Abstract
The inner surrounding of arterial vessels, the endothelium, is optimally located to detect changes in blood characteristics or blood flow that may result from changes in physical activity or from diseases. In response to physical stimuli, the endothelium varies its release of circulating vasoactive substances and serves as a source of local and systemic endothelium-derived dilator and vasoconstrictor factors. Endothelial dysfunction is one of the earliest markers of vascular abnormalities observed in cardiovascular disease and ageing. Exercise training is an efficient therapeutic strategy to improve endothelial function. Traditionally, studies on endothelial dysfunction and physical (in)activity-related effects on vascular adaptations are primarily focused on vasodilator substances (i.e. nitric oxide). One may suggest that augmentation of vasoconstrictor pathways (such as endothelin-1 and angiotensin II) contributes to the endothelial dysfunction observed after physical inactivity. Moreover, these pathways may also explain the exercise-induced beneficial cardiovascular adaptations. This review summarizes the current knowledge on the effects of physical (in)activity on several endothelium-derived vasoconstrictor substances.
The importance of physical inactivity as a modifiable behavioural risk factor for cardiovascular disease is widely recognized (Wannamethee & Shaper, 2001). The endothelial function is suggested to underlie the physical activity-induced vascular adaptations. Situated between the circulating blood and the surrounding tissue, the endothelium is optimally located to detect changes in blood contents or blood flow that may result from physical (in)activity. In response, the endothelium varies its release of substances that modulate vascular tone (e.g. vasodilators and vasoconstrictors), structure (proliferative) or blood characteristics (e.g. coagulation pathway, inflammatory control).
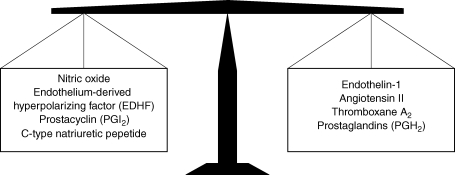
Vasodilators in general, and nitric oxide (NO) specifically, have been the primary focus in explaining the mechanisms of vascular changes resulting from activity and inactivity. Several animal studies and human in vivo invasive studies (using pharmacological blockade or stimulation of vasodilators) have assessed the role of these vasodilators in the regulation of vascular tone. In an excellent recent review for this journal, Green et al. (2004) summarized these studies and described the importance of the endothelium-derived NO pathway for exercise-induced cardiovascular adaptations. Whilst the effects of the vasoactive substances on vascular tone and vascular growth largely depend on a delicate balance between dilators and constrictors (Spieker et al. 2006) (Fig. 1), there is a predominance of studies focusing on vasodilators (primarily NO) to explain exercise-induced adaptations. It may well be that exercise-induced changes are, at least in part, related to other pathways than NO. In addition, physical inactivity results in cardiovascular adaptations that are the opposite of the effects of exercise training. Given the effects of exercise training on the NO pathway, vascular changes to physical inactivity were hypothesized to result from an inhibition of the NO pathway. However, we (de Groot et al. 2004; Bleeker et al. 2005) and others (Bonnin et al. 2001) found a preserved contribution of NO to vascular tone and preserved NO-dependent endothelial function during inactivity.
Figure 1. The delicate balance between endothelium-derived vasoactive substances contributing to the vascular tone.
The above results suggest that other pathways than solely vasodilator mechanisms may be involved in cardiovascular adaptation to changes in physical activity. In this review, we discuss findings regarding the contribution of endothelium-derived constricting factors in explaining cardiovascular adaptations during physical (in)activity in healthy subjects and in cardiovascular disease. Studies discussed in this review article related to (changes in) physical activity pertain to dynamic exercise rather than resistance exercise.
Endothelium-derived vasoconstricting factors
Endothelin-1
Endothelin-1 (ET-1) is the predominant isoform of the endothelin family and is mainly secreted by the endothelium (Yanagisawa et al. 1988) in response to a variety of stimuli (Table 1). The release of ET-1 results in activation of two receptors: ETA and ETB. Activation of the ETA and ETB receptors on the smooth muscle cell mediates a sustained constrictor action of ET-1. The ETB receptors on the endothelium mediate the release of the dilators NO and prostacyclin, but also mediate the rapid uptake of ET-1 (Haynes & Webb, 1998). Therefore, the endothelial ETB receptor largely opposes the vascular effect of smooth muscle cell-located ETA/B receptors. In addition to the direct vascular effects, ET-1 induces vascular smooth muscle cell proliferation and growth in a dose-dependent manner (Komuro et al. 1988).
