold and colleagues (de Bold et al. 1981; Flynn et al. 1983) has led to an entire new field of study of a group of related peptide hormones that are now known to have widespread effects in all vertebrates including humans. In addition to ANP, the NP family includes brain natriuretic peptide (BNP) (Sudoh et al. 1988), C-type natriuretic peptide (CNP) (Sudoh et al. 1990), Dendroaspis natriuretic peptide (Schweitz et al. 1992; Schirger et al. 1999) and urodilatin (an alternate splice variant of pro-ANP) (Schulz-Knappe et al. 1988). New members of the NP family continue to be discovered. For example, a recent report describes three novel natriuretic-like peptides from the venom of the Australian inland taipan (Oxyuranus microlepidotus). These have been denoted TNP-a, b and c (Fry et al. 2005).
Although best known for the important role they play in the regulation of blood pressure and cardiovascular homeostasis (Levin et al. 1998; D'Souza et al. 2004; Kuhn, 2004), NPs also affect many different tissues in a wide variety of organ systems. In addition to their ability to modulate blood volume and blood pressure through their effects on the kidney and the vasculature, NPs affect the electrophysiology of the heart (Rose et al. 2003, 2004) and central nervous system (Trachte et al. 1995, 2003; Rose et al. 2005) as well as muscular contraction in the gastrointestinal system (Murthy et al. 1998, 2000). The formation and maintenance of bone and musculoskeletal structures are also influenced by NPs (Matsukawa et al. 1999; Pejchalova et al. 2007).
NPs exert their biological effects by binding to three distinct cell surface receptors denoted NP receptors A, B and C (NPR-A, NPR-B and NPR-C) (Nakao et al. 1992; Levin et al. 1998). NPR-A and NPR-B mediate their cellular effects by altering intracellular cGMP levels following activation of a particulate guanylyl cyclase domain on the cytoplasmic side of these receptors. NPR-A primarily binds ANP and BNP, while NPR-B preferentially binds CNP (Lucas et al. 2000; Potter et al. 2006).
In contrast, NPR-C does not contain a guanylyl cyclase domain and has no direct effect on cGMP levels. NPR-C is known to bind ANP, BNP and CNP with similar affinity (Anand-Srivastava & Trachte, 1993). Furthermore, the affinity of NPR-C for the NPs is similar to the guanylyl cyclase-linked NPR-A and NPR-B receptors, suggesting that one class of receptor would not dominate the others on the basis of affinity for the peptides (Maack et al. 1987; Levin, 1993). Maack and colleagues demonstrated that the ring-deleted ANP analogue, cANF4-23 (cANF), can compete for the vast majority of ANP binding sites in the isolated perfused rat kidney without altering guanylyl cyclase activity (Maack et al. 1987). In this study the specific binding of radiolabelled ANP was almost completely inhibited by cANF (10−7m) demonstrating that cANF can occupy up to 99% of ANP binding sites. Despite its inability to stimulate guanylyl cyclase activity, cANF significantly increased sodium excretion and decreased blood pressure in conscious rats. These effects were attributed to a significant increase in plasma ANP levels in the presence of cANF (Maack et al. 1987). It was further concluded that the ability of ANP to bind NPR-C was occluded by cANF and that the majority of renal ANP receptors were ‘silent’. This appears to be the basis for the classification of NPR-C as a ‘clearance receptor’. Accordingly, it was suggested that the main function of NPR-C is to remove NPs from the circulation, thereby buffering the levels of NP available to alter guanylyl cyclase activity and intracellular cGMP levels via NPR-A and NPR-B. This hypothesis received further support from data derived from a transgenic mouse model in which NPR-C was genetically ablated (Matsukawa et al. 1999). These experiments showed that the half-life of radiolabelled ANP in the circulation of homozygote mice lacking NPR-C was 66% longer than in wild-type animals and it was concluded that NPR-C functioned mainly as a modulator of NP availability at target organs.
Although NPR-C is still commonly referred to as a clearance receptor, there now exists a wealth of experimental evidence that indicates NPR-C is coupled to an inhibitory heterotrimeric G protein, Gi. Both the α and βγ subunits of this Gi protein mediate a number of important physiological effects. The NPR-C receptor subtype is widely distributed in many tissues and cell types including cardiac myocytes and fibroblasts, vascular smooth muscle cells, platelets, gastrointestinal smooth muscle, cerebral cortex, brain striatum, hypothalamus, adrenal glands, zona glomerulosa, bone and chondrocytes. (Anand-Srivastava & Trachte, 1993; Anand-Srivastava, 2005).The main purpose of this review is to summarize the biological actions of NPs that are mediated specifically by NPR-C in the heart and vasculature.
The NPR-C receptor: crystal structure
NPR-C receptors are disulphide-linked homodimers of a single transmembrane 64–66 kDa protein. NPR-C has an extracellular domain of about 440 amino acids and a short 37 amino acid cytoplasmic domain or tail (Fuller et al. 1988; Anand-Srivastava et al. 1996). Initially, the crystal structure of the extracellular portion of both the unliganded NPR-C receptor and the CNP-bound NPR-C receptor were determined (He et al. 2001). The unliganded structure has been studied at 2.9 Å resolution. Each monomer is dumbbell-shaped, with each domain possessing an αβ fold made up of a β sheet surrounded by α helices. The NH2 and COOH termini are connected by three short peptides, which form a hinge at an angle of approximately 120 deg. This hinge creates a cleft between these two domains, and this motif is the ligand-binding site.
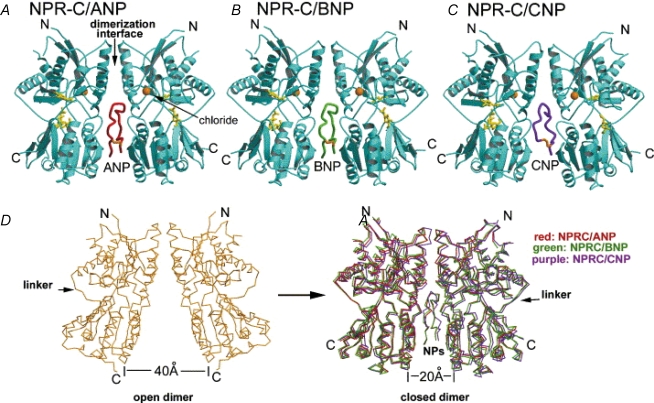
Crystallization of the CNP–NPR-C complex (at 2 Å resolution) revealed that a single CNP molecule can bind to the centre of the symmetrical dimeric receptors (Fig. 1). The NP assumes a disc-like conformation and intercalates between the two halves of the dimer. Following ligand binding the gap between the COOH termini closes by approximately 20 Å which results in the two monomers making contact with the opposing faces of the peptide. Contact between the peptide and membrane proximal domains causes the angle between the NH2 and COOH terminal domains to open by approximately 13.5 deg. Thus the NPR-C receptor is said to have open and closed states and the transition is regulated by peptide binding. What is unique about NPR-C is that NPs appear to interact with two non-identical hormone interaction sites on this receptor subtype. The determination of the crystal structure of NPR-C has shown that a single chloride ion is bound in the membrane distal domain of NPR-C, which is thought to be necessary for receptor activity (He et al. 2001, 2006). Very recently, the structure of the extracellular portion of NPR-C, when bound to ANP and BNP, has been determined and shown to be similar to the NPR-C–CNP complex described above (He et al. 2006) (Fig. 1).
Figure 1. Ribbon diagrams illustrating the structure of the extracellular domain of the NPR-C receptor when bound to ANP (A), BNP (B) and CNP (C).
