Abstract
NMDA receptors are present at glutamatergic synapses throughout the brain, and are important for the development and plasticity of neural circuits. Their subunit composition is developmentally regulated. We have investigated the developmental profile of functional synaptic NMDA receptor subunits in dopaminergic neurones of the substantia nigra pars compacta (SNc). In SNc dopaminergic neurones from rats aged postnatal day (P)7, ifenprodil inhibited NMDA-EPSCs with an estimated IC50 of 0.36 μm and a maximum inhibition of 73.5 ± 2.7% (10 μm), consistent with a substantial population of NR1/NR2B-containing diheteromeric receptors. UBP141, a novel NR2D-preferring antagonist, inhibited NMDA-EPSCs with an estimated IC50 of 6.2 μm. During postnatal development, the maximum inhibitory effect of 10 μm ifenprodil significantly decreased. However, NMDA-EPSCs were not inhibited by Zn2+ (200 nm) or potentiated by the Zn2+ chelator TPEN (1 μm), and the effect of UBP141 did not increase during development, indicating that NR2B subunits are not replaced with diheteromeric NR2A or NR2D subunits. The time course of the decay of NMDA-EPSCs was not significantly changed in ifenprodil at any age tested. Together, these data suggest that diheteromeric NR1/NR2A or NR1/NR2D receptors do not account for the ifenprodil-resistant component of the NMDA-EPSC. We propose that NR1/NR2B/NR2D triheteromers form a significant fraction of synaptic NMDA receptors during postnatal development. This is the first report of data suggesting NR2D-containing triheteromeric NMDA receptors at a brain synapse.
Dopaminergic neurones of the substantia nigra pars compacta (SNc) project to the striatum and are required for the normal physiology of the basal ganglia; this dopaminergic projection is lost in Parkinson's disease (PD). Excitatory afferents to SNc dopaminergic neurones activate NMDA glutamate receptors (Mereu et al. 1991; Wu & Johnson, 1996) to induce burst firing (Johnson et al. 1992; Johnson & Wu, 2004), and NMDA receptors are required for the induction of long-term potentiation at these synapses (Bonci & Malenka, 1999). NMDA receptors may contribute to excitotoxic death of dopaminergic neurones (Doble, 1999; Blandini et al. 2000), and NMDA receptor antagonists have therapeutic potential in PD (Hallett & Standaert, 2004; Paoletti & Neyton, 2007).
The functional and pharmacological properties of NMDA receptors are determined by their subunit composition (Stern et al. 1992; Wyllie et al. 1996; Vicini et al. 1998; see Cull-Candy & Leszkiewicz, 2004 and Paoletti & Neyton, 2007 for review). NR2 subunits are the main determinants of NMDA receptor functional diversity, and the four NR2 subunits, NR2A–NR2D, each confer different properties. In brain neurones, diheteromeric NR1/NR2A and NR1/NR2B receptors predominate; NR2C subunits are mainly expressed in the cerebellum and NR2D subunits have been sparsely reported (Hrabetova et al. 2000; Vicini & Rumbaugh, 2000; Cull-Candy et al. 2001; Brickley et al. 2003; Cull-Candy & Leszkiewicz, 2004; Jones & Gibb, 2005; Borgland et al. 2006). Triheteromeric NMDA receptors (Sheng et al. 1994) composed of NR1 subunits and more than one type of NR2 subunit show unique properties (Chazot & Stephenson, 1997; Cheffings & Colquhoun, 2000; Chazot et al. 2002; Brickley et al. 2003; Cull-Candy & Leszkiewicz, 2004; Hatton & Paoletti, 2005), providing scope for further diversity of function, but triheteromeric NMDA receptors have not been widely reported in CNS neurones (Brickley et al. 2003).
In some brain regions, NMDA receptor subunit expression changes during postnatal development (Monyer et al. 1994; Wenzel et al. 1997). For example, in cortical and hippocampal pyramidal neurones NR2B subunits initially predominate but are later replaced or joined by NR2A subunits (Williams et al. 1993; Flint et al. 1997; Okabe et al. 1998; Stocca & Vicini, 1998; Tovar & Westbrook, 1999; Nase et al. 1999). This is important for the development and possibly for the functional plasticity of neuronal circuits (Katz & Shatz, 1996; Liu et al. 2004a,b; Lozovaya et al. 2004; Massey et al. 2004; but see Berberich et al. 2005).
Although SNc dopaminergic neurones express functional synaptic NMDA receptors (Mereu et al. 1991; Wu & Johnson, 1996), their subunit composition during postnatal development is not known. NR2D subunit mRNA and protein is expressed in the developing and adult rat brain, particularly in brainstem and diencephalon structures, including the SNc (Monyer et al. 1994; Buller et al. 1994; Dunah et al. 1996). NR2A and NR2B subunit proteins are present in low levels in the SNc of adult rats (Albers et al. 1999). Recently, we found that functional NR2B and NR2D subunits form somatic NMDA receptors, possibly as triheteromeric receptors, while no somatic NR2A subunits are present in SNc dopaminergic neurones from rats aged postnatal day (P)14 (Jones & Gibb, 2005). The absence of NR2A subunits was intriguing, as they are present by the second postnatal week in cortex, hippocampus and cerebellum (Monyer et al. 1994).
Following this work, we asked whether SNc dopaminergic neuronal synapses express possible triheteromeric combinations of NR2B and NR2D subunits but no NR2A subunits. We also asked whether NR2 subunit expression is regulated in SNc dopaminergic neurones during early postnatal development, as postnatal changes are seen in other brain regions, and differentiation of dopaminergic neurones continues through the first three postnatal weeks in rats (Prakash & Wurst, 2006). Therefore, the objective of this study was to determine which NR2 subunits form synaptic NMDA receptors in SNc dopaminergic neurones, and whether synaptic NR2 subunit expression is developmentally regulated.