Table 1.
Physical stimuli that stimulate or inhibit the pathways of endothelin-1 and angiotensin II
| Humoral stimuli | Physical/exogenous stimuli | |||
|---|---|---|---|---|
| Endothelin-1 | Angiotensin II | Endothelin-1 | Angiotensin II | |
| Stimulators | Angiotensin | Endothelin | Pulsatile stretch | Pulsatile stretch |
| Insulin | Insulin | Shear stress (low) | (cardiomyocytes) | |
| Cytokines | Cytokines | Osmolarity | Volume depletion | |
| Interleukin-1 | Interleukin-1 | Hypoxia | ||
| Oxidized LDL | Oxidized LDL | |||
| Vasopressin | Progesterone | |||
| Adrenalin | ||||
| TGF-β | ||||
| Endotoxin | ||||
| Glucose | ||||
| Inhibitors | Nitric Oxide | Nitric oxide | Statins | Statins |
| Oestrogens | Oestrogens | Shear stress (high) | Atrial distension | |
| Prostacyclin | FGF | |||
| Heparin | Free radicals | |||
LDL, low-density lipoprotein; FGF, fibroblast growth factor.
Angiotensin II
After cleavage of angiotensinogen to angiotensin (Ang) I via renin, this peptide is cleaved by the angiotensin converting enzyme (produced by pulmonary and systemic vascular endothelium) into Ang II, which binds to its specific receptors on the vascular wall. Various stimuli alter the level of synthesis of Ang II (Table 1). Two well-described subtypes of the Ang II receptors, designated AT1 and AT2, have been identified. The smooth muscle cell-localized AT1 receptor subtype mediates the predominant action of Ang II: vasoconstriction. These vasoactive actions are partly counteracted by the AT2 receptor, which causes vasodilatation (Hernandez Schulman et al. 2007). Besides the vasoactive effects, Ang II leads to proliferation and growth of the vascular smooth muscle cells through activation of the AT1 receptor.
Thromboxane A2
Thromboxane A2 (TXA2) is one of the end products of arachidonic acid metabolism and is produced by TXA2 synthase. TXA2 is primarily produced by platelets, but also by the endothelium. The physiological role of TXA2 is platelet aggregation and vasoconstriction (Oates et al. 1988).
Prostaglandins
While prostaglandins have vasodilator effects, the prostaglandin H2 (PGH2) isoform is a vasoconstrictor substance. PGH2 is closely related to TXA2: both are formed during arachidonic acid metabolism, and PGH2 is the precursor of TXA2 and exerts its vascular effects through the same receptors on the vascular wall (Davidge, 2001).
We are not aware of any studies that have examined the potential role of TXA2 or PGH2 in cardiovascular changes during physical (in)activity. Therefore, the role of these two endothelium-derived vasoconstricting factors will not be discussed in this review.
Physical inactivity
Functional changes
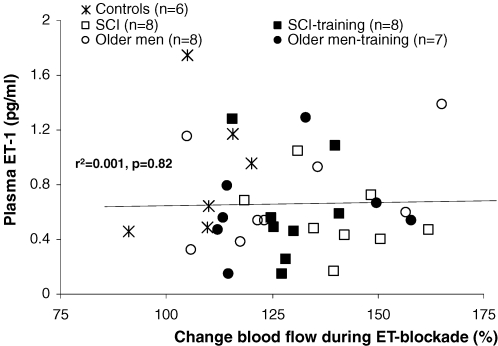
While 5–18 days of space flight did not alter ET-1 plasma concentrations in humans (Meck et al. 2004), increased ET-1 plasma concentrations were observed after hindlimb unloading in rats (Biondi et al. 1995) and detraining in humans (Maeda et al. 2001). Short-term bed rest increased concentrations of Ang II (Haruna et al. 1997; Bestle et al. 2001). Paralysed muscles of spinal cord-injured individuals are subject to extreme inactivity and can therefore serve as a ‘model of nature’ for localized deconditioning. This population demonstrated high concentrations of ET-1 (Robergs et al. 1993), which increased even further after a period of training. Interpreting these scattered results, one should realize that plasma concentrations do not necessarily indicate a functional change in these pathways. In our lab, we examined ET-1 plasma concentrations and the ET-1-mediated leg vascular tone after intra-arterial blockade of ETA/B receptors using BQ-123 and BQ-788 in the same subjects (Thijssen et al. 2007a,c). Combining the results of these studies, we found that ET-1 plasma concentrations do not correlate with the contribution of ET to baseline vascular tone (Fig. 2). However, baseline leg blood flow and ET-1-mediated vascular tone showed an inverse relation (r2= 0.12, P = 0.03), indicating that a low leg blood flow correlates with an elevated ET-1-mediated vascular tone. This advocates the use of local infusion to assess the role of ET-1 to regulate vascular function, rather than plasma concentrations.
