Abstract
The exercise pressor reflex (EPR) is an important neural mechanism that controls blood pressure and heart rate during static muscle contraction. It has been previously demonstrated that the EPR is exaggerated in cardiomyopathy. Both mechanically (group III) and metabolically (group IV) sensitive afferent neurons are important to this reflex in normal humans and animals. In cardiomyopathy, however, the metabolically sensitive afferents are less responsive to activation whereas the mechanically sensitive fibres are overactive. We have demonstrated that this overactivity is responsible for the exaggeration in the EPR. Of importance, we have also demonstrated that the reduced responsiveness in the group IV afferent neuron is an initiating factor in the development of the exaggerated EPR. To date, the mechanism mediating this reduced group IV responsiveness remains unclear. Given that group IV afferent neurons are activated via chemically sensitive receptors, it is logical to suggest that changes in receptor function are responsible for the blunted behaviour of group IV neurons in cardiomyopathy. In order to test this postulate, however, potential receptor candidates must first be identified. The transient receptor potential vanilloid 1 (TRPv1) receptor is a non-selective cation channel that serves as a marker of the group IV afferent neurons in the periphery. We have demonstrated that the TRPv1 is abnormal in cardiomyopathy. It has been shown that the TRPv1 receptor is colocalized with the cannabinoid 1 (CB1) receptor on group IV afferent neurons. Therefore, we hypothesized that the function of CB1 receptors is abnormal in cardiomyopathy. We explored this possibility by using anandamide (AEA), an endogenously produced cannabinoid that has been shown to control blood pressure via activation of the CB1 receptor. In these studies, we evaluated the cardiovascular responses to intra-arterial injection of AEA into the hindlimb of normal, cardiomyopathic and neonatally capsaicin-treated (NNCAP) rats (rats that lack group IV afferent neurons) to determine whether administration of AEA results in abnormal responses of group IV afferent neurons in cardiomyopathic rats. We determined that AEA controls changes in blood pressure, predominately via activation of the CB1 receptor in this preparation. We further observed that the blood pressure response to AEA is blunted in cardiomyopathic rats when compared to normal rats. We also observed a reduced blood pressure response to AEA in NNCAP animals, indicating that AEA is acting on group IV afferent neurons in this preparation. To determine whether programmed cell death could account for the decreased responsiveness that we observed during activation of the CB1 and TRPv1 receptors on group IV afferent neurons in heart failure, we performed terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) assay. We observed no evidence of cell death within the dorsal root ganglia in rats with cardiomyopathy. The data suggest that the responsiveness of CB1 receptors on group IV afferent neurons is blunted in cardiomyopathy. Importantly, these data indicate that group IV primary afferent neurons express multiple receptor defects in cardiomyopathy that may contribute to the decreased CB1 receptor sensitivity in this disease.
During exercise, the sympathetic nervous system is stimulated by the activation of a muscle-based reflex. This reflex has been termed the exercise pressor reflex (EPR) (Mitchell et al. 1983). The EPR is a mechanism whereby signals from contracting muscle activate two groups of primary afferent neurons that innervate the muscle: (1) group III fibres which are thinly myelinated and, predominately, sensitive to mechanical forms of stimulation (mechanoreflex); and (2) group IV fibres which are unmyelinated and are activated by metabolic and chemical forms of stimulation (metaboreflex) (Kaufman et al. 1983; Mense & Stahnke, 1983; Kaufman & Rybicki, 1987). These skeletal muscle afferent fibres provide feedback to the brainstem regarding the mechanical and metabolic conditions of skeletal muscle. This feedback results in reflexive increases in blood pressure and heart rate (Coote et al. 1971; McCloskey & Mitchell, 1972; Mitchell et al. 1983).
The EPR has been previously shown, in both humans and rats, to be exaggerated in cardiomyopathy (Piepoli et al. 1999; Middlekauff et al. 2000; Negrao et al. 2001; Smith et al. 2003; Sinoway & Li, 2005). It has been determined that the cardiovascular responses to stimulation of metabolically sensitive group IV afferent fibres (via activation of transient receptor potential vanilloid 1 (TRPv1) receptors localized on group IV afferent fibres) are reduced in cardiomyopathic animals (Li et al. 2004b; Smith et al. 2005b). Whereas the metaboreflex is blunted in cardiomyopathy, it is the mechanically sensitive, group III afferent neurons that mediate the exaggeration of the EPR in disease (Middlekauff et al. 2001, 2004; Smith et al. 2003, 2005a). Further, we have observed that selective ablation of the group IV afferent neurons (via neonatal capsaicin treatment) results in an exaggerated EPR, which is mediated by group III afferent neurons, similar to that which we observed in cardiomyopathic animals (Smith et al. 2005a,b). Based on these data, we believe that there is an abnormality in the group IV afferent neurons which initiates a cascade of events and results in overcompensation by the group III afferents and therefore an exaggeration in the pressor response.