Methods
Brain slice preparation
Brain slices were prepared from rats aged between P6–P22. Three age groups have been studied: rats aged around P7 (P6–P8), P14 (P13–P15) and P21 (P20–P22); the notations P7, P14 and P21 are subsequently used for clarity. Rats were killed by decapitation under halothane anaesthesia. All experiments were carried out in accordance with the Animals (Scientific Procedures) Act 1986 and with local ethical approval. Brains were rapidly removed into ice-cold sucrose-based Ringer solution of the following composition (mm): sucrose 206, KCl 2.5, NaH2PO4 1.25, NaHCO3 26, CaCl2 1, MgCl2 5, and d-glucose 25. Horizontal slices (300 μm) containing the midbrain were prepared (DTK-1000 microslicer, Dosaka, Japan) and held after cutting in a submersion chamber containing artificial cerebrospinal fluid (ACSF) of the following composition (mm): NaCl 119, KCl 2.5, NaH2PO4 1.25, NaHCO3 26, CaCl2 2.5, MgCl2 6, d-glucose 25, at 30°C and saturated with 95% O2 and 5% CO2.
Electrophysiological recordings
Slices were transferred to a recording chamber 1–6 h after preparation and continuously perfused at 2–3 ml min−1 with ACSF (as detailed above but containing 1.3 mm MgCl2 and 10 mm glucose) at 29–31°C, saturated with 95% O2 and 5% CO2. Slices and individual cells were visualized using an Olympus BX51W microscope with differential interference contrast optics. A bipolar stainless steel electrode (Frederick Haer and Co., USA) was placed just rostral to the SNc, and stimuli (100 μs duration; amplitude 100–400 μA) were applied at 10 s intervals. Whole-cell patch-clamp recordings were made using pipettes of 1–3 MΩ resistance when filled with intracellular solution (mm): caesium methane sulphonate 117, NaCl 2.8, MgCl2 3, Hepes 20, MgATP 2, Na3GTP 0.3, EGTA 5, CaCl2 0.5, pH 7.2. Dopaminergic neurones comprise ∼90% of the neuronal population in SNc (Margolis et al. 2006), and were identified in this study by the presence of a time-dependent, hyperpolarization-activated inward current in response to voltage steps from −60 mV to between −100 and −120 mV (Johnson & North, 1992; Jones & Gibb, 2005). Excitatory postsynaptic currents (EPSCs) recorded in SNc dopaminergic neurones were amplified using a HEKA EPC9 amplifier, low-pass filtered at 3 kHz and digitally sampled to a PC at 20 kHz using a Micro1401 interface (Cambridge Electronic Design, Cambridge, UK). The amplitude of the EPSC was measured at the peak unless otherwise stated, using the computer program Spike 2 (Version 4, Cambridge Electronic Design, Cambridge, UK).
The AMPA : NMDA ratio was determined by measuring the amplitude of the AMPA receptor-mediated EPSC (recorded in the presence of d-AP5) and the amplitude of the NMDA-EPSC (obtained by subtracting the AMPA-EPSC from the total EPSC), as shown in Fig. 1 (the average of 10 EPSCs in each condition was used for the calculation), and compared using one-way ANOVA. To determine the change in amplitude following addition of all other drugs, EPSCs were normalized to pre-drug control amplitudes. EPSC amplitudes before and after drug within experimental groups were compared using a paired t test, while for comparisons between experimental groups, EPSC amplitude values (measured during a 10 min plateau between 10 and 30 min after drug addition) were compared using one-way ANOVA (with post hoc tests if P < 0.05) or an unpaired t test (with Welch's correction if the variances between the groups were unequal). All statistical tests were performed using GraphPad Prism (Version 4, San Diego, CA, USA). Antagonist inhibition data were fitted with a single site hyperbola (in GraphPad Prism) of the form:
For measurement of the time constant of NMDA-EPSC decay, 10–20 EPSCs were averaged and the decay from the peak of the response to the plateau was fitted with a two-component exponential decay function (in GraphPad Prism):
The weighted time constant (τW) was calculated as:
All combined data are shown as mean ± standard error (s.e.m.), the ‘n’ values reported refer to the number of slices, and a critical P value of P < 0.05 was considered significant for the statistical tests used throughout this study.
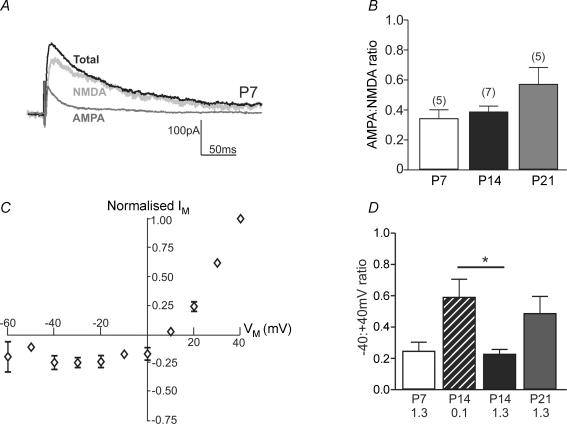
Figure 1. NMDA-EPSCs throughout early postnatal development.
A, example recording of EPSCs at +40 mV from a SNc dopaminergic neurone in a slice from a rat aged P7. Traces are the average of 10 EPSCs. ‘Total’ EPSC (black trace) was recorded in picrotoxin (50 μm) and glycine (10 μm), ‘AMPA’-EPSC (dark grey trace) was recorded in the presence of 50 μm d-AP5, and ‘NMDA’-EPSC was obtained by subtracting the AMPA-EPSC from the Total EPSC. B, bar graph showing the ratio of the AMPA-EPSC to the NMDA-EPSC (obtained by measuring the responses shown in A at the three developmental stages tested. There was no significant difference during the first 3 weeks of postnatal development (ANOVA P > 0.05; ‘n’ in parentheses). C, current–voltage relationship for NMDA-EPSCs recorded in 1.3 mm extracellular Mg2+ and normalized to the response at +40 mV (rat aged P7). D, bar graph showing the ratio of the NMDA-EPSC recorded at −40 mV to that recorded at +40 mV in slices from rats aged P7, P14 and P21. Data for P14 slices are shown at 1.3 mm and 0.1 mm extracellular Mg2+. ANOVA revealed a significant difference (P < 0.05), and the post hoc tests revealed a significant difference only between 0.1 and 1.3 mm Mg2+ at P14 (*P < 0.05, Bonferroni's multiple comparison post hoc test; ‘n’ in parentheses).
