Abstract
The physiological roles of constituitively expressed nitric oxide synthase (NOS) isoforms in humans, in vivo, are unknown. Cutaneous vasodilatation during both central nervous system-mediated, thermoregulatory reflex responses to whole-body heat stress and during peripheral axon reflex-mediated, local responses to skin warming in humans depend on nitric oxide (NO) generation by constituitively expressed NOS of uncertain isoform. We hypothesized that neuronal NOS (nNOS, NOS I) effects cutaneous vasodilatation during whole-body heat stress, but not during local skin warming. We examined the effects of the nNOS inhibitor 7-nitroindazole (7-NI) administered by intradermal microdialysis on vasodilatation induced by whole-body heat stress or local skin warming. Skin blood flow (SkBF) was monitored by laser–Doppler flowmetry (LDF). Blood pressure (MAP) was monitored and cutaneous vascular conductance calculated (CVC = LDF/MAP). In protocol 1, whole-body heat stress was induced with water-perfused suits. In protocol 2, local skin warming was induced through local warming units at LDF sites. At the end of each protocol, 56 mm sodium nitroprusside was perfused at microdialysis sites to raise SkBF to maximal levels for data normalization. 7-NI significantly attenuated CVC increases during whole-body heat stress (P < 0.05), but had no effect on CVC increases induced by local skin warming (P > 0.05). These diametrically opposite effects of 7-NI on two NO-dependent processes verify selective nNOS antagonism, thus proving that the nNOS isoform affects NO increases and hence vasodilatation during centrally mediated, reflex responses to whole-body heat stress, but not during locally mediated, axon reflex responses to local skin warming. We conclude that the constituitively expressed nNOS isoform has distinct physiological roles in cardiovascular control mechanisms in humans, in vivo.
Over the last decade, a myriad of functions for nitric oxide (NO) have been elucidated in human physiological and pathophysiological processes. Indeed, the apparent surfeit of functions attributed to NO raises the question of how such a simple molecule can exert such widespread, yet diverse control over numerous bodily functions. It is now appreciated that the plethora of mechanistic roles attributed to NO derives from its generation by the different nitric oxide synthase (NOS) isoforms: neuronal NOS (nNOS, NOS I), inducible NOS (iNOS, NOS II), and endothelial NOS (eNOS, NOS III). These isoforms are found in numerous tissues, in a variety of cellular locations, with multiple regulatory controls (Murad, 2006). Recently, efforts to differentiate the various roles for the NOS isoforms have come to the fore. For example, work in animal models has revealed that nNOS may be a key component in autonomic control mechanisms (Sears et al. 2004); however, to date, the mechanistic involvement of the different NOS isoforms in human physiology, in vivo, remains unknown.
The human skin represents a tissue with demonstrated roles for NO generation by constituitively expressed NOS in whole-body thermoregulatory reflexes and in local control of skin vessels (Kellogg, 2006). Thermoregulatory reflex control of the vasculature in non-glabrous regions of human skin is accomplished by dual innervation from the sympathetic nervous system (Lewis & Pickering, 1931; Grant & Holling, 1938). One branch is an active vasoconstrictor system that relies on noradrenaline and neuropeptide Y (NPY) as neurotransmitters and decreases skin blood flow (SkBF) in response to periods of whole-body cold stress to conserve body heat (Kellogg et al. 1989; Stephens et al. 2004; Kellogg, 2006). The second branch is a powerful active vasodilator system that is capable of directing 60% of cardiac output, or approximately 8 l min−1 of blood flow, to skin during periods of whole-body heat stress to dissipate body heat (Fox & Edholm, 1963; Rowell, 1977; Johnson & Proppe, 1996). This vasodilator system is made up of sympathetic cholinergic nerves that release co-transmitters to cause cutaneous active vasodilatation. The neurotransmitters involved in cutaneous active vasodilatation include acetylcholine (ACh), possibly vasoactive intestinal peptide (VIP), as well as others (Kellogg et al. 1995; Bennett et al. 2003; Wong et al. 2004). Studies with non-isoform specific NOS antagonists and direct measurements of NO demonstrated that NO generation by NOS mediates a significant portion of the increased SkBF induced by whole-body heating, demonstrating that cutaneous active vasodilatation is an NO-dependent process (Kellogg et al. 1998, 2003; Shastry et al. 1998, 2000; Wilkins et al. 2003). While it is clear that production of NO by NOS causes at least 30% of cutaneous active vasodilatation during whole-body heat stress (Dietz et al. 1994; Kellogg et al. 1998, 2003; Shastry et al. 1998; Wilkins et al. 2003), it is unclear which NOS isoforms are involved in the process.