D, unliganded and ligand-bound NPR-C dimers demonstrating the ‘open’ and ‘closed’ receptor conformations, which differ by approximately 20 Å (see text for details). Figure originally published in He et al. (2006); reprinted with permission from Elsevier.
The NPR-C receptor: intracellular signalling mechanisms
In addition to their well-characterized effects on guanylyl cyclase activity and cGMP signalling via NPR-A and NPR-B (Potter et al. 2006), there is substantial evidence that NPs can also inhibit adenylyl cyclase and its downstream signalling molecules via the activation of NPR-C. Anand-Srivastava and Cantin studied the effect of ANP on adenylyl cyclase activity in cultured atrial and ventricular myocytes from the neonatal rat heart (Anand-Srivastava & Cantin, 1986). In this study ANP significantly inhibited adenylyl cyclase in a concentration-dependent matter. Furthermore, ANP was shown to significantly inhibit the stimulatory effects of several activators of adenylyl cyclase, including isoproterenol and forskolin. As expected from an inhibitory effect on adenylyl cyclase, ANP application decreased intracellular cAMP levels in these neonatal cardiomyocytes.
More detailed studies of the mechanism through which ANP inhibits adenylyl cyclase activity have demonstrated the essential involvement of the inhibitory guanine nucleotide regulatory protein (Gi) family. The role of a Gi protein in the inhibition of adenylyl cyclase in rat aorta was confirmed by pretreatment with pertussis toxin (PT), a manoeuvre which antagonizes the GTP-dependent and receptor-mediated inhibition of adenylyl cyclase via the ADP ribosylation of the Gi protein (Katada & Ui, 1982; Katada et al. 1986). PT treatment strongly attenuated the ANP-mediated inhibition of adenylyl cyclase activity in rat aorta at all ANP concentrations tested and also abolished the inhibitory effect of ANP on isoproterenol and forskolin-stimulated adenylyl cyclase activity (Anand-Srivastava et al. 1987). These results now provide a firm basis for the hypothesis that some ANP receptors are coupled to adenylyl cyclase through a Gi protein.
Attempts to define the role of NPR-C in mediating an inhibition of adenylyl cyclase have been aided by the use of cANF as a selective NPR-C agonist (Maack et al. 1987). The effects of cANF have been studied in rat aorta, brain striatum, anterior pituitary and adrenal cortical membrane preparations where it was found to mimic the inhibition of adenylyl cyclase by ANP. Maximum inhibition was 50–60% with an apparent Ki of 0.1–1 nm (Anand-Srivastava et al. 1990). When cAMP and cGMP production were assayed in cultured vascular smooth muscle cells from the rat aorta it was found that ANP decreased cAMP levels and increased cGMP levels whereas cANF decreased cAMP levels but had no effect on cGMP levels. This pattern of results can be explained by the ability of ANP to bind to both NPR-A and NPR-C (and therefore activate guanylyl cyclase and inhibit adenylyl cyclase, respectively) whereas cANF only binds to NPR-C (and therefore only inhibits adenylyl cyclase). Applying cANF and ANP in combination caused the same amount of inhibition of adenylyl cyclase as either peptide alone. Moreover, the inhibitory effect of cANF on adenylyl cyclase was abolished by PT treatment, confirming a role for Gi in the inhibition of adenylyl cyclase (Anand-Srivastava et al. 1990). It is now accepted that cANF is a specific and selective agonist of NPR-C.
The NPR-C receptor is not a traditional G protein-coupled receptor in that it is not a ‘serpentine’ or ‘heptahelical’ receptor with seven transmembrane-spanning domains as is the case for the majority of receptors coupled to inhibitory and stimulatory G proteins (Birnbaumer et al. 1990; Birnbaumer, 1992). Nevertheless, it is clear that NPs can inhibit adenylyl cyclase following the activation of a pertussis toxin-sensitive G protein. Important features of the mechanism by which this interaction occurs have now been established. The cytoplasmic domain of NPR-C is thought to be responsible for the inhibition of adenylyl cyclase by ANP (Anand-Srivastava et al. 1996). In experiments performed using rat heart tissue, a polyclonal antibody raised against the 37 amino acid intracellular portion of NPR-C blocked the ANP-dependent inhibition of adenylyl cyclase. This finding was extended further by synthesizing a synthetic peptide corresponding to the 37 amino acids of the free cytoplasmic domain of NPR-C, and evaluating its effect on adenylyl cyclase activity (Anand-Srivastava et al. 1996). This synthetic peptide inhibited adenylyl cyclase activity in the rat heart by 35–45% with a Ki of approximately 1 nm. In contrast, a scrambled peptide control had no effect on adenylyl cyclase activity. The effect of this synthetic 37 amino acid peptide was dependent on the presence of guanine nucleotides and was antagonized by pertussis toxin, thus it was concluded that the cytoplasmic domain of NPR-C is able to directly activate Gi.
Examination of the cytoplasmic domain of NPR-C in search of the epitope responsible for the inhibition of adenylyl cyclase revealed the presence of Gi activator sequences (Murthy & Makhlouf, 1999; Pagano & Anand-Srivastava, 2001). Gi activator sequences were first described for the insulin-like growth factor receptor. These moieties are characterized by the presence of two NH2-terminal basic residues and a COOH-terminal BBXXB motif, where B and X are basic and non-basic residues, respectively (Okamoto et al. 1990). These Gi activator sequences have been identified in several single transmembrane domain receptors, including NPR-C (Okamoto & Nishimoto, 1992; Patel, 2004).
The cytoplasmic domain of NPR-C consists of the following 37 amino acids: 1RKKYRITIERRNHQEESNI-GKHRELREDSIRSHFSVA37 (single-letter amino acid abbreviations). Four regions within these 37 amino acids have been identified that exhibit similarities to the Gi activator sequence identified in the insulin-like growth factor receptor (Pagano & Anand-Srivastava, 2001). These four regions have been synthesized as short peptides and each can significantly inhibit adenylyl cyclase activity in rat heart and vascular smooth muscle cells. Neither scrambled versions of the peptides nor other short synthetic peptides lacking a full Gi activator sequence had any effect on adenylyl cyclase levels. The inhibition of adenylyl cyclase by these Gi activator peptides is comparable to that induced by both ANP and the NPR-C-selective agonist cANF and is strongly antagonized by pretreatment with PT (Pagano & Anand-Srivastava, 2001). Collectively, these findings demonstrate that the 37 amino acid cytoplasmic domain of NPR-C contains functional Gi-activator sequences that are able to inhibit adenylyl cyclase activity through a PT-sensitive Gi protein.
In addition to the inhibitory effect on adenylyl cyclase, the Gi proteins activated by the NPR-C receptor can also activate the β isoform of phospholipase C (PLCβ) (Pagano & Anand-Srivastava, 2001). Whereas the effect on adenylyl cyclase is mediated by the α subunit of the Gi protein, the activation of PLCβ is mediated by the βγ subunits of the same Gi protein (Murthy & Makhlouf, 1999). The βγ subunits of other G proteins are known to activate PLCβ in a similar fashion (Katz et al. 1992; Barr et al. 2000).
Most recently, a 17 amino acid sequence in the middle region of the 37 amino acid intracellular domain (R469–R485) has been identified as the functionally relevant Gi activator sequence. In these experiments amino acids R469–R485 were shown to be involved in the activation of Gαi1 and Gαi2, which inhibited adenylyl cyclase and activated PLC (Zhou & Murthy, 2003). The role of amino acids R469–R485 of NPR-C in activating Gi proteins was confirmed by mutating specific amino acids in this sequence and then examining the ability of the NPR-C-selective agonist, cANF, to inhibit adenylyl cyclase or activate PLC in COS-1 cells expressing the mutant receptors. Substitution of both NH2-terminal arginine residues (R469L and R470L) abolished PLCβ activity and significantly decreased the inhibition of adenylyl cyclase by approximately 30% (compared to the wild-type NPR-C receptor) in the presence of cANF. Substitution of single basic amino acid residues in the COOH-terminal motif H481RELR485 (mutants H481L, R482L and R485L) also abolished PLCβ activity. Adenylyl cyclase inhibition was decreased significantly by the H481L and R482L mutants (35% and 10%, respectively, compared to wild-type NPR-C) and completely abolished by the R485L mutation. Based on these results it is suggested that the 17 amino acid sequence R469–R485 is the functionally relevant Gi activator region of NPR-C.