Materials
All standard laboratory salts were obtained from BDH Laboratory Supplies (Poole, UK). Picrotoxin, DNQX (6,7-dinitroquinoxaline-2,3(1H,4H)-dione), d-AP5, ZnCl2, TPEN (N,N,N′,N′-tetrakis-[2-pyridylmethyl]-ethylenediamine) and ifenprodil were obtained from Sigma-Aldrich (Dorset, UK). NVP-AAM077 ([(R)-[(S)-1-(4-bromophenyl)-ethylamino]-(2,3-dioxo-1, 2,3,4-tetrahydro-quinoxalin-5-yl)methyl]-phosphonic acid) was a gift from Dr Y. Auberson, Novartis Institutes for BioMedical Research, Basel, Switzerland.
Results
NMDA-EPSCs are present throughout postnatal development
In order to determine the contribution of NMDA receptors to excitatory synaptic transmission during postnatal development, evoked EPSCs were recorded from individual SNc dopaminergic neurones in midbrain slices from rats of different postnatal ages (P7, P14 and P21), and the NMDA receptor antagonist d-AP5 (50 μm) was applied (Fig. 1A). The AMPA : NMDA ratios at each postnatal week were: P7, 0.34 ± 0.06 (n = 5); P14, 0.39 ± 0.04 (n = 7); P21, 0.57 ± 0.11 (n = 5). The AMPA : NMDA ratio did not significantly change in SNc dopaminergic neurones during the three developmental periods (ANOVA P > 0.05; Fig. 1B).
NMDA receptor-mediated EPSCs in SNc dopaminergic neurones showed the expected sensitivity to Mg2+ ions. The current–voltage relationship for NMDA-EPSCs at P7 is illustrated in Fig. 1C, showing the characteristic negative slope conductance in the presence of Mg2+. The ratio of the NMDA-EPSC recorded at −40 mV relative to the NMDA-EPSC recorded at +40 mV gives an indication of the voltage sensitivity of Mg2+-dependent block and is shown in Fig. 1D; there was no significant difference in the ratio at the three ages.
NR2B subunit expression is developmentally regulated
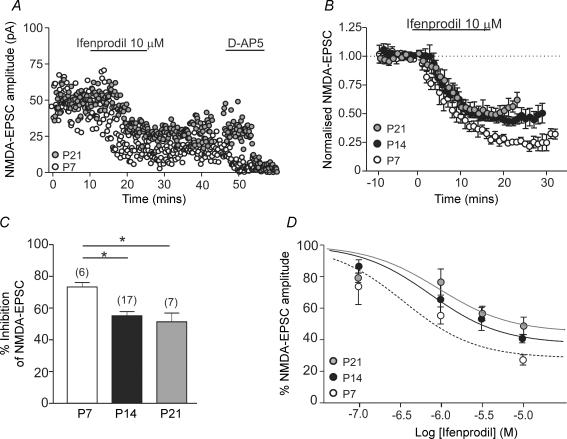
To determine the contribution of NR2B subunits to synaptic NMDA receptors during postnatal development of SNc dopaminergic neurones, the NR2B subunit-selective antagonist ifenprodil (0.1–10 μm) was used. At selective concentrations, ifenprodil causes a maximum inhibition of 80–90% of diheteromeric NR2B receptors (Williams, 1993; Neyton & Paoletti, 2006). In slices from P7 rats, ifenprodil caused a concentration-dependent inhibition of NMDA-EPSCs; the effect of a maximal concentration of ifenprodil (10 μm; 73.5 ± 2.7% inhibition; n = 6; P < 0.0001) is shown in Fig. 2A–D. At P14, the effect of 10 μm ifenprodil in inhibiting NMDA-EPSCs (55.1 ± 2.7% inhibition; n = 17; P < 0.0001) was significantly less than at P7 (P < 0.01); no further significant change occurred between P14 and P21 (Fig. 2B and C). These data suggest that there may be a reduction in the contribution of NR1/NR2B-containing diheteromeric receptors to NMDA-EPSCs during early postnatal development. The estimated IC50 values for ifenprodil at the three developmental ages, calculated from the fit of the inhibition data shown in Fig. 2D, were: P7, 0.36 μm; P14, 0.75 μm; P21, 0.94 μm. At lower ifenprodil concentrations, differences between age groups are not significant because the effect of ifenprodil is smaller while the variance between experiments is fairly constant.
Figure 2. Developmental change in functional NR2B receptors.
A, example recordings of NMDA-EPSC amplitude (pA) over time in a slice from a P7 rat (open circles) and a slice from a P21 rat (grey circles). Ifenprodil (10 μm) was added after a 10 min stable baseline was recorded, d-AP5 (50 μm) was added at the end of each experiment. B, combined data of NMDA-EPSCs over time showing the effect of ifenprodil (10 μm) at the three developmental stages tested (open circles, P7, n = 6; black circles, P14, n = 17; grey circles, P21, n = 7). In all three age groups ifenprodil caused a significant inhibition. C, bar graph comparing the mean inhibition (%) induced by 10 μm ifenprodil at P7 (measured 20–30 min post-drug), P14 and P21 (both measured 10–20 min post-drug). Significant difference detected with ANOVA; *P < 0.01, Bonferroni's multiple comparison post hoc test (‘n’ in parentheses). D, inhibition curves showing the effect of increasing concentrations of ifenprodil on NMDA-EPSCs at the three developmental stages. The fit of the data (see Methods) was used to obtain the IC50 values reported in the text. Numbers of slices at each concentration and age are as follows: P7 (open circles) 0.1 μm, n = 4; 1 μm, n = 5; 10 μm, n = 6; P14 (black circles) 0.1 μm, n = 6; 1 μm, n = 5; 3 μm, n = 6; 10 μm, n = 17; P21 (grey circles) 0.1 μm, n = 4; 1 μm, n = 5; 3 μm, n = 4; 10 μm, n = 7.
