Abstract
Microglia are the main immunocompetent cells of the mammalian central nervous system (CNS). Activation of cultured microglial cells and subsequent release of nitric oxide and cytokines critically depends on intracellular calcium levels. Since microglia undergo dramatic morphological, biochemical and electrophysiological changes in response to pathological events in the CNS, we investigated temporal changes in expression levels of ion channels involved in cellular calcium homeostasis in mouse cortical microglial cells in culture. Specifically, we assessed the inward and delayed outward rectifier potassium currents (IIRK and IDRK), calcium (Ca2+) release-activated Ca2+ currents (ICRAC) and Ca2+-activated TRPM4-like currents (ICAN) in non-activated microglia and cells that were activated by exposure to lipopolysaccharide (LPS) between 3 and 48 h. Unstimulated microglial cells, subcultured from an astrocyte coculture, typically exhibited a ramified, rod-shaped morphology. During the first 3 days of culture cell size and shape were maintained, but the percentage of cells showing prominent IIRK went up and those expressing IDRK went down. Cells retaining IDRK exhibited smaller amplitudes, whereas those of IIRK and ICRAC were not affected. However, after 24 h of exposure to 1 μg ml−1 LPS, most cells showed an amoeboid (‘fried egg’-shaped) morphology with a 62% increase in cell capacitance. At that point in time, only 14% of the cells revealed IIRK and 3% had IDRK exclusively, whereas the majority of cells expressed both currents. The amplitudes of ICRAC and IIRK progressively decreased after stimulation, whereas IDRK transiently reached a maximum after 6 h of LPS exposure and then returned to pre-stimulation expression levels. Cultured microglia also revealed TRPM4-like, Ca2+-activated non-selective currents (ICAN) with an EC50 of 1.2 μm[Ca2+]i. The expression levels of this current did not change significantly during and after 24 h of LPS exposure. We propose that LPS-induced down-regulation of IIRK and ICRAC will reduce the cell's capacity to produce significant calcium influx upon receptor activation and result in decreased sensitivity to exogenous stimulation. In this scenario, ICAN expression would remain constant, although its activity would automatically be reduced due to the diminished calcium influx capacity of the cell.
Microglia derive from cells of the monocytic lineage entering the brain from the bloodstream during early embryonic development. They differentiate into brain resident cells and become the main immune effector cells in the CNS. Microglia are recognized for their scavenging function in response to pathological events (McGeer et al. 1988; Dickson et al. 1991; Bo et al. 1994; McGeer & McGeer, 1995; Meda et al. 1995; Baker & Manuelidis, 2003; Streit et al. 2004; Jack et al. 2005; Sargsyan et al. 2005; Conde & Streit, 2006; Wang et al. 2007).
Microglia appear in three different shapes as ramified, amoeboid and intermediate cells (del Rio Hortega, 1932; Kershbaum, 1939) and three different physiological states as resting, activated and amoeboid-phagocytic cells (Streit et al. 1989; Sasaki et al. 1993). Ramified microglia, representing 5–20% of all glia in the normal CNS (Lawson et al. 1990), is often referred to as resting microglia, since they respond to insults such as infection, traumatic injury or ischaemia by converting into amoeboid macrophage-like cells that move towards the site of injury (Thomas, 1992; Vilhardt, 2005). Following activation, microglia change their shape and express a distinct pattern of ion channels linked to their activation state (Norenberg et al. 1994; Biro et al. 1998; Farber & Kettenmann, 2005). Cultured microglia mainly express voltage-independent inward and, depending on the state of activation, voltage-gated outward potassium currents (Norenberg et al. 1992, 1994; Pyo et al. 1997; Eder, 1998; Walz & Bekar, 2001). However, microglia of freshly isolated rat cortical brain slices reveal little, if any, inward potassium or voltage-gated membrane currents (Boucsein et al. 2000). Thus, cultured microglial cells might not represent the fully resting state of microglia in vivo, although they remain responsive to a variety of stimuli that induce further changes in microglial properties.
The by far most frequently used model stimulus to study microglia activation and inflammatory signalling is the Gram-negative bacterial cell-wall component lipopolysaccharide (LPS). LPS activates the microglial Toll-like receptor-4 (TLR-4) and induces the transcription of genes that are involved in initiation or regulation of the inflammatory response (Hoshino et al. 1999; Lee & Lee, 2002). As a result, microglial cells change their shape (abd-el Basset & Fedoroff, 1995; Kloss et al. 2001), express various secretory compounds such as cytokines/chemokines, growth factors, tumour necrosis factor-α (TNF-α), super-oxide, nitric-oxide (NO) and prostaglandin E2 (PGE2) (Nakamura et al. 1999; Ajmone-Cat et al. 2003; Rock et al. 2004), and show increased expression levels of glutamate transporter 1 (GLT-1) (Persson et al. 2005). They develop inward rectifying potassium currents (IIRK) in vivo (cultured miroglia already show IIRK, see above and present study) and voltage-gated, delayed rectifying potassium currents (IDRK) carried by Kv1.3 and/or Kv1.5 channels in culture and Kv1.5 in vivo (Kettenmann et al. 1990; Norenberg et al. 1992, 1994; Pyo et al. 1997; Jou et al. 1998; Boucsein et al. 2000). LPS also induces calcium (Ca2+) transients in cultured microglial cells, possibly resulting from caffeine-sensitive Ca2+ release (Bader et al. 1994) and/or dependent on Ca2+ influx (Herms et al. 1997; Choi et al. 2002; Yi et al. 2005). A permanent elevation of the basal Ca2+ concentration along with a suppression of UTP-evoked Ca2+ signalling has also been described for LPS-exposed cultured microglial cells (Hoffmann et al. 2003).
A recent model, first established in Jurkat T cells, proposes that several ion channels act in concert to shape calcium signals in immune cells (Launay et al. 2004). Here, TRPM4, a Ca2+-activated non-selective (CAN) channel and Ca2+ release-activated Ca2+ currents (ICRAC), as well as Kv1.3 and KCa control intracellular Ca2+ oscillations through oscillatory changes in membrane potential (Launay et al. 2004). Since LPS-induced chronic elevation of intracellular Ca2+ attenuates receptor-induced Ca2+ signals in activated microglia cells (Hoffmann et al. 2003), we wondered whether this would be achieved by an LPS-induced change not only in IIRK or IDRK, but also ICRAC and CAN channels, if the latter were expressed in cultured microglial cells. Since most studies have only assessed endpoint states of microglia activation, we also wanted to learn about the kinetics of ion channel expression, particularly with respect to early effects (< 24 h) of LPS.
We therefore set out to first confirm the presence of Ca2+-activated TRPM4-like currents in microglial cells. We then established a temporal profile of morphological and electrophysiological changes in cultured cortical mouse microglial cells over a period of 7 days in subculture and early responses to continuous exposure to 1 μg ml−1 LPS as a model of microglial cell activation. We used the patch-clamp technique to investigate LPS-dependent changes of inward and delayed outward rectifier potassium currents (IIRK and IDRK), Ca2+ release-activated Ca2+ currents (ICRAC) and Ca2+-activated TRPM4-like currents (ICAN) in non-activated and LPS-stimulated cultured mouse microglial cells.
