Abstract
Contraction in skeletal muscle fibres is governed by excitation of the transverse-tubular (t-) system, but the properties of the t-system and their importance in normal excitability are not well defined. Here we investigate the properties of the t-system chloride conductance using rat skinned muscle fibres in which the sarcolemma has been mechanically removed but the normal excitation–contraction coupling mechanism kept functional. When the t-system chloride conductance was eliminated, either by removal of all Cl− or by block of the chloride channels with 9-anthracene carboxylic acid (9-AC) or by treating muscles with phorbol 12,13-dibutyrate, there was a marked reduction in the threshold electric field intensity required to elicit a t-system action potential (AP) and twitch response. Calculations of the t-system chloride conductance indicated that it constitutes a large proportion of the total chloride conductance observed in intact fibres. Blocking the chloride conductance increased the size of the twitch response and was indicative that Cl− normally carries part of the repolarizing current across the t-system membrane on each AP. Block of the t-system chloride conductance also reduced tetanic force responses at higher frequency stimulation (100 Hz) and greatly reduced twitch responses in the period shortly after a brief tetanus, owing to rapid loss of t-system excitability during the AP train. Blocking activity of the Na+–K+ pump in the t-system membrane caused loss of excitability owing to K+ build-up in the sealed t-system, and this occurred ∼3–4 times faster when the chloride conductance was blocked. These findings show that the t-system chloride conductance plays a vital role during normal activity by countering the effects of K+ accumulation in the t-system and maintaining muscle excitability.
Contraction of vertebrate skeletal muscle is initiated by the spread of action potentials (APs) along the surface membrane (sarcolemma) and down into the network of invaginations known as the transverse tubular (t-) system. The APs activate voltage-sensor molecules (dihydropyridine receptors) in the t-system membrane, which in turn open the Ca2+ release channels (ryanodine receptors) in the adjacent sarcoplasmic reticulum (SR), leading to a rise in myoplasmic [Ca2+] and generation of force by the contractile apparatus (Melzer et al. 1995; Stephenson et al. 1998). The vital role of voltage-dependent Na+ channels and K+ channels in the excitation process is universally recognized, but the role and importance of chloride channels is much more poorly defined. In mammalian skeletal muscle fibres, the chloride conductance accounts for more than 80% of the total resting conductance (Bryant & Morales-Aguilera, 1971; Dulhunty, 1979; Kwiecinski et al. 1984; Bretag, 1987), and it clearly plays a key role in stabilizing the membrane potential during activity because when it is absent or markedly reduced the muscle fibres become myotonic (Bryant & Morales-Aguilera, 1971; Bretag, 1987; Lehmann-Horn & Jurkat-Rott, 1999).
In contrast to early reports on amphibian fibres (Eisenberg & Gage, 1969), experiments using osmotic shock to ‘detubulate’ mammalian muscle fibres found the almost complete loss of all chloride conductance, suggesting that the great majority of the total chloride conductance occurred across the t-system membrane (Dulhunty, 1979). However, some early reports were not fully consistent with this (see Bretag, 1987) and it is possible that the osmotic shock procedure reduced the chloride conductance by some other means, perhaps by activating protein kinase C, which is known to reduce the chloride conductance in mammalian fibres (Tricarico et al. 1991). Adding to the uncertainty on this issue, it is known that the great majority of the chloride conductance in mammalian muscle is due to the ClC-1 channel protein (Steinmeyer et al. 1991b), but this channel is putatively located exclusively on the sarcolemma and not in the t-system (Gurnett et al. 1995).
Here we use the mechanically skinned fibre preparation to investigate role and importance of the t-system chloride conductance in normal muscle function. In this preparation the sarcolemma is rolled back and completely removed, and the t-system seals off (Lamb et al. 1995) and can be repolarized by bathing the fibre in an appropriate ‘intracellular’ solution (Lamb & Stephenson, 1990). Electrical stimulation can then be used to trigger APs in the t-system, producing twitch and tetanic responses that are fully comparable with those in intact muscle fibres (Posterino et al. 2000; Dutka & Lamb, 2004). We have previously used this skinned fibre preparation to unequivocally demonstrate that there is an appreciable chloride conductance in the t-system of both mammalian and amphibian muscle fibres (Coonan & Lamb, 1998), and also to demonstrate that low intracellular pH reduces the t-system chloride conductance (Pedersen et al. 2004), which probably helps maintain muscle excitability during intense exercise (Nielsen et al. 2001). In this paper we examine the voltage-threshold behaviour and other properties of the skinned fibres in the absence and presence of the chloride conductance, and provide evidence that the majority of the total chloride conductance is probably located in the t-system and that its role is not just to lower excitability and prevent myotonia, but also to profoundly reduce the effects of K+ accumulation in the confined spaced of the t-system which would otherwise cause substantial depolarization and loss of excitability during normal muscle activity.
Methods
Preparations
With approval of the La Trobe University Animal Ethics Committee, male Long-Evans hooded rats (∼6–8 months old) were killed by overdose with fluothane (2% vol : vol) in a restricted air space. Both extensor digitorum longus (EDL) muscles were rapidly excised and pinned at resting length for 30 min at room temperature in an extracellular solution with or without Cl− (Cl−-containing solution (mm): NaCl, 135; KCl, 4; CaCl2, 2.5; MgCl2, 1; NaH2PO4, 0.3; Hepes, 10; pH to 7.2 with NaOH; zero-Cl− solution: sodium methylsulphate, 130; K2HDTA (hexamethylene-diamine-tetraacetic acid), 2; Ca HDTA, 2.5; Mg HDTA, 1; total HDTA2−, 10; pH to 7.2 with NaOH; the osmolality and concentrations of each cation were nearly identical in these two solutions). The muscles were then blotted dry and pinned out under paraffin oil and kept cool (∼10°C) on an ice pack. In control experiments other muscles were placed directly into paraffin oil after dissection and the properties of skinned fibres from these muscles were not noticeably different from those pre-soaked in the Cl−-based extracellular solution. In additional experiments one muscle was bathed for 30 min in the Cl−-based solution with 100 nm of phorbol 12,13-dibutyrate (added from a 100 μm stock in DMSO) and the contralateral muscle bathed in the same solution with DMSO only. Individual fibres from peripheral regions of the muscles were mechanically skinned by rolling back the sarcolemma with fine forceps (Lamb & Stephenson, 1994). A segment of the skinned fibre was then clamped at one end with fixed forceps and secured at the other end with fine silk thread to a force transducer (AME801, resonance frequency > 2 kHz; SensoNor, Horten, Norway) and set at 120% of resting length. The mounted skinned fibre segment (∼2 mm long, 30–50 μm diameter) was then transferred to a small Perspex well containing 2 ml of the appropriate K-HDTA-based ‘intracellular’ solution (see below) to equilibrate for 2 min. All experiments were conducted at ∼24°C.