NR2A subunits are absent throughout postnatal development
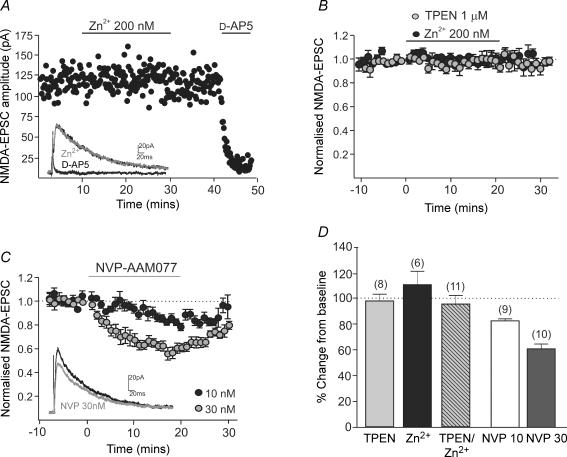
In cortical and hippocampal neurones, NR2A subunits replace or join NR2B subunits during early postnatal development (Williams et al. 1993; Flint et al. 1997; Stocca & Vicini, 1998; Tovar & Westbrook, 1999; Nase et al. 1999). The reduction in the inhibition by ifenprodil may be due to a concomitant increase in the contribution of NR2A subunits during postnatal development of SNc dopaminergic neurones. To address this, the effect of the NR2A subunit-preferring antagonists Zn2+ (Paoletti et al. 1997) and NVP-AAM077 (Auberson et al. 2002; but see Neyton & Paoletti, 2006; Frizelle et al. 2006), and the Zn2+ chelator, TPEN were investigated in slices from rats aged P13–P22. TPEN (1 μm) caused no significant potentiation of NMDA-EPSCs (101.7 ± 5.6% of baseline, n = 8; P = 0.59; Fig. 3B, D), indicating either that Zn2+ ions are not present in our solutions or that the concentration of endogenous Zn2+ ions is not sufficient to inhibit NMDA-EPSCs. Addition of Zn2+ (200 nm) had no effect on NMDA-EPSCs when applied alone (−8.3 ± 10% inhibition, n = 6; P = 0.13; Fig. 3B, D) or immediately following application of TPEN (3.9 ± 6.7% inhibition, n = 11; P = 0.21). In contrast, Zn2+ (200 nm) inhibited NMDA-EPSCs recorded from hippocampal pyramidal cell layer neurones in slices from rats aged P20–P22 (72.7 ± 11.8% inhibition, n = 4), serving as a positive control for Zn2+. These data suggest that NR2A subunits do not contribute to functional synaptic NMDA receptors in SNc dopaminergic neurones. Application of low concentrations of NVP-AAM077 inhibited NMDA-EPSCs in a manner consistent with their predicted effect on synaptic NR2B-containing receptors (Frizelle et al. 2006), with 10 nm causing 15 ± 2% (n = 9; P < 0.001) and 30 nm causing 38.7 ± 3.8% inhibition (n = 10; P < 0.001). These data are summarized in Fig. 3C,D.
Figure 3. NR2A-containing receptors are absent during early postnatal development.
A, example recording of NMDA-EPSC amplitude (pA) over time in a slice from a P14 rat. Zn2+ (200 nm) was added after a 10 min stable baseline, d-AP5 (50 μm) was added at the end of the experiment. Inset shows NMDA-EPSCs in control, Zn2+ and d-AP5. B, combined data of NMDA-EPSCs over time showing the lack of effect of TPEN (1 μm; grey circles, n = 8) and Zn2+ (200 nm; black circles, n = 6) in slices from rats aged P13–P22. C, combined data of NMDA-EPSCs over time showing the effect of NVP-AAM077 (10 nm; open circles, n = 9; grey circles, 30 nm, n = 10) in slices from rats aged P13–P22. Inset shows NMDA-EPSCs in control and 30 nm NVP-AAM077. D, bar graph summarizes the lack of effect of TPEN and Zn2+ alone or Zn2+ following a baseline in TPEN, and the modest effects of NVP-AAM077, suggestive of an effect on NR2B-containing receptors.
NR2D subunits form functional synaptic NMDA receptors
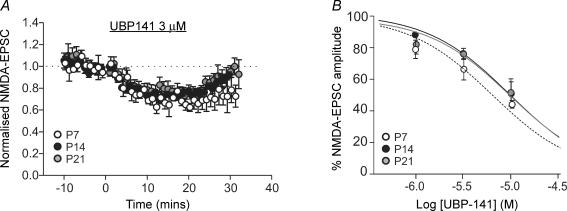
We have previously reported that NR2D-containing NMDA receptors are present at extrasynaptic sites in SNc dopaminergic neurones (Jones & Gibb, 2005). In order to determine whether NR2D subunits also contribute to synaptic NMDA receptors, and whether the contribution of NR2D subunits is developmentally regulated, the NR2D subunit-preferring antagonist UBP141 (1–10 μm) was applied. Reported KD values for UBP141 at NMDA receptor subtypes are: NR2A, 14.2 μm; NR2B, 19.3 μm; NR2C, 4.2 μm; NR2D, 2.8 μm (Morley et al. 2005). At a concentration (3 μm) which should have minimal effect on NR1/NR2B receptors, UBP141 inhibited the peak of NMDA-EPSCs in slices from P7 rats by 33.7 ± 6.8% (n = 6; Fig. 4A), and the slow NMDA-EPSC component (∼200 ms after the stimulus) by 46.1 ± 8.3% (n = 6; P = 0.28 compared with peak). In slices from rats aged P14 or P21, the amount of inhibition with 3 μm UBP141 was not significantly different to that seen at P7 (Fig. 4A). The estimated IC50 values for UBP141, obtained from the fit of the inhibition data shown in Fig. 4B, were: P7, 6.2 μm; P14, 9.6 μm; P21, 9.7 μm. Together, these data suggest that NR2D subunits form synaptic NMDA receptors in SNc dopaminergic neurones, and that the functional contribution of NR2D-containing receptors to NMDA-EPSCs is not obviously developmentally regulated.