In summary, a series of elegant studies from a number of laboratories have made use of selective pharmacological agents, as well as biochemical and molecular techniques to clearly demonstrate the ability of NPs to activate Gi proteins via NPR-C receptor-mediated signalling. NPR-C contains a specific Gi-activator domain (R469–R485). This is essential for the activation of Gi and the subsequent inhibition of adenylyl cyclase activity (via the α subunit) as well as for the stimulation of PLCβ signalling (via the βγ subunits).
The role of NPR-C signalling in cardiac myocytes
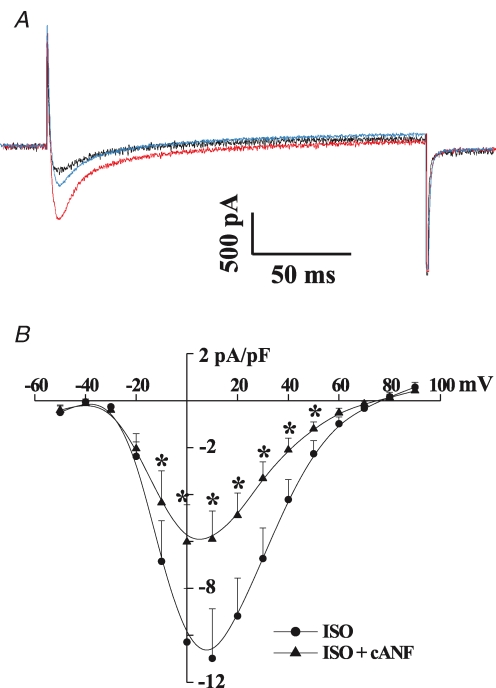
The elucidation of the biochemical and signalling pathways modulated by NPR-C (many of which were characterized in cardiac tissue as described above) provided a basis for our studies of the electrophysiological effects of NPR-C in the heart. Application of CNP (10−8m) to enzymatically isolated bullfrog atrial myocytes significantly shortened the action potential and reduced the peak amplitude following pretreatment with isoproterenol (ISO) (Rose et al. 2003). Voltage-clamp studies investigating the ionic mechanism for this effect first revealed the ability of CNP, as well as the NPR-C agonist cANF, to significantly inhibit L-type Ca2+ current (ICa,L). This effect is selective as there was no change in the inward rectifier K+ current in these cells. Additional evidence for the role of NPR-C in this inhibition of ICa,L was provided by applying HS-142-1, a selective antagonist of the guanylyl cyclase-linked NPR-A and NPR-B receptors (Matsuda & Morishita, 1993; Matsuda, 1997). CNP inhibited ICa,L by approximately 50% in the presence of HS-142-1. HS-142-1 removes the ability of NPs to increase cGMP levels, thus the CNP effects on ICa,L are mediated by NPR-C. This effect on ICa,L is also present in the working myocardium of mammalian hearts, where CNP and cANF potently reduced ISO-stimulated ICa,L amplitude in mouse ventricular myocytes (Figs 2 and 3).
Figure 2. Effects of the NPR-C-selective agonist cANF on L-type Ca2+ current in ventricular myocytes from the adult mouse heart.
A, representative ICa,L recordings made during a voltage-clamp step to 0 mV in control conditions (black trace), in the presence of isoproterenol (ISO, 1 × 10−6m, red trace) and following the application of cANF (1 × 10−8m, blue trace). B, summary I–V curves illustrating that cANF potently reduces ISO-stimulated ICa,L. Data are presented as mean ± s.e.m., *P < 0.05; n = 7 myocytes. R. A. Rose & W. R. Giles, previously unpublished data.
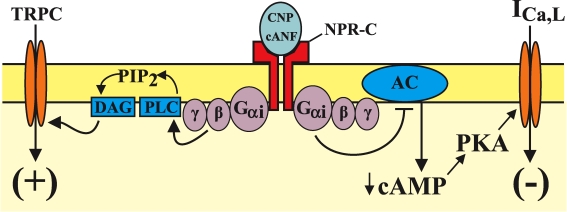
Figure 3. Schematic representation of the effects of CNP, acting via the NPR-C receptor, on cardiac myocytes and fibroblasts.
The right side of the figure shows that binding of NPR-C by CNP or cANF activates Gi proteins, which inhibit adenylyl cyclase (AC) activity via the α subunit of the G protein. This results in a reduction in intracellular cAMP levels and a significant inhibition of ICa,L in cardiomyocytes. The left side of the diagram illustrates the different signalling pathway activated by NPR-C in cardiac fibroblasts. In fibroblasts, the βγ subunits of Gi proteins activate phospholipase C (PLC), which converts PIP2 to diacylglycerol (DAG) (and IP3; not shown). DAG directly activates members of the TRPC subfamily, independently of protein kinase C.
Subsequently, in an effort to define the role of NPs in cardiac pacemaker mechanisms, the effects of CNP and NPR-C signalling were explored in the mouse sinoatrial node (SAN) (Rose et al. 2004). Isolated myocytes from the SAN express several cAMP-sensitive currents, including ICa,L and the hyperpolarization-activated current If (DiFrancesco, 1993; Accili et al. 2002). Somewhat surprisingly, CNP and cANF inhibited ICa,L by about 50%, but had no significant effect on If. Application of a synthetic ‘Gi-activator peptide’ consisting of the 17 amino acid sequence from NPR-C that stimulates Gi (R469–R485) mimicked the effect of CNP and cANF on ICa,L. Although If was not altered by CNP, it was decreased by application of acetylcholine as expected from previous publications (DiFrancesco & Tromba, 1988a,b). This result confirmed that If was modulated by cAMP levels in these SAN myocytes while also demonstrating that this conductance is insensitive to CNP or cANF. These results suggest that the adenylyl cyclase enzymes and/or intracellular cAMP pools that are modulated by the NPR-C receptor may be compartmentalized in the mouse SAN. Compartmentation of cAMP (the process whereby different hormones activate distinct pools of cAMP) is known to occur in ventricular myocytes (Bers & Ziolo, 2001; Steinberg & Brunton, 2001; Kerfant et al. 2006) and is thought to be due, at least in part, to the actions and/or locations of key intracellular molecules including phosphodiesterases, phosphatases and anchoring proteins for protein kinase A (AKAPs) (Bauman & Scott, 2002; Georget et al. 2003; Wong & Scott, 2004). The molecular mechanism(s) by which CNP–NPR-C effects are compartmentalized in mouse SAN myocytes is not clear and will require further investigation.
To determine if this NPR-C-mediated selective inhibition of ICa,L in SAN myocytes had an effect on heart rate, cANF was applied to Langendorff-perfused mouse hearts (Rose et al. 2004). Electrocardiograms (ECGs) were measured in control conditions, during pretreatment with ISO, and following the application of cANF. As expected, ISO decreased R–R intervals on the ECG (which corresponds to an increase in heart rate). This effect was strongly attenuated by cANF, indicating that NPR-C can mediate this reduction in heart rate. In addition to decreasing heart rate, cANF also significantly increased the P–R interval of the ECG, suggesting that conduction is slowed within the atrioventricular conduction system following the activation of NPR-C. These data are consistent with other studies demonstrating a key role for L-type Ca2+ channels in the intrinsic regulation of SAN pacemaker function and the determination of heart rate (Zhang et al. 2002; Mangoni et al. 2003).