Figure 2. Correlation between the relative change in blood flow of the infused leg during ET blockade (representing the contribution of ET-1 to leg vascular tone) and baseline plasma concentrations of ET-1.
Recently, we examined the contribution of ET-1 to baseline blood flow in extremely inactive legs of spinal cord-injured (SCI) individuals, using an intrafemoral administration of selective ETA/B receptor blockers (Thijssen et al. 2007a). We demonstrated that ET-1 importantly contributes to the increased vascular tone observed during physical inactivity. This is supported by the reversed ET-1-mediated vascular tone in these subjects after 6 weeks of exercise training.
Regarding Ang II, it was demonstrated that significantly lower dosages are necessary in SCI individuals compared with able-bodied controls to achieve a similar increase in blood pressure (Krum et al. 1992). This suggests the presence of an exaggerated pressor response to Ang II in SCI individuals.
Structural changes
To date, no studies have examined the role of endothelium-derived vasoconstricting factors in the regulation of physical inactivity-induced structural changes, such as an inward remodelling of conduit arteries during inactivity.
Physcial activity as an intervention
Functional changes
Using a cross-sectional design, it was demonstrated that the ET-1-sensitivity, ex vivo examined using the concentration of ET-1 necessary to cause a 50% response (EC50), of the aorta and coronary artery is reduced after a period of exercise training in swine (Jones et al. 1999). In addition, aortic and cerebellar arteries in exercise-trained rats have a diminished sensitivity to the actions of ET-1 on lipid metabolism compared with sedentary rats (Latorre et al. 2002). Moreover, the postischaemic sensitivity to ET-1 in coronary arteries was significantly lower in endurance-trained rats than in sedentary rats (Symons et al. 2000). Regarding the Ang II pathway, an exercise-induced decrease in Ang II-induced pulmonary vasoconstriction was present in rats that trained for 6 weeks (Kashimura et al. 1995). In humans, only one single study examined potential differences in the regulation of vascular tone by Ang II between healthy athletic and sedentary men. A similar response is reported in forearm vascular bed for Ang II (but also for NO), between elite athletic and sedentary healthy men (Kingwell et al. 1996).
Structural changes
Based on their potent proliferative acivity, both ET-1 and Ang II contribute to pathological structural changes. This is supported by the inhibited formation of atherosclerotic lesions during prolonged ETA receptor blockade (Barton et al. 1998) and by the accelerated atherosclerotic process during overexpression of the AT1 receptor (Nickenig & Harrison, 2002). Regarding the effects of exercise, lower ET-1-mediated DNA expression in arteries was found in exercise trained swine compared with sedentary peers (Wamhoff et al. 2002). Because amounts of DNA synthesis are suggested to correlate with proliferative activity (and therefore atherosclerosis), decreased proliferative responses of constrictor pathways may contribute to the exercise-induced cardioprotection. In addition, ET-1 and Ang II are hypothesized to contribute to angiogenesis. Under hypoxic conditions, ET-1 induces angiogenesis via activation of the ETB receptors (Goligorsky et al. 1999) and via enhanced expression of NO synthase (Liu et al. 2003), while Ang II results in angiogenesis through the actions of the vascular endothelial growth factor (Amaral et al. 2001).