NO generation by constituitively expressed NOS has also been found to be requisite for the vasodilatation induced by local application of heat to the skin. Local warming of the skin causes a local vasodilatation in two phases: an initial phase that appears to be dependent on antidromic release of neurotransmitter release by afferent sensory nerves (Minson et al. 2001) and a second prolonged plateau phase mediated by NO generation (Kellogg et al. 1999; Minson et al. 2001). The vasodilatation induced by local skin warming thus involves both local axon reflex mechanisms and NO generation by NOS and is capable or increasing SkBF to maximal levels (Pérgola et al. 1993; Minson et al. 2001; Kellogg, 2006). As with whole-body heat stress, it is unclear which NOS isoforms are involved in the vasodilatation induced by local skin warming because all studies have relied on non-isoform-specific NOS antagonists (Kellogg, 2006).
Of the three isoforms of NOS, two are constituitively expressed in human skin: eNOS and nNOS (Bruch-Gerharz et al. 1998; Cas-Grierson & Ormerod, 2004); thus either or both of these isoforms could generate the NO required for active vasodilatation during thermoregulatory responses to heat stress or to local warming of the skin. The eNOS isoform has been found in the endothelial cells of the cutaneous microvasculature (Zancanaro et al. 1999). The nNOS isoform has been found co-localized with VIP in cholinergic nerves (Ventura et al. 1997) as well as in sweat glands (Zancanaro et al. 1999). It is highly unlikely that iNOS mediates any increase in NO in skin during heat stress because while this isoform has been detected in human skin, it is present in miniscule amounts when compared with nNOS and eNOS (Wang et al. 1996). Only inflammatory processes, such as skin burns or wounds, increase iNOS levels to those of nNOS and eNOS (Levin et al. 1996). In addition, 24 h or longer are required for sufficient expression of iNOS to generate increases in NO levels (Seo et al. 2002). These observations argue against a role for iNOS in cutaneous active vasodilatation. In addition, iNOS is a calcium-independent enzyme that produces large amounts of NO continuously from the time of expression until the enzyme is degraded (Kleinhart et al. 2004). This relatively uncontrolled aspect of iNOS contrasts greatly with the highly regulated production of NO through the calcium-activated nNOS and eNOS isoforms (Kleinhart et al. 2004). Therefore, of the three NOS isoforms, nNOS and eNOS are the two isoforms with potential vasomotor control roles in human skin.
Prior observations show that abolition of neurotransmitter release from cutaneous nerves by cutaneous nerve blockade or botulinum toxin eliminates cutaneous vasodilatation induced by whole-body heat stress (Pérgola et al. 1993; Kellogg et al. 1995; Minson et al. 2001). In direct contrast, neither cutaneous nerve blockade nor botulinum toxin altered skin vasodilatation induced by local skin warming (Pérgola et al. 1993; Kellogg et al. 1995; Minson et al. 2001). As functioning cutaneous cholinergic nerves are requisite for cutaneous active vasodilatation, but not for increasing SkBF during local skin warming, these vasomotor responses are probably mediated by fundamentally different mechanisms. Given that both cutaneous active vasodilator and local warming responses require NO generation by NOS, it is likely that the NOS isoforms involved in the two distinct types of cutaneous vasodilatation are different. As cutaneous active vasodilatation is mediated by cholinergic nerve co-transmission (Kellogg et al. 1995), the foregoing data suggest the involvement of nNOS in cutaneous vascular responses to whole-body heat stress. Since the vasodilatation induced by local skin heating is not mediated by cholinergic nerve co-transmission, the foregoing also suggests that nNOS is not involved in locally mediated, axon reflex responses. We sought to test the hypotheses that the nNOS isoform generates NO during thermoregulatory reflex vasodilatation, centrally mediated responses to whole-body heat stress, but not during increases in SkBF effected by local warming of the skin.