Figure 4. NR2D receptors are present throughout early postnatal development.
A, combined data of NMDA-EPSCs over time showing the effect of UBP141 (3 μm) at the three developmental stages tested (open circles, P7, n = 6; black circles, P14, n = 5; grey circles, P21, n = 4). In all three age groups UBP141 caused a significant inhibition. B, inhibition curves showing the effect of increasing concentrations of UBP141 on NMDA-EPSCs at the three developmental stages. The fit of the data was used to obtain the IC50 values reported in the text. Numbers of slices at each concentration and age are as follows: P7 (open circles) 1 μm, n = 6; 3 μm, n = 6; 10 μm, n = 4; P14 (black circles) 1 μm, n = 5; 3 μm, n = 5; 10 μm, n = 4; P21 (grey circles) 1 μm, n = 5; 3 μm, n = 4; 10 μm, n = 4.
NR2B and NR2D subunits may form triheteromeric synaptic NMDA receptors
Rather than NR2B subunits being replaced with NR2A subunits or more NR2D subunits, the pharmacological properties of NMDA-EPSCs suggest that NR2B and NR2D subunits may increasingly form triheteromeric receptors that show a decrease in the maximum inhibition by ifenprodil. Previously, we have reported evidence for triheteromeric NR1/NR2B/NR2D receptors at extrasynaptic sites in P14 SNc dopaminergic neurones (Jones & Gibb, 2005). To investigate this further, we have looked at two properties of NMDA-EPSCs.
Firstly, we have measured the time constant of the decay of NMDA-EPSCs before and after the addition of ifenprodil. If the sizeable ifenprodil-insensitive component at P14–P21 was dominated by NR2A receptors, the rate of decay should accelerate in ifenprodil, while if NR2D subunits dominate the rate of decay should be slowed by ifenprodil (Hestrin, 1992; Flint et al. 1997; Vicini et al. 1998; Stocca & Vicini, 1998; Cull-Candy & Leszkiewicz, 2004). Figure 5A shows example NMDA-EPSCs at P14 recorded in control conditions and in the presence of ifenprodil (10 μm), scaled for comparison. At all three ages, the weighted decay time constant of control NMDA-EPSCs was not significantly different in the presence of ifenprodil (Fig. 5B), suggesting that the ifenprodil-insensitive NMDA-EPSC at P14 is mediated by a similar NMDA receptor population to the total NMDA-EPSC. Consistent with this, the weighted decay time constant of NMDA-EPSCs remaining in 3 μm UBP141 at P14 (115 ± 38 ms, n = 5) was not significantly different to control values (106 ± 26 ms, n = 5, P = 0.55, paired t test). On the other hand, there was a trend towards a decrease in the weighted decay time constant of control NMDA-EPSCs during development, with a significant difference between P7 (152 ± 23 ms, n = 6) and P21 (68 ± 8 ms, n = 6; P < 0.05, Bonferroni's multiple comparison post hoc test).
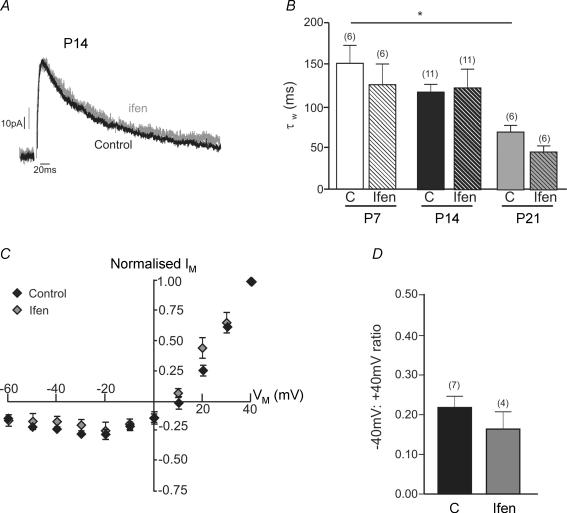
Figure 5. NR2B and NR2D subunits may form triheteromeric NMDA receptors.
A, example recording of NMDA-EPSCs at +40 mV from a SNc dopaminergic neurone in a slice from a rat aged P14. Traces are the average of 20 NMDA-EPSCs. ‘Control’ EPSC (black trace) was recorded in picrotoxin, glycine and DNQX (10 μm), ‘Ifenprodil’ EPSC (grey trace; Ifen) was recorded in the presence of 10 μm ifenprodil, and the two traces have been scaled for comparison of the decay. B, bar graph showing the time constant of the decay (weighted from a two-component exponential fit of the control NMDA-EPSC at P7, P14 and P21 and of the NMDA-EPSC component remaining in the presence of 10 μm ifenprodil at each age. ANOVA revealed a significant difference (P < 0.05); post hoc tests revealed a significant difference between control τW at P7 and P21 (*P < 0.05, Bonferroni's multiple comparison post hoc test; ‘n’ in parentheses). C, current–voltage relationship for NMDA-EPSCs recorded in 1.3 mm extracellular Mg2+ (normalized to the response at +40 mV) in control conditions (n = 7) and in the presence of 10 μm ifenprodil (n = 4) in slices from P14 rats. D, bar graph showing the ratio of the NMDA-EPSC recorded at −40 mV to that recorded at +40 mV in control and ifenprodil.