Methods
Preparation and culture of mouse microglial cells
Microglial cells were obtained from cortex of mouse pups (postnatal days 0–2) as previously described (Giulian & Baker, 1986). In brief, pups were decapitated in accordance with the University of Hawaii Institutional Animal Care and Use Committee guidelines after anaesthesia with enflurane (5–10 drops onto tissue in 200 ml beaker), and their brains were collected under sterile conditions in basal Eagle's medium (BME, Gibco). Cortices were dissected, the meninges removed, and triturated after enzymatic digestion with 0.25% trypsin/5.26 mM EGTA and washing in glial medium (BME supplemented with 1%l-glutamine and 2 ml l−1 penicillin–streptomycin). The homogenate was then forced through a 20 nm filter and the resulting suspension was plated in 75 cm3 poly l-lysine-coated culture flasks containing glial medium with 10% fetal bovine serum (FBS). The cells were kept in an incubator at 37°C and 5% CO2, and the medium was changed 3 days following preparation and once per week thereafter. After about 3–4 weeks in culture, the flasks showed a confluent layer of astrocytes with microglial cells growing on its surface. Microglial cells were harvested and isolated by shaking the flasks to release the loosely attached microglia into the supernatant. Microglia from the supernatant were washed and plated on poly l-lysine-coated glass coverslips in glial medium with 5% FBS. Plated cells were used for patch clamp experiments 1–7 days after harvest. To allow the cells to adapt to the subculture conditions, LPS experiments started 3 days after harvest. For microglia activation, 1 μg ml−1 lipopolysaccharide (LPS) was added to the media 3–48 h before starting patch-clamp experiments.
Salines
All salines were freshly prepared from refrigerated stock solutions. The external saline to measure inward and delayed outward rectifying potassium currents (IIRK, IDRK) was composed of (mm): NaCl 140, KCl 10, CaCl2 1, MgCl2 2, glucose 10, Hepes-NaOH 10, pH 7.2. The external solution for TRPM4-like currents contained 2.8 mm KCl and for ICRAC recordings, 2.8 mm KCl and 10 mm CaCl2. Intracellular pipette-filling solutions for IIRK and IDRK contained (mm): potassium glutamate 140, NaCl 8, MgCl2 1, Hepes-KOH 10, pH 7.2; for ICRAC (mm): caesium glutamate 140, NaCl 8, MgCl2 1, Cs-BAPTA 10, InsP3 0.02, Hepes-CsOH, pH 7.2. Pipette-filling solutions for TRPM4-like currents were the same as for IIRK and IDRK, but [Ca2+]i was buffered to elevated levels by adding 10 mm K-BAPTA and an appropriate amount of CaCl2 (calculated with WebMaxC http://www.stanford.edu/~cpatton/webmaxcS.htm). The osmolarity of all solutions ranged between 285 and 305 mosmol l−1. All salts were obtained from Sigma.
Patch-clamp experiments
Coverslips with subcultured microglial cells were transferred to a recording chamber and kept in the modified Ringer solution described above. Patch pipettes were prepared from borosilicate glass capillaries (inner diameter 1.5 mm, Kimble products) using a horizontal puller (Sutter Instruments, Modell P-97), fire-polished, and had resistances between 2 and 4 MΩ. Patch-clamp experiments were performed in the tight-seal whole-cell configuration at room temperature (22–24°C). High-resolution current recordings were acquired by a computer-based patch-clamp amplifier system (EPC-9, HEKA). For ICRAC recordings, voltage ramps of 50 ms duration spanning a voltage range of −130 to 100 mV over 50 ms were delivered from a holding potential (Vh) of 0 mV every 2 s over a period of 200 s. For IIRK and IDRK measurements, ramps from −130 to 100 mV were applied with Vh=−60 mV. Calcium-activated (TRPM4-like) currents were measured using ramps from −100 to 100 mV at a Vh of 0 mV and for 350 s. All voltages were corrected by a 10 mV liquid junction potential. Currents were filtered at 2.9 kHz and digitized at 100 μs intervals. Capacitive currents and series resistance were determined and corrected before each voltage ramp using the automatic capacitance compensation of the EPC-9. In ICRAC experiments, the first one to three ramps were subtracted from all following ramps to subtract background conductances. The low-resolution temporal development of currents at a given potential was extracted from individual ramp current recordings by measuring the current amplitudes at voltages of −110 mV for IIRK and ICRAC, +80 mV for IDRK and −80 mV and +80 mV for TRPM4-like currents. All currents were normalized to cell size to obtain current densities (pA pF−1).
Fura-2 Ca2+ measurements
For Ca2+ measurements, cells were loaded with 1 μm Fura-2 AM (acetoxymethylester, Molecular Probes) for 60 min in media at 37°C, washed and kept in external solution containing (mm): NaCl 140, KCl 2.8, CaCl2 1, MgCl2 2, glucose 10, Hepes-NaOH 10, pH 7.2. The Ca2+-dependent Fura-2 fluorescence of several cells was monitored at a rate of 0.2 Hz with a dual excitation fluorometric imaging system (TILL-Photonics, Gräfelfingen, Germany) controlled by TILLvisION software, using a Zeiss Axiovert 100 fluorescence microscope equipped with a 40× Plan-NeoFluar objective. Fura-2 AM-loaded cells were excited by wavelengths of 340 and 380 nm and the fluorescence emission, detected at 450–550 nm, was computed into relative ratio units (F340/F380).
Statistics
Where applicable, statistical errors of averaged data are given as means ± s.e.m. with n determinations and statistical significance assessed by Student's t test.
Results
Properties of non-activated and LPS-exposed mouse microglia
In acutely isolated brain slices, resting microglia are initially highly ramified and transform to amoeboid-like morphology within several hours (Stence et al. 2001). Here we studied shape and size of microglial cells harvested and replated from a primary coculture with astrocytes. The subcultured microglia exhibited either a ramified, rod-shaped appearance or a ‘fried egg’-shaped morphology (Fig. 1A, left and right, respectively). Some of the cells showed a more round intermediate morphology, but with clear ramification (Fig. 1A, middle) and were therefore classified as ramified cells. From day 1 to day 7 after subculturing, about 90% of the cells represented the ramified and 10% the ‘fried-egg’-shaped type (Fig. 1B). The cell membrane capacitance, which is proportional to cell surface area, was on average 12 ± 0.6 pF (n = 76) at day 1 and did not change significantly during the first 4 days. Thereafter the cell capacitance increased to 18.1 ± 1 pF (n = 38) at day 6 and 17.5 ± 1 pF (n = 35) at day 7 (Fig. 1D). After adding 1 μg ml−1 lipopolysaccharide (LPS), a widely used activator of microglial cells, 34% of the cells revealed a ‘fried egg’-shaped morphology already within 3 h. Within 48 h, the ratio of cell types completely reversed to just 6% ramified and 94%‘fried egg’-shaped cells (see Fig. 1C). The cell size increased to 23 ± 0.6 pF (n = 155) after 24 h and to 43.9 ± 2.6 pF (n = 43) after 48 h of incubation with LPS (Fig. 1E). Since LPS experiments were performed later than 3 days after harvest, control cells not exposed to LPS (Fig. 1C and E) represent the average data from day 3 to day 7. Bath application of 1 μg ml−1 LPS induced a prolonged Ca2+ signal (Fig. 1F) that set in after a marked delay of 40–50 min. After 3 h, intracellular Ca2+ concentration reached basic levels again, but further decreased significantly and reached a minimum after 12 h before settling in to values slightly higher than before LPS application (Fig. 1G).
Figure 1. Morphology, cell size and [Ca2+]i.