Methods
To test our hypotheses, we examined whether increases in SkBF during heat stress (Protocol 1) and local skin warming (Protocol 2) were altered by blockade of nNOS with the selective antagonist 7-nitroindazole (7-NI). 7-NI is minimally soluble in purely aqueous solution and therefore it is often dissolved in vehicles that contain dimethyl sulfoxide (DMSO) (Kurihara et al. 1998). For example, Shastry et al. (Shastry & Joyner, 2002) used the addition of DMSO to microdialysis perfusates to enhance aqueous solubility of geldanamycin successfully, although they did find DMSO to possess minor vasodilating properties. In a series of preliminary experiments, we tested the effect of 5% DMSO in Ringer solution on SkBF under normothermic, cold stress, and heat stress conditions identical to those used subsequently in protocol 1 (see below). Our findings confirmed that DMSO possessed some vasodilating effects in normothermia (Shastry & Joyner, 2002). Under such conditions, the mean SkBF increase induced by DMSO was 10 ± 2%. This concentration of DMSO did not alter reductions in SkBF in response to cold stress. Heat stress responses were also unaltered by the addition of 5% DMSO to the microdialysis perfusate as evidenced by SkBF reaching 65 ± 6% of maximal cutaneous vascular conductance (%max CVC) at sites perfused with Ringer solution alone and 64 ± 5%max CVC at sites perfused with 5% DMSO in Ringer solution in our preliminary studies. As 5% DMSO allowed significantly higher concentrations of 7-NI in Ringer solution than were otherwise possible, yet did not alter thermoregulatory reflex responses, we chose to use 5% DMSO in Ringer solution as our vehicle.
Based on our preliminary experiments, we chose a 2 mm concentration of 7-NI for our studies; the maximal concentration of 7-NI possible in solution with 5% DMSO in Ringer solution. We did not increase our concentration of 7-NI further because this would have required increased concentrations of DMSO, consequently increasing the potential for confounding vasodilator effects of this agent on baseline blood flow. The 7-NI was administered by intradermal microdialysis, a technique that permits the local administration of pharmacological agents into a small volume of skin. This technique allows a relatively large local drug concentration to exert purely local effects at the site of administration with no risk of systemic effects. Intradermal microdialysis probes of our own manufacture were used. Each probe was made from polyimide tubing with a 1 cm length of capillary microdialysis membrane (regenerated cellulose, 200 μm diameter, molecular cut-off 20 kDa) with reinforcement by a 51 μm diameter stainless steel wire inserted through the lumen of the membrane and tubing. Microdialysis probes were placed approximately 5 cm apart in the ventral forearm skin so that manipulations at one site did not influence the others. To place the microdialysis probe, a 25 gauge needle was inserted through the dermis using sterile technique after the topical application of ice for anaesthesia. Entry and exit points for each probe were approximately 2–3 cm apart. The microdialysis probe was threaded through the lumen of the needle that was then withdrawn, leaving the microdialysis probe in place (Kellogg et al. 1998). The microdialysis membrane was situated entirely within the dermis, with entry and exit points through the skin via the polyimide tubing. In prior studies, ultrasound measurements revealed that probes placed with such a technique were 0.3–1.0 mm under the epidermal surface, and thus were within the dermal layer (Kellogg et al. 1999). The microdialysis probes were perfused with Ringer solution at a rate of 2 μl min−1 using a microinfusion pump (Harvard Apparatus, South Natick, MA, USA). Subjects then waited 140 min or more to allow for insertion trauma to resolve before additional instrumentation was placed (Anderson et al. 1994). After insertion trauma resolved, subjects were placed in the supine position and instrumented to monitor SkBF by laser–Doppler flowmetry (LDF) at all microdialysis sites (Moorlab Flowmeter, Moor Instruments Ltd, Devon, UK). A Finapres cuff was placed on a finger for pulse rate (PR) and mean arterial pressure (MAP) measurement by photoplethysmography (Ohmeda, Madison, WI, USA).
All subjects who volunteered for the protocols were in good health, were non-smokers, and were taking no medications. Prior to participation, written, informed consent was obtained from all subjects. The studies conformed to the standards set by the Declaration of Helsinki and institutional ethics committees approved all procedures. Subjects were instructed to forego caffeinated products on the day of the study. The menstrual phase of the female subjects was not assessed. All studies were performed in an air-conditioned laboratory with a constant ambient temperature of 24°C. Studies were done at the same time of day to control for any circadian variations in responses (Aoki et al. 2001).
Protocol 1
Ten subjects (3 male, 7 female) participated in the first protocol of the study. Their average age was 34 ± 3 years, average weight was 66 ± 5 kg, and average height was 171 ± 3 cm.