Secondly, we have examined the current–voltage relationship to obtain the ratio of the NMDA-EPSC at −40 mV relative to +40 mV (an indicator of Mg2+ sensitivity) before and after application of ifenprodil, because NR2A and NR2B receptors have a higher Mg2+ sensitivity and a steeper voltage-dependent Mg2+ block than NR2C and NR2D receptors (Monyer et al. 1994; Kuner & Schoepfer, 1996; Qian & Johnson, 2006). No significant change was observed (Fig. 5C and D), indicating that the ifenprodil-insensitive component has a similar Mg2+ sensitivity to the total NMDA-EPSC.
Discussion
We have investigated the developmental profile of functional synaptic NMDA receptors present in SNc dopaminergic neurones. We propose that NR2B and NR2D subunits but not NR2A subunits are present throughout early postnatal development, and that the properties of synaptic NMDA receptors are developmentally regulated. Our data suggest that triheteromeric NMDA receptors composed of NR1/NR2B/NR2D subunits are important during postnatal development.
A developmental change in synaptic NR2B subunit expression
Ifenprodil is a non-competitive antagonist of NR2B-containing NMDA receptors that acts by enhancing proton inhibition (Mott et al. 1998). Ifenprodil has a higher affinity for recombinant receptors containing NR2B subunits and, at the concentrations used here (0.1–10 μm), has little effect on recombinant diheteromeric receptors composed of NR1 and NR2A, NR2C or NR2D subunits (Williams, 1993, 1995, 2001; Cull-Candy et al. 2001; Paoletti & Neyton, 2007). In SNc dopaminergic neurones, 0.1–10 μm ifenprodil reduced NMDA-EPSCs at all stages of postnatal development, indicating that NR2B-containing receptors are persistently present. However, ifenprodil caused a greater maximal effect in the first postnatal week; subsequently, the maximum effect of ifenprodil decreased, suggesting either that NR2B-containing receptors were replaced with NMDA receptors composed of other subunits, or that during development NR2B subunits form different proportions of triheteromeric receptors that show a lower maximal inhibition with ifenprodil (Chazot & Stephenson, 1997; Tovar & Westbrook, 1999; Chazot et al. 2002; Hatton & Paoletti, 2005).
The persistent presence of NR2B subunits during development could contribute to slow NMDA receptor-dependent signalling and coincidence detection (Nevian & Sakmann, 2004) until at least the third postnatal week, and may also be important for NMDA receptor-mediated excitotoxicity (Zhou & Baudry, 2006), as NR2B-selective NMDA receptor antagonists protect SNc dopaminergic neurones against neurotoxicity in experimental models of PD (Blanchet et al. 1999; Nash et al. 1999; Steece-Collier et al. 2000; Nash et al. 2000; Hallett & Standaert, 2004).
Functional synaptic NR2A subunits are absent
NMDA-EPSCs in SNc dopaminergic neurones were not affected by NR2A-selective pharmacological agents, Zn2+ ions and the Zn2+ chelator TPEN (Paoletti et al. 1997; Rachline et al. 2005). On the basis of these data, we conclude that NR2A subunits are absent in SNc dopaminergic neurones. This is consistent with our previous study of extrasynaptic NMDA receptors (Jones & Gibb, 2005). NVP-AAM077 is a NR2A-preferring antagonist at concentrations < 100 nm (Auberson et al. 2002; Neyton & Paoletti, 2006); 10 nm is predicted to cause > 50% inhibition of synaptic NR2A-containing receptors and < 20% inhibition of synaptic NR2B-containing receptors (Frizelle et al. 2006). In SNc dopaminergic neurones, 10 nm NVP-AAM077 caused only about 15% inhibition of the NMDA-EPSC, suggestive of an effect on NR2B- but not NR2A- containing receptors. NVP-AAM077 (50 nm) caused ∼27% block of NMDA responses mediated by NR2B receptors expressed in HEK293 cells, and 19% inhibition of NMDA-EPSCs in hippocampal slices from mice lacking NR2A subunits (and therefore expressing only NR2B subunits; Berberich et al. 2005), effects smaller than that observed in our experiments. NVP-AAM077 also inhibits NR2D-containing receptors, with higher affinity than NR2B-containing receptors (Feng et al. 2004), and therefore NVP-AAM077 acting at NR2D-containing di- or triheteromeric receptors (see below) could contribute to the inhibitory effect in SNc dopaminergic neurones.
NR2D-containing synaptic NMDA receptors
Current commercial pharmacological antagonists have limited selectivity for NR2C or NR2D compared with the other subunits; however, when measured in electrophysiological assays against agonist binding to subunits expressed in Xenopus oocytes (with both agonist and antagonist binding to NMDA receptors at equilibrium), UBP141 shows 7-fold selectivity for NR2C and/or NR2D-containing receptors over NR2B-containing NMDA receptors and 5-fold selectivity over NR2A-containing receptors (Feng et al. 2004; Morley et al. 2005). NMDA-EPSCs in P7 SNc dopaminergic neurones were inhibited by UBP141 with an IC50 close to KD values for NR2C- or NR2D-containing receptors. It should be noted that all of our experiments test antagonists against synaptic currents; thus, the binding of glutamate is not at equilibrium, which will cause an apparent increase in potency where the drug is a competitive antagonist. NR2D subunit mRNA (Monyer et al. 1994) and protein (Dunah et al. 1996) is expressed at high levels in the brain stem, while NR2C expression is mainly limited to the cerebellum; therefore, the presence of NR2D seems more likely given that we have previously found evidence that NR2D subunits form the low conductance NMDA receptors present at extrasynaptic sites in SNc dopaminergic neurones (Jones & Gibb, 2005). Reports of NR2D subunits at brain synapses are sparse (Hrabetova et al. 2000; Vicini & Rumbaugh, 2000; Cull-Candy & Leszkiewicz, 2004; Borgland et al. 2006).