Physical activity in specific groups
Ageing
Animal studies demonstrated that ET-1 (possibly through ETA receptors) and Ang II (possibly through AT2 receptors) contribute to the age-related increase in vascular tone in coronary arteries (Goodwin et al. 1999; Korzick et al. 2005), mesenteric vessels (Pinaud et al. 2007), gastrocnemius vascular bed (Donato et al. 2005), total vascular bed (Asai et al. 2001), and renal arteries (Tank et al. 1994). Recently, the pivotal role of ET-1 in the age-related increase in vascular tone was confirmed with human in vivo experiments in the lower (Thijssen et al. 2007c) as well as in the upper extremities (Van Guilder et al. 2007). In the forearm, this was possibly regulated via ETA receptors (Van Guilder et al. 2007). Examining the potential beneficial effects of exercise training in ageing, it was demonstrated that 12 weeks of exercise in old rats did not change ETA/B receptor-mediated responsiveness, examined ex vivo using the EC50 value, of muscle arterioles (Donato et al. 2005). This finding is in contrast with two recent human in vivo studies, which reported a partly reversed ET-1-mediated vascular tone after exercise training in older men in the leg (Thijssen et al. 2007c) and forearm (Van Guilder et al. 2007) vascular bed. Based on these recent findings, it is hypothesized that the negative effects of ET-1 in cardiovascular disease, predominantly occurring in the ageing population, may be due to inactivity rather than to senesence (Thijssen et al. 2007b).
Coronary artery disease
It has been demonstrated that exercise training in patients with stable coronary artery disease leads to a 49% reduction in Ang II-induced vasoconstriction. Moreover, this adaptation is accompanied by lower expression of the AT1 receptor and increased expression of the AT2 receptor (Adams et al. 2005).
Pulmonary hypertension
Only one study so far has examined the vascular effects after 5 weeks of exercise training in pulmonary hypertensive rats on the ET pathway. While the pulmonary vasomotor function improved, the pulmonary vasoreactivity to vasoactive agents (e.g. ET-1) did not change (Goret et al. 2005). Pulmonary hypertension is the only widely accepted cardiovascular pathology that is treated with ET receptor blockers, so a large potential exists for exercise training to attenuate the central and peripheral vasoactive effects of the ET pathway in this disease.
Heart failure
Decreased plasma concentrations of Ang II have been reported after exercise training in rabbits with heart failure (Liu et al. 2000), while the significant up-regulation in AT1 receptor mRNA in heart failure in rats is normalized after exercise training (Zucker et al. 2004). Also in patients with heart failure, improving physical fitness results in suppressing circulating concentrations of Ang II (Braith et al. 1999) and lowering of plasma concentrations of ET-1 (Kubanek et al. 2006).
Conclusions
The studies discussed in the present review suggest that inhibition of endothelium-derived vasoconstricing pathways contribute to exercise-induced vascular changes. Accordingly, cardiovascular adaptations to a change in physical activity are likely to be regulated through tight interactions between vasodilator (e.g. NO) and vasoconstrictor pathways (e.g. ET-1, Ang II). This may even be of special interest in disease states characterized by altered endothelium-derived constrictor pathways. Better insight into the underlying mechanisms (e.g. the role of the receptors and of the post receptor signalling pathways) will help us to understand the vascular changes observed in physical (in)activity. In addition, little is known regarding the role of endothelium-derived vasoconstictors in structural changes after exercise or inactivity. Also the field of vasoconstrictor prostanoids (TXA2 and PGH2) is relatively unexplored.
With respect to cardiovascular diseases, several scientific lines of evidence are present that support a central role for endothelium-derived vasoconstricting factors. Based on the summarized findings in this review, one should realize that the negative effects of ET-1 and Ang II in cardiovascular disease may be importantly confounded by the degree of inactivity. Therefore, inactivity, rather than the pathology of these specific cardiovascular diseases, is emerging as a strong candidate to explain the increased vascular tone. However, only a few studies examined the effect of exercise training on the role of these vasoconstricting factors. The sparse data at present suggest that exercise training potentially improves cardiovascular function in these patients (at least partly) through inhibition of the constrictor pathways. We strongly advocate that future studies should examine the potential for exercise training as a non-pharmacological intervention in cardiovascular diseases, and take particular interest in vasoconstrictor-related mechanisms to explain the possible beneficial cardiovascular effect.
Acknowledgments
We apologize for the failure to cite many important publications because of space limitations. D.H.J.T. is financially supported by the Netherlands Organization for Scientific Research (NWO-grant 82507010).
