Abstract
The α2/δ1 subunit forms part of the dihydropyridine receptor, an essential protein complex for excitation–contraction (EC) coupling in skeletal muscle. Because of the lack of a viable knock-out animal, little is known regarding the role of the α2/δ1 subunit in EC coupling or in other cell functions. Interestingly, the α2/δ1 appears before the α1 subunit in development and contains extracellular conserved domains known to be important in cell signalling and inter-protein interactions. These facts raise the possibility that the α2/δ1 subunit performs vital functions not associated with EC coupling. Here, we tested the hypothesis that the α2/δ1 subunit is important for interactions of muscle cells with their environment. Using confocal microscopy, we followed the immunolocalization of α2/δ1 and α1 subunits with age. We found that in 2-day-old myotubes, the α2/δ1 subunit concentrated towards the ends of the cells, while the α1 subunit clustered near the centre. As myotubes aged (6–12 days), the α2/δ1 became evenly distributed along the myotubes and co-localized with α1. When the expression of α2/δ1 was blocked with siRNA, migration, attachment and spreading of myoblasts were impaired while the L-type calcium current remained unaffected. The results suggest a previously unidentified role of the α2/δ1 subunit in skeletal muscle and support the involvement of this protein in extracellular signalling. This new role of the α2/δ1 subunit may be crucial for muscle development, muscle repair and at times in which myoblast attachment and migration are fundamental.
Calcium channels are critical mediators of many diverse processes such as neurotransmitter and hormone release, activation of intracellular signalling pathways, pacemaker activity or changes in gene expression. In skeletal muscle, the L-type calcium channel or dihydropyridine receptor (DHPR) is involved in the excitation–contraction (EC) coupling mechanism. However, the relative contribution of the individual subunits of the DHPR complex (α1, α2/δ1, β and γ) to EC coupling varies widely. Muscle from dysgenic mice, which lack the α1 subunit, or from β-null mice has a complete loss of EC coupling and L-type calcium current (Tanabe et al. 1988; Gregg et al. 1996). In contrast, muscle from γ-null mice does not show changes in EC coupling and only modest effects of L-type calcium current (Freise et al. 2000).
Little is known about the contribution of the α2/δ1 subunit because absence of this protein is lethal in α2/δ1-null embryos (Joshi & Taylor, 2006). However, blockade of α2/δ1 expression with siRNA in the dysgenic muscle cell line GLT had no effect on EC coupling and caused only an acceleration of the calcium current (Obermair et al. 2005). That the deletion of the α2/δ1 subunit has such disparate results as lethality in one set of circumstances and little effect in another is intriguing and suggests that this protein may perform vital functions not related to EC coupling. The hypothesis that the α2/δ1 performs other functions outside EC coupling is supported by other evidence. First, the α2/δ1 subunit appears earlier than the α1 subunit and its levels remain high during skeletal muscle development. This has been demonstrated both at the mRNA (Varadi et al. 1989) and the protein levels (Morton & Froehner, 1989). Second, the α2/δ1 protein shares a strikingly similar structure with other proteins involved in other processes such as cell adhesion and molecule recognition.
The α2/δ1 subunit is a dimer protein product of a single gene (DeJongh et al. 1990; Jay et al. 1991). Post-translation cleavage results in an extracellular α2 protein containing ∼950 N-terminal residues and a δ protein with ∼130 C-terminal residues. Although the precise protein topology is not known, evidence suggests that a small portion of the δ peptide is a transmembrane segment with a short cytoplasmic tail. The rest of the δ peptide is located outside the cell and interacts with the α2 protein via disulphide bridges. Thus, about 90% of the α2/δ1 subunit is located outside the cell. In addition to carbohydrates on the extracellular portion of the α2/δ1 subunit, other conserved domains thought to mediate cell signalling are present in this region.
The N-terminal half of α2 contains a von Willebrand A (VWA) domain (Bork & Rohde, 1991; Whittaker & Hynes, 2002). The VWA domain is found in cell adhesion and extracellular matrix proteins and is thought to be involved in protein–protein interactions requiring divalent cations. The VWA occurs most notably in integrins and extracellular matrix proteins. Included in the VWA domain of α2 is a metal ion-dependent adhesion site (MIDAS), which is putatively important for mediating protein–protein interaction (Whittaker & Hynes, 2002), such as ligand-receptor binding. Interestingly, mutations in VWA domains result in a number of human diseases (Hanks et al. 2003; Himmelfarb et al. 2004). Immediately adjacent to the C-terminal of the VWA domain, there are two CACHE domains. These domains have been found only in this CAlcium channel subunit and prokaryotic CHEmotaxis receptors (Anantharaman & Aravind, 2000). The predicted membrane topology and the requirement of this domain in prokaryotes for sensing various molecules (i.e. amino acids, glucose, citrate), led Anantharaman & Aravind (2000) to suggest that the CACHE domain is involved in regulation of calcium channels by endogenous ligands. Mutations in the first CACHE domain of α2/δ1 result in loss of gabapentin binding (Wang et al. 1999). The presence of these conserved domains on α2 suggests that this subunit is important for mediating signalling from extracellular sources. However, the possible contribution of the α2/δ1 subunit in processes other than EC coupling has not been investigated.
We postulate that interactions of muscle cells with the extracellular matrix are mediated by the α2/δ1 subunit due to the presence of those conserved domains involved in adhesion. We report here experiments examining the localization of α2/δ1 subunits in muscle cells and the ability of the cells to interact with the substrate in the presence and the absence of the protein. We show that the α2/δ1 subunit localizes at the end of the cells and separate from the α1 subunit in young myotubes and that the absence of this protein impairs migration, attachment and spreading of myoblasts. The results support the idea that the α2/δ1 subunit's involvement in extracellular signalling is crucial for muscle development and muscle repair, and at times in which myoblast attachment and migration are fundamental.