The presence of NR2D subunits in synaptic NMDA receptors might be expected to confer a slow decay time constant (∼1.5–4 s at room temperature; Monyer et al. 1994; Wyllie et al. 1998; Cull-Candy & Leszkiewicz, 2004), whereas the decay time constants at all ages in SNc dopaminergic neurones were substantially faster. If the proportion of NR2D-containing receptors was less than 30% then their low open probability (0.04; Wyllie et al. 1998) and slow activation (τact≈ 45 ms; Wyllie et al. 1998) would mean that the NMDA-EPSC may be dominated by the NR2B-containing receptors. At room temperature, NR1/NR2B receptors have a weighted decay time constant of 287 ms; (Vicini et al. 1998); assuming a Q10 of 3.5, the weighted decay time constant for NR1/NR2B receptors at 30°C (the temperature for recordings in this study) would be 96 ms, similar to our measured values.
Interestingly, in most brain regions, levels of NR2D mRNA peak after the first postnatal week and then decrease (Monyer et al. 1994), but in SNc dopaminergic neurones, the effect of UBP141 did not change with development.
Functional synaptic NMDA receptors in SNc dopaminergic neurones may be triheteromeric receptors composed of NR1, NR2B and NR2D subunits
One possible interpretation of our data is that NR2B and NR2D subunits form triheteromeric receptors, and that the proportions of triheteromers change during postnatal development. The interaction of ifenprodil-type ligands with the NMDA receptor is affected by the presence of other NR2 subunits in the complex, including NR2A (Chazot & Stephenson, 1997; Tovar & Westbrook, 1999; Chazot et al. 2002; Hatton & Paoletti, 2005) and NR2D (Brickley et al. 2003), and triheteromeric receptors have been shown to exhibit a lower maximum inhibition with ifenprodil than their diheteromeric counterparts. If all of the receptors (at any age) were triheteromeric, we would expect a maximal inhibition by ifenprodil of around 50% (Tovar & Westbrook, 1999; Hatton & Paoletti, 2005; although a triheteromeric NR1/NR2B/NR2D receptor has not been directly tested in this way). However, ifenprodil causes ∼74% inhibition at P7, making it likely that a combination of diheteromers and triheteromers contribute to the NMDA-EPSC at this age since if all receptors were diheteromeric NR2B receptors, we would expect a maximum of ∼90% inhibition by ifenprodil (Williams, 1993). At P14–P21, our maximum inhibition with ifenprodil was 50–55%, which is more consistent with a triheteromeric receptor (Tovar & Westbrook, 1999; Hatton & Paoletti, 2005). The decrease in the maximum effect of ifenprodil could also be explained by a decrease in triheteromers, if triheteromers are replaced by non-NR2B containing diheteromers. However, there is no apparent increase in NR2A-containing or NR2D-containing diheteromers, and so we feel that this is unlikely. Table 1 presents the results of a global least-squares fit of our proposed scheme to our data. The results suggest that there are few, if any, diheteromeric NR1/NR2D receptors at these synapses even though the proportion of these has been allowed to vary freely during fitting. In contrast, the proportion of diheteromeric NR1/NR2B receptors is predicted to decrease with development, while the proportion of triheretomeric NR1/NR2B/NR2D receptors increases from around 50% at P7 to 90% by P21.
Table 1.
Fit of observed block of synaptic currents at P7, P14 and P21 to a model assuming synaptic receptors are a mixture of diheteromeric NR1/NR2B receptors, triheteromeric NR1/NR2B/NR2D receptors, and diheteromeric NR1/NR2D receptors
| P7 | P14 | P21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predicted % of current | % EPSC block by ifenprodil | % block by UBP141 | Predicted % of current | % EPSC block by ifenprodil | % block by UBP141 | Predicted % of current | % EPSC block by ifenprodil | % block by UBP141 | |
| NR2B diheteromer | 46.6 | 42.0 | 6.3 | 21.4 | 19.2 | 2.9 | 5.2 | 4.7 | 0.7 |
| NR2B/NR2D triheteromer | 53.4 | 26.7 | 18.6 | 78.6 | 39.3 | 27.4 | 92.6 | 46.3 | 32.3 |
| NR2D diheteromer | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 | 0.0 | 1.1 |
| Predicted block | — | 68.7 | 24.9 | — | 58.6 | 30.3 | — | 51.0 | 34.1 |
| Observed block | — | 73.5 | 33.7 | — | 55.1 | 24.6 | — | 51.4 | 23.4 |
The model assumes that diheteromeric NR2B receptors are inhibited by 90% (Williams, 1993), triheteromeric NR2B/NR2D receptors are inhibited by 50% (Hatton & Paoletti, 2005), while diheteromeric NR2D receptors will be inhibited by 2% by 10 μm ifenprodil (Williams, 1995). The model assumes that 3 μm UBP141 will block diheteromeric NR2B receptors by 13.5% and diheteromeric NR2D receptors by 51.7% (Kd values of 19.3 and 2.8 μm respectively; Morley et al. 2005) and triheteromeric NR2B/NR2D receptors by 34.9%. The model was fitted to the data by minimizing the sum of squares of the differences between predicted and observed block.
The shapes of the current–voltage relationships for control and ifenprodil-insensitive NMDA-EPSCs are similar at P14, suggesting that the ifenprodil-insensitive NMDA-EPSC is not mediated by diheteromeric NR1/NR2D receptors with lower Mg2+ sensitivity. It should be noted that the reversal potential for NMDA-EPSCs was typically around +10 mV and therefore there is an uneven driving force on the inward currents at −40 mV compared with the outward currents at +40 mV such that the true Mg2+ sensitivity is underestimated in this study. In addition, the ifenprodil-insensitive component at P14 and P21 does not have a significantly accelerated or slowed decay time constant, suggesting that NMDA receptors in control and ifenprodil have similar kinetics and are composed of similar subunits.