To induce thermoregulatory reflexes, subjects wore a tube-lined suit. The suit was used to control skin temperature (Tsk) and hence whole-body temperature by perfusion with water of different temperatures and thus effect periods of normothermia, cold stress and heat stress (Rowell et al. 1969; Johnson & Park, 1981). The suit was perfused with 18°C cold water to decrease Tsk and induce cold stress, and 48°C warm water to raise Tsk to 38–39°C during heating periods. Over the suit, subjects wore a water-impermeable plastic garment to insulate them thoroughly from the normothermic room environment and also to prevent evaporation of sweat. The suit and garment covered the entire body with the exception of the head and the forearm from which LDF measurements were made. The hands and feet were also uncovered.
Internal temperature (Tor) was monitored with a thermocouple placed in the sublingual sulcus. Subjects were instructed not to speak during studies so as not to interfere with this measurement. Tsk was recorded as the weighted electrical average from six thermocouples taped on the skin surface (Rowell et al. 1969; Johnson & Park, 1981).
After arriving in the laboratory on the morning of the study, two intradermal microdialysis probes were placed on the ventral surface of one forearm. Subjects were prepared for the specific protocol with instrumentation as outlined above. Finally, subjects were placed in a supine position for data collection.
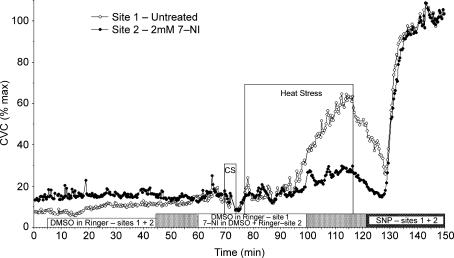
Data collection began with a 5–10 min normothermic control period during which Tsk was maintained at a normothermic level and all microdialysis sites were perfused with Ringer solution at 2 μl min−1. After this period, the perfusates at both microdialysis sites were changed to 5% DMSO in Ringer solution. Perfusion was maintained at 2 μl min−1 for 30–35 min. Following this period, the perfusate of one microdialysis site was changed to 7-NI (2 mm) dissolved in 5% DMSO and Ringer solution which served as the treated experimental site. Perfusion at a second microdialysis site was continued with 5% DMSO in Ringer solution which served as the untreated control site for any unanticipated effects of DMSO. Perfusion rates at both sites were maintained at 2 μl min−1 throughout subsequent periods of cold and heat stress. After perfusing for 30–35 min to allow for the antagonist to enter the intradermal space, Tsk was decreased to induce cold stress for 3 min after which Tsk was returned to a normothermic level. Tsk was then raised to 38–39°C and maintained at that level for 35–50 min to induce heat stress and thus activate the vasodilator system. Whole-body heating was maintained until Tor had increased by approximately 1°C and SkBF had stabilized at an elevated level. After heat stress, subjects were cooled to normothermic levels. All microdialysis sites were then perfused with 56 mm nitroprusside (SNP) to effect maximal vasodilatation by an endothelium-independent mechanism at each site (Kreidstein et al. 1992; Kellogg et al. 2005). SNP perfusion was maintained for 30–40 min until a clear plateau in LDF had been achieved for data normalization. The protocol is summarized in Fig. 1.
Figure 1. Protocol 1. Example of nNOS antagonism and whole-body heat stress.
Two microdialysis sites were used. After an initial normothermic control period when both microdialysis sites were perfused with Ringer solution, both perfusates were changed to 5% DMSO in Ringer solution. Perfusate at one microdialysis site was then changed to a 2 mm solution of 7-NI in 5% DMSO in Ringer solution while 5% DMSO in Ringer solution was maintained at the other site that served as an untreated control. After 30–35 min of perfusion with the 7-NI, skin temperature (Tsk) was decreased to induce whole-body cold stress for 3 min. Tsk was then increased to induce whole-body heat stress for 35–50 min. Finally, subjects were cooled to normothermia and the perfusates at both sites changed to 56 mm nitroprusside to cause maximal vasodilatation.