In these studies, we wanted to evaluate whether the abnormalities that we observed in group IV afferent neurons were limited to the TRPv1 receptor, as previously determined (Smith et al. 2005b), or whether other receptors were altered in cardiomyopathy. Administration of anandamide (AEA), an endogenously produced cannabinoid, has been shown to control blood pressure via activation of the cannabinoid 1 (CB1) receptor (Lake et al. 1997a,b; Malinowska et al. 2001) and in some preparations, by activation of the TRPv1 receptor as well (Malinowska et al. 2001; Hogestatt & Zygmunt, 2002; Ralevic et al. 2002; Randall et al. 2002; Pacher et al. 2004, 2005). The CB1 receptor is colocalized with the TRPv1 receptor on group IV afferent neurons (Ahluwalia et al. 2000, 2002, 2003; Bridges et al. 2003; Sagar et al. 2004). Therefore, we administered AEA intra-arterially in normal and cardiomyopathic animals to determine the responses to this agonist in cardiomypathy. The ultimate goal of this study was to determine whether receptors other than TRPv1, that also reside on group IV afferent neurons, are affected by cardiomyopathy. As alterations in group IV neuronal activity may initiate the development of EPR dysfunction in cardiomyopathy, determining the receptor mechanisms responsible for this abnormal behaviour are important for understanding the pathology of this disorder.
Methods
Experiments were performed in age-matched male Sprague–Dawley rats (Harlan) divided into the following distinct experimental groups: normal, neonatal capsaicin treated (NNCAP) and dilated cardiomyopathy (DCM). Animals were housed in standard rodent cages with 12 h light–dark cycles and were given food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. In addition, all studies were conducted in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Model of dilated cardiomyopathy
A subset of animals within the weight range of 150–175 g underwent thoracic surgery. Anaesthesia was induced with isoflurane gas (2–5% in 100% oxygen), and subsequently animals were intubated and ventilated. A thoracotomy was performed exposing the heart, and the left anterior descending (LAD) coronary artery was ligated to produce a dilated cardiomyopathy (DCM group) as previously described (Smith et al. 2003). The analgesic buprenorphine was given (20 μg (kg body weight)−1i.m.) every 6 h as needed, up to 48 h after surgery. Animals were observed for signs of distress to determine whether extended medication (Tylenol/Codeine; 0.24 mg ml−1/0.024 mg ml−1 in drinking water) was needed. None of the animals in this study required extended medication. Under isoflurane anaesthesia, transthoracic echocardiography (Vivid 7 Pro, GE Medical Systems) was performed 9 weeks post surgery and physiological experiments were performed a minimum of 11 weeks after ligation as previously described (Smith et al. 2003). Excluding thoracotomy, all procedures were also performed in age-matched normal rats. In this study, we use the term ‘cardiomyopathy’ to indicate a significantly decreased left ventricular dysfunction.
Neonatal capsaicin treatment
To determine the effect of destruction of group IV primary afferent neurons on the cardiovascular response to AEA, we administered capsaicin to neonatal rats. Neonatal capsaicin treatment is a well-established model for the selective and permanent destruction of group IV primary afferent neurons (Jancso et al. 1977) which does not significantly affect larger diameter primary afferent neurons (Scadding, 1980; Nagy et al. 1983; Nagy & Hunt, 1983; Matsumoto et al. 2006). In this experiment, 2-day-old neonatal rat pups (NNCAP group) received a 50 mg kg−1 subcutaneous injection of capsaicin. Upon initial injection, acute respiratory distress can occur. Each pup received manual stimulation of its chest wall by brisk stroking. Using this technique, the pups survived the brief (30–45 s) period of respiratory distress. Six weeks after injection, male animals were challenged by application of a 0.01% capsaicin solution to the cornea. Rats displaying 10 or fewer protective eye wipings were considered capsaicin-insensitive and were included in this study. A lack of bowel and bladder control can occur following neonatal capsaicin treatment. We monitored, on a regular basis, capsaicin-treated animals for faecal and urine retention though manual expression of bowel and bladder was not required for animals included in this study. Capsaicin-treated animals that displayed more than 10 eye wipings (n = 3) were eliminated from the study and were killed with an overdose of sodium pentobarbital (120 mg kg−1). Echocardiography and physiological testing were performed in adult NNCAP rats at time points and ages matched to normal and DCM animals.
Acute physiological preparations
General
Physiological experimentation was performed in adult normal (body weight, 418 ± 5 g), cardiomyopathic (body weight, 422 ± 7 g), and NNCAP (body weight, 408 ± 16 g) animals. Rats were initially anaesthetized with isoflurane gas and instrumented as previously described (Smith et al. 2001). Briefly, animals were intubated for mechanical ventilation and cannulated with jugular venous and carotid arterial catheters. Blood pressure (BP) was recorded by connecting the arterial catheter to a pressure transducer (Model DTX plus-DT-NNIZ, Ohmeda). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 1–4 s. Heart rate (HR) was derived from the BP pulse wave with use of a biotachometer (Gould Instruments). Subsequently, animals were rendered insentient by precollicular decerebration as previously described (Smith et al. 2001). Animals were allowed to recover from the decerebration procedure for a minimum of 1 h prior to beginning experimental protocols. Following all physiological experiments, animals were immediately killed with an intracardiac injection of 4 m saturated KCl.