A, composite image of scanning electron micrographs of microglial cells at various stages of activation with 1 μg ml−1 LPS from non-activated (left cell, arborized) to fully activated (right cell, ‘fried egg’-shaped). B and C, percentage of ramified (^, ± s.e.m.) and ‘fried egg’-shaped (•, ± s.e.m.) cells per coverslip depending on time in subculture (B, day 1 n = 12, 2 n = 10, 3 n = 9, 4 n = 5, 5 n = 5, 6 n = 8, 7 n = 5 coverslips, 2142 cells) and incubation time in 1 μg ml−1 LPS (C, 0 h n = 32, 3 h n = 6, 6 h n = 8, 12 h n = 4, 24 h n = 9, 48 h n = 8 coverslips, 2211 cells). D and E, cell capacitance (in pF, ± s.e.m.) of microglial cells depending on time in subculture (D, day 1 n = 76, 2 n = 63, 3 n = 110, 4 n = 117, 5 n = 35, 6 n = 38, 7 n = 35 cells) and incubation time in 1 μg ml−1 LPS (E, 0 h n = 335, 3 h n = 40, 6 h n = 49, 12 h n = 29, 24 h n = 155, 48 h n = 43 cells). The controls (0 h LPS) in C and E represent the average data from day 3 to day 7 in B and D, respectively. F and G, intracellular Ca2+ changes (F340/F380) during the first 3 h (F, n = 22, 2 coverslips) and basic internal Ca2+ up to 48 h (G) after incubation with 1 μg ml−1 LPS. (G, 0 h n = 47, 3 h n = 57, 6 h n = 78, 12 h n = 54, 24 h n = 26, 48 h n = 45).
LPS-dependent down- and up-regulation of IIRK and IDRK
The delayed outward rectifier (DRK) and inward rectifier (IRK) potassium channels are the dominant and best-studied ion channels in cultured microglial cells. Their down- and up-regulation often is used as a marker for the microglial activation status (Norenberg et al. 1994; Eder, 1998; Walz & Bekar, 2001). We determined the changes of inward and outward potassium currents of subcultured microglia over a period of 1–7 days after harvest and 3–48 h after incubation and activation with 1 μg ml−1 LPS. On the first day after harvest, 2.8 ± 2.8% of the cells revealed expression of IIRK only, 26.1 ± 5.7% expressed IDRK only, and a majority of 71.1 ± 7.4% showed both types of potassium currents (n = 4 coverslips with 36 cells; Fig. 2A). Figure 2C–E shows sample current–voltage (I–V) relationships of microglial cells expressing IIRK only (Fig. 2C), IDRK only (Fig. 2D), or both IIRK plus IDRK (Fig. 2E). During the first 4 days in culture, the percentage of cells showing IIRK only increased dramatically to 58.2 ± 15.2% (n = 4 coverslips with 32 cells) at the expense of cells expressing IDRK only (0%) or IIRK+DRK (41.8 ± 15.2%). These relative proportions remained stable even after 7 days (n = 3 coverslips with 19 cells; Fig. 2A). With respect to average current amplitudes, IDRK strongly decreased from 25.9 ± 3.4 pA pF−1 (n = 36) at day 1 to 5.2 ± 0.9 pA pF−1 (n = 18) at day 7, whereas IIRK increased from 15.5 ± 2.2 pA pF−1 (n = 36) at day 1 to 27.7 ± 4.1 pA pF−1 (n = 18) at day 7 (Fig. 2F).
Figure 2. IIRK and IDRK.
A and B, percentage of microglial cells in culture showing just IIRK (^, ± s.e.m.), just IDRK (▵, ± s.e.m.) or IIRK+DRK (•, ± s.e.m.) depending on time in subculture (A, day 1 n = 4, 2 n = 3, 3 n = 4, 4 n = 4, 5 n = 2, 6 n = 3, 7 n = 3 coverslips, 183 cells) and incubation time in 1 μg ml−1 LPS (B, 0 h n = 16, 3 h n = 4, 6 h n = 4, 12 h n = 2, 24 h n = 4, 48 h n = 3 coverslips, 247 cells). C, D and E, typical current–voltage relationships (I–V) of whole-cell currents in microglial cells revealing just IIRK (C), just IDRK (D) and both, IIRK+DRK (E). F and G, average inward (IRK, •, ± s.e.m.) and outward (DRK, ^, ± s.e.m.) currents (in pA pF−1) measured at −110 and +80 mV, respectively, of microglial cells depending on time in subculture (F, day 1 n = 36, 2 n = 22, 3 n = 37, 4 n = 32, 5 n = 17, 6 n = 19, day 7 n = 18 cells) and (G) incubation time in 1 μg ml−1 LPS (circles, 0 h n = 123, 3 h n = 18, 6 h n = 27, 12 h n = 15, 24 h n = 28, 48 h n = 25 cells). The controls (0 h LPS) in B and G represent the average data from day 3 to day 7 in A and F, respectively.
Since the most dramatic changes in potassium currents took place from day 1 to day 3, further experiments investigating early effects of LPS induction were performed on microglial cells at least 3 days in subculture. For these experiments, the unstimulated control data in Fig. 2B and G represent the average of data from day 3 to day 7. Within just 3 h, exposure of cells to 1 μg ml−1 LPS dramatically changed the ratio between cells expressing IIRK only, IDRK only and IIRK+DRK (see Fig. 2B). After 6 h of LPS exposure, the number of cells revealing IIRK+DRK more than doubled to 87.5 ± 5.1%, cells showing IDRK increased about 4-fold to 12.5 ± 5.1% and at the same time, none of the cells expressed IIRK (n = 4 coverslips with 28 cells; Fig. 2B). However, within 48 h of LPS exposure the percentage of cells expressing IIRK only recovered to 30.3 ± 15.1%, cells presenting IIRK+DRK to 66 ± 13% and cells with IDRK only to 3.7 ± 3.7% (n = 3 coverslips with 28 cells; Fig. 2B).
Acute LPS exposure not only affected potassium channel expression levels, but also their current amplitudes. IDRK significantly increased and reached its peak after 6 h of exposure to 1 μg ml−1 LPS (43.9 ± 5.8 pA pF−1, n = 27), before leveling off to control values again after 24 h of LPS exposure (10 ± 2.3 pA pF−1, n = 28; Fig. 2G). In contrast, the current size of IIRK gradually decreased from control levels of 22.2 ± 1.5 pA pF−1 (n = 123) to 5.6 ± 0.6 pA pF−1 (n = 28) after 24 h of incubation with LPS (Fig. 2G).
LPS-dependent down-regulation of ICRAC
Ca2+ release from intracellular stores plays a critical role in intracellular signalling in many cell types. In non-excitable cells, depletion of stores activates store-operated (capacitative) Ca2+ entry (SOCE) to induce cellular responses and refill the Ca2+ stores (Putney, 1986). The calcium release-activated calcium current (ICRAC) (Hoth & Penner, 1992) is the best-characterized SOCE current and the main Ca2+ influx pathway in immunocompetent cells such as T-lymphocytes and mast cells, but little is known about CRAC in microglial cells. We measured IP3-induced CRAC currents in non-stimulated microglial cells over 1–7 days in subculture and during a period of 3–48 h of exposure to 1 μg ml−1 LPS. Figure 3A shows the average development of ICRAC over time, normalized to cell capacitance, in microglia from day 1, 3 and 6 after replating and subculturing. The normalized amplitude of CRAC currents, measured at its plateau phase after 150–200 s into the experiment, ranged between 1.2 ± 0.13 pA pF−1 (n = 11, day 2) to 1.29 ± 0.08 pA pF−1 (n = 12, day 4), and did not change significantly during the 7 days in subculture (Fig. 3A and B). However, after exposure to 1 μg ml−1 LPS, current sizes of ICRAC decreased with time of incubation from a control level (mean amplitude of ICRAC from day 3 to day 7) of 1.25 ± 0.04 pA pF−1 (n = 60, set as 100%) to about 58% (0.73 ± 0.11 pA pF−1, n = 7) within 12 h and further to 41% (0.51 ± 0.06 pA pF−1, n = 11) after 48 h of exposure (Fig. 3C and D). Figure 3E shows the typical inward rectifying average I–V relationships of leak-subtracted CRAC currents after 100 s into the experiment from unstimulated control cells and cells exposed to LPS (1 μg ml−1) for 24 h and 48 h.