Methods
Muscle cell preparation
All experiments using animals were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago, which is fully accredited by AAALAC (Association for Assesment and Accreditation of Laboratory Animal Care International). Mice were deeply anaesthetized by methoxyflurane inhalation then decapitated. Experiments were performed on muscle cells in culture as described previously (García et al. 1994). Skeletal muscle of newborn mice (at postnatal day 0) was dissociated in Ca2+- and Mg2+-free rodent Ringer solution (mm): 155 NaCl, 5 KCl, 11 glucose, 10 Hepes, pH 7.4 containing collagenase type IA (1 mg ml−1) (Sigma). Dissociated cells were plated on collagen I-coated glass coverslips in Dulbecco's modified Eagle's medium (DMEM) supplemented with 4.5 g l−1 glucose, 10% horse serum and 10% fetal calf serum. Fusion and differentiation were promoted after 2 days in culture by reducing horse serum concentration to 5% and removing fetal calf serum. This day corresponds to experimental day 0.
C2C12 cells (ATCC) were plated on collagen I-coated glass coverslips in DMEM with 4 mm l-glutamine, 1.5 g l−1 of sodium bicarbonate and 4.5 g l−1 glucose with 10% fetal bovine serum. Cells were grown to about 80% confluence to avoid depleting the myoblast population.
siRNA plasmids
The sequences of the five α2/δ gene 1 isoforms were analysed with the program RNAstructure 4.1 (Mathews et al. 2004). The secondary structure of the different RNAs was predicted and compared to select the best targets for siRNA. Five consensus sites present in all five sequences were chosen as the targets and tested: 252–270, 536–554, 763–781, 1167–1185 and 1462–1480. The random control sequence AAGGACGAATGAGTTGAAC was examined with BLAST and found to bear no significant similarities with the mouse genomic sequences. The sense oligonucleotide strand contained the 19-nt target sequence, which was preceded by a Bgl II site and followed by the hairpin sequence TCAAGAGA, a 19-nt antisense sequence, and a Hind III site. The antisense strand was complementary to the sense strand and had the Hind III site on the 5′ end and the Bgl II on the 3′ end. For each target, the pair of oligonucleotides was annealed and cloned into the pSUPER.neo vector (OligoEngine, Seattle, WA, USA). C2C12 cells were transfected with one of several plasmids containing the neomycin resistance gene using TransIT LT-1 reagent (Mirus, Madison, WI, USA): an empty vector (pS.neo), a vector containing a target sequence (pS-α2/δ1) or a vector with the control sequence (pS-Ctr). Transfected cells were selected with G418 (500 μg ml−1). α2/δ1 mRNA and protein were measured with RT-PCR and Western blots, respectively, to determine levels of this subunit.
RT-PCR
Total RNA was isolated from untreated cells, or cells treated with the pSUPER vectors using the QIAGEN RNeasy system. The RNA eluate was reverse-transcribed using 50 U of MuLV reverse transcriptase and reverse primers specific for α2/δ genes 1–4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (for sequences see below). PCR was performed with AmpliTaq DNA Polymerase (PerkinElmer, Norwalk, CT, USA) and gene-specific primers, as described in Nabhani et al. (2005). The total PCR volume was 100 μl, including 20 μl of RT reaction and 2.5 units of AmpliTaq DNA polymerase. The reaction consisted of 40 cycles and an annealing temperature of 60°C. PCR products were size-fractionated by 2% agarose gel electrophoresis. DNA fragments of the expected lengths were sequenced using an ABI Prism big dye terminator cycle sequencing reaction kit (PE Applied Biosystems, Norwalk, CT, USA), AmpliTaq DNA polymerase, and the reverse or forward primer. The primers for the α2/δ1 gene were the following (Alden & Garcia, 2002): (forward) GGC CGG ATC CGC AAT TGA TCC TAA TGG and (reverse) GAA GGC TGC AGA TCA TTG CAG TAT TC. Primers for the α2/δ2 gene were the following (Alden & García, 2002): (forward) AAC TTC TTC TAC ACC CGA AAG and (reverse) TTA TAG GAT GCG TTC ACC GAG. The primer sequence for the α2/δ3 gene was taken from Klugbauer et al. (1999): (forward) GGC ACA GAT GTC CCA GTT AAA GA and (reverse) TGT ATA GTA GTA GTC ATT GGT CAT. Primer sequence for α2/δ4 was (Obermair et al. 2005): (forward) CTA ACA ATG GCT ACA TCC TCT CT and (reverse) GAG AGG TCC ACA CTG TTG TAG TT. Positive controls included adult skeletal muscle for α2/δ1 and brain tissue for α2/δ2–4. Samples were normalized against GAPDH expression. Primers used for GAPDH amplification were: (forward) TAT GAC AAT GAA TAC GGC T and (reverse) CTC CTG TTA TTA TGG GGG.
Immunolocalization
Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton X-100. Cells were simultaneously labelled with the α2/δ1 affinity-purified anti-1a isoform antibody (1 : 500, Bethyl Laboratories) and either the α1 monoclonal antibody mAb 1A (1 : 1000; Morton & Froehner, 1987) or the α2/δ1 monoclonal antibody mAb 20A (1 : 1000; Morton & Froehner, 1989). Labelling of muscle cells with both α2/δ1 antibodies resulted in the same immunolocalization of the protein, indicating that both antibodies recognize the same protein. The secondary antibodies for immunocytochemistry were Alexa-Fluor 488 and Alexa-Fluor 555, both at 1 : 1000 (Molecular Probes). Controls omitting the primary antibody or using mismatched secondary antibodies were routinely performed. Cells were examined with a Bio-Rad Radiance 2100 confocal microscope attached to a Nikon TE300 inverted microscope and using a 60× water-immersion objective with 1.2 NA. Alexa-Fluor 488 was excited with the 488 line of a krypton–argon laser and the emission was measured at wavelengths of 515 ± 30 nm. Alexa-Fluor 555 was excited with the 568 nm line of the laser and the emission measured at 600 ± 40 nm. To label the nuclei, cells were stained with 3 μm TO-PRO3 (Molecular Probes), which was excited at 637 nm and the emission measured at wavelengths > 660 nm. Images were analysed using LaserPix image analysis software (Bio-Rad, Hercules, CA, USA).
COS7 cells (Invitrogen) were also used as a control to test the α2/δ1 affinity-purified anti-1a isoform antibody. COS7 cells were plated on collagen I-coated glass coverslips in media of the same composition as C2C12 cells. Cells were grown to 90% confluency and transfected with calcium phosphate with a clone containing the full length α2/δ1a subunit in pcDNA3.1. Cells were fixed 24 h later and labelled with the α2/δ1a polyclonal antibody and Alexa-Fluor 488 as the secondary antibody.