References
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol Heart Circ Physiol. 2001;281:H1163–H1169. doi: 10.1152/ajpheart.2001.281.3.H1163. [DOI] [PubMed] [Google Scholar]
- Asai K, Kudej RK, Takagi G, Kudej AB, Natividad F, Shen YT, Vatner DE, Vatner SF. Paradoxically enhanced endothelin-B receptor-mediated vasoconstriction in conscious old monkeys. Circulation. 2001;103:2382–2386. doi: 10.1161/01.cir.103.19.2382. [DOI] [PubMed] [Google Scholar]
- Barton M, Haudenschild CC, d'Uscio LV, Shaw S, Munter K, Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestle MH, Norsk P, Bie P. Fluid volume and osmoregulation in humans after a week of head-down bed rest. Am J Physiol Regul Integr Comp Physiol. 2001;281:R310–R317. doi: 10.1152/ajpregu.2001.281.1.R310. [DOI] [PubMed] [Google Scholar]
- Biondi R, Capodicasa E, Tassi C, Mezzasoma L, Benedetti C, Valiani M, Marconi P, Rossi R. Cardiovascular and organ responses and adaptation responses to hypogravity in an experimental animal model. Acta Astronaut. 1995;37:373–377. doi: 10.1016/0094-5765(95)00055-5. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, Kooijman M, Rongen GA, Hopman MT, Smits P. Preserved contribution of nitric oxide to baseline vascular tone in deconditioned human skeletal muscle. J Physiol. 2005;565:685–694. doi: 10.1113/jphysiol.2005.085936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol. 2001;85:420–426. doi: 10.1007/s004210100483. [DOI] [PubMed] [Google Scholar]
- Braith RW, Welsch MA, Feigenbaum MS, Kluess HA, Pepine CJ. Neuroendocrine activation in heart failure is modified by endurance exercise training. J Am Coll Cardiol. 1999;34:1170–1175. doi: 10.1016/s0735-1097(99)00339-3. [DOI] [PubMed] [Google Scholar]
- Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–660. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2004;287:H374–H380. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Goligorsky MS, Budzikowski AS, Tsukahara H, Noiri E. Co-operation between endothelin and nitric oxide in promoting endothelial cell migration and angiogenesis. Clin Exp Pharmacol Physiol. 1999;26:269–271. doi: 10.1046/j.1440-1681.1999.03029.x. [DOI] [PubMed] [Google Scholar]
- Goodwin AT, Amrani M, Marchbank AJ, Gray CC, Jayakumar J, Yacoub MH. Coronary vasoconstriction to endothelin-1 increases with age before and after ischaemia and reperfusion. Cardiovasc Res. 1999;41:554–562. doi: 10.1016/s0008-6363(98)00253-3. [DOI] [PubMed] [Google Scholar]
- Goret L, Reboul C, Tanguy S, Dauzat M, Obert P. Training does not affect the alteration in pulmonary artery vasoreactivity in pulmonary hypertensive rats. Eur J Pharmacol. 2005;527:121–128. doi: 10.1016/j.ejphar.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna Y, Bonde-Petersen F, Takenaka K, Suzuki Y, Kawakubo K, Gunji A. Effects of the renin-angiotensinaldosterone system on the cardiovascular system during 20-days bed rest. J Gravit Physiol. 1997;4:S62–S68. [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16:1081–1098. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- Hernandez Schulman I, Zhou MS, Raij L. Cross-talk between angiotensin II receptor types 1 and 2: potential role in vascular remodeling in humans. Hypertension. 2007;49:270–271. doi: 10.1161/01.HYP.0000253966.21795.d3. [DOI] [PubMed] [Google Scholar]
- Jones AW, Rubin LJ, Magliola L. Endothelin-1 sensitivity of porcine coronary arteries is reduced by exercise training and is gender dependent. J Appl Physiol. 1999;87:1172–1177. doi: 10.1152/jappl.1999.87.3.1172. [DOI] [PubMed] [Google Scholar]
- Kashimura O, Sakai A, Yanagidaira Y. Effects of exercise-training on hypoxia and angiotensin II-induced pulmonary vasoconstrictions. Acta Physiol Scand. 1995;155:291–295. doi: 10.1111/j.1748-1716.1995.tb09976.x. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Tran B, Cameron JD, Jennings GL, Dart AM. Enhanced vasodilation to acetylcholine in athletes is associated with lower plasma cholesterol. Am J Physiol Heart Circ Physiol. 1996;270:H2008–H2013. doi: 10.1152/ajpheart.1996.270.6.H2008. [DOI] [PubMed] [Google Scholar]
- Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Korzick DH, Muller-Delp JM, Dougherty P, Heaps CL, Bowles DK, Krick KK. Exaggerated coronary vasoreactivity to endothelin-1 in aged rats: role of protein kinase C. Cardiovasc Res. 2005;66:384–392. doi: 10.1016/j.cardiores.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Krum H, Louis WJ, Brown DJ, Howes LG. Pressor dose–responses and baroreflex sensitivity in quadriplegic spinal cord injury patients. J Hypertens. 1992;10:245–250. doi: 10.1097/00004872-199203000-00007. [DOI] [PubMed] [Google Scholar]