Skinned fibre solutions
Skinned fibres were bathed in ‘intracellular’ solutions with or without Cl− in accordance with whether the whole muscle had been pre-equilibrated with or without Cl− present extracellularly. The standard K-HDTA intracellular solution with no Cl− contained (mm): HDTA2− (Fluka, Buchs, Switzerland), 48.5; total ATP, 8; creatine phosphate (CrP), 10; Na+, 36; K+, 126; total Mg2+, 8.5; total EGTA, 0.075; Hepes, 90; pH 7.1 and pCa (=−log10[Ca2+]) 6.9. This solution had an osmolality of 295 ± 5 mosmol (kg water)−1 and a calculated free [Mg2+] of 1 mm (Lamb & Stephenson, 1994). The matching intracellular solution with Cl− had 3 mm Cl− (with appropriate reduction in HDTA). The pCa of solutions (for pCa < 7.2) was measured with a Ca2+-sensitive electrode (Orion Research, Cambridge, MA, USA). In solutions where there was no Na+, it was replaced isosmotically by 20 mm Tris and 16 mm additional K+; Tris itself had no noticeable effects on excitation–contraction (EC) coupling. Where required, 100 μm 9-AC was added to the appropriate K-HDTA solution from a 100 mm stock made in DMSO, with the same amount of DMSO (0.1%) added to the matching control solutions. All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless specified otherwise.
Contractile apparatus properties
For examination of contractile apparatus properties, solutions similar to the zero-Cl− K-HDTA solution were made with all HDTA replaced by 50 mm EGTA (pCa > 10, ‘relaxing’ solution) or 50 mm CaEGTA (pCa ∼4.7, ‘max’ solution), with total Mg2+ adjusted to maintain 1 mm free (Stephenson & Williams, 1981). These two solutions were mixed in appropriate ratio to produce solutions with pCa in the range 6.7–4.7, and then this was added in a 1 : 9 ratio to the appropriate HDTA-based solution in order to strongly buffer the [Ca2+] at the desired level (with 5 mm total EGTA-CaEGTA). The skinned fibre segment was treated with Triton X-100 (1% v/v) in relaxing solution for 5 min to disrupt the SR and then thoroughly washed in relaxing solution. The fibre was then activated by exposure to sequences of solutions with progressively higher free [Ca2+] (pCa 9 to pCa 4.7), firstly in a set made with the zero-Cl− solution, and then a set with the 3 mm Cl− solution, and finally a set with the 3 mm Cl− solution with 100 μm 9-AC, with bracketing measurements made under the zero-Cl− conditions. Force elicited at each pCa in a sequence was expressed as a percentage of the corresponding maximum Ca2+-activated force, plotted against pCa and fitted with a Hill curve using GraphPad Prism 3 (GraphPad Software, San Diego, CA, USA), to determine the pCa producing 50% of maximum Ca2+-activated force (pCa50) and the Hill coefficient (h). The values for a given test condition were then expressed relative to the average of the bracketing examinations under the zero-Cl− conditions, in order to eliminate any changes associated with repeated activation or time.
Electrical stimulation
The skinned fibre segment was positioned parallel to, and midway between, two platinum electrodes in a stimulating chamber containing 130 μl of the appropriate K-HDTA solution. An in-house stimulator was used to apply rectangular electric field pulses of different strength and duration (0–125 V cm−1; duration, 1–5 ms) in order to generate APs in the sealed t-system and a resulting force response (Posterino et al. 2000). The voltage threshold at a given pulse duration for triggering an AP in the sealed t-system was defined as the lowest field strength needed to evoke > 50% of the maximum twitch force and the voltage threshold was determined for each individual fibre preparation for at least four stimulus durations. Stimulus strength–duration curves were then constructed by fitting rectangular hyperbolas to the data points:
| (1) |
where R, known as the rheobase, is the minimum strength of an indefinitely long rectangular stimulus capable of producing t-system excitation sufficient to elicit > 50% of maximum twitch force, and σ, known as the chronaxie, is the duration of the stimulating pulse when the threshold stimulus strength for producing excitation is twice the rheobase. Assuming that the time constant of the stimulus strength–duration curve for triggering an AP in the t-system is a good approximation of the time constant of the t-system membrane (Stephenson, 2006), the t-system membrane conductance (gts) can be estimated from the following relation (Stephenson, 2006):
| (2) |
where Cts is the t-system membrane capacitance per unit area assumed to be similar to that of other biological membranes (≈1 μF cm−2). The chloride conductance of the t-system, gCl, can then be calculated as:
| (3) |
where σ and σNCl are the chronaxie values for conditions when a chloride conductance was present and absent (or blocked), respectively.
Other examinations of twitch and tetanic force responses were conducted with the field strength set ∼2.5-fold above that required for maximum twitch force. To quantify the ability of the t-system to propagate two successive closely spaced APs (AP repriming measurement), pairs of pulses (duration, 1 ms; 75 V cm−1) were applied with the interpulse interval ranging between 1 and 20 ms, as previously described (Verburg et al. 2006; Dutka & Lamb, 2007b). If the second pulse in a pair was applied too soon for the t-system to sustain another AP, the twitch response would be little different to that for single pulse stimulation, indicating that the t-system membrane was still refractory at that time. If the second pulse did elicit an AP then the twitch response, a sensitive indicator of the amount of Ca2+ released, should increase substantially in size. The ‘repriming period’ was defined as the minimum time needed between two pulses for the twitch force to display > 50% of the maximum incremental increase in force obtainable with two successive pulses.
Caffeine-activation of Ca2+ release
The effect of 9-AC on the responsiveness to caffeine was examined in some fibres. The skinned fibre segment was first treated with saponin to permeabilize the t-system and prevent the caffeine responsiveness from being influenced by any effect of the 9-AC treatment on t-system properties (fibre treated with 50 μg ml−1 saponin in standard K-HDTA solution with 0.2 mm EGTA for 30 s, followed by extensive washing out of saponin). In a manner similar to that previously described (Lamb et al. 2001), the skinned fibre was then subjected to repeated cycles in which (i) the SR was fully depleted of releasable Ca2+ (by a 1 min exposure to a solution with 30 mm caffeine and 0.05 mm free Mg2+, with 0.5 mm free EGTA (pCa 8.5) to chelate the released Ca2+), (ii) the SR was reloaded to a set level (by 30 s in a solution with 1 mm CaEGTA-EGTA at pCa 6.7), (iii) the fibre was equilibrated for 20 s in the K-HDTA solution (3 mm Cl−, 50 μm EGTA, pCa 6.9, 1 mm free Mg2+) with or without 9-AC, and finally (iv) the responsiveness was tested by applying the same solution with 30 mm caffeine and measuring the resulting force response. The presence of 1 mm free Mg2+ greatly reduces the ability of caffeine to trigger Ca2+ release in mammalian skeletal muscle fibres (Fryer & Stephenson, 1996; Lamb et al. 2001), which is why the test responses to 30 mm caffeine were only ∼30–40% of maximum Ca2+-activated force whereas the SR could be fully depleted by caffeine if the free [Mg2+] was simultaneously lowered to 0.05 mm.
Statistics
Data are expressed as mean ± standard error of the mean (s.e.m.), with the number of fibres studied denoted as ‘n’. One way ANOVA with Bonferroni's multiple comparison post hoc test was used to test significance in the unpaired data sets in Tables 1 and 2. Student's paired t test was used to determine statistical significance in paired data sets. Significance was accepted at a probability value (P) < 0.05.
Table 1.