Figure 3. ICRAC.
A, average membrane current (ICRAC, in pA pF−1) measured at −110 mV 0–200 s after break-in from cultured microglial cells at day 1 (^, n = 14, ± s.e.m.), day 3 (•, n = 14, ± s.e.m.) and day 6 (▪, n = 14, ± s.e.m.) in subculture, and C, after 6 h (•, n = 8, ± s.e.m.), 12 h (^, n = 8, ± s.e.m.) and 48 h (□, n = 11, ± s.e.m.) incubation in 1 μg ml−1 LPS. B, average ICRAC amplitude (in −pA pF−1), measured at the plateau current (150 s to 200 s), in dependency of time in subculture (day 1 n = 13, 2 n = 11, 3 n = 14, 4 n = 12, 5 n = 12, 6 n = 15, 7 n = 7 cells). D, ICRAC (current in percentage of control ± s.e.m.) after 3 h (n = 8), 6 h (n = 7), 12 h (n = 7), 24 h (n = 9), and 48 h (n = 11) incubation with 1 μg ml−1 LPS. E, averaged current–voltage relationships (I–Vs) of whole-cell currents after 100 s into the experiment measured from a resting (control, n = 8), a 24 h (n = 8) and a 48 h (n = 10) activated microglial cell. The control (100%, 0 h LPS) in D represents the average data from day 3 to day 7 in B.
Microglia express Ca2+-activated TRPM4-like currents
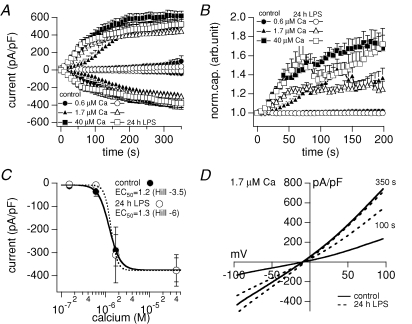
Ca2+-activated non-selective cation channels, like the melastatin-related transient receptor potential channels TRPM4 and TRPM5, are widely expressed and proposed to be involved in membrane depolarization (Launay et al. 2002; Prawitt et al. 2003; Guinamard et al. 2004). In the present study we show that perfusing microglial cells with increasing intracellular Ca2+ concentrations through the patch pipette, recruits a TRPM4-like current in non-activated as well as LPS-induced (24 h exposure) cells in a dose-dependent manner (see Fig. 4A and C). The dose–response curve calculated for the inward current at −80 mV yields an EC50 of 1.2 μm Ca2+ for control and 1.3 μm Ca2+ for 24 h LPS-exposed microglial cells (Fig. 4C). As seen with TRPM4 (Cheng et al. 2007), the Ca2+-activated current in microglia (Fig. 4A) developed in parallel with an increase in cell capacitance (Fig. 4B), indicating that a proportion of channels might be recruited to the plasma membrane through exocytosis. The dose–response for inward and outward (data not shown) currents did not show any significant difference between non-activated and 24 h LPS-exposed microglial cells. However, 1.7 μm intracellular Ca2+ induced a faster recruitment of the TRPM4-like current in 24 h LPS-activated cells (see Fig. 4A). In addition, higher concentrations of Ca2+ (see Fig. 4A and 40 μm) resulted in a slightly larger outward current in non-activated (618 ± 55 pA pF−1, n = 19) compared with 24 h LPS-exposed microglia (520 ± 96 pA pF−1, n = 7). As the inward current revealed the same amplitude for control (375 ± 52 pA pF−1, n = 19) and LPS-activated cells (377 ± 68 pA pF−1, n = 7; see Fig. 4A and C) this results in a slightly stronger outward rectification of the TRPM4-like current in non-activated migroglial cells. Figure 4D shows the average I–V relationships of TRPM4-like currents induced by 1.7 μm intracellular Ca2+ in control (n = 9) and 24 h LPS-exposed (n = 9) microglial cells after 100 s and 350 s into the experiment.
Figure 4. Calcium-activated (TRPM4-like) current.
A, average inward and outward currents (in pA pF−1) measured for 350 s, and B, normalized cell capacitance measured for 200 s after break-in from resting (filled) and activated (24 h 1 μg ml−1 LPS, open) microglial cells with 0.6 μm (circles, n = 7/5, ± s.e.m.), 1.7 μm (triangles, n = 13/10, ± s.e.m.) and 40 μm (squares, n = 19/7, ± s.e.m.) free calcium ([Ca2+]i) in the patch pipette. C, plateau inward currents (in pA pF−1) after 350 s versus log [Ca2+]i of resting (•) and activated (24 h 1 μg ml−1 LPS, ^) microglial cells. The sigmoidal fits of the inward current amplitudes revealed an EC50 of 1.2 μm Ca2+ (Hill coefficient =−3.5) for resting and 1.3 μm Ca2+ (Hill =−6) for activated microglial cells. D, average current–voltage relationships (I–Vs) of whole-cell currents (in pA pF−1) activated by 1.7 μm internal Ca2+ from resting (control, continuous traces, n = 9) and activated (24 h 1 μg ml−1 LPS, dotted traces, n = 9) microglial cells at 100 s and 350 s into the experiment.
Discussion
Character of non-activated microglial cells in culture
In the present study more than 90% of the mouse microglial cells appeared in a ramified, rod- or round-shaped morphology from day 1 to day 7 after harvest from an astrocyte coculture and the shape of the cells remained relatively stable. Resting microglia in vivo are thought to primarily express inward rectifying potassium currents (IIRK) and no significant delayed outward rectifying potassium currents (IDRK), increasing the expression of IDRK in favour of IIRK upon activation (Norenberg et al. 1994; Eder, 1998; Walz & Bekar, 2001). In our cultures, the initial expression pattern of potassium currents revealed that the majority of cells expressed both types of currents and about some 30% of cells had only IDRK, indicating that the microglia were already somewhat preactivated in the astrocyte coculture (Schmidtmayer et al. 1994) and/or the harvesting procedure induced some activation. We suspect that serum levels are an important factor, since initial experiments in which we kept the levels of FBS at 10% in both co- and subculture, most of the cells showed already significant signs of activation such as amoeboid ‘fried egg’-shaped morphology, often with prominent IDRK (data not shown). However, coculturing cells with 10% FBS in basal modified Eagle's medium (BME) and reducing FBS to 5% in the subculture improved the yield of resting cells and most microglia were ramified rod-shaped within 3 days; most cells had little or no IDRK but prominent IIRK, indicating largely resting cultured microglia (Kettenmann et al. 1990, 1993). In contrast, Eder et al. (1999) and Nakamura et al. (1999) obtained amoeboid-shaped microglia using Dulbecco's modified Eagle's medium (DMEM) plus 10% FBS for microglia/astrocyte coculture and DMEM plus 10% or 2% FBS for the subculture (Eder et al. 1999; Nakamura et al. 1999). These cells revealed prominent IIRK but no IDRK (Eder et al. 1999). Lombardi et al. (2003) used BME instead of DMEM under serum-free conditions (BME also lacks l-glycine (Gly) and l-serine (Ser)). Under Gly–Ser-free serum-free conditions, microglia displayed a round morphology, whereas in the presence of 5 μm Gly and 25 μm Ser they revealed ramifications (Lombardi et al. 2003). These and our results show that the morphological and electrophysiological character of cultured microglial cells depend on the environmental condition, and the electrophysiological properties do not necessarily correlate completely with the morphology in culture.