Western blotting
Protein was extracted from transfected cells selected with G418. Cells were rinsed twice with phosphate-buffered saline, scraped from the culture dishes and pelleted. The pellet was resuspended in 100 μl of sample buffer (2% SDS, 100 mm dithiothreitol, 60 mm Tris, 0.01% bromophenol blue, pH 6.8). The suspension was heated for 5 min at 95°C and later centrifuged at 14 000 g for 10 min. The supernatant was collected and protein was quantified by Bradford assay. Proteins (10 μg lane−1) were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidenefluoride (PVDF) membranes. Protein transfer was verified with Pounceau S staining. The membranes were incubated with the primary antibody, washed and blocked overnight. Membranes were then incubated with a secondary antibody conjugated to horseradish peroxidase at a 1 : 20 000 dilution. Antibody binding was detected with chemiluminescence using the SuperSignal West Femto Detection Kit (Pierce) following manufacturer's instructions.
Electrophysiological measurements
The whole-cell configuration of the patch-clamp technique (Hamill et al. 1981) was used for measurement of calcium currents in C2C12 cells. Membrane linear components were digitally subtracted by appropriate scaling and subtracting negative control currents that did not activate ionic conductances. Cell capacitance was measured by integrating the area under the capacity transient before series resistance compensation and was used to normalize calcium currents. Data acquisition and processing were performed with pCLAMP 8.0 software (Axon Instruments). Recording electrodes were pulled from borosilicate glass and had resistances between 1.6 and 2.0 MΩ when filled with a solution containing (mm): 140 caesium aspartate, 3 Mg2Cl, 2.5 Mg-ATP, 10 Cs-EGTA and 10 Hepes, pH 7.4 adjusted with CsOH. The extracellular solution contained (mm): 145 TEACl, 10 CaCl2, 10 Hepes and 0.003 TTX, pH 7.4 adjusted with CsOH. To isolate L-type currents and minimize contributions of gating currents from other voltage-dependent channels, a 1 s depolarizing pulse to −30 mV was delivered followed by a 25 ms repolarization to −50 mV and test pulses of varying amplitude. Test pulses ranged from −40 to 40 mV in 10 mV increments.
Statistics
Data are expressed as means ±s.e.m. Student's t test or ANOVA were used to determine statistical significance when appropriate. A value of P < 0.05 was considered to be significant.
Results
Immunolocalization of α2/δ1 in skeletal muscle cells changes with development
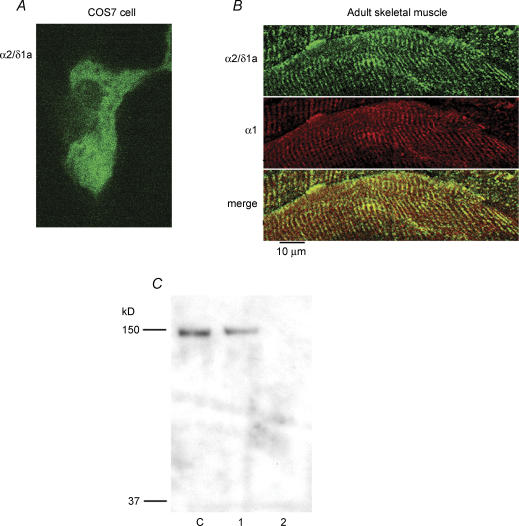
Since the α1 and the α2/δ1 subunits are part of the DHPR complex, our working hypothesis was that the α2/δ1 isoform would co-localize with the α1 subunit. Previous studies examining the localization of these two subunits used the same monoclonal antibodies as used in the present report to label the proteins in skeletal muscle cells (Protasi et al. 1997; Obermair et al. 2005). However, the interpretation of the results from those studies was complicated by the fact that only one protein was labelled at a time. In order to label the α1 and a2/δ1 subunits simultaneously in the same cell, we developed and affinity-purified a rabbit antibody that could be used with either of the mouse monoclonal antibodies 1A or 20A. The affinity-purified antibody recognized the α2/δ1a isoform expressed in COS7 cells after transfection with a clone encoding the full length protein (Fig. 1A). In addition, simultaneous double labelling of adult skeletal muscle with the α1 antibody and the affinity-purified α2/δ1a antibody showed co-localization of the proteins when the tissue sections were examined with confocal microscopy (Fig. 1B). There was no labelling of COS7 cells or adult skeletal muscle when the affinity-purified antibody was omitted or pre-adsorbed with the peptide used to generate the antibody. The α2/δ1a polyclonal antibody was further tested by Western blotting using whole-cell homogenate of myotubes, naive COS7 cells or COS7 cells transfected with a plasmid encoding α2/δ1a. As shown in Fig. 1C, the α2/δ1a polyclonal antibody reacted with a band of about 140 kDa in myotubes and transfected COS7 cells, but not in untransfected COS7 cells. These controls indicate that the antibody we generated specifically recognizes the α2/δ1 subunit.
Figure 1. Identification of α2/δ1a isoform by a polyclonal antibody.
A, COS7 cells were transfected with a plasmid containing the full sequence of the α2/δ1a subunit, kindly provided by Drs N. Klugbauer and F. Hofmann and fixed 48 h later. Cells were examined for expression of the α2/δ1a protein by labelling with the α2/δ1 affinity-purified anti-1a isoform primary antibody (1 : 500) and Alexa-Fluor 488 goat anti-rabbit secondary antibody (1 : 1000). Confocal microscopy revealed diffuse staining of transfected cells. Control, untransfected, cells did not show any labelling. B, the tibialis anterior muscle was isolated from adult mice, frozen in liquid nitrogen, and sectioned. Sections of tissue were simultaneously labelled with the α2/δ1 affinity-purified anti-1a isoform antibody (1 : 500) and the α1 monoclonal antibody mAb 1A (1 : 1000; Morton & Froehner, 1987). The secondary antibodies were Alexa-Fluor 488 goat anti-rabbit and Alexa-Fluor 555 goat anti-mouse, both at 1 : 1000 (Molecular Probes). Examination of the sections with confocal microsopy showed co-localization of α2/δ1a and α1 labelling in a striated pattern, as would be expected for adult skeletal muscle. Note that α2/δ1a is the only isoform expressed in adult skeletal muscle. C, Western blot of whole-cell homogenate probed with the α2/δ1a polyclonal antibody. Two-day skeletal myotubes (C), COS7 cells transfected with α2/δ1a plasmid (1), and naïve COS7 cells (2). The affinity-purified antibody specifically recognizes both expressed and native α2/δ1a subunits. There was no signal in naïve COS7 cells.