- Kubanek M, Malek I, Bytesnik J, Fridl P, Riedlbauchova L, Karasova L, Lanska V, Kautzner J. Decrease in plasma B-type natriuretic peptide early after initiation of cardiac resynchronization therapy predicts clinical improvement at 12 months. Eur J Heart Fail. 2006;8:832–840. doi: 10.1016/j.ejheart.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Latorre E, Moran M, Aragones MD, Saborido A, Fernandez I, Delgado J, Catalan RE, Megias A. Exercise training-induced changes in sensitivity to endothelin-1 and aortic and cerebellum lipid profile in rats. Lipids. 2002;37:43–52. doi: 10.1007/s11745-002-0862-x. [DOI] [PubMed] [Google Scholar]
- Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- Liu S, Premont RT, Kontos CD, Huang J, Rockey DC. Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein βγ subunit signaling to protein jinase B/Akt. J Biol Chem. 2003;278:49929–49935. doi: 10.1074/jbc.M306930200. [DOI] [PubMed] [Google Scholar]
- Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 2001;69:1005–1016. doi: 10.1016/s0024-3205(01)01192-4. [DOI] [PubMed] [Google Scholar]
- Meck JV, Waters WW, Ziegler MG, deBlock HF, Mills PJ, Robertson D, Huang PL. Mechanisms of postspaceflight orthostatic hypotension: low α1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am J Physiol Heart Circ Physiol. 2004;286:H1486–H1495. doi: 10.1152/ajpheart.00740.2003. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT1 receptor regulation. Circulation. 2002;105:530–536. doi: 10.1161/hc0402.102619. [DOI] [PubMed] [Google Scholar]
- Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (1) N Engl J Med. 1988;319:689–698. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- Pinaud F, Bocquet A, Dumont O, Retailleau K, Baufreton C, Andriantsitohaina R, Loufrani L, Henrion D. Paradoxical role of angiotensin II type 2 receptors in resistance arteries of old rats. Hypertension. 2007;50:96–102. doi: 10.1161/HYPERTENSIONAHA.106.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robergs RA, Appenzeller O, Qualls C, Aisenbrey J, Krauss J, Kopriva L, DePaepe J. Increased endothelin and creatine kinase after electrical stimulation of paraplegic muscle. J Appl Physiol. 1993;75:2400–2405. doi: 10.1152/jappl.1993.75.6.2400. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Flammer AJ, Luscher TF. The vascular endothelium in hypertension. Handb Exp Pharmacol. 2006;176:249–283. doi: 10.1007/3-540-36028-x_8. [DOI] [PubMed] [Google Scholar]
- Symons JD, Rendig SV, Stebbins CL, Longhurst JC. Microvascular and myocardial contractile responses to ischemia: influence of exercise training. J Appl Physiol. 2000;88:433–442. doi: 10.1152/jappl.2000.88.2.433. [DOI] [PubMed] [Google Scholar]
- Tank JE, Vora JP, Houghton DC, Anderson S. Altered renal vascular responses in the aging rat kidney. Am J Physiol Renal Physiol. 1994;266:F942–F948. doi: 10.1152/ajprenal.1994.266.6.F942. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Ellenkamp R, Kooijman M, Pickkers P, Rongen GA, Hopman MT, Smits P. A causal role for endothelin-1 in the vascular adaptation to skeletal muscle deconditioning in spinal cord injury. Arterioscler Thromb Vasc Biol. 2007a;27:325–331. doi: 10.1161/01.ATV.0000253502.83167.31. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Hopman MT, Levine BD. Endothelin and aged blood vessels: one more reason to get off the couch? Hypertension. 2007b;50:292–293. doi: 10.1161/HYPERTENSIONAHA.107.091686. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol. 2007c;103:852–857. doi: 10.1152/japplphysiol.00357.2007. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK, Dietz NJ, Hu Q, Sturek M. Exercise training attenuates coronary smooth muscle phenotypic modulation and nuclear Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2002;283:H2397–H2410. doi: 10.1152/ajpheart.00371.2001. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease: an epidemiological perspective. Sports Med. 2001;31:101–114. doi: 10.2165/00007256-200131020-00003. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev. 2004;32:107–111. doi: 10.1097/00003677-200407000-00006. [DOI] [PubMed] [Google Scholar]