Resting microglia in vivo are highly ramified and convert into amoeboid macrophage-like cells when activated (Thomas, 1992; Vilhardt, 2005). As tissue preparations represent traumatic injuries that result in activated microglia, physiological aspects of resting microglial function and early activation in vivo remains largely elusive. The probably closest representation of truly resting microglia in vivo are ramified microglial cells found in acutely isolated brain slices. A few hours after preparation, microglial cells of rat hippocampal brain slices change their morphology from ramified to amoeboid (Stence et al. 2001). Interestingly, and in contrast to cultured cells, microglia in rat brain slices revealed almost no inward and voltage-gated outward potassium currents (Boucsein et al. 2000). Within 12 h after facial nerve axotomy, microglia in the facial nucleus expressed a prominent IIRK and thus acquired the physiological properties of cultured microglial cells. Within 24 h of axotomy, the cells expressed IDRK, which is typical for lipopolysaccharide (LPS)-exposed cultured microglia. Similar results have been obtained after a permanent middle cerebral artery occlusion (MCAO) in the rat brain (Lyons et al. 2000). It thus appears that the activation of microglial cells in vivo proceeds in two steps, first characterized by the expression of IIRK and later by IDRK. Since cultured non-activated microglial cells reveal almost exclusively IIRK they represent a similar physiology as the early activated stage (Boucsein et al. 2000; Lyons et al. 2000), which is also similar to the highly motile microglia invading the brain in early development, as these cells also express just IIRK (Brockhaus et al. 1993). Thus, cultured microglial cells might represent an early intermediate stage between resting and activated microglia that still allows the study of further microglia activation.
Potassium currents (IIRK and IDRK) after LPS exposure
Microglial cells appear in different shapes and express a distinct pattern of ion channels according to their status of activation. In our study, non-activated cultured microglia appeared in a ramified, rod- or round-shaped morphology with prominent IIRK. After exposure to 1 μg ml−1 LPS, cells responded with an increase in cell capacitance (surface area) and exhibited an amoeboid, ‘fried egg’-shaped morphology (see Fig. 1C and D). While IIRK constantly decreased, IDRK reached maximal expression within 6 h but decreased thereafter again (see Fig. 2B and G). This result is in agreement with the general consensus that resting cultured microglia primarily express IIRK and recruit IDRK after exposure to LPS (Kettenmann et al. 1990, 1993; Norenberg et al. 1992, 1994; Illes et al. 1996; McLarnon et al. 1997; Draheim et al. 1999). The IIRK is suggested to be of the Kir2.1-like subtype (Newell & Schlichter, 2005) and LPS-activated microglia express mRNA and proteins for the delayed outward rectifier potassium channels Kv1.3 and Kv1.5 (Khanna et al. 2001). While pharmacological evidence indicates that the channel might be of the Kv1.3 subtype (Pyo et al. 1997; Visentin & Levi, 1998; Cayabyab et al. 2000; Khanna et al. 2001), immunological data by Pyo et al. (1997) suggested that the LPS-induced outward current is probably due to the Kv1.5 subtype and more recent studies revealed that LPS-induced IDRK is reduced in Kv1.5−/− knock-out mice. Since K+ currents in antisense oligonucleotide-treated cultured mouse microglial cells are affected (Pannasch et al. 2006), it seems possible that both Kv1.5 as well as Kv1.3 might be expressed in microglia.
Less is known about the physiological functions of IIRK and IDRK in microglial cells. IDRK seems to be somehow correlated to NO release, since knock-out or block of Kv1.5, but not Kv1.3, resulted in a significant decrease in LPS-induced NO release in culture (Pyo et al. 1997; Pannasch et al. 2006), and Kv1.5 expression in vivo precedes the expression of inducible nitric oxide synthase (iNOS) (Jou et al. 1998). In addition, the Kv1.3 inhibitor agitoxin-2 reduced the microglial respiratory burst, a metabolic cascade that generates antimicrobial superoxide and other reactive oxygen intermediates (Khanna et al. 2001). IIRK and IDRK are mostly discussed to stabilize the membrane potential and are involved in microglial proliferation (Chung et al. 1999; Kotecha & Schlichter, 1999; Eder, 2005; Pannasch et al. 2006). Since resting microglia in vivo are devoid of the potassium currents, their membrane potential is relatively positive (Boucsein et al. 2000). In a first step of LPS-induced activation, IIRK appears (Boucsein et al. 2000; Lyons et al. 2000) and the membrane potential becomes more negative. Hence, microglia cell activation correlates with an increase of the driving force for Ca2+ into the cell. This also applies to non-activated microglial cells in culture, as they already express IIRK. In the absence of IDRK, even a small inward current can lead to a large membrane depolarization. In a second step, IDRK appears in microglia in vivo (Boucsein et al. 2000; Lyons et al. 2000) as well as in cultured microglia (Kettenmann et al. 1990, 1993; Norenberg et al. 1992, 1994; Illes et al. 1996; Newell & Schlichter, 2005), stabilizing the negative membrane potential and the driving force for Ca2+ into the cell. Franchini et al. (2004) observed that inhibition of IIRK with Ba2+ significantly reduced the Ca2+ influx following receptor activation (Franchini et al. 2004). Our results reveal that following an initial peak after 6 h of LPS exposure, the amplitude of IDRK decreased again within 24 h to almost basal level. There was also a parallel decrease in the amplitude of IIRK. Both effects would favour a more depolarized membrane potential and decreased driving force for Ca2+ into the cell. After about 48 h, the expression pattern returned to that observed in microglia prior to LPS exposure in culture, with most cells expressing both IIRK and IDRK, but with relatively small amplitudes (see Fig. 2B and G). The cell shape, however, remained amoeboid. The transient increase in IDRK and the associated potential for enhanced Ca2+ driving force into the cell indicates some importance for Ca2+ signalling, especially during the first 6 h after LPS-induced activation.
Time course of LPS-induced effects
Most studies in cultured microglia assess the effects of LPS 24 h or later after exposure, a time where the most prominent electrophysiological change in our study, the increase of IDRK, has already declined (see Fig. 2G). In addition, the most prominent changes in [Ca2+]i caused by LPS occur over a time window that is not typically studied by most investigations. After a delay of 40–50 min, LPS induces a prolonged increase in the intracellular Ca2+ levels, followed by a transient undershoot lasting several hours, before returning to normal levels (see Fig. 1F and G). Thus, our results show that the most dramatic changes in terms of basic Ca2+ take place in the first 3 h and in terms of potassium currents in the first 3–6 h after LPS exposure. Intracerebroventricular injection of LPS in mice induced a sharp peak of interleukin-6 (IL-6) and monocyte chemotactic protein-1 (MCP-1) production at about 4 h, returning to baseline after about 16 h (Lund et al. 2006). Nakamura et al. (1999) also reported the most dramatic production of IL-6 as well as IL-1-β and TNF-α in the first 6 h after LPS exposure (Nakamura et al. 1999). Interestingly, IL-6 and IL-1-β induction remained elevated, TNF-α expression declined after 12 h. These LPS-induced events thus occur in a similar time frame as IDRK and the resulting increase in Ca2+ signalling ability seen in our study. Nakamura et al. (1999) also showed that LPS induced a transient change of morphology from round amoeboid to process-bearing bipolar and back to round cells, where the cells reached maximum size after 6 h and returned to baseline after 24 h (Nakamura et al. 1999). In contrast, we observed a continuous increase of cell capacitance and a consistent morphological change from ramified to amoeboid over a period of 48 h after LPS exposure (see Fig. 1C and E). These differences in morphology and time course might be due to the fact that Nakamura et al. (1999) studied rat microglial cells grown in DMEM and we studied mouse microglial cells grown in BME medium.