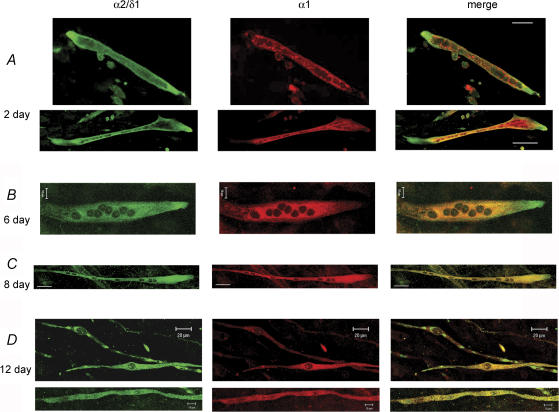
Primary myotubes were fixed at different days after fusion and differentiation were promoted and simultaneously labelled with the α2/δ1a antibody and antibody 1A. Cells from at least three different cultures were examined with confocal microscopy and images from random fields were recorded for quantification and analysis. In 2-day-old myotubes the α2/δ1 was found predominantly at the ends of the cells and little in other parts. In contrast, the α1 subunit was found closer to the centre of the cells (Fig. 2A). An intriguing localization for α1, since t-tubules are practically absent in this region at this age (Zhou et al. 2006). In 435 cells examined with double labelling, α1 labelling appeared in the form of clusters in 224 cells and only 54 of those showed clusters of α2/δ1 labelling, which co-localized with α1. The appearance of clusters was similar to that found in other reports (Protasi et al. 1997; Obermair et al. 2005). The rest of the cells (211) displayed more diffuse staining of α1. Of the same 435 cells, 66% (286 cells) had preferential localization of α2/δ1 labelling at the ends without α1. In contrast, only 8.5% (37 cells) had α1 labelling at the ends at this age.
Figure 2. Immunolocalization of α2/δ1 and α1 subunits in primary myotubes.
Myotubes were simultaneously labelled with an affinity-purified α2/δ1 antibody and the α1 monoclonal antibody 1A at different times after fusion and differentiation were promoted. A, representative 2-day-old myotubes from different cultures showed a strong α2/δ1 labelling at the ends of the cells where α1 labelling was weak or absent. Bars, 10 μm. B–D, representative 6-, 8- and 12-day-old myotubes, respectively, showing a progressively more homogeneous localization of α2/δ1 and α1 subunits along the myotubes than the 2-day-old myotubes. The 12-day-old myotubes belong to different cultures. There were patches of α2/δ1 staining without α1 labelling in some myotubes still at 12 days. Calibration bars: B, 10 μm; C, 20 μm; D, 20 μm top and 10 μm bottom.
When cells were examined at 6, 8 and 12 days after fusion and differentiation were promoted, a gradual change in the localization of the α2/δ1 subunit was found as cells aged. α2/δ1 became more homogeneously localized along the myotubes. In 12-day-old cells, the α2/δ1 subunit co-localized almost entirely with the α1 subunit (Fig. 2B). In 269 myotubes examined at 12 days, α1 labelling resulted in clusters in 261 cells, which co-localized with α2/δ1 clusters in 253 cells. In addition, there were far fewer myotubes with α2/δ1 labelling at the ends at 12 days compared to 2 days. At 12 days, 3.3% (9 cells) of the myotubes had α2/δ1 labelling at the ends with no α1 labelling. The localization of the α2/δ1 subunit at the ends of the cells with little association to α1 subunits suggests that the α2/δ1 subunit is important for other cellular functions independent from EC coupling. It further suggests that the assembly of the DHPR as a complex occurs at later times.
Block of α2/δ1 expression with siRNA
The localization of the α2/δ1 subunit at the ends of the cells in young myotubes together with the presence of conserved domains involved in signalling on the extracellular portion of the protein (VWA, MIDAS, CACHE), suggests that the α2/δ1 subunit may be involved in signalling mechanism(s) other than EC coupling. To address this issue, levels of α2/δ1 were reduced with siRNA in the mouse skeletal muscle cell line C2C12. As recommended (Elbashir et al. 2001; Kurreck, 2003; Ui-Tei et al. 2004), five siRNA targets specific for the mouse α2/δ1 sequence were tested. The levels of α2/δ1 mRNA and protein were quantified with RT-PCR and Western blotting, respectively, in cells selected with G418. The siRNA probe targeting sequence 536–554 showed the highest degree of inhibition of α2/δ1 expression as shown in Fig. 3A and therefore this probe was used for all the experiments. Levels of α2/δ1 were not modified in C2C12 cells transfected with an empty vector (pS.neo) or a vector with the random control sequence (pS-Ctr). The mRNA levels of α2/δ2 and α2/δ3 were substantially lower (< 5%) than those of α2/δ1, while α2/δ4 could not be detected. The mRNA levels of α2/δ2–4 subunits were not affected when α2/δ1 expression was reduced, indicating that these genes are not up-regulated under these conditions. Figure 3B shows the quantification of α2/δ1 protein expression levels with the different siRNA probes in five separate assays. Results obtained with the random control sequence were used as a reference for comparison with the test siRNA probe.
Figure 3. Measurement of siRNA-induced silencing in C2C12 cells.