Intra-arterial hindlimb AEA injections
In normal (n = 12), DCM (n = 7) and NNCAP (n = 6) animals, a catheter was placed in the left common iliac artery and its tip advanced to the bifurcation of the abdominal aorta. Injections were directly into the arterial supply of the right hindlimb via the right common iliac artery. To limit drug delivery to the hindlimb being tested, a reversible ligature was placed around the common iliac vein emptying the right hindlimb. Upon injection of drug, the ligature was pulled for 2 min to trap the injectate in the leg. Each animal was injected, first, with saline and/or the vehicle for AEA followed by five increasing doses of AEA (0.01–1.00 mg (100 μl)−1, Tocris Cookson Inc.) with an interval of 15 min between each injection. In preliminary studies, we randomized the drug doses of AEA and found no diminution or exaggeration of drug effect based on order of drug administration (data not shown). Peak MAP responses to these injections were recorded. To prevent muscle contractions or twitches as a result of injection of AEA, brief neuromuscular blockade (2 min; 1 mg ml−1 vecuronium bromide, Oregan) was induced prior to delivery of each injectate. To rule out the possibility that AEA was acting upon cutaneous afferent neurons, we performed a separate set of experiments in which we infused increasing doses of AEA (identical to those described above) in normal rats with skinned hindlimbs (n = 6). We observed no significant difference when we injected AEA in intact rats versus rats with skinned hindlimbs at any dose (data not shown). Therefore, these data indicate that cutaneous afferent neurons innervating the hindlimb do not significantly contribute to the responses to AEA in this preparation.
Site of action of AEA
Using the methods described, subsets of normal rats received intra-arterial AEA together with selective antagonists to either the TRPv1 (n = 13), CB1 (n = 12) or CB2 receptors (n = 4) (the CB2 receptor is also activated by AEA but primarily localized in peripheral immune tissues and glia (Devane et al. 1992; Munro et al. 1993; Pertwee, 1999) or AEA transporter inhibitors (n = 10). Normal rats received intra-arterial injections of AEA within the hindlimb in combination with either capsazepine (CPZ; a selective TRPv1 competitive antagonist, 100 μg (100 μl)−1, Tocris Cookson Inc.), AM281 (a selective CB1 antagonist, 100 (μg 100 μl)−1, Tocris Cookson Inc.) or AM630 (a selective CB2 antagonist, 10 (μg 100 μl)−1, Tocris Cookson Inc.), in order to determine the site of AEA action in this preparation. We also administered AEA in the presence of the AEA transport inhibitor VDM11 (10 (μg 100 μl)−1, Tocris Cookson Inc.) in order to determine whether AEA transport across the cell membrane affects the cardiovascular response to AEA.
TUNEL assay
To determine whether cell death occurs in the primary afferent neurons during heart failure, we used terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) assay to evaluate programmed cell death in the dorsal root ganglia (location of the cell bodies of the primary afferent neurons) in normal and DCM rats. Rats were deeply anaesthetized with sodium pentobarbital (120 mg kg−1) and were transcardially perfused with 4% paraformaldehyde. Cryosections were evaluated for TUNEL staining using the in situ apoptosis detection kit NeuroTACS II (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. All slides were counterstained with blue counterstain for 30 s. A nuclease-generated positive control was prepared using TACS-Nuclease (50 μl) in sections from normal rat. In the negative control, the dorsal root ganglia of normal rat were untreated.
Data analysis and statistics
Cardiovascular data were acquired and analysed using hardware and software for the CED micro 1401 system (Cambridge Electronic Design Ltd). Baseline values were determined using 30 s of data prior to a given manoeuvre. The peak response was defined as the greatest change from baseline elicited after the administration of the injectate. On all data sets, statistics were performed using ANOVA (with repeated measures for dose–response data) with Student–Newman–Keuls post hoc test as used appropriate.
Results
Left ventricular function
Fractional shortening is an index of left ventricular systolic function. As determined by echocardiography, animals with LAD coronary artery ligation had an average fractional shortening of 29.7 ± 1% which was significantly less than that measured for normal (48 ± 1%) or NNCAP animals (49.5 ± 1%; P < 0.01). All echocardiography was performed on animals anaesthetized with 1% isoflurane gas. We determined previously that the EPR is exaggerated in anaesthetized rats with a fractional shortening of less than 35% (Smith et al. 2003). Therefore, a fractional shortening of greater than 35% was used to exclude animals with coronary artery ligation from this study.