Role of NPR-C signalling in cardiac fibroblasts
In the mammalian heart fibroblasts comprise roughly 90% of the non-myocyte cell population. Fibroblasts are responsible for the synthesis and secretion of the extracellular matrix proteins collagen (types I and II) and matrix metalloproteinases, as well as various hormonal factors (Brilla & Maisch, 1994; Brilla et al. 1995; Baudino et al. 2006). Cardiac fibroblasts secrete NPs, including BNP and CNP, and express all three NP receptors (Tsuruda et al. 2002; Horio et al. 2003; Huntley et al. 2006).
NPs have been shown to act as paracrine factors that exert antifibrotic and antiproliferative effects on cardiac fibroblasts (Tsuruda et al. 2002; Horio et al. 2003; Kapoun et al. 2004; Kawakami et al. 2004; Huntley et al. 2006). These effects may be of particular importance during pathophysiological conditions that result in abnormal cardiac fibrosis, which is characterized by the inappropriate proliferation of fibroblasts and increased deposition of collagen that results in increased myocardial stiffness and diastolic dysfunction (Brilla & Maisch, 1994).
Although some of the antifibrotic effects of NPs are attributed to the NPR-A and NPR-B receptors, recent studies have begun to elucidate a role for NPR-C as well. Specifically, BNP has been shown to exert an antiproliferative action on human fibroblasts based on a BrdU cell proliferation assay (Huntley et al. 2006). This effect was not affected by application of HS-142-1 (which blocks the NPR-A and NPR-B receptors), but was antagonized by application of cANF, suggesting a distinct role for NPR-C in the regulation of fibroblast proliferation. NPR-C has also been implicated in antiproliferative processes in other cell types such as smooth muscle cells (Cahill & Hassid, 1994) and glia (Levin & Frank, 1991).
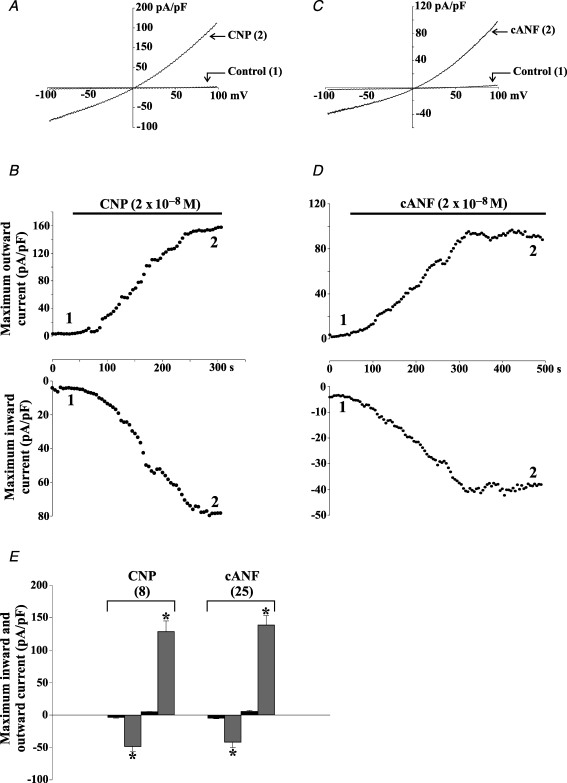
Relatively little is known about the electrophysiological properties of cardiac fibroblasts, but they do express a number of ion channels and are known to display oscillations in membrane potential termed ‘mechanically induced potentials’ (reviewed in Baudino et al. 2006). The electrophysiological effects of CNP on acutely isolated adult rat ventricular fibroblasts have been examined (Rose et al. 2007). Application of CNP and cANF (2 × 10−8m) potently activated a weakly outwardly rectifying non-selective cation current, which has an apparent reversal potential near 0 mV (Fig. 4). This cANF-activated current was antagonized by several compounds including Gd3+, SKF 96365 and 2-aminoethoxydiphenyl borate (2-APB), indicating the possible involvement of transient receptor potential (TRP) channels. RT-PCR on these acutely isolated fibroblasts revealed the expression of several TRP channel transcripts from the TRPC, TRPM and TRPV subfamilies. Additional electrophysiological studies demonstrated that the cANF-activated current was abolished by pretreatment with PT and blocked by the PLC antagonist U73122. Finally, the effects of cANF were mimicked by intracellular application of 1-oleyl-2-acetyl-sn-glycerol (OAG), independently of protein kinase C activity, which is a defining characteristic of specific members of the TRPC family of ion channels, including TRPC2, 3, 6 and 7 (Hofmann et al. 1999; Lucas et al. 2003; Zufall et al. 2005). In summary, these data demonstrate that CNP, acting via the NPR-C receptor and the activation of Gi, stimulates a non-selective cation current in cardiac fibroblasts that is partially carried by TRPC channels (summarized in Fig. 3).
Figure 4. CNP and the natriuretic peptide C receptor agonist cANF activate an outwardly rectifying non-selective cation current in acutely isolated rat cardiac fibroblasts.
A, representative recordings of the transmembrane current response during a 1 s voltage ramp from −100 to +100 mV in an isolated rat ventricular fibroblast. Holding potential was 0 mV. Note that CNP (2 × 10−8m) activates a weakly outwardly rectifying current that reverses at approximately 0 mV. B, time course of the effects of CNP on peak inward and outward current recorded in the same cardiac fibroblast. The numerals correspond to the time points at which the representative traces in A were recorded and black bars indicate application of an experimental compound. Note that both the inward and outward currents activate slowly, reaching a steady state in approximately 4–5 min. C and D, cANF (2 × 10−8m) mimics the effect of CNP on isolated rat cardiac fibroblasts. E, summary of the effects of CNP and cANF on cardiac fibroblasts (mean ± s.e.m., n values indicated in parentheses). Black bars denote control data, shaded bars depict results following the application of the indicated compound. *The value in the presence of CNP or cANF is significantly different from control. The effects of CNP and cANF were indistinguishable, which strongly suggests that the CNP effect is mediated by the NPR-C receptor. Figure modified from Rose et al. (2007).
The precise identity of the TRPC channel(s) modulated by CNP remains to be determined. The lack of specific pharmacological tools is a major limitation to the study of these channels in native tissues (Clapham et al. 2001; Clapham, 2003); therefore, molecular interventions such as RNA interference may be necessary to more definitively determine the molecular identity of the TRP channels involved in this response. The functional implications of the activation of a non-selective cation conductance by CNP–NPR-C also require further investigation. Cardiac fibroblasts are non-excitable cells that do not appear to express time- and voltage-gated cation currents such as INa or ICa (Rose et al. 2007); therefore, TRP channels may be key regulators of the secretory state of this important paracrine cell and could potentially be involved in the antifibrotic/antiproliferative effects of NPs in the heart. Also, fibroblasts are known to couple to atrial and ventricular myocytes through well-defined connexin-mediated mechanisms. The resulting electrotonic influences could have important implications on the electrophysiology of the working myocardium (Baudino et al. 2006; Chilton et al. 2007; MacCannell et al. 2007). Therefore, in addition to the direct electrophysiological effects of CNP–NPR-C on cardiomyocytes (discussed in previous section), it is possible that there could be indirect effects on myocyte electrical activity via electrotonic coupling to cardiac fibroblasts/myofibroblasts (Gaudesius et al. 2003).