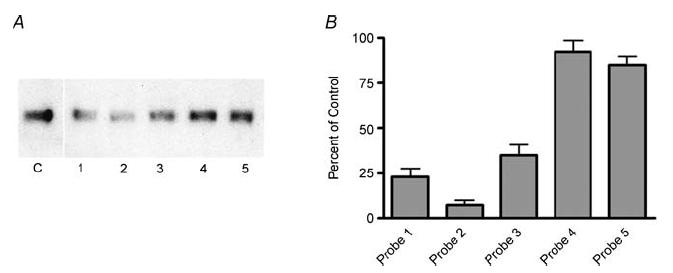
A, Western blot analysis of α2/δ1 subunit from whole-cell homogenate using monoclonal antibody mAb 20A. Control samples (C) were obtained from pS-Ctr-transfected cells. Numbers below the lanes correspond to the targets in the α2/δ1 sequence; (1) 252–270, (2) 536–554, (3) 763–781, (4) 1167–1185, and (5) 1462–1480. All samples were obtained from cells that had been selected with G418 after transfection of siRNA vectors. B, quantification of α2/δ1 protein reduction by siRNA expressed as per cent of control (pS-Ctr). Data correspond to the average of five measurements.
Reduced migration of α2/δ1-deficient C2C12 cells
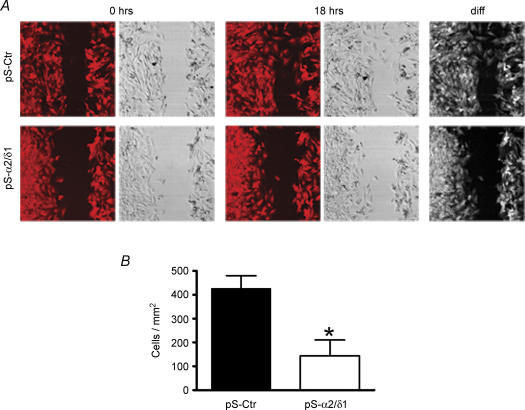
To investigate whether the α2/δ1 subunit is important for interaction of the cells with the extracellular matrix, we examined myoblast migration using C2C12 cells. Cells were plated on glass coverslips coated with collagen type I (6 μg cm−2) and grown to 80% confluency in DMEM plus 10% fetal bovine serum. Myoblasts were transfected with the siRNA plasmids, as described above. Treatment with G418 started 24 h later. An area along the coverslip was denuded of myoblasts and subsequently examined at different times (Huttenlocher et al. 1998; Shiraha et al. 1999). Figure 4 shows images of cells transfected with pS-Ctr or pS-α2/δ1 immediately after clearing an area of the coverslip (0 h) and 18 h later. siRNA-induced block of α2/δ1 subunit expression significantly reduced the number of C2C12 cells that migrated to the denuded area. On average, there were 66 ± 7.8% (n = 5, P < 0.01) fewer cells at 18 h after G418 treatment in the denuded area in pS-α2/δ1-transfected dishes compared to pS-Ctr dishes. These results indicate that migration of myoblasts was impaired when the expression of the α2/δ1 subunit was blocked and suggest that this protein is involved in extracellular signalling.
Figure 4. Migration is impaired in α2/δ1-deficient C2C12 cells.
Images recorded right after the central area of the coverslip was cleared of myoblasts (0 h) and 18 h later. The last column represents the difference of the fields between 18 and 0 h. Images in each row were taken from the same place in the dish. Images in each pair correspond to Rhod-2 fluorescence or transmitted light. A, myoblasts deficient in α2/δ1 subunit (pS-α2/δ1) showed impaired migration to the denuded area, compared to control cells (pS-Ctr). Images are 625 μm × 625 μm. B, a significantly lower number of α2/δ1-deficient cells migrated to the denuded area of the coverslip after 18 h compared to control cells (P < 0.05).
Reduced attachment and spreading of α2/δ1-deficient C2C12 cells
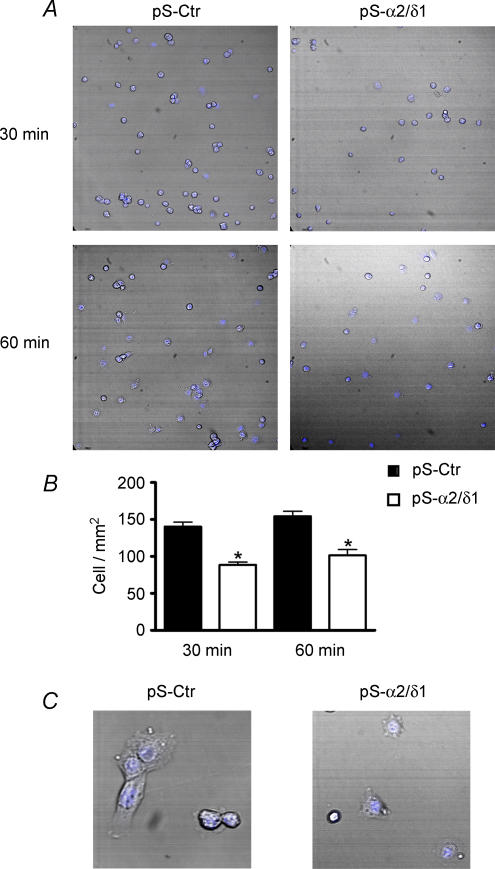
To gain further insight into the possible role of the α2/δ1 subunit in extracellular signalling and the impairment of migration, we measured the proportion of cells able to attach to the substrate in the presence and absence of α2/δ1 subunit. C2C12 cells were grown and transfected as described above. After 48 h of G418 treatment, cells were detached from the dishes by incubation with a mixture of trypsin (0.025%)–EGTA (0.1%) for 10 min. Cells were pelleted and resuspended in DMEM plus 10% fetal bovine serum. They were plated at a density of 15 × 103 cells cm−2 on collagen I-coated coverslips and allowed to attach for 30 or 60 min. After these times, the dishes were rinsed to remove the non-adherent cells. Cells that remained attached were immediately fixed and stained with TO-PRO3 to facilitate measurements. Random images of the coverslips were saved for quantification of cells attached. As shown in Fig. 5, there were significantly less α2/δ1-deficient cells attached compared to control cells at either time point. The number of α2/δ1-deficient cells attached was 36 ± 3.5% less than control cells at 30 min and 32 ± 4.1% less at 60 min. Non-adherent cells were stained with trypan blue and counted in a haemocytometer to assess viability by exclusion of the dye. There was no significant difference in the number of viable cells between control (89 ± 6.2%) and α2/δ1-deficient cells (91 ± 7.5%) in four different assays. This indicates that the reduced attachment of α2/δ1-deficient cells was due to the absence of the protein and not because the number of viable cells was lower than control.