Cardiovascular effects of AEA in normal and cardiomyopathic rats
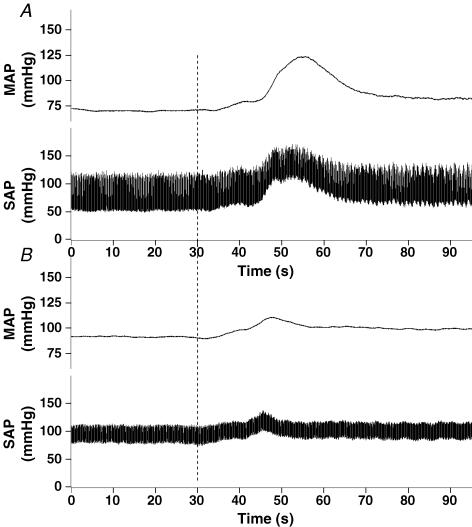
Figure 1 shows representative tracings of the effect of AEA (1 mg (100 μl)−1) on blood pressure in both normal (Fig. 1A) and cardiomyopathic (Fig. 1B) rats. Note that the response in the DCM rat is blunted when compared to the normal animal. We observed little or no effect of AEA on HR in either normal or DCM rats (data not shown).
Figure 1. Original tracing showing the mean arterial blood pressure response to injection of anandamide in both a normal (A) and DCM (B) rat.
The baseline blood pressure was recorded for 30 s prior to administration of anandamide (1.0 (mg 100 μl)−1). The point of anandamide administration is indicated by the vertical dashed line in the graph.
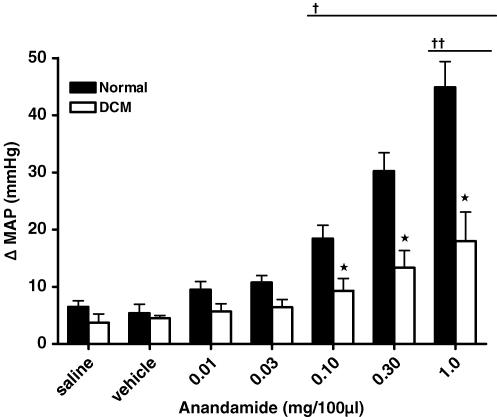
Figure 2 illustrates the cardiovascular responses to increasing doses of AEA in normal and DCM rats. Baseline MAP was significantly different between normal (134 ± 9 mmHg) and DCM (99 ± 9 mmHg; P < 0.05) groups, consistent with the decreased left ventricular function in the cardiomyopathic rats. We observed that intra-arterial administration of AEA into the hindlimb resulted in a dose-related increase in MAP as compared to saline or vehicle administration in normal rats. There were no significant effects on baseline MAP in response to injection of saline or vehicle. We observed a dose-related increase in MAP in DCM rats in response to AEA; however, this response was significantly blunted when compared to the response in normal rats (P < 0.05).
Figure 2. Effect of hindlimb intra-arterial injection of anandamide on MAP in normal and DCM rats.
*Significantly different from normal; †significantly different from saline and vehicle trials within normal group; ††significantly different from saline and vehicle trials within the DCM group (P < 0.05).
Effect of AEA is blunted in NNCAP animals
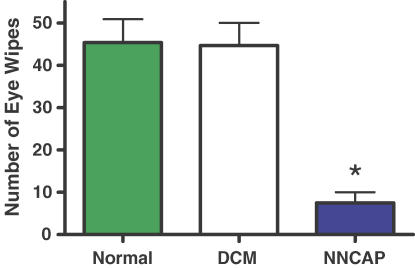
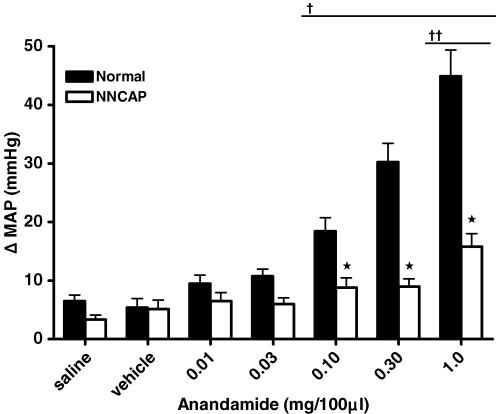
The destruction of group IV primary afferent neurons was achieved by the administration of capsaicin to neonatal rats. We have previously demonstrated that NNCAP animals display an exaggerated EPR similar to that which we observe in heart failure (Smith et al. 2005a,b). In this study, we confirmed destruction of group IV afferent neurons by delivering dilute capsaicin drops to the surface of the cornea in NNCAP-treated, DCM and normal rats. NNCAP rats were significantly less responsive to this treatment when compared to DCM and normal rats (Fig. 3). This decreased responsiveness is consistent with an absence of the TRPv1 protein and significantly reduced MAP responses to capsaicin (Smith et al. 2005b). The effect of destruction of group IV capsaicin-sensitive afferent neurons on MAP responses to intra-arterial injection of AEA into the hindlimb is shown in Fig. 4. Baseline MAP was not different between normal (134 ± 9 mmHg) and NNCAP (129 ± 16 mmHg) animals. There was a dose-related increase in MAP in response to AEA in normal rats; however, we did not observe a dose-related increase in MAP when AEA was administered to the NNCAP rats. Therefore, destruction of group IV capsaicin-sensitive neurons attenuated the MAP responses to AEA administration compared with those elicited in normal animals (P < 0.05).
Figure 3. Behavioural assessment of animals receiving neonatal capsaicin treatment.
NNCAP rats showed a significant decrease in the number of eye wipes in response to the intraocular administration of dilute capsaicin drops, verifying successful abolition of capsaicin-sensitive fibres. *Significantly different from normal and DCM (P < 0.001).