Effect of t-system Cl− conductance on voltage threshold, twitch size and repriming period
| Treatment | Threshold (V cm−1) | Twitch (%) | RP (ms) |
|---|---|---|---|
| 3 mm Cl− | 33.8 ± 1.2 (12)a,b | 19.4 ± 2.2 (13)a,b | ;4.8 ± 0.1 (13)a,b |
| 0 mm Cl− | 26.8 ± 2.1 (9)a | 33.9 ± 3.1 (17)a | 4.3 ± 0.2 (16)b,c |
| 3 mm Cl−+ phorbol | 26.0 ± 0.7 (5)b | 38.5 ± 2.9 (5)b | 5.8 ± 0.1 (5)a,c |
| Paired data | |||
| 3 mm Cl− | 32.5 ± 1.4 (6)* | 19.6 ± 4.5 (6)* | 5.0 ± 0.0 (6)* |
| 3 mm Cl−+ 9-AC | 25.5 ± 1.6 (6)* | 29.0 ± 5.3 (6)* | 4.3 ± 0.2 (6)* |
| 0 mm Cl− | 30.0 ± 2.3 (4) | 33.4. ± 2.7 (4) | 4.8 ± 0.3 (4) |
| 0 mm Cl−+ 9-AC | 28.1 ± 1.6 (4) | 33.0 ± 3.7 (4) | 4.8 ± 0.3 (4) |
| Phorbol-treated | |||
| 3 mm Cl− | 26.0 ± 0.7 (5) | 38.5 ± 2.9 (5) | 5.8 ± 0.1 (5) |
| 3 mm Cl−+ 9-AC | 24.8 ± 1.3 (5) | 39.8 ± 2.3 (5) | 5.6 ± 0.2 (5) |
Values are the mean (±s.e.m.), with number of fibres shown in parentheses. Twitch responses were elicited in skinned fibres with normal extracellular solution in the t-system and 3 mm Cl− in the intracellular bathing solution (3 mm Cl−) or with no Cl− in either the t-system or intracellular solution (0 mm Cl−) (see Methods). Voltage threshold determined with 1 ms pulses. Twitch force expressed as a percentage of maximum Ca2+-activated force in that fibre. Repriming period (RP) indicates the time between a pair of pulses required for the second pulse to also elicit an AP. Where indicated, measurements were made in same fibre both before and after addition of 100 μm 9-AC. Fibres obtained from muscles pre-exposed to phorbol ester where indicated. Values for the three unpaired treatments (3 mm Cl−, no Cl− and 3 mm Cl− in phorbol-treated muscle) were compared by one-way ANOVA followed by Bonferroni's multiple comparison post hoc test; values significantly different between two treatments are labelled with the same superscript (a, b or c).
Significant difference from indicated paired case (paired t test). Eliminating the chloride conductance by removing Cl−, or by treatment with 9-AC or phorbol ester, caused a decrease in voltage threshold and increase in twitch size, and, except for the phorbol treatment, a decrease in repriming period.
Table 2.
Effect of t-system Cl− conductance on response to tetanic stimulation
| Treatment | 50 Hz peak (% max) | 100 Hz/50 Hz (%) | 50 Hz fade (%) | 100 Hz fade (%) |
|---|---|---|---|---|
| 3 mm Cl− | 95.4 ± 1.3 (13) | 94.5 ± 1.1 (13) | 2.6 ± 0.7 (13)a | 12.8 ± 3.4 (13)a,b |
| 0 mm Cl− | 96.3 ± 2.0 (17) | 89.6 ± 1.9 (13) | 11.4 ± 3.0 (17)a | 36.7 ± 6.4 (13)a |
| 3 mm Cl−+ phorbol | 100 (5) | 87.5 ± 2.2 (5) | 9.2 ± 0.9 (5) | 38.9 ± 5.9 (5)b |
| Paired data | ||||
| 3 mm Cl− | 93.1 ± 4.9 (6) | 92.0 ± 2.1 (6)* | 4.3 ± 0.8 (6)* | 8.0 ± 1.8 (6)* |
| 3 mm Cl−+ 9-AC | 95.6 ± 1.4 (6) | 74.2 ± 6.6 (6)* | 8.0 ± 2.3 (6)* | 44.0 ± 7.8 (6)* |
| 0 mm Cl− | 99.7 ± 1.8 (4) | 88.6 ± 3.8 (4) | 11.3 ± 2.6 (4) | 34.8 ± 4.0 (4) |
| 0 mm Cl−+ 9-AC | 98.3 ± 3.2 (4) | 89.7 ± 3.1 (4) | 13.4 ± 2.7 (4) | 41.5 ± 3.5 (4) |
| Phorbol-treated | ||||
| 3 mm Cl− | 100 (5) | 87.5 ± 2.2 (5) | 9.2 ± 0.9 (5)* | 38.9 ± 5.9 (5)* |
| 3 mm Cl−+ 9-AC | 100 (5) | 84.6 ± 2.3 (5) | 13.3 ± 1.2 (5)* | 67.1 ± 4.8 (5)* |
Values are the mean (±s.e.m.), with number of fibres shown in parentheses, and similar arrangement as in Table 1. Responses elicited as in Fig. 3. Peak tetanic force at 50 Hz is expressed as a percentage of the maximum Ca2+-activated force measured in the same fibre following completion of all electrical stimulation; no statistical comparisons of this variable were made between different fibres. In the phorbol-treated fibres the 50 Hz tetanic force is given as 100% because the subsequently measured Ca2+-activated maximum was slightly lower. Peak tetanic force at 100 Hz was expressed relative to that at 50 Hz (denoted as 100 Hz/50 Hz); all of these values were significantly less than 100% (P < 0.05). ‘Fade’ was defined as the extent of force decline at the end of the 400 ms stimulation period relative to the peak of the tetanus. Values labelled with same superscript (a or b) were significantly different from each other by one-way ANOVA (as in Table 1); there were no significant differences between the cases with 0 mm Cl− and with 3 mm Cl−+ phorbol.
Significant difference from indicated paired case (paired t test).
Results
Effect of Cl− conductance on voltage threshold
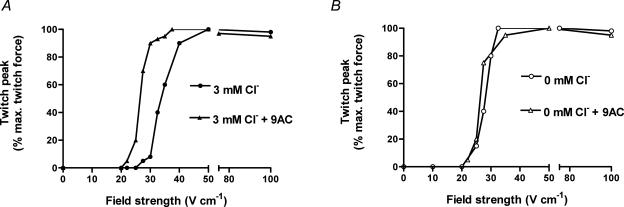
Twitch and tetanic force responses could be elicited by electric field stimulation in skinned fibres both with and without Cl− present. When Cl− was present in the t-system the intracellular [Cl−] was set at 3 mm, and in the zero-Cl− case there was no Cl− present in either the t-system or the intracellular solution, with Cl− replaced isosmotically by methylsulphate (in t-system) and HDTA2− (in intracellular solution). When Cl− was absent, the electric field strength needed to elicit a twitch response was significantly (P < 0.05) reduced; the mean threshold for 1 ms field pulses was 33.8 ± 1.2 V cm−1 (n = 12) in the presence of Cl− and 26.8 ± 2.1 V cm−1 (n = 9) in the absence of Cl− (Table 1). A similar effect was observed in individual fibres when the t-system Cl− channels were blocked by applying 100 μm 9-AC with Cl− still present (e.g. Fig. 1A and Table 1). Control experiments demonstrated that application of 9-AC in the absence of Cl− had no significant effect on the field strength threshold for twitch generation (e.g. Fig. 1B; Table 1). Skinned fibres obtained from muscles pre-treated with phorbol 12,13-dibutyrate, which blocks the chloride conductance in rat skeletal muscle by activating PKC (Tricarico et al. 1991), also had a significantly lower field strength threshold with Cl− present than did fibres from untreated muscles (Table 1). Furthermore, the threshold in these phorbol-treated fibres was not significantly different from that seen in fibres from untreated muscles in the absence of Cl−.