Figure 5. Attachment and spreading is reduced in α2/δ1-deficient C2C12 cells.
Control and α2/δ1-deficient cells were plated at 15 × 103 cells cm−2 and allowed to attach for 30 or 60 min to collagen I-coated coverslips. Unattached cells were rinsed. Cells were fixed and labelled with TO-PRO3. Random fields were recorded to quantify number of cells attached and area of the cells. A, a significantly lower number of α2/δ1-deficient C2C12 cells attached to the coverslips at both 30 and 60 min. Images are 625 μm × 625 μm. B, quantification of cell attachment for control (filled bars) or α2/δ1-deficient cells (open bars) (P < 0.05). C, spreading and consequently the area of α2/δ1-deficient cells was considerably smaller than in control cells. Images are 110 μm × 110 μm. (The online version of this figure is in colour.)
In addition to reduced attachment, spreading of α2/δ1-deficient cells was also diminished compared to control cells. Spreading was measured from the digital images. A cell was considered spreading if the difference between the total area of the cell and the area of TO-PRO3 staining was 25% or more. Control cells spread out further and in larger numbers that α2/δ1-deficient cells. This difference was evident at 60 min. The number of cells that spread in four attachment assays ranged from 57 to 69% of control cells attached, while in α2/δ1-deficient cells the range was 38–46% of cells attached. The average area calculated from the digital images was on average 1116 ± 25 μm2 for control cells (682 cells) and 581 ± 73 μm2 for α2/δ1-deficient cells (455 cells), both at 60 min. This difference was statistically significant (P < 0.01). The reduced attachment and spreading of the cells deficient in α2/δ1 is consistent with the impairment of cell migration under the same conditions and provides additional support to the idea of the α2/δ1 subunit having a role in extracellular signalling.
Calcium currents in α2/δ1-deficient C2C12 cells
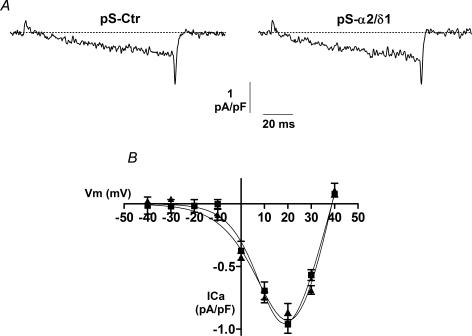
Calcium ions mediate a myriad of cellular functions, including differentiation of skeletal muscle. In neurons, L-type calcium currents can directly activate signalling pathways leading to transcriptional activation (Dolmetsch et al. 2001). Thus, to determine whether the reduced attachment, spreading and migration of α2/δ1-deficient cells was indeed due to the lack of the α2/δ1 protein or to an effect on L-type calcium currents, we recorded calcium currents from C2C12 cells under the same experimental conditions as described above for the attachment and migration assays. Calcium currents were recorded after 48 h of G418 treatment. The amplitude of the L-type current from three different transfections was on average −0.96 ± 0.07 pA pF−1 (n = 18) in control cells and −0.86 ± 0.06 pA pF−1 (n = 19) in α2/δ1-deficient cells (Fig. 6). This difference was not statistically significant. The maximum value of L-type current amplitude found here is smaller than that previously reported for C2C12 cells using the same extracellular calcium concentration of 10 mm (Schuhmeier & Melzer, 2004; Gouadon et al. 2006). This discrepancy can be explained by the use of cells at different stages of development. While other studies have used fully mature myotubes (1–2 weeks after promotion of differentiation), here we used myoblasts, which express little or no α1 subunit (Bidaud et al. 2006) and a robust expression of α2/δ1 as shown in Fig. 3. Interestingly, this pattern of calcium channel subunit expression is not unique to C2C12 cells as it has been described in native skeletal muscle as well (Morton & Froehner, 1989). In addition to the similar amplitude of the calcium current, we found that other parameters of voltage dependence were also unchanged between control and α2/δ1-deficient cells. Current data were fit to the equation:
where I(V) is the maximum calcium current at a given test potential, GmaxL is the maximum L-type channel conductance, Vr is the reversal potential for calcium, V is the test potential, VL is the half-maximal activation potential for the L-type channel, and kL is the slope factor. The average values for each of these parameters in control cells (n = 18) were GmaxL= 8.1 ± 1.0 pS pF−1, Vr= 38.9 ± 0.7 mV, VL= 14.0 ± 2.1 mV, and kL= 6.7 ± 0.9 mV. The corresponding values in α2/δ1-deficient cells (n = 19) were GmaxL= 7.7 ± 1.2 pS pF−1, Vr= 38.8 ± 0.5 mV, VL= 18.4 ± 2.6 mV, and kL= 8.6 ± 0.8 mV. These results suggest that the L-type calcium current is not involved in the reduced migration and attachment of α2/δ1-deficient cells.
Figure 6. L-type calcium current is not modified in α2/δ1-deficient cells.
Calcium currents recorded from C2C12 cells under the same experimental conditions as in Figs 4 and 5. A, representative L-type calcium currents from a control cell (pS-Ctr) and an α2/δ1-deficient cell (pS-α2/δ1). B, current–voltage relationship for all control (█) and α2/δ1-deficient (▴) cells. The smooth curves correspond to the fit of all data in each group to the equation described in the text.
Our results demonstrate a novel aspect of cellular signalling involving the α2/δ1 subunit of voltage-activated calcium channels. The fact that the α2/δ1 subunit is localized at the ends of immature muscle cells combined with defective migration, attachment and spreading of cells devoid of this protein, indicates that it is important for interactions of the cell with the environment.