The role of NPR-C signalling in EDHF responses in the vasculature
NPs are highly expressed in vascular endothelial cells and NPRs are located on the adjacent vascular smooth muscle cells. Furthermore, CNP is a potent vasodilator of blood vessels in humans (Wiley & Davenport, 2001), rats (Drewett et al. 1995; Brunner & Wolkart, 2001), dogs (Wennberg et al. 1999), mice (Madhani et al. 2003; Steinmetz et al. 2004) and pigs (Barber et al. 1998). It is well known that acetylcholine can cause smooth muscle hyperpolarization and vasodilatation by stimulating the release of an endothelium-dependent factor that functions independently of nitric oxide and prostacyclins (Cohen & Vanhoutte, 1995; Cohen, 2005). For more than two decades investigators have sought to identify this so-called endothelium-derived hyperpolarizing factor (EDHF).
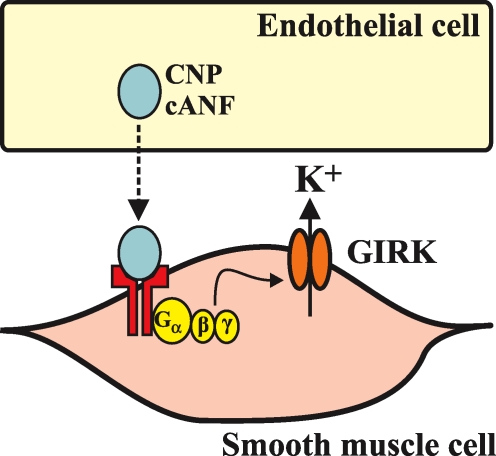
Because CNP is stored in endothelial cells and causes vasorelaxation by hyperpolarization of smooth muscle cells it is an interesting candidate molecule for this elusive EDHF. In a study of rat mesenteric arteries, a direct comparison was made between the vasorelaxant activity of EDHF (elicited by the administration of acetylcholine) and CNP (Chauhan et al. 2003). These experiments were done in the presence of l-NAME and indomethacin to block the actions of nitric oxide and prostacyclins, respectively, and to ensure that only EDHF-dependent relaxations were being investigated. Under these experimental conditions, acetylcholine and CNP produced identical hyperpolarizations of mesenteric vascular smooth muscle. Both responses were reduced by pharmacological inhibitors known to antagonize EDHF activity. These effects of CNP (and acetylcholine) were also attenuated by PT, which strongly implicated mediation by Gi and suggested the involvement of NPR-C. Importantly, the effects of CNP were not affected by endothelial denudation indicating that CNP is acting directly on the smooth muscle cells. The specific role of NPR-C in mediating smooth muscle relaxation was further supported by the finding that the effect was not altered by the NPR-A and NPR-B antagonist HS-142-1, but was mimicked by cANF. The effects of CNP, cANF and acetylcholine on smooth muscle relaxation were also antagonized by application of a putative K+ channel blocker, tertiapin. It was concluded that CNP can act as an EDHF by binding to NPR-C, stimulating Gi, and activating a K+ conductance (of the GIRK family of channels) following agonist-stimulated release of the βγ subunits of the G proteins (Fig. 5). Activation of this K+ conductance would lead to hyperpolarization and relaxation of vascular smooth muscle cells. An important result from this study was the finding that acetylcholine can stimulate the release of CNP from the vascular endothelium (Chauhan et al. 2003). The acetylcholine-elicited EDHF response was associated with this release of CNP suggesting that CNP may be a physiologically relevant EDHF. Most recently, the roles of CNP and NPR-C in EDHF responses in mesenteric arteries were substantiated functionally with the demonstration that the selective NPR-C antagonist M372049 blocked the vasorelaxant activity of CNP (Villar et al. 2007).
Figure 5. Schematic representation of the mechanism by which CNP, acting via the NPR-C receptor, can function as an endothelium-derived hyperpolarizing factor (EDHF).
The working hypothesis is that CNP is released from endothelial cells and then binds to NPR-C on vascular smooth muscle cells. βγ subunits of the Gi protein are then released and activate an inwardly rectifying K+ channel of the GIRK family. The K+ efflux hyperpolarizes the cell and elicits a relaxation response. See text for additional details.
The role of CNP (and NPR-C) as an EDHF has also been evaluated in the coronary circulation (Hobbs et al. 2004). In this study, CNP and cANF elicited very similar dose-dependent decreases in coronary perfusion pressure in Langendorff-perfused rat hearts. The effect was antagonized by a combination of Ba2+ and ouabain as well as by the application of the NPR-C antagonist M372049 (Veale et al. 2000), which supports the hypothesis of CNP acting as an EDHF via the NPR-C receptor.
Despite these interesting new findings which suggest that CNP, acting via the NPR-C receptor, can act as an EDHF molecule, important questions remain unanswered (Sandow & Tare, 2007). Although the pathway described for CNP–NPR-C in the EDHF response (Fig. 5) appears consistent in mesenteric and coronary arteries, similar measurements from other vessels do not yield the same pattern of results. For example, the rat aorta does display a relaxant response to CNP (and ANP); however, this response is not altered by application of the NPR-C antagonist M372049 (Villar et al. 2007). Similarly, a recent study investigating CNP effects in guinea pig carotid arteries (a common model used to study EDHF responses) concludes that CNP does not contribute to EDHF responses in this vessel (Leuranguer et al. 2007). These data indicate that the role of CNP as an EDHF may be vessel-specific. Also, it is proposed that CNP elicits its hyperpolarizing effect by activating an inwardly rectifying K+ current from the GIRK family in vascular smooth muscle cells. Definitive evidence for this is still lacking; therefore, patch-clamp and molecular studies on isolated vascular smooth cells are necessary to confirm this hypothesis. Such isolated cell studies could confirm the expression of NPR-C on vascular smooth muscle cells and identify the specific ionic conductance involved in the NPR-C-mediated effect.
The work of Hobbs and colleagues has also demonstrated that NPR-C signalling can protect against ischaemia/reperfusion injury (Hobbs et al. 2004). Hearts were pretreated with CNP or cANF (or a vehicle control), and then subjected to global ischaemia for 25 min. This was followed by 120 min of reperfusion. In some experiments CNP was not administered until the time of reperfusion. Hearts were then examined for the development of zones of infarcted tissue. In the presence of CNP or cANF, coronary perfusion pressure and left ventricular diastolic pressure were maintained at pre-ischaemic levels. In addition, the size of the infarct produced by the ischaemia/reperfusion event was reduced by up to 50% in CNP and cANF treated animals. This pattern of results suggests that CNP may prove to be an effective post-ischaemia agent to rescue the injured heart.
Other signalling cascades associated with NPR-C have also been shown to be present in vascular smooth muscle cells. For example, cANF both inhibits adenylyl cyclase activity and stimulates PLC activity in cultured smooth muscle cells. It has been suggested that there is cross-talk between these two pathways (Mouawad et al. 2004). Recently, cANF and synthetic NPR-C Gi-activator peptides have been found to inhibit vascular smooth muscle cell proliferation and smooth muscle cell hypertrophy via pathways involving MAP kinase and phosphatidylinositol 3-kinase (Hashim et al. 2006; Li et al. 2006).
The conclusions on the role of NPR-C in mediating the effects of NPs in the heart and vasculature described in the previous sections depend on the selectivity and effectiveness of the pharmacological compounds used to activate or antagonize specific NPRs. These findings would be strengthened by demonstrating the loss of these effects in mice in which NPR-C has been genetically ablated. A whole-body NPR-C knockout mouse has been generated (Matsukawa et al. 1999). This mouse displays profound skeletal abnormalities, consistent with a role for NPs in bone formation, and about 50% of the homozygous knockouts die before weaning. It would be useful to examine NP effects in these NPR-C knockout mice in the context of the above studies; however, due to the profound effects of whole-body NPR-C deletion, it may be necessary to generate tissue-specific NPR-C knockouts to properly assess the role of NPR-C in the heart and vasculature.