Data are presented as group means ±s.e.m. For data analysis, CVC was indexed as LDF (in mV) divided by MAP (in mmHg). CVC data were normalized to their respective maxima as elicited by SNP at each site. This normalization allowed for comparisons between 7-NI-treated and untreated control sites within and between subjects. The vasomotor and NO responses to heat stress were analysed by comparing the internal temperature thresholds for the initial increases in CVC during whole-body heating at the different microdialysis sites. The internal temperature threshold for the onset of vasodilatation for each site was defined as the level of Tor at which a sustained increase in CVC began, after Tsk had been increased to 38°C. Tor thresholds were chosen from individual graphs of CVC versus Tor by an investigator blinded as to the condition, subject and antagonist treatment. The thresholds for cutaneous vasodilatation were compared by paired t test that compared Tor thresholds at untreated and 7-NI-treated sites. For each site, the rate of rise of CVC with Tor during heat stress was calculated as the CVC change from onset of vasodilatation to the peak of heat stress divided by the corresponding Tor change. CVC values during normothermia, the final minute of cold stress, and the final 3 min of heat stress were compared by ANOVA followed by planned means comparisons (Kellogg et al. 1998). P values < 0.05 were deemed statistically significant.
Protocol 2
Seven subjects participated in the local skin warming protocol (4 male, 3 females). Their average age was 39 ± 3 years, average weight was 75 ± 5 kg, and average height was 169 ± 2 cm. Subjects were fully clothed with the exception of the instrumented forearm and the head so as to be comfortably normothermic throughout the experiment.
Upon arrival in the laboratory, each subject had three microdialysis probes placed on the ventral aspect of one forearm and were instrumented for LDF and MAP monitoring as previously described. Unlike in protocol 1, each LDF probe was equipped with a special probe holder equipped with heating elements to permit simultaneous LDF measurements and control of Tloc at each site (Kellogg et al. 1999). After the LDF probes were placed, the Tloc at each site was increased to 34°C to ensure identical local thermal conditions at the start of the study. Perfusion of the microdialysis probes was then initiated with Ringer solution, each at a rate of 2 μl min−1.
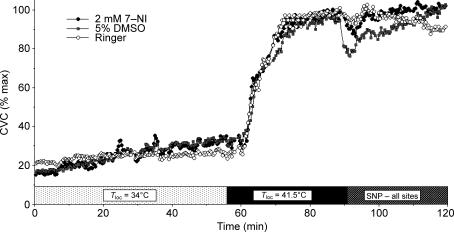
Data collection began with a 5–10 min control period with Tloc maintained at 34°C. The perfusate at one site was then changed to 5% DMSO in Ringer solution and served as a control for any unanticipated effects of DMSO on local warming responses. Another probe's perfusate was changed to 2 mm 7-NI in the 5% DMSO in Ringer vehicle. Perfusion with Ringer solution was maintained at the third site, which served as an untreated control site. Tloc was maintained at 34°C for at least 45 min during perfusion with these solutions, after which Tloc was increased to 41.5°C at all sites for 45 min. This temperature was chosen to preclude activation of pain fibres as can happen with warming to greater temperatures and probably causes vasodilatation through mechanisms other than NO alone (Kellogg et al. 1999). Finally, the perfusates at all sites were changed to 56 mm sodium nitropusside (SNP) in Ringer solution for 30–40 min to effect the maximal vasodilatation mechanism for data normalization (Kreidstein et al. 1992; Kellogg et al. 2005). SNP perfusion was maintained for 30–40 min until a clear plateau in LDF had been achieved. The protocol is summarized in Fig. 2.
Figure 2. Protocol 2. Example of nNOS antagonism and local skin warming.
Three microdialysis sites were used. One site was perfused with Ringer solution alone. A second site was perfused with 5% DMSO in Ringer. The third site was perfused with 7-NI in 5% DMSO in Ringer. During the initial part of the protocol, the local temperature (Tloc) of all microdialysis sites was held at 34°C. Local skin warming was then performed to raise Tloc to 41.5°C and maintained at that level for 45 min. Finally, the perfusates at all sites changed to 56 mm nitroprusside to cause maximal vasodilatation.
Data are presented as group means ±s.e.m. For data analysis, CVC was calculated as LDF/MAP to control for any fluctuations in blood pressure during data collection. Vasomotor responses were analysed by comparing the mean levels of CVC during the final 3 min with a Tloc of 34°C with the final 3 min of the 41.5°C Tloc period. CVC responses were analysed by repeated measures ANOVA, followed by specific means comparisons (Kellogg et al. 1999). P values < 0.05 were deemed statistically significant.