Figure 1. Relationship between force response and electric field strength in skinned EDL fibres.
A, force response elicited by a 1 ms pulse at the indicated field strength in a skinned fibre with Cl− present in the t-system and 3 mm Cl− in the ‘intracellular’ solution bathing the fibre, both before and after addition of 100 μm 9-AC. B, responses to similar pulses in another skinned EDL fibre in the absence of any Cl−. Addition of 9-AC, a Cl− channel blocker, reduced the field strength needed to elicit force in the presence of Cl− (A), but had little if any effect on responses in the absence of Cl− (B).
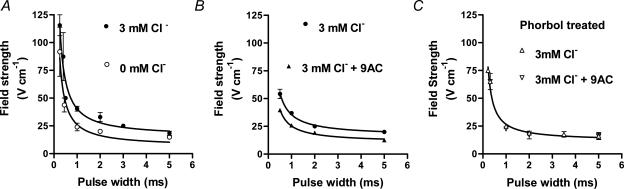
Threshold field strength was determined over a range of different pulse widths (0.2–5 ms) to produce strength–duration curves for the various conditions (Fig. 2), which were fitted by eqn (1) as described in Methods. For the fibres from the phorbol-treated muscles, the data with and without 9-AC were almost indistinguishable, and so both sets of data were fitted with the same curve (Fig. 2C). For the cases where Cl− was present and the channels unblocked, the overall values obtained for the rheobase (R) and chronaxie (σ), respectively, were 14.5 ± 5.4 V cm−1 and 1.55 ± 0.71 ms (n = 10), and for the cases where Cl− was absent or the channels blocked with 9-AC the values, respectively, were 7.5 ± 1.8 V cm−1 and 2.51 ± 0.72 ms (n = 15). Thus, the average rheobase value decreased by a factor of ∼1.9 when Cl− was absent or the Cl− channels blocked with 9-AC, indicating a markedly decreased t-system membrane conductance in the latter cases. Consistent with this, the average value of the t-system membrane conductance, calculated as described in the Methods, decreased from 447 μS cm−2 to 276 μS cm−2, indicating that under our conditions with 3 mm intracellular Cl− present, the t-system membrane Cl− conductance was ∼171 μS cm−2 (note that the values are expressed per square centimetre of t-system membrane, not fibre surface area – see Discussion).
Figure 2. Strength–duration curves in skinned EDL fibres in various conditions.
A, mean (±s.e.m.) threshold field strength for pulses of indicated width in fibres with Cl− present (•) or absent (^); n = 6 in both cases, with fibres dissected alternately from contralateral muscles bathed with or without Cl−. B, threshold field strength data for fibres with Cl− present, before (•) and after (^) addition of 100 μm 9-AC (n = 4). C, similar data with Cl− present in fibres obtained from muscles pre-treated with 100 nm phorbol 12,13-dibutyrate, before (▵) and after (∇) addition of 100 μm 9-AC (n = 5). Where Cl− was present the intracellular bathing solution contained 3 mm Cl−. In many instances the error bar is smaller than the symbol. Curves show the least-squares fits of eqn (1). R is in V cm−1 and σ in ms, respectively. A, 3 mm Cl−: 15.2 ± 4.0 and 1.66 ± 0.54 (r2= 0.989); 0 mm Cl−: 6.3 ± 4.4 and 3.17 ± 2.49 (r2= 0.986). B, 3 mm Cl−: 16.4 ± 1.1 and 1.18 ± 0.13 (r2= 0.999); 3 mm Cl−+ 100 μm 9-AC: 10.6 ± 1.0 and 1.39 ± 0.21 (r2= 0.998). C, 12.0 ± 2.1 and 1.24 ± 0.28 (r2= 0.994).
Effect of t-system chloride conductance on twitch responses
The peak size of the twitch response to supramaximal field stimulation was substantially higher when Cl− was absent compared to when Cl− was present (33.9 ± 3.1% and 19.4 ± 2.2% of maximum Ca2+-activated force, respectively; Table 1). This was not due to differences in the properties of the contractile apparatus in the two slightly different intracellular solutions, as maximum Ca2+-activated force was unchanged in the zero-Cl− solution (100.1 ± 0.3% of that in 3 mm Cl− solution, n = 5) and the Ca2+ sensitivity was in fact very slightly lower in the zero-Cl− solution (changes in pCa50 and h: −0.020 ± 0.002 and −0.3 ± 0.1, n = 5).
Blocking the t-system Cl− channels with 9-AC also caused a substantial increase in twitch size (e.g. Fig. 3A) (twitch increase from 19.6 ± 4.5% to 29.0 ± 5.3% of maximum Ca2+-activated force, Table 1), similar to that seen in the absence of Cl−. This effect of 9-AC was not attributable to an action on the contractile apparatus, the properties of which were altered only to a small degree and in the opposite way (with 9-AC present maximum Ca2+-activated force declined to 97.6 ± 0.4%, changes in pCa50 and h: −0.037 ± 0.006 and −0.6 ± 0.1, n = 5). Furthermore, the potentiating effect of 9-AC on the twitch response was evidently not due to direct effects of the drug on the Ca2+ release channels or on the SR, because additional experiments showed no significant effect of 9-AC on the force response when activating the release channels directly with caffeine (peak force: 32 ± 2% and 33 ± 4% of maximum Ca2+-activated force, without and with 9-AC, respectively, n = 3; relative change in paired data 1.04 ± 0.04, not significantly different). This conclusion is further supported by the fact that application of 9-AC caused no significant change in twitch size either when there was no Cl− present or when the fibres were from muscles pre-treated with phorbol 12,13-dibutyrate (Table 1). The twitch response in fibres from muscles pre-treated with phorbol 12,13-dibutyrate was also found to be significantly greater than in untreated muscles (38.5 ± 2.9% and 19.4 ± 2.2% of maximum Ca2+-activated force, respectively; Table 1). All the above data are fully consistent with the twitch response being potentiated in situations where the chloride conductance is eliminated, either by the absence of Cl− or by the block of the Cl− channels by 9-AC or pre-treatment with the phorbol ester.
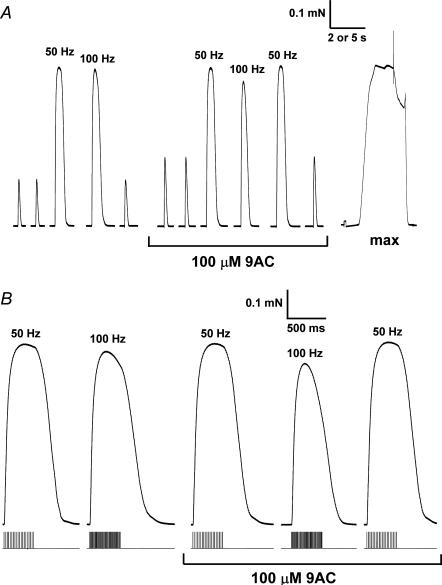
Figure 3. Effect of 100 μm 9-AC on twitch and tetanic responses in a skinned EDL fibre with Cl− present.