Figure 4. Effect of hindlimb intra-arterial injection of anandamide on MAP in normal and capsaicin treated rats.
*Significantly different from normal; †significantly different from saline and vehicle trials within normal group; ††significantly different from saline and vehicle trials within the NNCAP group (P < 0.05).
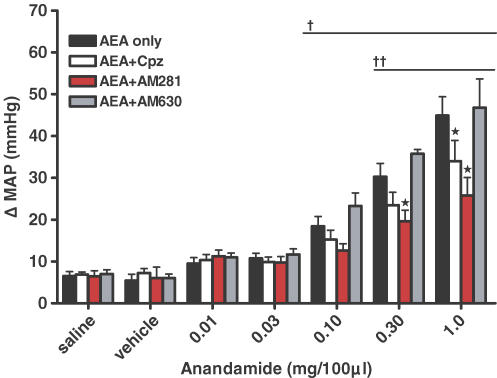
AEA acts predominately at the CB1 receptor to increase MAP
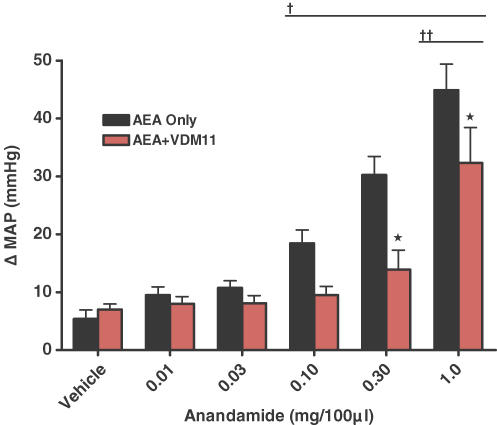
Next, we sought to determine the receptors at which AEA was acting to affect blood pressure in normal rats. AEA works predominately at the CB1 receptor, but can also act as an agonist at the TRPv1 and the CB2 receptors. Therefore, we injected AEA in the absence or presence of either: (1) CPZ, a selective TRPv1 antagonist; (2) AM281, a selective CB1 antagonist; (3) AM630, a selective CB2 antagonist; or (4) VDM11, an inhibitor of AEA membrane transport. We observed that the AEA-induced pressor response was significantly reduced in the presence of the selective CB1 antagonist AM281 (Fig. 5; P < 0.05). We further observed that although there was a reduction of MAP in the presence of CPZ (at a dose that significantly attenuates the MAP responses to capsaicin (Smith et al. 2005b)), it only occurred at the highest dose of AEA (P < 0.001). By contrast, we observed that AM630 did not alter the effects of AEA on MAP. In the presence of the AEA transport inhibitor VDM11, the AEA-induced MAP response was significantly reduced compared to control responses (Fig. 6; P < 0.01).
Figure 5. Effect of intra-arterial injection of anandamide.
Anandamide was administered in normal animals alone or in the presence of: capsazepine (CPZ; a selective TRPv1 receptor antagonist, 100 (μg 100 μl)−1); AM281 (a selective CB1 antagonist, 100 mg (100 μl)−1); or AM630 (a selective CB2 antagonist, 10 μm). *Significantly different from AEA alone group; †significantly different from saline and vehicle trials within the AEA alone, CPZ and AM630 groups; ††significantly different from saline and vehicle trials within the AM281 group (P < 0.05).
Figure 6. Effect of intra-arterial injection of anandamide in normal animals alone or in the presence of the selective anandamide transport inhibitor, VDM11 (10 μm).
*Significantly different from AEA alone; †significantly different from vehicle trials within the AEA alone group; ††significantly different from vehicle trials within the VDM11 group (P < 0.05).
Evaluation of apoptosis
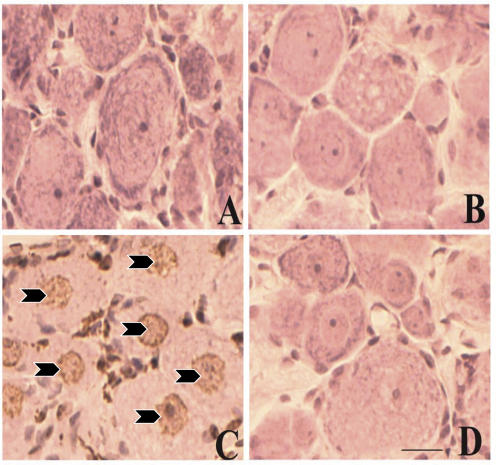
As we have established that the responsiveness of group IV afferent neurons is reduced in cardiomyopathy, we wanted to determine whether the occurrence of cell death could explain this phenomenon. To determine whether cell death is occurring in the primary afferent neurons, we used TUNEL assay to evaluate programmed cell death in the dorsal root ganglia in normal and DCM rats. We observed no TUNEL-positive neurons in sections from either normal (Fig. 7A) or DCM (Fig. 7B) rats. Positive (arrowheads in Fig. 7C) and negative controls (Fig. 7D) were performed in dorsal root ganglia from normal rats to ensure the quality of the assay.