ICRAC in cultured microglial cells
Ca2+ signalling is widely used as a central mechanism for numerous cellular functions in other immunocompetent cells. However, the involvement of Ca2+ and the role of different Ca2+ signalling mechanisms in microglial cells are largely unknown. Microglial cells express several receptors such as purinergic P2Y (James & Butt, 2002; McLarnon, 2005; Light et al. 2006), adrenergic α1 (Mori et al. 2002), muscarinic (Whittemore et al. 1993; Zhang et al. 1998) endothelin (Moller et al. 1997; McLarnon et al. 1999), thrombin (Moller et al. 2000), platelet-activating factor (Mori et al. 1996) and cytokine/chemokine receptors (Boddeke et al. 1999; Franciosi et al. 2002; Shideman et al. 2006), coupled to the second messenger inositol 1,4,5-trisphosphate (IP3) and mediating release of Ca2+ from intracellular stores. Ca2+ release often is followed by a prolonged secondary phase that is due to store-operated (capacitative) Ca2+ entry (SOCE) (Putney, 1986). The best characterized SOCE current and probably the main Ca2+ influx pathway in immunocompetent cells is the Ca2+ release-activated Ca2+ current (ICRAC) (Hoth & Penner, 1992). In microglial cells, SOCE has so far mainly been studied via Ca2+ imaging techniques (Toescu et al. 1998; Khoo et al. 2001; Choi et al. 2003), only few publications showing microglial ICRAC measured with the whole-cell patch-clamp technique (Norenberg et al. 1997; Hahn et al. 2000). In the present study we show that the amplitude of ICRAC in cultured microglial cells decreases significantly with time of activation and LPS exposure. In contrast to the transient increase in IDRK with the peak after 6 h, ICRAC continuously decreased, indicating an immediate down-regulation of Ca2+ influx after LPS-induced activation. This suggests that either increasing the driving force for Ca2+ is not the major purpose of LPS-induced increase of IDRK, or ICRAC is not its most important addressee. SOCE is probably the major but not the only way of Ca2+ influx into microglial cells. There is no evidence for voltage-gated Ca2+ channels in microglial cells, but they reportedly express several other Ca2+-permeable ion channels such as the AMPA (α-amino-3-hydroxy-4-methyl-4-isoxazole propionate) receptor channel, a subtype of the ionotropic glutamate receptor, with a relatively low Ca2+ permeability (Noda et al. 2000), TRPM2 and TRPM7, members of the melastatin-related transient potential receptor channels (Wang et al. 2000; Jiang et al. 2003) and purinergic P2X receptors (Visentin et al. 1999; Wang et al. 2000; Farber & Kettenmann, 2006). In terms of intracellular Ca2+, LPS exposure also induces Ca2+ transients in cultured microglial cells (see Fig. 1F, unidentified mechanism), possibly resulting from caffeine-sensitive Ca2+ release (Bader et al. 1994) and/or dependent on unidentified Ca2+ influx channels (Herms et al. 1997; Choi et al. 2002; Yi et al. 2005).
ICAN in LPS-induced activation of cultured microglial cells
Besides voltage-gated potassium channels, other ion channels such as Ca2+-activated K+ channels (Kaushal et al. 2007), H+ channels (Klee et al. 1998, 1999), non-selective cation channels (Schilling et al. 2004), and Cl− channels (Schlichter et al. 1996; Eder et al. 1998) have also been shown to be involved in further activation or proliferation of cultured microglial cells, although their temporal expression patterns during the activation process have not yet been characterized. Our study additionally reveals a Ca2+-activated TRPM4-like current in non-activated cultured mouse microglial cells. Ca2+-activated cation channels such as TRPM4 and TRPM5 are widely expressed and proposed to be involved in membrane depolarization (Launay et al. 2002; Prawitt et al. 2003; Guinamard et al. 2004). While IIRK and IDRK support the driving force for Ca2+ through negative membrane potentials, incoming Ca2+ might depolarize the microglial cells via activation of the TRPM4-like current, thus reducing the driving force and regulating the Ca2+ influx negatively. In electrically non-excitable Jurkat T lymphocytes, suppression of TRPM4 disregulates receptor-induced Ca2+ oscillations leading to enhanced IL-2 production due to sustained increases in cellular Ca2+ levels (Launay et al. 2004). In microglia, however, besides a slightly faster activation and a slightly stronger outward rectification, LPS exposure did not significantly change the character or size of the Ca2+-activated TRPM4-like ICAN. We therefore propose that LPS-induced down-regulation of IIRK and ICRAC reduces the cell's capacity to produce significant Ca2+ influx upon receptor stimulation and results in decreased sensitivity to additional exogenous stimulation. In this scenario, ICAN does not need to be affected, as its activity would automatically be adjusted due to the diminished calcium influx capacity of the cell.
The mechanism(s) of LPS-induced down-regulation of IIRK and ICRAC and transient up-regulation of IDRK are not known yet. However, the activation of microglial Toll-like receptor-4 (TLR-4) by LPS results in the induction of an array of genes that are involved in initiation or regulation of the inflammatory response (Hoshino et al. 1999; Lee & Lee, 2002). LPS-induced CD40 expression involves activation of the transcription factor nuclear factor-κB (NF-κB) and signal transducer and activator of transcription 1α (STAT-1α), which is activated by the endogenous production of interferon-β (IFN-β) (Qin et al. 2005). The extended delayed transient Ca2+ increase after LPS exposure observed in this study (see Fig. 1F) might also be involved in nuclear gene regulation, since it is known that intracellular Ca2+ signals can trigger gene expression.
Acknowledgments
We thank Carolyn E. Oki-Idouchi, Ka'ohimanu Dang and Mahealani K. Monteilh-Zoller for excellent technical support. This work was supported by NIH grant R01 NS40927 (R.P.) and Deutsche Forschungsgemeinschaft fellowship BE-2616/1-1 (A.B.).