A, responses to supramaximal single pulse stimuli and trains of pulses at 50 or 100 Hz, before and after addition of 9-AC. Force response to maximum Ca2+-activation solution (‘max’) shown at right. Time scale: 2 s for twitch and tetani and 5 s for maximum Ca2+ activation. B, tetanic responses from A on an expanded time scale to highlight the fade of the response at 50 and 100 Hz (stimulus timing shown beneath each response).
The repriming time, which is the time interval needed between successive closely spaced stimuli for the t-system to be able to sustain a second AP and trigger additional Ca2+ release and a larger force response (Posterino et al. 2003; Dutka & Lamb, 2007b) (see Methods), was slightly reduced when there was no Cl− present or the Cl− channels were blocked with 9-AC (Table 1). This is consistent with the repriming time being briefer when the total t-system conductance is reduced by eliminating the Cl− conductance. The repriming time in fibres from muscles pre-treated with the phorbol ester was unaffected by applying 9-AC (Table 1), consistent with both treatments blocking the Cl− conductance; the mean values, however, were ∼1 ms longer than in fibres from untreated muscles, possibly indicating an additional action of the phorbol treatment on the t-system membrane properties.
Effect of t-system chloride conductance on tetanic responses
Tetanic stimulation at 50 Hz for 400 ms always gave close to maximum Ca2+-activated force (∼96% of maximum), both when Cl− was present or absent (e.g. Fig. 3; Table 2). Because maximum Ca2+-activated force was measured with heavily Ca2+-buffered solutions it could only be examined after all electrical stimulation had been completed. Consequently, as there was sometimes a small level of deterioration of the responses over the course of the whole experiment in some fibres, and as the treatment regimes differed between some groups of fibres, no particular significance should be attached to small differences in this measure between fibres or treatments. Instead, the key aspects are whether the time course and relative size of 100 Hz and 50 Hz tetanic responses in a given fibre were affected by the various treatments (columns 2–4 in Table 2). It appeared that tetanic responses were indeed adversely affected by the absence of Cl− conductance, particularly with 100 Hz stimulation. Blocking the t-system Cl− channels with 9-AC reduced the relative peak force at 100 Hz despite the twitch force being potentiated (e.g. Fig. 3) (100 Hz/50 Hz: 92.0 ± 2.1% and 74.2 ± 6.6%, respectively, before and after addition of 9-AC; Table 2), with 9-AC having no significant effect on this parameter in the absence of Cl− or in fibres from phorbol-treated muscles (Table 2). A similar trend was also apparent when comparing unpaired data for different treatments, with the mean value of the 100 Hz/50 Hz ratio being lower in the absence of Cl− (89.6 ± 1.9%) and in phorbol-treated fibres (87.5 ± 2.2%) than in control fibres with Cl− (94.5 ± 1.1%) (Table 2), though these differences did not reach the significance level (P only < 0.1).
When the 50 Hz stimulation was maintained for a total of 20 pulses (380 ms), the tetanic force generated by the skinned fibres reached a peak and then declined slightly by the end of the stimulation period (by 2.6 ± 0.7%, Cl− present, Table 2) (see Fig. 3B). This decline was defined as ‘fade’. Stimulation at 100 Hz for a similar duration (40 pulses, 390 ms) resulted in greater fade (12.8 ± 3.4%, Cl− present), probably in part because K+ built-up in the sealed t-system, causing some level of depolarization, which interfered with the ability of the t-system to sustain repeated APs separated by only 10 ms (Dutka & Lamb, 2007b). When Cl− was absent, the extent of fade was significantly increased for both 50 Hz and 100 Hz stimulation (11.4 ± 3.0% and 36.7 ± 6.4%, respectively, Table 2). Blocking the Cl− channels with 9-AC had a very similar effect if Cl− was present (Fig. 3B), but had no significant additional effect if Cl− was absent (Table 2). Fibres from phorbol-treated muscles showed levels of fade at 50 Hz and 100 Hz (9.2 ± 0.9% and 38.9 ± 5.9%, respectively) that were very similar to those observed both in the absence of Cl− and when 9-AC was added (Table 2). Addition of 9-AC in these fibres increased the level of fade, particularly with 100 Hz stimulation.
Effect of tetanic stimulation on following twitch responses
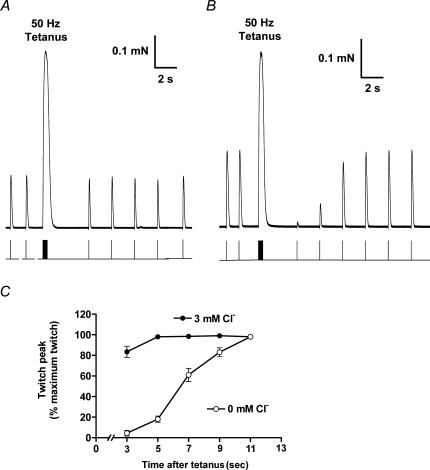
The effect on excitability of the changes in t-system ions occurring during a train of APs was further examined by subjecting a fibre to a single 50 Hz train lasting 400 ms and examining the responsiveness to single pulse stimulation over the following 10–20 s (e.g. Fig. 4). When Cl− was present, a single pulse applied ∼3 s after the 50 Hz train elicited a twitch response that was only slightly reduced compared to the pre-tetanus level (Fig. 4A), but when Cl− was absent the twitch response was almost fully abolished initially and only recovered to the pre-tetanus level after a total of ∼11 s (Fig. 4B and C). Very similar behaviour was also found when the chloride conductance was blocked by addition of 9-AC, with the twitch response being reduced to 15 ± 8% (of its pre-tetanus level) 3 s after the tetanus and recovering to > 95% after ∼15 s (n = 5).
Figure 4. Effect of one tetanic stimulation period on subsequent twitch responses.
Each fibre was subjected to a 400 ms period of 50 Hz stimulation (20 pulses) and then the twitch response examined ∼3 s later and then successively at 2 s intervals. When Cl− was present (A), the first twitch response after the 50 Hz train was only slightly reduced, but when Cl− was absent (B) the twitch response initially was almost completely abolished and then recovered to the pre-stimulation level only after a total of ∼11 s. C, mean (±s.e.m.) of twitch size for the sequence of twitch stimuli following the 50 Hz stimulation, normalized by the twitch size beforehand, in 5 fibres with Cl− present and 5 fibres with Cl− absent, examined with fibres from contralateral muscles alternately.