There is no unequivocal test for triheteromeric receptors in native neurones, and so we cannot state with absolute certainty that triheteromers are present and that their expression is developmentally regulated. However, it is known that native triheteromeric NMDA receptors can arise from NR1, NR2B and NR2D subunits in rat brain (Buller et al. 1994; Buller & Monaghan, 1997; Dunah et al. 1998; Brickley et al. 2003; Cull-Candy & Leszkiewicz, 2004), and it has been proposed that the rat midbrain does not contain NR1/NR2D diheteromeric NMDA receptors but instead has NR2D subunits present as triheteromeric receptors (Dunah et al. 1998); thus, we propose that NR1/NR2B/NR2D-containing triheteromeric NMDA receptors may be present at all ages tested.
In summary, we have shown that NMDA receptors at excitatory synapses on SNc dopaminergic neurones contain NR2B and NR2D subunits, and exhibit properties that may reflect a triheteromeric molecular composition, a relatively rare synaptic NMDA receptor. The observed developmental changes in synaptic NMDA receptors may have important consequences for the function and plasticity of the dopaminergic circuitry.
Acknowledgments
We thank Dr Y. Auberson (Novartis Institutes for BioMedical Research, Basel, Switzerland) for the gift of NVP-AAM077 and Mr M. Smith for technical assistance. This work was funded by the BBSRC, UK (BB/D015286/1), the National Institutes of Health, USA (MH060252-05A2), and a Medical Research Council UK studentship to S.L.C.B.
References
- Albers DS, Weiss SW, Ladarola MJ, Standaert DG. Immunohistochemical localization of N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor subunits in the substantia nigra pars compacta of the rat. Neuroscience. 1999;89:209–220. doi: 10.1016/s0306-4522(98)00328-5. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet PJ, Konitsiotis S, Whittemore ER, Zhou ZL, Woodward RM, Chase TN. Differing effects of N-methyl-D-aspartate receptor subtype selective antagonists on dyskinesias in levodopa-treated 1-methyl-4-phenyl-tetrahydropyridine monkeys. J Pharmacol Exp Ther. 1999;290:1034–1040. [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AL, Monaghan DT. Pharmacological heterogeneity of NMDA receptors: characterization of NR1a/NR2D heteromers expressed in Xenopus oocytes. Eur J Pharmacol. 1997;320:87–94. doi: 10.1016/s0014-2999(96)00880-1. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Lawrence S, Thompson CL. Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B-selective NMDA receptor antagonists. Neuropharmacology. 2002;42:319–324. doi: 10.1016/s0028-3908(01)00191-5. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Stephenson FA. Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem. 1997;69:2138–2144. doi: 10.1046/j.1471-4159.1997.69052138.x. [DOI] [PubMed] [Google Scholar]
- Cheffings CM, Colquhoun D. Single channel analysis of a novel NMDA channel from Xenopus oocytes expressing recombinant NR1a, NR2A and NR2D subunits. J Physiol. 2000;526:481–491. [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004 doi: 10.1126/stke.2552004re16. re16. [DOI] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB. Subunit composition of N-methyl-D-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol. 1998;53:429–437. doi: 10.1124/mol.53.3.429. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang YH, Luo J, Davila-Garcia M, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J Neurochem. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure–activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Wu YN. Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Res. 2004;1019:293–296. doi: 10.1016/j.brainres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Jones S, Gibb AJ. Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J Physiol. 2005;569:209–221. doi: 10.1113/jphysiol.2005.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004a;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004b;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G, Costa E, Armstrong DM, Vicini S. Glutamate receptor subtypes mediate excitatory synaptic currents of dopamine neurons in midbrain slices. J Neurosci. 1991;11:1359–1366. doi: 10.1523/JNEUROSCI.11-05-01359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE. Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci. 1998;1:659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- Nase G, Weishaupt J, Stern P, Singer W, Monyer H. Genetic and epigenetic regulation of NMDA receptor expression in the rat visual cortex. Eur J Neurosci. 1999;11:4320–4326. doi: 10.1046/j.1460-9568.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, Maneuf Y, Hille C, Brotchie JM, Crossman AR. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol. 2000;165:136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- Nash JE, Hill MP, Brotchie JM. Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Exp Neurol. 1999;155:42–48. doi: 10.1006/exnr.1998.6963. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Single spine Ca2+ signals evoked by coincident EPSPs and backpropagating action potentials in spiny stellate cells of layer 4 in the juvenile rat somatosensory barrel cortex. J Neurosci. 2004;24:1689–1699. doi: 10.1523/JNEUROSCI.3332-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Collin C, Auerbach JM, Meiri N, Bengzon J, Kennedy MB, Segal M, McKay RDG. Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J Neurosci. 1998;18:4177–4188. doi: 10.1523/JNEUROSCI.18-11-04177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1–NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006;63:187–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian A, Johnson JW. Permeant ion effects on external Mg2+ block of NR1/2D NMDA receptors. J Neurosci. 2006;26:10899–10910. doi: 10.1523/JNEUROSCI.3453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-D-aspartate receptors. Exp Neurol. 2000;163:239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Rumbaugh G. A slow NMDA channel: in search of a role. J Physiol. 2000;525:283. doi: 10.1111/j.0021-3751.2000.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N-methyl-D-aspartate (NMDA) receptors containing the epsilon 4 (NR2D) subunit. Neurosci Lett. 1995;184:181–184. doi: 10.1016/0304-3940(94)11201-s. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2:285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Wu YN, Johnson SW. Pharmacological characterization of inward current evoked by N-methyl-D-aspartate in dopamine neurons in the rat brain slice. J Pharmacol Exp Ther. 1996;279:457–463. [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc Biol Sci. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26:2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]