References
- abd-el-Basset E, Fedoroff S. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. J Neurosci Res. 1995;41:222–237. doi: 10.1002/jnr.490410210. [DOI] [PubMed] [Google Scholar]
- Ajmone-Cat MA, Nicolini A, Minghetti L. Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signaling pathways and selectively promotes prostaglandin E2 synthesis. J Neurochem. 2003;87:1193–1203. doi: 10.1046/j.1471-4159.2003.02087.x. [DOI] [PubMed] [Google Scholar]
- Bader MF, Taupenot L, Ulrich G, Aunis D, Ciesielski-Treska J. Bacterial endotoxin induces [Ca2+]i transients and changes the organization of actin in microglia. Glia. 1994;11:336–344. doi: 10.1002/glia.440110406. [DOI] [PubMed] [Google Scholar]
- Baker CA, Manuelidis L. Unique inflammatory RNA profiles of microglia in Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 2003;100:675–679. doi: 10.1073/pnas.0237313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro T, Brodie C, Modarres S, Lewin NE, Acs P, Blumberg PM. Specific vanilloid responses in C6 rat glioma cells. Brain Res Mol Brain Res. 1998;56:89–98. doi: 10.1016/s0169-328x(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Bo L, Mork S, Kong PA, Nyland H, Pardo CA, Trapp BD. Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol. 1994;51:135–146. doi: 10.1016/0165-5728(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Boddeke EW, Meigel I, Frentzel S, Gourmala NG, Harrison JK, Buttini M, Spleiss O, Gebicke-Harter P. Cultured rat microglia express functional β-chemokine receptors. J Neuroimmunol. 1999;98:176–184. doi: 10.1016/s0165-5728(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Boucsein C, Kettenmann H, Nolte C. Electrophysiological properties of microglial cells in normal and pathologic rat brain slices. Eur J Neurosci. 2000;12:2049–2058. doi: 10.1046/j.1460-9568.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ilschner S, Banati RB, Kettenmann H. Membrane properties of ameboid microglial cells in the corpus callosum slice from early postnatal mice. J Neurosci. 1993;13:4412–4421. doi: 10.1523/JNEUROSCI.13-10-04412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayabyab FS, Khanna R, Jones OT, Schlichter LC. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur J Neurosci. 2000;12:1949–1960. doi: 10.1046/j.1460-9568.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic β-cells. Cell Calcium. 2007;41:51–61. doi: 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HB, Hong SH, Ryu JK, Kim SU, McLarnon JG. Differential activation of subtype purinergic receptors modulates Ca2+ mobilization and COX-2 in human microglia. Glia. 2003;43:95–103. doi: 10.1002/glia.10239. [DOI] [PubMed] [Google Scholar]
- Choi HB, Khoo C, Ryu JK, van Breemen E, Kim SU, McLarnon JG. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-α and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem. 2002;83:546–555. doi: 10.1046/j.1471-4159.2002.01122.x. [DOI] [PubMed] [Google Scholar]
- Chung S, Jung W, Lee MY. Inward and outward rectifying potassium currents set membrane potentials in activated rat microglia. Neurosci Lett. 1999;262:121–124. doi: 10.1016/s0304-3940(99)00053-1. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- del Rio Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. Vol. 2. New York: Paul B Hoeber; 1932. pp. 481–534. [Google Scholar]
- Dickson DW, Mattiace LA, Kure K, Hutchins K, Lyman WD, Brosnan CF. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991;64:135–156. [PubMed] [Google Scholar]
- Draheim HJ, Prinz M, Weber JR, Weiser T, Kettenmann H, Hanisch UK. Induction of potassium channels in mouse brain microglia: cells acquire responsiveness to pneumococcal cell wall components during late development. Neuroscience. 1999;89:1379–1390. doi: 10.1016/s0306-4522(98)00407-2. [DOI] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am J Physiol Cell Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- Eder C. Regulation of microglial behavior by ion channel activity. J Neurosci Res. 2005;81:314–321. doi: 10.1002/jnr.20476. [DOI] [PubMed] [Google Scholar]
- Eder C, Klee R, Heinemann U. Involvement of stretch-activated Cl− channels in ramification of murine microglia. J Neurosci. 1998;18:7127–7137. doi: 10.1523/JNEUROSCI.18-18-07127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C, Schilling T, Heinemann U, Haas D, Hailer N, Nitsch R. Morphological, immunophenotypical and electrophysiological properties of resting microglia in vitro. Eur J Neurosci. 1999;11:4251–4261. doi: 10.1046/j.1460-9568.1999.00852.x. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48:133–143. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:656–665. doi: 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- Franchini L, Levi G, Visentin S. Inwardly rectifying K+ channels influence Ca2+ entry due to nucleotide receptor activation in microglia. Cell Calcium. 2004;35:449–459. doi: 10.1016/j.ceca.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. Interferon-γ acutely induces calcium influx in human microglia. J Neurosci Res. 2002;69:607–613. doi: 10.1002/jnr.10331. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P. Functional characterization of a Ca2+-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004;558:75–83. doi: 10.1113/jphysiol.2004.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Jung W, Kim N, Uhm DY, Chung S. Characterization and regulation of rat microglial Ca2+ release-activated Ca2+ (CRAC) channel by protein kinases. Glia. 2000;31:118–124. [PubMed] [Google Scholar]
- Herms JW, Madlung A, Brown DR, Kretzschmar HA. Increase of intracellular free Ca2+ in microglia activated by prion protein fragment. Glia. 1997;21:253–257. doi: 10.1002/(sici)1098-1136(199710)21:2<253::aid-glia8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23:4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Illes P, Norenberg W, Gebicke-Haerter PJ. Molecular mechanisms of microglial activation. B. Voltage- and purinoceptor-operated channels in microglia. Neurochem Int. 1996;29:13–24. doi: 10.1016/0197-0186(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. J Neurosci Res. 2005;81:363–373. doi: 10.1002/jnr.20482. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Jiang X, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem. 2003;278:42867–42876. doi: 10.1074/jbc.M304487200. [DOI] [PubMed] [Google Scholar]
- Jou I, Pyo H, Chung S, Jung SY, Gwag BJ, Joe EH. Expression of Kv1.5 K+ channels in activated microglia in vivo. Glia. 1998;24:408–414. [PubMed] [Google Scholar]
- Kaushal V, Koeberle PD, Wang Y, Schlichter LC. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J Neurosci. 2007;27:234–244. doi: 10.1523/JNEUROSCI.3593-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershbaum J. Genesis of microglia in the human brain. Arch Neurol Psychiatry. 1939;41:24–50. [Google Scholar]
- Kettenmann H, Banati R, Walz W. Electrophysiological behavior of microglia. Glia. 1993;7:93–101. doi: 10.1002/glia.440070115. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hoppe D, Gottmann K, Banati R, Kreutzberg G. Cultured microglial cells have a distinct pattern of membrane channels different from peritoneal macrophages. J Neurosci Res. 1990;26:278–287. doi: 10.1002/jnr.490260303. [DOI] [PubMed] [Google Scholar]
- Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. Am J Physiol Cell Physiol. 2001;280:C796–C806. doi: 10.1152/ajpcell.2001.280.4.C796. [DOI] [PubMed] [Google Scholar]
- Khoo C, Helm J, Choi HB, Kim SU, McLarnon JG. Inhibition of store-operated Ca2+ influx by acidic extracellular pH in cultured human microglia. Glia. 2001;36:22–30. doi: 10.1002/glia.1092. [DOI] [PubMed] [Google Scholar]
- Klee R, Heinemann U, Eder C. Changes in proton currents in murine microglia induced by cytoskeletal disruptive agents. Neurosci Lett. 1998;247:191–194. doi: 10.1016/s0304-3940(98)00322-x. [DOI] [PubMed] [Google Scholar]