Effect on stopping the Na+–K+ pump
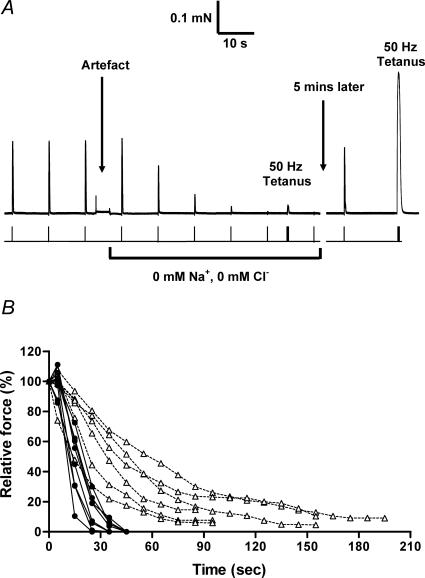
The importance of t-system Na+–K+ pump activity in maintaining fibre excitability, in both the presence and absence of Cl−, was examined by rapidly stopping all Na+–K+ pump function by changing the solution bathing the inside of the fibre to one with no Na+. The solution change should have lowered the [Na+] to extremely low levels within ∼2–3 s (Lamb & Stephenson, 1990; Nielsen et al. 2004). Single supramaximal pulses were applied 5 s after the solution change and then subsequently every 10 s. When Cl− was absent, the twitch response to single pulse stimulation was completely abolished in every case (e.g. Fig. 5A), dropping to less than 10% of the initial level after 3.6 ± 0.3 pulses (n = 9), that is, after a mean time of ∼31 s. The responses could be restored by reapplying the standard intracellular solution which contained some Na+ (see Methods). In contrast, in cases where Cl− was always present in t-system and intracellular solution, the twitch response to successive stimuli decreased much more slowly when the Na+–K+ pump was stopped (Fig. 5B), only dropping to less than 10% of the initial level after a mean of 12.7 ± 1.9 pulse applications (n = 6), that is, after ∼130 s, and in one case the twitch response was still not fully abolished after > 3 min. In some experiments the same fibre was subjected to two successive trials, one in which the test stimulus was applied every 10 s (as above) and the other in which the first stimulus was applied only 30 s or 60 s after stopping the pump; the rate of decline of the twitch response was found to be ∼25–30% faster when the fibre was stimulated every 10 s compared to when it was left unstimulated (for both Cl− and zero-Cl− cases), suggesting that the repeated AP stimulation increased the rate of accumulation of K+ in the t-system by only ∼25–30% above that occurring by time alone.
Figure 5. Effect on twitch responses of stopping the t-system Na+–K+ pump.
A, in a skinned fibre without Cl− present, the twitch response to successive stimuli, applied 10 s apart, declined to less than 10% of the control level within 30 s after stopping the Na+–K+ pump; the pump was stopped by substitution of a bathing solution with no Na+ (force artefact produced by this solution change). Twitch and tetanic response returned to close to initial levels after return of Na+. Stimulus timing shown beneath force trace. B, data of the relative size of the twitch response after stopping the Na+–K+ pump in fibres with (Δ) and without (•) Cl− present.
Discussion
t-system chloride conductance
By using skinned fibres in which the sarcolemma was removed but EC coupling retained, this study was able to investigate the properties and functional importance of the t-system chloride conductance. The threshold field strength for eliciting a twitch response in the skinned fibres was lowered substantially by each of the three distinct procedures designed to eliminate or greatly reduce the t-system chloride conductance: removal of all Cl−, addition of 9-AC (Bretag, 1987) or pre-treatment with phorbol 12,13-dibutyrate (Tricarico et al. 1991) (see Figs 1 and 2, and Table 1). Addition of 9-AC had no significant effect in the absence of Cl− or in phorbol-treated fibres, nor did it affect direct activation of Ca2+ release by caffeine, strongly suggesting that its action in lowering the threshold was due specifically to its blocking of the chloride conductance.
Fits to the strength–duration curves showed that the rheobase decreased approximately 2-fold when the chloride conductance was eliminated, and calculations based on the accompanying change in the chronaxie (see Methods) (Stephenson, 2006) gave a value for the t-system chloride conductance of ∼171 μS cm−2 of t-system membrane. This represents the t-system chloride conductance when the t-system was very well polarized (probably ∼−90 mV; Pedersen et al. 2004) and when there was a high [Cl−] in the t-system lumen (∼145 mm) and with the cytoplasmic [Cl−] set at 3 mm by the bathing solution. Measurements of the chloride conductance in intact fibres typically are expressed relative to the apparent surface area of the fibre, and the values reported for adult rat EDL muscle fibres are in the range of ∼1730 μS cm−2 (Pedersen et al. 2005) to ∼2730 μS cm−2 (Pierno et al. 2002). Thus, taking into account that there is approximately 5 cm2 of t-system membrane per square centimetre of apparent fibre surface in rat EDL fibres (Dulhunty et al. 1984, 1986), the t-system chloride conductance in the present study corresponds to ∼855 μS cm−2 of apparent fibre surface. In addition, the intracellular chloride concentration in intact muscle fibres is expected to be at or above the level determined by its passive electrochemical distribution (Dulhunty, 1978; Donaldson & Leader, 1984; McCaig & Leader, 1984), and consequently it was likely to be ∼10 mm or higher in the studies of Pedersen et al. (2005) and Pierno et al. (2002). Thus, given that the chloride conductance should increase approximately in proportion with the intracellular chloride concentration, the chloride conductance found in the skinned fibres here would correspond to > 2800 μS cm−2 under the conditions prevailing in the intact fibre experiments. There is substantial uncertainty in the accuracy of this extrapolation, however, and it is reasonable to conclude only that the chloride conductance measured in the t-system of the fibres here appears to account for a large proportion of the total chloride conductance in rat EDL fibres. This fits well with the findings of a study in which rat EDL fibres were detubulated by osmotic shock, where it was concluded that the great majority of the total chloride conductance occurred across the t-system membrane (Dulhunty, 1979).
Role of chloride in AP repolarization current
When all movement of Cl− across the t-system membrane was stopped, either by removing Cl− or blocking the Cl− channels with 9-AC or phorbol-treatment, the twitch force to a single AP stimulus was substantially potentiated, from ∼20% to ∼34% of maximum Ca2+-activated force (Table 1; Fig. 3A). This effect was not due to the changes in the properties of the contractile apparatus and was evidently due to the release of a larger amount of Ca2+ from the SR. Based on our previous studies using this same skinned fibre preparation in which the amount of Ca2+ release per AP was matched to the size of the twitch response (Dutka & Lamb, 2004; Dutka et al. 2005), the increase in twitch force found here corresponds to approximately a 5–7% increase in Ca2+ release per AP. This increase in AP-induced Ca2+ release when the Cl− conductance was abolished is only readily explained by prolongation of the time course of the AP, and this indicates that part of the current involved in the repolarization phase of the t-system AP in the EDL fibres is normally carried by Cl− ions moving out of the t-system. This is similar to the conclusion reached in a study using potentiometric dyes to monitor the t-system AP in frog muscle fibres (Heiny et al. 1990). From the present results, however, it is not possible to say what proportion of the repolarization current is normally carried by Cl−. This is because the Ca2+ release to an AP stimulus is to a substantial extent self-limiting, as the released Ca2+ (i) shortens the duration of the AP, probably by activating Ca2+-activated K+ channels in the t-system, (ii) speeds deactivation of the voltage-sensors, and also (iii) inactivates the Ca2+-release channels themselves (Pape et al. 1998). Hence, eliminating the Cl− repolarization current would be expected to cause only a relatively small increase in the amount of Ca2+ released by an AP.