Figure 7. TUNEL assay in dorsal root ganglia.
A, normal animals. B, DCM animals. C, positive control in normal animal. Arrowheads indicate positive staining. D, negative control in normal animal. Scale, 25 μm.
Discussion
AEA evokes a pressor response when administered into the arterial supply of the hindlimb in decerebrate rats
A key finding of the present study was that the local, hindlimb intra-arterial injection of AEA evokes a dose-related increase in MAP when administered in the decerebrate rat preparation. These results are consistent with the results of Li et al. (2004a) though, generally, the reported cardiovascular actions of endogenous cannabinoids, such as AEA, are varied. Studies on whole animals or isolated tissues have shown that AEA induces a variety of effects in the cardiovascular system, including vasodilatation, bradycardia and hypotension (Hogestatt & Zygmunt, 2002). The mechanisms that mediate these diverse effects are not clearly understood but seem to depend on the route of administration and the state of consciousness of the animal (Hogestatt & Zygmunt, 2002).
In anaesthetized rats, it has been reported that intravenous AEA causes a triphasic response consisting of an initial bradycardia and depressor action (phase I) followed by a transient pressor effect (phase II) and a longer-lasting hypotensive response (phase III) (Varga et al. 1996; Lake et al. 1997b). Activity at the TRPv1 and the CB1 receptors, both of which are localized on the afferent neuron (Ahluwalia et al. 2000, 2002, 2003; Bridges et al. 2003; Sagar et al. 2004), suggest that AEA may be activating small diameter afferent neurons to affect BP and HR. Further evidence for the potential participation of sensory nerves in mediating the cardiovascular responses to AEA comes from studies in anaesthetized rats which showed that intra-arterial injection of AEA led to hypotension and increased ventilation (Smith & McQueen, 2001). By contrast, work performed in conscious rats demonstrated that intravenous administration of AEA caused a profound bradycardia with a short-lived depressor effect but this was followed by a longer-lasting pressor effect as well as vasoconstriction (Stein et al. 1996; Lake et al. 1997a,b; Gardiner et al. 2002). In our studies in the decerebrate rat model (in the absence of anaesthesia), we have demonstrated that intra-arterial administration of AEA results in a dose-related increase in MAP similar to that observed in a conscious rat preparation. Similar pressor responses to AEA have previously been demonstrated (Li et al. 2004a). We have also previously demonstrated that this decerebrate rat model is reliable for the study of the EPR as the changes in BP and HR, in response to exercise, mimic those observed in conscious animals (Smith et al. 2001).
The response to AEA is blunted in cardiomyopathic and NNCAP rats
To determine whether AEA was acting upon receptors located on small diameter primary afferent neurons, we performed selective ablation of capsaicin-sensitive (predominately group IV) afferent neurons in normal rats. It is well documented that the capsaicin receptor, TRPv1, is a marker for group IV afferent neurons in the periphery (Guo et al. 1999; Michael & Priestley, 1999). Therefore, stimulation of this receptor provides a selective means to activate the group IV afferent neurons. We believe that stimulation of the TRPv1 receptor provides an excellent means by which to artificially stimulate the metaboreflex because TRPv1 receptors are expressed on a significant subpopulation of the group IV afferent neurons. Previously we observed a significant reduction in the MAP responses to hindlimb intra-arterial capsaicin administration in NNCAP rats (Smith et al. 2005b). It is interesting that the responses to capsaicin administration to the hindlimb were also significantly blunted in the cardiomyopathic rats (Li et al. 2004b; Smith et al. 2005b). In the present study using the decerebrate rat preparation, the intra-arterial injection of AEA resulted in a dose-dependent pressor response in the cardiomyopathic rats but these responses were significantly blunted when compared to those in the normal rats. Additionally, the response to AEA was significantly attenuated in NNCAP rats, similar to previously reported responses to resiniferatoxin (Li et al. 2004a). These data suggest that AEA is acting on the capsaicin-sensitive, group IV afferent neuron to cause a change in BP. From these data we infer that capsaicin-sensitive, group IV afferent neurons display blunted responsiveness to other substances (e.g. AEA) in addition to capsaicin during heart failure.
AEA evokes increases in MAP via activation of the CB1 and TRPv1 receptors
Endogenous cannabinoids have been implicated in the control of a large number of physiological processes, and most of their effects appear to by mediated by CB1 or CB2 receptors (Zygmunt et al. 1999; Szallasi & Di Marzo, 2000; Malinowska et al. 2001). There is, however, sufficient evidence to suggest the involvement of the TRPv1 receptors in mediating some of the cardiovascular effects of endogenous cannabinoids (Zygmunt et al. 1999; Szallasi & Di Marzo, 2000; Malinowska et al. 2001; Li et al. 2004a). In the present study, we addressed the question of whether the endocannabinoid, AEA, acts at the TRPv1, CB1 or CB2 receptor to elicit its cardiovascular responses. In our study, we observed significant effects of both CPZ and AM281 on the AEA-induced increases in MAP whereas the CB2 antagonist was ineffective. We observed, however, that CPZ (at doses that are effective in blocking the actions of capsaicin (Smith et al. 2005b)) was only effective at the highest dose of AEA used in this study. Although the interpretation of this observation requires further investigation, it is a possibility that the TRPv1 receptor may not be activated until the CB1 receptor is fully occupied. Therefore, in the decerebrate rat preparation, AEA elicits its cardiovascular response via activation of the CB1 receptor and, possibly, the TRPv1 receptor but not at the CB2 receptor.