- Klee R, Heinemann U, Eder C. Voltage-gated proton currents in microglia of distinct morphology and functional state. Neuroscience. 1999;91:1415–1424. doi: 10.1016/s0306-4522(98)00710-6. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Schlichter LC. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J Neurosci. 1999;19:10680–10693. doi: 10.1523/JNEUROSCI.19-24-10680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1:181–191. doi: 10.2174/1568010023344698. [DOI] [PubMed] [Google Scholar]
- Light AR, Wu Y, Hughen RW, Guthrie PB. Purinergic receptors activating rapid intracellular Ca increases in microglia. Neuron Glia Biol. 2006;2:125–138. doi: 10.1017/S1740925X05000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi VR, Etcheverria I, Fernandez-Novoa L, Cacabelos R. In vitro regulation of rat derived microglia. Neurotox Res. 2003;5:201–212. doi: 10.1007/BF03033140. [DOI] [PubMed] [Google Scholar]
- Lund S, Christensen KV, Hedtjarn M, Mortensen AL, Hagberg H, Falsig J, Hasseldam H, Schrattenholz A, Porzgen P, Leist M. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Pastor A, Ohlemeyer C, Kann O, Wiegand F, Prass K, Knapp F, Kettenmann H, Dirnagl U. Distinct physiologic properties of microglia and blood-borne cells in rat brain slices after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:1537–1549. doi: 10.1097/00004647-200011000-00003. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- McLarnon JG. Purinergic mediated changes in Ca2+ mobilization and functional responses in microglia: effects of low levels of ATP. J Neurosci Res. 2005;81:349–356. doi: 10.1002/jnr.20475. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Wang X, Bae JH, Kim SU. Endothelin-induced changes in intracellular calcium in human microglia. Neurosci Lett. 1999;263:9–12. doi: 10.1016/s0304-3940(99)00082-8. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Xu R, Lee YB, Kim SU. Ion channels of human microglia in culture. Neuroscience. 1997;78:1217–1228. doi: 10.1016/s0306-4522(96)00680-x. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Moller T, Hanisch UK, Ransom BR. Thrombin-induced activation of cultured rodent microglia. J Neurochem. 2000;75:1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- Moller T, Kann O, Prinz M, Kirchhoff F, Verkhratsky A, Kettenmann H. Endothelin-induced calcium signaling in cultured mouse microglial cells is mediated through ETB receptors. Neuroreport. 1997;8:2127–2131. doi: 10.1097/00001756-199707070-00008. [DOI] [PubMed] [Google Scholar]
- Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express α1, α2, β1 and β2 adrenergic receptors. Neuropharmacology. 2002;43:1026–1034. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- Mori M, Aihara M, Kume K, Hamanoue M, Kohsaka S, Shimizu T. Predominant expression of platelet-activating factor receptor in the rat brain microglia. J Neurosci. 1996;16:3590–3600. doi: 10.1523/JNEUROSCI.16-11-03590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Si QS, Kataoka K. Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res. 1999;35:95–100. doi: 10.1016/s0168-0102(99)00071-1. [DOI] [PubMed] [Google Scholar]
- Newell EW, Schlichter LC. Integration of K+ and Cl− currents regulate steady-state and dynamic membrane potentials in cultured rat microglia. J Physiol. 2005;567:869–890. doi: 10.1113/jphysiol.2005.092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Cordes A, Blohbaum G, Frohlich R, Illes P. Coexistence of purino- and pyrimidinoceptors on activated rat microglial cells. Br J Pharmacol. 1997;121:1087–1098. doi: 10.1038/sj.bjp.0701241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Gebicke-Haerter PJ, Illes P. Inflammatory stimuli induce a new K+ outward current in cultured rat microglia. Neurosci Lett. 1992;147:171–174. doi: 10.1016/0304-3940(92)90587-w. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Gebicke-Haerter PJ, Illes P. Voltage-dependent potassium channels in activated rat microglia. J Physiol. 1994;475:15–32. doi: 10.1113/jphysiol.1994.sp020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Farber K, Nolte C, Blonski M, Yan Chiu S, Messing A, Kettenmann H. The potassium channels Kv1.5 and Kv1.3 modulate distinct functions of microglia. Mol Cell Neurosci. 2006;33:401–411. doi: 10.1016/j.mcn.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Hansson E, Ronnback L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-α. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Pyo H, Chung S, Jou I, Gwag B, Joe EH. Expression and function of outward K+ channels induced by lipopolysaccharide in microglia. Mol Cells. 1997;7:610–614. [PubMed] [Google Scholar]
- Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-γB and STAT-1α in macrophages and microglia. Blood. 2005;106:3114–3122. doi: 10.1182/blood-2005-02-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Hirato J, Nakazato Y. Immunohistochemical study of microglia in the Creutzfeldt-Jakob diseased brain. Acta Neuropathol. 1993;86:337–344. doi: 10.1007/BF00369445. [DOI] [PubMed] [Google Scholar]
- Schilling T, Lehmann F, Ruckert B, Eder C. Physiological mechanisms of lysophosphatidylcholine-induced de-ramification of murine microglia. J Physiol. 2004;557:105–120. doi: 10.1113/jphysiol.2004.060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl− channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–236. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schmidtmayer J, Jacobsen C, Miksch G, Sievers J. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: membrane currents. Glia. 1994;12:259–267. doi: 10.1002/glia.440120403. [DOI] [PubMed] [Google Scholar]
- Shideman CR, Hu S, Peterson PK, Thayer SA. CCL5 evokes calcium signals in microglia through a kinase-, phosphoinositide-, and nucleotide-dependent mechanism. J Neurosci Res. 2006;83:1471–1484. doi: 10.1002/jnr.20839. [DOI] [PubMed] [Google Scholar]
- Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989;105:115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Moller T, Kettenmann H, Verkhratsky A. Long-term activation of capacitative Ca2+ entry in mouse microglial cells. Neuroscience. 1998;86:925–935. doi: 10.1016/s0306-4522(98)00123-7. [DOI] [PubMed] [Google Scholar]
- Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Visentin S, Levi G. Arachidonic acid-induced inhibition of microglial outward-rectifying K+ current. Glia. 1998;22:1–10. [PubMed] [Google Scholar]
- Visentin S, Renzi M, Frank C, Greco A, Levi G. Two different ionotropic receptors are activated by ATP in rat microglia. J Physiol. 1999;519:723–736. doi: 10.1111/j.1469-7793.1999.0723n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, Bekar LK. Ion channels in cultured microglia. Microsc Res Tech. 2001;54:26–33. doi: 10.1002/jemt.1117. [DOI] [PubMed] [Google Scholar]
- Wang X, Kim SU, van Breemen C, McLarnon JG. Activation of purinergic P2X receptors inhibits P2Y-mediated Ca2+ influx in human microglia. Cell Calcium. 2000;27:205–212. doi: 10.1054/ceca.2000.0110. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Yan ZQ, Lu GQ, Stuart S, Chen SD. Parkinson disease IgG and C5a-induced synergistic dopaminergic neurotoxicity: role of microglia. Neurochem Int. 2007;50:39–50. doi: 10.1016/j.neuint.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Korotzer AR, Etebari A, Cotman CW. Carbachol increases intracellular free calcium in cultured rat microglia. Brain Res. 1993;621:59–64. doi: 10.1016/0006-8993(93)90297-z. [DOI] [PubMed] [Google Scholar]
- Yi HA, Yi SD, Jang BC, Song DK, Shin DH, Mun KC, Kim SP, Suh SI, Bae JH. Inhibitory effects of glucosamine on lipopolysaccharide-induced activation in microglial cells. Clin Exp Pharmacol Physiol. 2005;32:1097–1103. doi: 10.1111/j.1440-1681.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, McLarnon JG, Goghari V, Lee YB, Kim SU, Krieger C. Cholinergic agonists increase intracellular Ca2+ in cultured human microglia. Neurosci Lett. 1998;255:33–36. doi: 10.1016/s0304-3940(98)00706-x. [DOI] [PubMed] [Google Scholar]