Discussion
The extracellular portion of the α2/δ1 subunit contains conserved domains involved in cellular signalling in other proteins. The VWA domain, present in integrin molecules, binds to laminin and collagen. This property makes it an ideal domain candidate for the α2/δ1 to interact with the substratum. Mutations in the VWA domain cause disruption of the interaction of the proteins with extracellular components, resulting in diseases such as von Willebrand disease (Fressinaud et al. 2002), infantile systemic hyalinosis (Hanks et al. 2003), and breast cancer development (Himmelfarb et al. 2004) for example. Interestingly, the MIDAS domain found within the VWA domain in other proteins is a receptor for the anthrax toxin (Scobie et al. 2003) indicating a complex interaction of these domains with extracellular molecules. The presence of α2/δ1 subunit at the ends of muscle cells suggests that this protein may be involved in directing the movement of the cell. Similarly to the disruption of those interactions above, the absence of the α2/δ1 subunit in C2C12 cells resulted in an impairment of the interaction of the cells with the substratum as evidenced by reduced attachment and spreading, which in turn would decrease the migration of the cells. In addition, the fact that the L-type calcium current remained unaltered in the absence of α2/δ1 suggests that those changes are not due to calcium influx through this channel but rather to a direct action of the α2/δ1 protein. The effects of a deficiency in the α2/δ1 subunit are probably more important during muscle development and muscle repair rather than in fully differentiated skeletal muscle since cell migration is absolutely necessary during these two conditions.
The role of the α2/δ1 subunit as a cell adhesion molecule as proposed here is similar to that described for the β subunit of sodium channels (Srinivasan et al. 1998; Xiao et al. 1999; Malhotra et al. 2000). The external portion of the sodium channel β subunit has high homology to immunoglobulins. This region of the β subunit confers the protein the ability to interact with the extracellular matrix molecules tenascin-C and tenascin-R (Srinivasan et al. 1998; Xiao et al. 1999). Additionally, β subunits promote recruitment of ankyrin to points of contact between cells presumably by a direct mechanism or through its interaction with NrCAM or neurofascin (Malhotra et al. 2000). Although β subunits of potassium channels have not been described to interact with the extracellular matris, other functions in cellular signalling have recently been reported. For example, it has been shown that potassium channel β subunits have enzymatic properties and are able to regulate gene transcription (Weng et al. 2006; Zeng et al. 2004; Zhang et al. 2003; Buxbaum et al. 1998; Carrion et al. 1999; Savignac et al. 2005). Thus, an emerging theme is the identification of signalling roles of the auxiliary subunits independent of the function of ion channels as transporters, which is consistent with the role of the α2/δ1 subunit in extracellular signalling. Based on our findings, we propose that the extracellular portion of the α2/δ1 subunit interacts with collagen, a major constituent of the extracellular matrix (Kovanen, 2002; Kjaer, 2004), as shown in the schematic representation in Fig. 7. This interaction is perhaps mediated by the VWA domain since, as indicated above, this region binds collagen. It will be interesting to determine interaction(s) of the α2/δ1 subunit with other components, such as focal adhesion proteins, in the future.
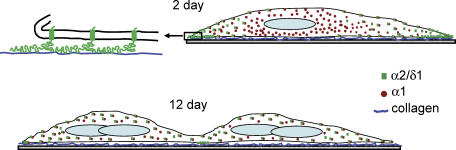
Figure 7. Schematic representation of the interaction of α2/δ1 with the substratum and differential localization during myotube differentiation.
Young myotubes (2 day) showed strong α2/δ1 labelling at the ends of the cells where α1 labelling was weak or absent. The top left represents a magnification of the end of the cell to illustrate the interaction of α2/δ1 with collagen. Older myotubes (12 day) showed homogeneous distribution of α2/δ1 along the cells and co-localization with α1. α2/δ1 is represented by squares and α1 by circles.
Another interesting aspect of the work presented here is that the α2/δ1 subunit was not always found in association with the α1 subunit, in contrast with previous studies (Protasi et al. 1997; Obermair et al. 2005). Experimental conditions that may account for these differences include our earlier time (2 days) for examining the cells, the use of simultaneous labelling of both proteins in the same cell, and the use of confocal microscopy versus wide-field microscopy (Protasi et al. 1997). The spatial separation of these two calcium channel subunits in muscle cells at early times suggest that the assembly of the DHPR complex occurs after cells have migrated and made contact with each other. In fact, it has been demonstrated previously that the α2/δ1 subunit is expressed earlier than the α1 subunit during development and that its levels remain practically constant, while the levels of α1 subunit increase during the same time course (Varadi et al. 1989; Morton & Froehner, 1989). Furthermore, Bidaud et al. (2006) have reported the absence of α1 subunit and L-type calcium current in C2C12 cells and primary muscle cells prior to fusion and differentiation. Our measurement of a small L-type calcium current in undifferentiated C2C12 cells, even in the presence of 10 mm extracellular calcium, and a lack of change of this current in the absence of α2/δ1 agree with the studies by Bidaud et al. (2006) and Morton & Froehner (1989). As muscle cells in culture differentiate α1 and α2/δ1 show higher degrees of co-localization (Figs 2 and 6). Interestingly, the L-type calcium current experiences changes in voltage-dependent inactivation and amplitude (Adams et al. 1996; Nabhani et al. 2005) that parallel the time course of co-localization of these two subunits. Although the role of the α2/δ1 subunit as a cell adhesion/extracellular signalling protein is not apparently related to the function calcium channels, more experiments need to be done to determine whether the α2/δ1 subunit is also important for localization and/or interactions of calcium channels with the environment. Additionally, further experiments are necessary to examine a possible involvement of the α2/δ1 subunit in intracelluar signalling, similar to those mediated by chemotactic receptors.
Acknowledgments
This work was supported by a grant from the Muscular Dystrophy Association, Inc. USA (to J.G.).