Role of t-system Cl− conductance in maintaining excitability
The major role of the t-system Cl− conductance in maintaining excitability was evident when the fibres were subjected to trains of closely spaced stimuli. When there was no t-system chloride conductance and the skinned fibre was stimulated at 100 Hz, tetanic force failed to reach the maximum level and declined substantially before the end of the train (‘fade’) (Fig. 3B and Table 2), indicative that the Ca2+ release was markedly reduced at later stages of the 40 pulse stimulus train. Similar results were found with each of the three methods used to abolish the chloride conductance (Table 2). Furthermore, when there was no chloride conductance, a 20 pulse train of stimuli at 50 Hz rendered the skinned fibres largely unresponsive to stimulation for several seconds, with the response only fully recovering after ∼11 s (Fig. 4). When there was no Cl− conductance all the repolarization current for each AP must have been carried by K+, and it appears that sufficient K+ accumulated within the t-system during a 20 pulse train so as to depolarize the t-system to a level where APs could no longer be elicited, that is, to more positive than ∼−55 mV (Filatov et al. 2005). This indicates that the 20 pulse train must have raised the [K+] in the t-system from its resting level of ∼3 mm (when the t-system was polarized to ∼−90 mV) to ∼12 or 13 mm, which implies that the K+ influx upon each AP must have been at least ∼0.5 mm. Additional experiments (not shown) indicated that some Ca2+ release was still occurring on the tenth stimulus in the train, which puts the maximum K+ influx per AP at < 1 mm. This amount of K+ influx (0.5–1 mm per AP) is quite comparable with the value of ∼0.4 mm per AP calculated to occur in the t-system of frog muscle fibres (Kirsch et al. 1977), particularly given that the cross-sectional area of the t-tubules in rat EDL fibres is approximately half of that in amphibian fibres (Dulhunty, 1984).
In comparison when the chloride conductance was functional, t-system excitability was little affected during and after the trains of stimuli, and the tetanic force responses was appreciably greater, particularly at higher stimulus frequency (Figs 3 and 4, and Table 2). This clearly demonstrates that the t-system chloride conductance normally plays a vital role in preventing the t-system from becoming depolarized during a train of APs. The presence of the chloride conductance would help preserve excitability in several ways. Firstly, the fact that some of the repolarizing current was carried by Cl− would reduce the amount carried by K+, and hence reduce the rate of accumulation of K+ in the t-system. Secondly, and very importantly, the relatively high chloride permeability of the t-system membrane means that membrane potential will be strongly biased towards the chloride equilibrium potential (Hodgkin & Horowicz, 1959), which would change little in the short term, as the Cl− efflux out of the t-system during AP repolarization would not greatly change the [Cl−] in either the t-system or the intracellular space. Thus, the depolarizing effect of any accumulation of K+ in the t-system would be greatly reduced. Thirdly, whenever K+ did build up appreciably in the t-system the mismatch between its equilibrium potential and the t-system potential would result in K+ being driven back into the intracellular space through the inward rectifier K+ channels (Wallinga et al. 1999), which are present in high density in the t-system membrane (Kristensen et al. 2006). In this way the Cl− electrochemical gradient built-up over a prolonged period in a resting muscle fibre acts to buffer any acute changes in [K+] occurring in the t-system, thereby avoiding the substantial depolarizing effects that would otherwise ensue, and helping maintain t-system excitability and tetanic force responses. The overall importance of the chloride conductance in preserving muscle excitability during intense activity is also suggested by the findings of Cairns et al. (2004), though that study did not examine whether the effects were predominantly due to chloride conductance in the surface membrane or to that in the t-system.
Chloride conductance and the Na+–K+ pump
The effectiveness of the t-system Cl− conductance in maintaining t-system excitability was also evident when the Na+–K+ pumps were rapidly stopped by removal of all intracellular Na+. When there was no Cl− conductance to help reduce K+ accumulation in the t-system and to shunt its depolarizing action, the skinned fibre became inexcitable after only ∼30 s, whereas when the chloride conductance was functional this occurred ∼3–4 times more slowly (Fig. 5). In intact fibres the t-system membrane properties play a very large role in determining the membrane potential, and the same factors as described above would be operating in the intact fibre, and in addition the [K+] and [Cl−] concentrations in the t-system would also be influenced by the extra-fibre concentrations through the open t-system connections to the extracellular space. In the long term the continued ability of chloride movements to help maintain fibre excitability depends on the maintenance of the [Cl−] gradient, which in turn depends on whether the Na+–K+ pump is able to maintain the [K+] gradient in the face of the continued overall muscle activity. The activity of the Na+–K+ pump is strongly stimulated by elevated intracellular [Na+] and also by muscle activity (Clausen, 2003). Approximately half of all the Na+–K+ pumps in a rat EDL fibre are located in the t-system, and this density of pumps is estimated to be able to clear K+ out of the t-system at a maximal rate of ∼4 mm s−1 (Nielsen et al. 2006). In the experiment in Fig. 4 where Cl− was absent, the recovery of excitability depended solely on the ability of the Na+–K+ pump to remove K+ from the t-system, and these data allow the rough estimate that ∼10 mm K+ is transported out of the t-system in ∼11 s (i.e. the [K+] decreased from ∼13 mm to 3 mm to fully restore excitability). This is ∼25% of the estimated maximum pump rate, and this seems entirely plausible because the pumps in the skinned fibre may not be operating at the maximal rate owing to the possible absence of normal endogenous cofactors and pump stimuli (Clausen, 2003) and also, as shown recently, because the pumps in the t-system require local glycolytically produced ATP, not just ATP from the cytoplasm, in order to operate at their maximum rate (Dutka & Lamb, 2007a).
ClC-1 and the chloride conductance
The findings here suggest that the channel responsible for the t-system chloride conductance is likely to be ClC-1 or some closely related protein. The properties of the conductance here are fully consistent with those of ClC-1. We have previously shown that the t-system chloride conductance is reduced ∼2-fold when the pH is lowered from 7.1 to 6.6 (Pedersen et al. 2004), and this is very similar to that found recently for expressed ClC-1 channels in comparable conditions with physiological levels of ATP (Bennetts et al. 2007; Tseng et al. 2007). Further we show here that the chloride conductance was blocked by 100 μm 9-AC and also by pre-treating the muscle with 100 nm phorbol 12,13-dibutyrate to stimulate PKC, both of which are known properties of ClC-1 (Steinmeyer et al. 1991b; Rosenbohm et al. 1999). Finally, mutation of ClC-1 leads to the loss of most of the chloride conductance in muscle fibres (Mehrke et al. 1988; Steinmeyer et al. 1991a) and hence, given that most of the total chloride conductance in mammalian fibres appears to be located in the t-system (results here and Dulhunty, 1979), this implies that ClC-1 must be responsible for much, if not all, of the t-system chloride conductance. Gurnett et al. (1995) reported finding evidence of ClC-1 only in the sarcolemma and not in the t-system. However, that study used an antibody to the C-terminal of ClC-1 and the authors noted that their experiments would not have detected splice variants of ClC-1 with a different or truncated C-terminal.
Concluding remarks
This study has provided evidence of the existence of a substantial chloride conductance in the t-system of rat EDL fibres, which probably constitutes much of the total chloride conductance in the muscle fibres and which has characteristics consistent with it arising from ClC-1 or closely related channel proteins. Furthermore, this study unequivocally demonstrates that the chloride conductance of the t-system plays a vital role in maintaining normal muscle excitability, most likely by reducing both the accumulation of K+ in the t-system and its depolarizing effect.
Acknowledgments
We thank Mr Brian Taylor for designing and building the in-house stimulator used here. This work was supported by the National Health & Medical Research Council of Australia (grants 280623 and 433034).