The AEA membrane transporter also mediates the AEA-induced pressor response
AEA activates the G-coupled receptors, CB1, CB2 and TRPv1, in a variety of systems (Munro et al. 1993; Pertwee, 1997, 2005; Howlett, 1998). AEA signalling is limited by cellular uptake through an AEA membrane transporter (AMT) which is responsible for the uptake of extracellular AEA by facilitated diffusion (Di Marzo et al. 1994; Beltramo et al. 1997; Hillard et al. 1997). In the present study, the administration of the selective AEA transport inhibitor VDM11 resulted in a reduced blood pressure response to intra-arterial injections of AEA. These results suggest that primary afferent neurons express the AMT and are consistent with the findings of Price et al. (2005) who observed that AEA transport inhibitors reduced capsaicin-stimulated calcitonin gene related peptide (CGRP) release from trigeminal neurons in vitro. These data further indicate that this transporter is important to the blood pressure response to AEA in this preparation.
Apoptosis is not occurring in the dorsal root ganglia in cardiomyopathic rats
Results from this study indicate that the response to AEA is blunted in heart failure. Therefore, these data indicate that the abnormality in the group IV afferent neuron is not limited to the TRPv1 receptor as AEA acts predominately at the CB1 receptor in this preparation. We hypothesized that the blunted cardiovascular responses to both capsaicin (Smith et al. 2005b) and AEA may suggest that programmed cell death is occurring and that the group IV afferent neurons are dying in cardiomyopathy. We performed TUNEL assay in order to evaluate the occurrence of apoptosis in sensory neurons of cardiomyopathic rats. The results from this study showed no evidence of programmed cell death or of necrotic cell death in the DRG of cardiomyopathic rats. These studies indicate that cell death is not occurring in the primary afferent neurons in cardiomyopathy. Taken collectively, our data indicate that the group IV, capsaicin-sensitive, afferent neuron undergoes changes involving a number of receptors in cardiomyopathic rats.
Multiple defects occur on primary afferent neurons in cardiomyopathic rats
CB1 receptors are present on group IV primary afferent fibres (Ross et al. 2001a,b) and are co-expressed with TRPv1 receptors (Ahluwalia et al. 2000, 2002, 2003; Bridges et al. 2003; Sagar et al. 2004). Previously, we have observed that TRPv1 mRNA levels were reduced in the dorsal root ganglia and soleus muscle and that MAP and HR responses to activation of group IV fibres via capsaicin were significantly blunted in cardiomyopathic animals (Smith et al. 2005b). The data in the present study indicate that AEA binds to and activates the CB1 receptor (at doses that do not activate the TRPv1 receptor) to cause an increase in MAP in this preparation. Though the responsiveness to AEA is blunted in cardiomyopathy, it is unlikely that the reduction in the AEA-induced pressor response is due to a defect in the TRPv1 receptor as we show that this receptor contributes minimally to the BP response to AEA. Instead, our data suggest that defects occur both at the TRPv1 (Smith et al. 2005b) and at the CB1 receptor as evidenced by the decreased cardiovascular responses to AEA on group IV afferent neurons in heart failure that we have observed in this study.
Limitations of the study
We recognize that neonatal capsaicin treatment has effects on both group III and group IV afferent neurons. The effect on group III afferent neurons, however, is minimal. For example, 50 mg kg−1 capsaicin (the dose used in our study) administered neonatally to rats results in a significant decrease in small-diameter, unmyelinated afferent fibres (1–2.5 μm) whereas fibres that are 2.5–10 μm are not significantly affected by this dose as determined by electron microscopy (Nagy et al. 1983). The data indicate that 90% of the unmyelinated fibres were destroyed whereas only a very small proportion of the finest (smallest diameter) Aδ fibres were affected. In a separate study, no effect of 50 mg kg−1 capsaicin was observed on the number or the size distribution of myelinated fibres in the rat (Scadding, 1980). In another study (Nagy & Hunt, 1983), the termination of primary afferent neurons within the superficial dorsal horn was examined using anterograde tracing in normal rats and those treated neonatally with capsaicin (50 mg kg−1). It was determined that capsaicin treatment destroyed up to 90% of unmyelinated fibres in laminae I and IIinner whereas lamina IIouter was spared. Lamina IIouter is known to receive projections from both C (unmyelinated) and Aδ (thinly myelinated) fibres whereas laminae I and IIinner receive projections from C fibres only. Moreover, we have demonstrated that group III afferent neurons mediate the exaggeration in the EPR that we observe in NNCAP animals. We determined this by infusing gadolinium (a selective blocker of group III afferent neurons in this preparation (Hayes & Kaufman, 2001)) prior to performing contraction and stretch in NNCAP animals (Smith et al. 2005a). In addition, it has previously been demonstrated that group III afferent neurons are primarily responsive to passive stretch (Hayes et al. 2005). We have observed that neonatally capsaicinized animals are responsive to passive stretch (Smith et al. 2005b), supporting the concept that the group III afferents are intact in this preparation. All of these data, taken together, support the fact that group III afferents are largely spared in the presence of neonatal capsaicin treatment though we do wish to emphasize that we cannot rule out an effect of capsaicin on this population of neurons.