References
- Adams BA, Tanabe T, Beam KG. Ca2+ current activation rate correlates with α1 subunit density. Biophys J. 1996;71:156–162. doi: 10.1016/S0006-3495(96)79212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alden KJ, Garcia J. Dissociation of charge movement from calcium release and calcium current in skeletal myotubes by gabapentin. Am J Physiol Cell Physiol. 2002;283:C941–C949. doi: 10.1152/ajpcell.00004.2002. [DOI] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. Cache – a signaling domain common to Ca2+ channel subunits and a class of prokaryotic chemotaxic receptors. Trends Biochem Sci. 2000;25:535–537. doi: 10.1016/s0968-0004(00)01672-8. [DOI] [PubMed] [Google Scholar]
- Bidaud I, Montiel A, Nargeot J, Lory P. Properties and role of voltage-dependent calcium channels during mouse skeletal muscle differentiation. J Muscle Res Cell Motility. 2006;27:75–81. doi: 10.1007/s10974-006-9058-5. [DOI] [PubMed] [Google Scholar]
- Bork P, Rohde K. More von Willebrand factor type A domains? Sequence similarities with malaria thrombospondin-related anonymous protein, dihydropyridine sensitive calcium channel and inter-alpha-trypsin inhibitor. Biochem J. 1991;279:908–910. doi: 10.1042/bj2790908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nature Med. 1998;4:1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. Ca2+-dependent transcriptional repression and derepression: DREAM, a direct effector. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Freise D, Held B, Wissenbach U, Pfeifer A, Trost C, Himmerkus N, Schweig U, Freichel M, Biel M, Hofmann F, Hoth M, Flockerzi V. Absence of the γ subunit of the skeletal muscle dihydropyridine receptor increases L-type Ca2+ currents and alters channel inactivation properties. J Biol Chem. 2000;275:14476–14481. doi: 10.1074/jbc.275.19.14476. [DOI] [PubMed] [Google Scholar]
- Fressinaud E, Mazurier C, Meyer D. Molecular genetics of type 2 von Willebrand disease. Int J Hematol. 2002;75:9–18. doi: 10.1007/BF02981973. [DOI] [PubMed] [Google Scholar]
- García J, Tanabe T, Beam KG. Relationship of calcium transients to calcium currents and charge movements in myotubes expressing skeletal and cardiac DHP receptors. J Gen Physiol. 1994;103:125–147. doi: 10.1085/jgp.103.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouadon E, Schuhmeier RP, Ursu D, Anderson AA, Treves S, Zorzato F, Lehmann-Horn F, Melzer W. A possible role of the junctional face protein JP-45 in modulating Ca2+ release in skeletal muscle. J Physiol. 2006;572:269–280. doi: 10.1113/jphysiol.2005.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci U S A. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanks S, Adams S, Douglas J, Arbour L, Atherton DJ, Balci S, et al. Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:791–800. doi: 10.1086/378418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelfarb M, Klopocki E, Grube S, Staub E, Klaman I, Hinzmann B, Kristiansen G, Rosenthal A, Durst M, Dahl E. ITIH5, a novel member of the inter-α-trypsin inhibitor heavy chain family is downregulated in breast cancer. Cancer Lett. 2004;204:69–77. doi: 10.1016/j.canlet.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T, Knudsen KA, Horwitz AF. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol. 1998;141:515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- Joshi I, Taylor CP. Pregabalin action at a model synapse: binding to presynaptic calcium channel α2-δ subunit reduces neurotransmission in mice. Eur J Pharmacol. 2006;553:82–88. doi: 10.1016/j.ejphar.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel α2δ subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev. 2002;30:20–25. doi: 10.1097/00003677-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- Mathews DH, Disney MD, Childs JL, Schroeder SJ, Zuker M, Turner DH. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc Natl Acad Sci U S A. 2004;101:7287–7292. doi: 10.1073/pnas.0401799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton ME, Froehner SC. Monoclonal antibody identifies a 200-kDa subunit of the dihydropyridine-sensitive calcium channel. J Biol Chem. 1987;262:11904–11907. [PubMed] [Google Scholar]
- Morton ME, Froehner SC. The α1 and α2 polypeptides of the dihydropyridine-sensitive calcium channel differ in developmental expression and tissue distribution. Neuron. 1989;2:1499–1506. doi: 10.1016/0896-6273(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Nabhani T, Shah T, García J. Skeletal muscle cells express different isoforms of the calcium channel α2/δ subunit. Cell Biochem Biophys. 2005;42:13–20. doi: 10.1385/CBB:42:1:013. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel α2δ-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1s or excitation-contraction coupling. J Biol Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- Protasi F, Franzini-Armstrong C, Flucher BE. Coordinated incorporation of skeletal muscle dihydropyridine receptors and ryanodine receptors in peripheral couplings of BC3H1 cells. J Cell Biol. 1997;137:859–870. doi: 10.1083/jcb.137.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac M, Pintado B, Gutierrez-Adan A, Palczewska M, Mellstrom B, Naranjo JR. Transcriptional repressor DREAM regulates T-lymphocyte proliferation and cytokine gene expression. EMBO J. 2005;24:3555–3564. doi: 10.1038/sj.emboj.7600810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmeier RP, Melzer W. Voltage-dependent Ca2+ fluxes in skeletal myotubes determined using a removal model analysis. J Gen Physiol. 2004;123:33–51. doi: 10.1085/jgp.200308908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–254. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, Schachner M, Catterall WA. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc Natl Acad Sci U S A. 1998;95:15753–15757. doi: 10.1073/pnas.95.26.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, Ueda R, Saigo K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucl Acids Res. 2004;32:936–948. doi: 10.1093/nar/gkh247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi G, Orlowski J, Schwartz A. Developmental regulation of expression of the α1 and α2 subunits mRNAs of the voltage-dependent calcium channel in a differentiating myogenic cell line. FEBS Lett. 1989;250:515–518. doi: 10.1016/0014-5793(89)80787-2. [DOI] [PubMed] [Google Scholar]
- Wang M, Offord J, Oxender DL, Su T-Z. Structural requirement of the calcium-channel subunit α2δ for gabapentin binding. Biochem J. 1999;342:313–320. [PMC free article] [PubMed] [Google Scholar]
- Weng J, Cao Y, Zhou M. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J Biol Chem. 2006;281:15194–15200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/Integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13:3369–3387. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZC, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL. Tenascin-R is a functional modulator of sodium channel β subunits. J Biol Chem. 1999;274:26511–26517. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- Zeng H, Fei H, Levitan IB. The slowpoke channel binding protein Slob from Drosophila melanogaster exhibits regulatable protein kinase activity. Neurosci Lett. 2004;65:33–38. doi: 10.1016/j.neulet.2004.04.043. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Oliva R, Gisselmann G, Hatt H, Guckenheimer J, Harris-Warrick RM. Overexpression of a hyperpolarization-activated cation current (Ih) channel gene modifies the firing activity of identified motor neurons in a small neural network. J Neurosci. 2003;23:9059–9067. doi: 10.1523/JNEUROSCI.23-27-09059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yi J, Launikonis B, González A, García J, Ríos E. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol. 2006;290:C539–C553. doi: 10.1152/ajpcell.00592.2004. [DOI] [PubMed] [Google Scholar]