References
- Bennetts B, Parker MW, Cromer BA. Inhibition of skeletal muscle ClC-1 chloride channels by low intracellular pH and ATP. J Biol Chem. 2007;282:32780–32791. doi: 10.1074/jbc.M703259200. [DOI] [PubMed] [Google Scholar]
- Bretag AH. Muscle chloride channels. Physiol Rev. 1987;67:618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Bryant SH, Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J Physiol. 1971;219:367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP, Ruzhynsky V, Renaud JM. Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C762–C770. doi: 10.1152/ajpcell.00589.2003. [DOI] [PubMed] [Google Scholar]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Coonan JR, Lamb GD. Effect of transverse-tubular chloride conductance on excitability in skinned skeletal muscle fibres of rat and toad. J Physiol. 1998;509:551–564. doi: 10.1111/j.1469-7793.1998.551bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson PJ, Leader JP. Intracellular ionic activities in the EDL muscle of the mouse. Pflugers Arch. 1984;400:166–170. doi: 10.1007/BF00585034. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J Physiol. 1978;276:67–82. doi: 10.1113/jphysiol.1978.sp012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF. Distribution of potassium and chloride permeability over the surface and T-tubule membranes of mammalian skeletal muscle. J Membr Biol. 1979;45:293–310. doi: 10.1007/BF01869290. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. Heterogeneity of T-tubule geometry in vertebrate skeletal muscle fibres. J Muscle Res Cell Motil. 1984;5:333–347. doi: 10.1007/BF00713111. [DOI] [PubMed] [Google Scholar]
- Dulhunty A, Carter G, Hinrichsen C. The membrane capacity of mammalian skeletal muscle fibres. J Muscle Res Cell Motil. 1984;5:315–332. doi: 10.1007/BF00713110. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Gage PW, Lamb GD. Differential effects of thyroid hormone on T-tubules and terminal cisternae in rat muscles: an electrophysiological and morphometric analysis. J Muscle Res Cell Motil. 1986;7:225–236. doi: 10.1007/BF01753555. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Effect of low cytoplasmic [ATP] on excitation–contraction coupling in fast-twitch muscle fibres of the rat. J Physiol. 2004;560:451–468. doi: 10.1113/jphysiol.2004.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Na+–K+ pumps in the transverse-tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am J Physiol Cell Physiol. 2007a;293:C967–C977. doi: 10.1152/ajpcell.00132.2007. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Transverse tubular system depolarization reduces tetanic force in rat skeletal muscle fibers by impairing action potential repriming. Am J Physiol Cell Physiol. 2007b;292:C2112–C2121. doi: 10.1152/ajpcell.00006.2007. [DOI] [PubMed] [Google Scholar]
- Eisenberg RS, Gage PW. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969;53:279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov GN, Pinter MJ, Rich MM. Resting potential-dependent regulation of the voltage sensitivity of sodium channel gating in rat skeletal muscle in vivo. J Gen Physiol. 2005;126:161–172. doi: 10.1085/jgp.200509337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, Kahl SD, Anderson RD, Campbell KP. Absence of the skeletal muscle sarcolemma chloride channel ClC-1 in myotonic mice. J Biol Chem. 1995;270:9035–9038. doi: 10.1074/jbc.270.16.9035. [DOI] [PubMed] [Google Scholar]
- Heiny JA, Valle JR, Bryant SH. Optical evidence for a chloride conductance in the T-system of frog skeletal muscle. Pflugers Arch. 1990;416:288–295. doi: 10.1007/BF00392065. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch GE, Nichols RA, Nakajima S. Delayed rectification in the transverse tubules: origin of the late after-potential in frog skeletal muscle. J Gen Physiol. 1977;70:1–21. doi: 10.1085/jgp.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M, Hansen T, Juel C. Membrane proteins involved in potassium shifts during muscle activity and fatigue. Am J Physiol Regul Integr Comp Physiol. 2006;290:R766–R772. doi: 10.1152/ajpregu.00534.2004. [DOI] [PubMed] [Google Scholar]
- Kwiecinski H, Lehmann-Horn F, Rudel R. The resting membrane parameters of human intercostal muscle at low, normal, and high extracellular potassium. Muscle Nerve. 1984;7:60–65. doi: 10.1002/mus.880070110. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol. 2001;531:715–728. doi: 10.1111/j.1469-7793.2001.0715h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation–contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- McCaig D, Leader JP. Intracellular chloride activity in the extensor digitorum longus (EDL) muscle of the rat. J Membr Biol. 1984;81:9–17. doi: 10.1007/BF01868805. [DOI] [PubMed] [Google Scholar]
- Mehrke G, Brinkmeier H, Jockusch H. The myotonic mouse mutant ADR: electrophysiology of the muscle fiber. Muscle Nerve. 1988;11:440–446. doi: 10.1002/mus.880110505. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Macdonald WA, Stephenson DG, Clausen T. Distribution of Na+–K+ pumps in skeletal muscle and its significance for maintenance of T-tubular K+ homeostasis. Proc Physiol Soc. 2006;4:C4. [Google Scholar]
- Nielsen OB, Ortenblad N, Lamb GD, Stephenson DG. Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+–K+ pump activity. J Physiol. 2004;557:133–146. doi: 10.1113/jphysiol.2003.059014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape PC, Jong DS, Chandler WK. Effects of partial sarcoplasmic reticulum calcium depletion on calcium release in frog cut muscle fibers equilibrated with 20 mM EGTA. J Gen Physiol. 1998;112:263–295. doi: 10.1085/jgp.112.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol. 2005;125:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Pierno S, Desaphy JF, Liantonio A, De Bellis M, Bianco G, De Luca A, Frigeri A, Nicchia GP, Svelto M, Leoty C, George AL, Jr, Camerino DC. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain. 2002;125:1510–1521. doi: 10.1093/brain/awf162. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Cellini MA, Lamb GD. Effects of oxidation and cytosolic redox conditions on excitation–contraction coupling in rat skeletal muscle. J Physiol. 2003;547:807–823. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD, Stephenson DG. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J Physiol. 2000;527:131–137. doi: 10.1111/j.1469-7793.2000.t01-2-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbohm A, Rudel R, Fahlke C. Regulation of the human skeletal muscle chloride channel hClC-1 by protein kinase C. J Physiol. 1999;514:677–685. doi: 10.1111/j.1469-7793.1999.677ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K, Klocke R, Ortland C, Gronemeier M, Jockusch H, Grunder S, Jentsch TJ. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991a;354:304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991b;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Stephenson DG. Tubular system excitability: an essential component of excitation–contraction coupling in fast-twitch fibres of vertebrate skeletal muscle. J Muscle Res Cell Motil. 2006;27:259–274. doi: 10.1007/s10974-006-9073-6. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Lamb GD, Stephenson GM. Events of the excitation-contraction-relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D, Conte Camerino D, Govoni S, Bryant SH. Modulation of rat skeletal muscle chloride channels by activators and inhibitors of protein kinase C. Pflugers Arch. 1991;418:500–503. doi: 10.1007/BF00497778. [DOI] [PubMed] [Google Scholar]
- Tseng PY, Bennetts B, Chen TY. Cytoplasmic ATP inhibition of ClC-1 is enhanced by low pH. J Gen Physiol. 2007;130:217–221. doi: 10.1085/jgp.200709817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg E, Dutka TL, Lamb GD. Long-lasting muscle fatigue: partial disruption of excitation–contraction coupling by elevated cytosolic Ca2+ concentration during contractions. Am J Physiol Cell Physiol. 2006;290:C1199–C1208. doi: 10.1152/ajpcell.00469.2005. [DOI] [PubMed] [Google Scholar]
- Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED, Ypey DL. Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J. 1999;28:317–329. doi: 10.1007/s002490050214. [DOI] [PubMed] [Google Scholar]