It is also possible that the blunted response to AEA in the cardiomyopathic rats is a reflection of decreased vascular responsiveness and not an abnormal reflex. Although we have not directly tested this possibility, we think it is unlikely because we have previously demonstrated that cardiomyopathic rats display exaggerated pressor responses to both static muscle contraction and passive stretch (Smith et al. 2003, 2005a,b). These data indicate that the ability to vasoconstrict in these animals is not impaired.
Finally, we wish to point out that we did not test the effect of endogenous cannabinoids in this system. Instead, we used AEA as a tool to activate another receptor that resides on group IV afferent neurons in which we identified significant differences in the responsiveness between normal and cardiomyopathic animals. Therefore, our observations are pharmacological in nature. Further studies will need to be conducted to determine whether these observations will translate into physiological differences between these groups of animals.
Conclusions
The present findings indicate that AEA elicits predominately CB1 receptor-mediated pressor responses in the decerebrate rat preparation. CB2 receptors do not appear to contribute to these effects, but the AEA membrane transporter is involved in these pressor responses. Further, these data suggest that the responsiveness of CB1 receptors on group IV afferent neurons is blunted in cardiomyopathy. As a result, the findings of this study indicate that cardiomyopathy results in multiple functional and molecular abnormalities of the group IV primary afferent neurons that affect the EPR.
Acknowledgments
This research was supported by a grant from the National Institutes of Health HL-070242 and the American Heart Association 0640007N to M.G.G. The authors thank Margaret Robledo and Martha Romero for their expert technical assistance. M.G.G is an Established Investigator of the American Heart Association.
References
- Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience. 2002;110:747–753. doi: 10.1016/s0306-4522(01)00601-7. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Yaqoob M, Urban L, Bevan S, Nagy I. Activation of capsaicin-sensitive primary sensory neurones induces anandamide production and release. J Neurochem. 2003;84:585–591. doi: 10.1046/j.1471-4159.2003.01550.x. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Bridges D, Rice ASC, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol. 2002;135:1889–1896. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol. 2001;280:H2153–H2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–6. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Hogestatt ED, Zygmunt PM. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot Essent Fatty Acids. 2002;66:343–351. doi: 10.1054/plef.2001.0346. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The CB1 cannabinoid receptor in the brain. Neurobiol Dis. 1998;5:405–416. doi: 10.1006/nbdi.1998.0215. [DOI] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–I65. [PubMed] [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol. 2004a;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004b;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Inoue M, Hald A, Yamaguchi A, Ueda H. Characterization of three different sensory fibers by use of neonatal capsaicin treatment, spinal antagonism and a novel electrical stimulation-induced paw flexion test. Mol Pain. 2006;2006:16–24. doi: 10.1186/1744-8069-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101:784–789. doi: 10.1161/01.cir.101.7.784. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol. 2001;90:1714–1719. doi: 10.1152/jappl.2001.90.5.1714. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Hunt SP. The termination of primary afferents within the rat dorsal horn: evidence for rearrangement following capsaicin treatment. J Comp Neurol. 1983;218:145–158. doi: 10.1002/cne.902180203. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Iversen LL, Goedert M, Chapman D, Hunt SP. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci. 1983;3:399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrao CE, Rondon MU, Tinucci T, Alves MJ, Roveda F, Braga AM, Reis SF, Nastari L, Barretto AC, Krieger EM, Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart Circ Physiol. 2001;280:H1286–H1292. doi: 10.1152/ajpheart.2001.280.3.H1286. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–1138. doi: 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handbook of Experimental Pharmacology. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the ‘muscle hypothesis’ of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- Price TJ, Patwardhan AM, Flores CM, Hargreaves KM. A role for the anandamide membrane transporter in TRPV1-mediated neurosecretion from trigeminal sensory neurons. Neuropharmacology. 2005;49:25–39. doi: 10.1016/j.neuropharm.2005.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Smart D. Cannabinoid modulation of sensory neurotransmission via cannabinoid and vanilloid receptors: roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001a;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001b;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Smith PA, Millns PJ, Smart D, Kendall DA, Chapman V. TRPV1 and CB1 receptor-mediated effects of the endovanilloid/endocannabinoid N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur J Neurosci. 2004;20:175–184. doi: 10.1111/j.1460-9568.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- Scadding JW. The permanent anatomical effects of neonatal capsaicin on somatosensory nerves. J Anat. 1980;131:471–482. [PMC free article] [PubMed] [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- Smith PJ, McQueen DS. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br J Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005a;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005b;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br J Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Srgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]