Significance of NPR-C signalling in the cardiovascular system
NP levels in the circulation are significantly increased in several pathological states including heart failure and myocardial ischaemia (Wei et al. 1993; Baxter, 2004; Richards, 2004). Under these conditions sustained myocardial stretch leads to the up-regulation of peptide synthesis so that both tissue and circulating levels of NPs increase significantly. This increased production and release of NPs is an important compensatory response as demonstrated in studies of experimental models of heart failure. For example, preventing the secretion of ANP or antagonizing its receptors significantly enhances the development of heart failure in an animal model of the disease. Similarly, knockout models in which animals lack ANP, BNP or their receptors show much greater loss of cardiac function and increased fibrosis compared to wild-type controls (Wada et al. 1994; Oliver et al. 1997; Tamura et al. 2000). Infusion of CNP prior to or following an ischaemic event reduces the size of ventricular infarction by up to 50% (Hobbs et al. 2004). This cardioprotective effect may be the result of increased coronary dilation, an inhibition of ICa,L and a reduction in heart rate (Hobbs et al. 2004; Rose et al. 2004). These CNP effects, which are mediated by the NPR-C receptor, may significantly increase myocardial perfusion (Scotland et al. 2005) and optimize metabolism due to the coincident bradycardia.
These changes in NP expression and secretion in the setting of cardiovascular disease have resulted in the use of assays for BNP production in determining the prognosis of patients with heart failure (Richards, 2004). These data demonstrate that patients diagnosed with heart failure have a strong association between elevated levels of plasma BNP and the risk of mortality (Cheng et al. 2001; Koglin et al. 2001). Furthermore, in randomized controlled trials of β blocker therapy in heart failure, plasma BNP has been shown to be a predictor of the probability of mortality, recurrent heart failure, and the likelihood of effective responses to therapy (Richards et al. 1999).
Based on the ability of NPs to elicit vasodilatation, natriuresis and antifibrotic effects, clinical trials have begun to examine the effects of administration of BNP to heart failure patients (Mills et al. 1999; Colucci et al. 2000; Strain, 2004). These studies have demonstrated that BNP infusion at a dose of approximately 0.01 μg kg−1 min−1 can reduce pulmonary capillary wedge pressure, cause vasodilatation and increase cardiac output. This results in the reduction or cessation of parenteral therapy as effectively and safely as conventional drug treatments such as dobutamine. Partly on this basis, human recombinant BNP (a synthetic form of the peptide known as ‘Nesiritide’) has been approved as a treatment for patients with decompensated heart failure in the United States (Richards, 2004). Nevertheless, many questions remained unanswered regarding the clinical applications of NPs (Carrillo-Jimenez et al. 2007; Sackner-Bernstein & Aaronson, 2007). It is anticipated that as our understanding of the roles of NPR-C (as well as NPR-A and NPR-B) in the cardiovascular system develop the clinical uses for NPs may be refined and improved.
In conclusion, the intracellular signalling cascades that are modulated by the NPR-C receptor have been the focus of a number of recent studies using cardiovascular tissues and cells. Although it was once denoted as a clearance receptor with no known signalling function it is now clear that NPR-C is functionally linked to an inhibitory G protein, Gi. The α subunit of this Gi protein inhibits adenylyl cyclase activity and lowers intracellular cAMP while the βγ subunits activate PLCβ in the heart and vasculature (as well as in other tissues outside the cardiovascular system). It is expected that new roles and functional implications for NPR-C signalling will continue to be elucidated. As many tissues express NPR-C as well as the guanylyl cyclase-linked NPR-A and NPR-B receptors it now seems prudent to begin questioning how these two kinds of receptors work in concert to regulate the physiology of the organism and to recognize that NPR-C has functional significance in addition to its classical clearance receptor role.
Acknowledgments
W.R.G. is supported by funding from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. R.A.R. is supported by awards from The Heart and Stroke Foundation of Canada and The Alberta Heritage Foundation for Medical Research.
References
- Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. doi: 10.1152/physiologyonline.2002.17.1.32. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB, Cantin M. Atrial natriuretic factor receptors are negatively coupled to adenylate cyclase in cultured atrial and ventricular cardiocytes. Biochem Biophys Res Commun. 1986;138:427–436. doi: 10.1016/0006-291x(86)90299-8. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB, Sairam MR, Cantin M. Ring-deleted analogs of atrial natriuretic factor inhibit adenylate cyclase/cAMP system. Possible coupling of clearance atrial natriuretic factor receptors to adenylate cyclase/cAMP signal transduction system. J Biol Chem. 1990;265:8566–8572. [PubMed] [Google Scholar]
- Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271:19324–19329. doi: 10.1074/jbc.271.32.19324. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB, Srivastava AK, Cantin M. Pertussis toxin attenuates atrial natriuretic factor-mediated inhibition of adenylate cyclase. Involvement of inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1987;262:4931–4934. [PubMed] [Google Scholar]
- Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev. 1993;45:455–497. [PubMed] [Google Scholar]
- Barber DA, Burnett JC, Jr, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. 1998;32:5–11. doi: 10.1097/00005344-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Barr AJ, Ali H, Haribabu B, Snyderman R, Smrcka AV. Identification of a region at the N-terminus of phospholipase C-β3 that interacts with G protein βγ subunits. Biochemistry. 2000;39:1800–1806. doi: 10.1021/bi992021f. [DOI] [PubMed] [Google Scholar]
- Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Scott JD. Kinase- and phosphatase-anchoring proteins: harnessing the dynamic duo. Nat Cell Biol. 2002;4:E203–E206. doi: 10.1038/ncb0802-e203. [DOI] [PubMed] [Google Scholar]
- Baxter GF. Natriuretic peptides and myocardial ischaemia. Basic Res Cardiol. 2004;99:90–93. doi: 10.1007/s00395-004-0458-7. [DOI] [PubMed] [Google Scholar]
- Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res. 2001;89:373–375. [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for βγ dimers as well as α subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Brilla CG, Maisch B. Regulation of the structural remodelling of the myocardium: from hypertrophy to heart failure. Eur Heart J. 1994;15(Suppl. D):45–52. doi: 10.1093/eurheartj/15.suppl_d.45. [DOI] [PubMed] [Google Scholar]
- Brilla CG, Maisch B, Zhou G, Weber KT. Hormonal regulation of cardiac fibroblast function. Eur Heart J. 1995;16(Suppl. C):45–50. doi: 10.1093/eurheartj/16.suppl_c.45. [DOI] [PubMed] [Google Scholar]
- Brunner F, Wolkart G. Relaxant effect of C-type natriuretic peptide involves endothelium and nitric oxide-cGMP system in rat coronary microvasculature. Cardiovasc Res. 2001;51:577–584. doi: 10.1016/s0008-6363(01)00283-8. [DOI] [PubMed] [Google Scholar]
- Cahill PA, Hassid A. ANF-C-receptor-mediated inhibition of aortic smooth muscle cell proliferation and thymidine kinase activity. Am J Physiol Regul Integr Comp Physiol. 1994;266:R194–R203. doi: 10.1152/ajpregu.1994.266.1.R194. [DOI] [PubMed] [Google Scholar]
- Carrillo-Jimenez R, Borzak S, Hennekens CH. Brain natriuretic peptide: clinical and research challenges. J Cardiovasc Pharmacol Ther. 2007;12:85–88. doi: 10.1177/1074248407302764. [DOI] [PubMed] [Google Scholar]
- Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V, Kazanagra R, Garcia A, Lenert L, Krishnaswamy P, Gardetto N, Clopton P, Maisel A. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37:386–391. doi: 10.1016/s0735-1097(00)01157-8. [DOI] [PubMed] [Google Scholar]
- Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583:225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Cohen RA. The endothelium-derived hyperpolarizing factor puzzle: a mechanism without a mediator? Circulation. 2005;111:724–727. doi: 10.1161/01.CIR.0000156405.75257.62. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988a;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D, Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988b;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewett JG, Fendly BM, Garbers DL, Lowe DG. Natriuretic peptide receptor-B (guanylyl cyclase-B) mediates C-type natriuretic peptide relaxation of precontracted rat aorta. J Biol Chem. 1995;270:4668–4674. doi: 10.1074/jbc.270.9.4668. [DOI] [PubMed] [Google Scholar]
- D'Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–129. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Flynn TG, de Bold ML, de Bold AJ. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun. 1983;117:859–865. doi: 10.1016/0006-291x(83)91675-3. [DOI] [PubMed] [Google Scholar]
- Fry BG, Wickramaratana JC, Lemme S, Beuve A, Garbers D, Hodgson WC, Alewood P. Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): isolation, chemical and biological characterisation. Biochem Biophys Res Commun. 2005;327:1011–1015. doi: 10.1016/j.bbrc.2004.11.171. [DOI] [PubMed] [Google Scholar]
- Fuller F, Porter JG, Arfsten AE, Miller J, Schilling JW, Scarborough RM, Lewicki JA, Schenk DB. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988;263:9395–9401. [PubMed] [Google Scholar]
- Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- Georget M, Mateo P, Vandecasteele G, Lipskaia L, Defer N, Hanoune J, Hoerter J, Lugnier C, Fischmeister R. Cyclic AMP compartmentation due to increased cAMP-phosphodiesterase activity in transgenic mice with a cardiac-directed expression of the human adenylyl cyclase type 8 (AC8) FASEB J. 2003;17:1380–1391. doi: 10.1096/fj.02-0784com. [DOI] [PubMed] [Google Scholar]
- Hashim S, Li Y, Anand-Srivastava MB. Small cytoplasmic domain peptides of natriuretic peptide receptor-C attenuate cell proliferation through Giα protein/MAP kinase/PI3-kinase/AKT pathways. Am J Physiol Heart Circ Physiol. 2006;291:H3144–H3153. doi: 10.1152/ajpheart.00327.2006. [DOI] [PubMed] [Google Scholar]
- He X, Chow D, Martick MM, Garcia KC. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science. 2001;293:1657–1662. doi: 10.1126/science.1062246. [DOI] [PubMed] [Google Scholar]
- He XL, Dukkipati A, Garcia KC. Structural determinants of natriuretic peptide receptor specificity and degeneracy. J Mol Biol. 2006;361:698–714. doi: 10.1016/j.jmb.2006.06.060. [DOI] [PubMed] [Google Scholar]
- Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–1235. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, Burnett JC., Jr BNP-induced activation of cGMP in human cardiac fibroblasts: Interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209:943–949. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- Kapoun AM, Liang F, O'Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-β in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- Katada T, Oinuma M, Ui M. Mechanisms for inhibition of the catalytic activity of adenylate cyclase by the guanine nucleotide-binding proteins serving as the substrate of islet-activating protein, pertussis toxin. J Biol Chem. 1986;261:5215–5221. [PubMed] [Google Scholar]
- Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Wu D, Simon MI. Subunits βγ of heterotrimeric G protein activate β2 isoform of phospholipase C. Nature. 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Nakagawa Y, Nakanishi M, Tanimoto K, Usami S, Yasuno S, Kinoshita H, Chusho H, Tamura N, Ogawa Y, Nakao K. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation. 2004;110:3306–3312. doi: 10.1161/01.CIR.0000147829.78357.C5. [DOI] [PubMed] [Google Scholar]
- Kerfant BG, Rose RA, Sun H, Backx PH. Phosphoinositide 3-kinase γ regulates cardiac contractility by locally controlling cyclic adenosine monophosphate levels. Trends Cardiovasc Med. 2006;16:250–256. doi: 10.1016/j.tcm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934–1941. doi: 10.1016/s0735-1097(01)01672-2. [DOI] [PubMed] [Google Scholar]
- Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99:76–82. doi: 10.1007/s00395-004-0460-0. [DOI] [PubMed] [Google Scholar]
- Leuranguer V, Vanhoutte PM, Verbeuren T, Feletou M. C-type natriuretic peptide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br J Pharmacol. 2007. DOI 10.1038/sj.bjp.0707476. [DOI] [PMC free article] [PubMed]
- Levin ER. Natriuretic peptide C-receptor: more than a clearance receptor. Am J Physiol Endocrinol Metab. 1993;264:E483–E489. doi: 10.1152/ajpendo.1993.264.4.E483. [DOI] [PubMed] [Google Scholar]
- Levin ER, Frank HJ. Natriuretic peptides inhibit rat astroglial proliferation: mediation by C receptor. Am J Physiol Regul Integr Comp Physiol. 1991;261:R453–R457. doi: 10.1152/ajpregu.1991.261.2.R453. [DOI] [PubMed] [Google Scholar]
- Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- Li Y, Hashim S, Anand-Srivastava MB. Intracellular peptides of natriuretic peptide receptor-C inhibit vascular hypertrophy via Gqα/MAP kinase signaling pathways. Cardiovasc Res. 2006;72:464–472. doi: 10.1016/j.cardiores.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–4132. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani M, Scotland RS, MacAllister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol. 2003;139:1289–1296. doi: 10.1038/sj.bjp.0705365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y. Design and utilization of natriuretic peptide antagonists. In: Samson WK, Levin ER, editors. Contemporary Endocrinology: Natriuretic Peptides in Health and Disease. Totawa, NJ, USA: Humana Press Inc.; 1997. pp. 289–307. [Google Scholar]
- Matsuda Y, Morishita Y. HS-142-1: a novel nonpeptide atrial natriuretic peptide antagonist of microbial origin. Cardiovasc Drug Rev. 1993;11:45–59. [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RM, LeJemtel TH, Horton DP, Liang C, Lang R, Silver MA, Lui C, Chatterjee K. Sustained hemodynamic effects of an infusion of nesiritide (human b-type natriuretic peptide) in heart failure: a randomized, double-blind, placebo-controlled clinical trial. Natrecor Study Group. J Am Coll Cardiol. 1999;34:155–162. doi: 10.1016/s0735-1097(99)00184-9. [DOI] [PubMed] [Google Scholar]
- Mouawad R, Li Y, Anand-Srivastava MB. Atrial natriuretic peptide-C receptor-induced attenuation of adenylyl cyclase signaling activates phosphatidylinositol turnover in A10 vascular smooth muscle cells. Mol Pharmacol. 2004;65:917–924. doi: 10.1124/mol.65.4.917. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Identification of the G protein-activating domain of the natriuretic peptide clearance receptor (NPR-C) J Biol Chem. 1999;274:17587–17592. doi: 10.1074/jbc.274.25.17587. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Teng B, Jin J, Makhlouf GM. G protein-dependent activation of smooth muscle eNOS via natriuretic peptide clearance receptor. Am J Physiol Cell Physiol. 1998;275:C1409–C1416. doi: 10.1152/ajpcell.1998.275.6.C1409. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Teng BQ, Zhou H, Jin JG, Grider JR, Makhlouf GM. Gi−1/Gi−2-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am J Physiol Gastrointest Liver Physiol. 2000;278:G974–G980. doi: 10.1152/ajpgi.2000.278.6.G974. [DOI] [PubMed] [Google Scholar]
- Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. II: Natriuretic peptide receptors. J Hypertens. 1992;10:1111–1114. doi: 10.1097/00004872-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Katada T, Murayama Y, Ui M, Ogata E, Nishimoto I. A simple structure encodes G protein-activating function of the IGF-II/mannose 6-phosphate receptor. Cell. 1990;62:709–717. doi: 10.1016/0092-8674(90)90116-v. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Nishimoto I. Detection of G protein-activator regions in M4 subtype muscarinic, cholinergic, and α2-adrenergic receptors based upon characteristics in primary structure. J Biol Chem. 1992;267:8342–8346. [PubMed] [Google Scholar]
- Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Anand-Srivastava MB. Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J Biol Chem. 2001;276:22064–22070. doi: 10.1074/jbc.M101587200. [DOI] [PubMed] [Google Scholar]
- Patel TB. Single transmembrane spanning heterotrimeric G protein-coupled receptors and their signaling cascades. Pharmacol Rev. 2004;56:371–385. doi: 10.1124/pr.56.3.4. [DOI] [PubMed] [Google Scholar]
- Pejchalova K, Krejci P, Wilcox WR. C-natriuretic peptide: An important regulator of cartilage. Mol Genet Metab. 2007;92:210–215. doi: 10.1016/j.ymgme.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Richards AM. The natriuretic peptides in heart failure. Basic Res Cardiol. 2004;99:94–100. doi: 10.1007/s00395-004-0461-z. [DOI] [PubMed] [Google Scholar]
- Richards AM, Doughty R, Nicholls MG, Macmahon S, Ikram H, Sharpe N, Espiner EA, Frampton C, Yandle TG. Neurohumoral prediction of benefit from carvedilol in ischemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. Circulation. 1999;99:786–792. doi: 10.1161/01.cir.99.6.786. [DOI] [PubMed] [Google Scholar]
- Rose RA, Anand-Srivastava MB, Giles WR, Bains JS. C-type natriuretic peptide inhibits L-type Ca2+ current in rat magnocellular neurosecretory cells by activating the NPR-C receptor. J Neurophysiol. 2005;94:612–621. doi: 10.1152/jn.00057.2005. [DOI] [PubMed] [Google Scholar]
- Rose RA, Hatano N, Ohya S, Imaizumi Y, Giles WR. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol. 2007;580:255–274. doi: 10.1113/jphysiol.2006.120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RA, Lomax AE, Giles WR. Inhibition of L-type Ca2+ current by C-type natriuretic peptide in bullfrog atrial myocytes: an NPR-C-mediated effect. Am J Physiol Heart Circ Physiol. 2003;285:H2454–H2462. doi: 10.1152/ajpheart.00388.2003. [DOI] [PubMed] [Google Scholar]
- Rose RA, Lomax AE, Kondo CS, Anand-Srivastava MB, Giles WR. Effects of C-type natriuretic peptide on ionic currents in mouse sinoatrial node: a role for the NPR-C receptor. Am J Physiol Heart Circ Physiol. 2004;286:H1970–H1977. doi: 10.1152/ajpheart.00893.2003. [DOI] [PubMed] [Google Scholar]
- Sackner-Bernstein J, Aaronson KD. Nesiritide for acute decompensated heart failure: does the benefit justify the risk? Curr Cardiol Rep. 2007;9:187–193. doi: 10.1007/BF02938349. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Schirger JA, Heublein DM, Chen HH, Lisy O, Jougasaki M, Wennberg PW, Burnett JC., Jr Presence of Dendroaspis natriuretic peptide-like immunoreactivity in human plasma and its increase during human heart failure. Mayo Clin Proc. 1999;74:126–130. doi: 10.4065/74.2.126. [DOI] [PubMed] [Google Scholar]
- Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of ‘urodilatin’, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- Scotland RS, Ahluwalia A, Hobbs AJ. C-type natriuretic peptide in vascular physiology and disease. Pharmacol Ther. 2005;105:85–93. doi: 10.1016/j.pharmthera.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- Steinmetz M, Potthast R, Sabrane K, Kuhn M. Diverging vasorelaxing effects of C-type natriuretic peptide in renal resistance arteries and aortas of GC-A-deficient mice. Regul Pept. 2004;119:31–37. doi: 10.1016/j.regpep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Strain WD. The use of recombinant human B-type natriuretic peptide (nesiritide) in the management of acute decompensated heart failure. Int J Clin Pract. 2004;58:1081–1087. doi: 10.1111/j.1368-5031.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachte GJ. Natriuretic peptides suppress protein kinase C activity to reduce evoked dopamine efflux from pheochromocytoma (PC12) cells. Endocrinology. 2003;144:94–100. doi: 10.1210/en.2002-220494. [DOI] [PubMed] [Google Scholar]
- Trachte GJ, Kanwal S, Elmquist BJ, Ziegler RJ. C-type natriuretic peptide neuromodulates via ‘clearance’ receptors. Am J Physiol Cell Physiol. 1995;268:C978–C984. doi: 10.1152/ajpcell.1995.268.4.C978. [DOI] [PubMed] [Google Scholar]
- Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC., Jr Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- Veale CA, Alford VC, Aharony D, Banville DL, Bialecki RA, Brown FJ, Damewood JR, Jr, Dantzman CL, Edwards PD, Jacobs RT, Mauger RC, Murphy MM, Palmer W, Pine KK, Rumsey WL, Garcia-Davenport LE, Shaw A, Steelman GB, Surian JM, Vacek EP. The discovery of non-basic atrial natriuretic peptide clearance receptor antagonists. Part 1. Bioorg Med Chem Lett. 2000;10:1949–1952. doi: 10.1016/s0960-894x(00)00387-5. [DOI] [PubMed] [Google Scholar]
- Villar IC, Panayiotou CM, Sheraz A, Madhani M, Scotland RS, Nobles M, Kemp-Harper B, Ahluwalia A, Hobbs AJ. Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res. 2007;74:515–525. doi: 10.1016/j.cardiores.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A, Tsutamoto T, Matsuda Y, Kinoshita M. Cardiorenal and neurohumoral effects of endogenous atrial natriuretic peptide in dogs with severe congestive heart failure using a specific antagonist for guanylate cyclase-coupled receptors. Circulation. 1994;89:2232–2240. doi: 10.1161/01.cir.89.5.2232. [DOI] [PubMed] [Google Scholar]
- Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, Edwards WD, Schaff HV, Burnett JC., Jr Natriuretic peptide system in human heart failure. Circulation. 1993;88:1004–1009. doi: 10.1161/01.cir.88.3.1004. [DOI] [PubMed] [Google Scholar]
- Wennberg PW, Miller VM, Rabelink T, Burnett JC., Jr Further attenuation of endothelium-dependent relaxation imparted by natriuretic peptide receptor antagonism. Am J Physiol Heart Circ Physiol. 1999;277:H1618–H1621. doi: 10.1152/ajpheart.1999.277.4.H1618. [DOI] [PubMed] [Google Scholar]
- Wiley KE, Davenport AP. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery. Br J Pharmacol. 2001;133:568–574. doi: 10.1038/sj.bjp.0704119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional roles of Cav1.3 (α1D) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- Zhou H, Murthy KS. Identification of the G protein-activating sequence of the single-transmembrane natriuretic peptide receptor C (NPR-C) Am J Physiol Cell Physiol. 2003;284:C1255–C1261. doi: 10.1152/ajpcell.00520.2002. [DOI] [PubMed] [Google Scholar]
- Zufall F, Ukhanov K, Lucas P, Leinders-Zufall T. Neurobiology of TRPC2: from gene to behavior. Pflugers Arch. 2005;451:61–71. doi: 10.1007/s00424-005-1432-4. [DOI] [PubMed] [Google Scholar]