Abbreviations
ACh, acetylcholine; CVC, cutaneous vascular conductance; DMSO, dimethyl sulfoxide; eNOS, endothelial nitric oxide synthase, NOS 3; HSP90, heat shock protein 90; iNOS, inducible nitric oxide synthase, NOS 2; LDF, laser–Doppler flowmetry; l-NAME, NG.-nitro-l-arginine-methyl ester; l-NMMA, NG.-monomethyl-l-arginine; MAP, mean arterial pressure; NO, nitric oxide; NOS, nitric oxide synthase; nNOS, neuronal nitric oxide synthase, NOS 1; NPY, neuropeptide Y; PR, pulse rate; SkBF, skin blood flow; SNP, sodium nitroprusside; Tloc, local skin temperature; Tor, oral temperature; Tsk skin temperature; VIP, vasoactive intestinal peptide; 7-NI, 7-nitroindazole.
Results
The physical characteristics (age, height and weight) of the subject groups in protocols 1 and 2 were not statistically different. Within each protocol, no differences were noted between responses of male or female subjects, therefore data from the two were combined for statistical analysis.
Protocol 1
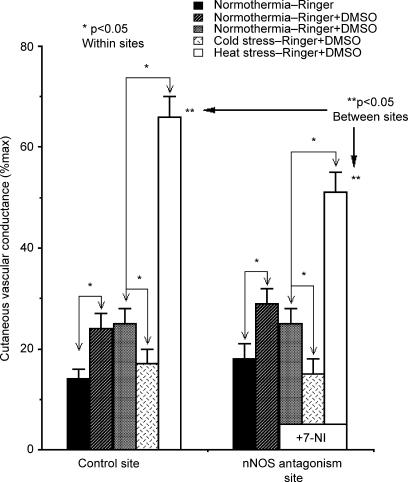
Under normothermic conditions at the beginning of the protocol, Tor averaged 36.67 ± 0.07°C while Tloc at the LDF microdialysis sites averaged 32 ± 1°C. During this period, when all microdialysis sites were perfused with Ringer solution only, there were no statistical differences between CVC values at the two microdialysis sites (14 ± 2 versus 18 ± 3%max CVC, P > 0.05). CVC increased to 24 ± 3 and 28 ± 3%max CVC at the two respective microdialysis sites during perfusion with 5% DMSO in Ringer solution (P < 0.05 Ringer only versus DMSO), a result consistent with our preliminary findings and those of Shastry et al. (Shastry & Joyner, 2002). The addition of 7-NI to the 5% DMSO in Ringer solution did not cause any significant change in CVC (P > 0.05) (see Fig. 3).
Figure 3. Cutaneous vascular conductance (CVC) responses to whole-body heat stress with and without nNOS antagonism.
During normothermia, when all sited were perfused with Ringer solution only, CVC did not differ between microdialysis sites (P > 0.05 between sites). During perfusion of 5% DMSO in Ringer, these values increased at both sites (P > 0.05 between sites). Perfusion of the latter site with 2 mm 7-NI in 5% DMSO in Ringer did not change CVC values significantly (P > 0.05 7-NI versus 5% DMSO in Ringer). No such trend was present at the untreated site where 5% DMSO perfusion was maintained. During whole-body cold stress, CVC fell at both sites. The CVC responses to cold stress did not differ between sites (P > 0.05 between sites). During whole-body heating, CVC rose at both sites. The CVC responses to whole-body heat stress differed significantly between untreated and 7-NI-treated sites, thus nNOS antagonism attenuated the cutaneous active vasodilator response to whole-body heat stress. (*P < 0.05 within sites; **P < 0.05 between sites).
In response to whole-body cooling, CVC fell at both microdialysis sites. Average CVC at untreated sites fell to 17 ± 3%max and to 15 ± 3%max at 7-NI-treated sites (P < 0.05 versus pre-cold stress, control versus 7-NI-treated sites). These responses did not differ between the two sites (P > 0.05).
During whole-body heating, CVC began to rise at untreated sites when Tor reached 36.86 ± 0.10°C. CVC began to increase at 7-NI-treated sites when Tor reached 36.87 ± 0.10°C. These Tor values did not differ between sites (P > 0.05). At the peak of heat stress, Tor achieved a maximal level of 37.82 ± 0.18°C (P < 0.01 versus normothermia).
Under normothermic conditions, MAP averaged 75 ± 3 mmHg and tended to fall during heating (71 ± 2 mmHg, P > 0.10, normothermia versus heat stress). PR averaged 62 ± 8 in normothermia and increased significantly to 97 ± 6 over the course of whole-body heating (P < 0.01, normothermia versus heat stress).
As heat stress responses developed, CVC rose significantly at both untreated and 7-NI-treated sites (P < 0.05, normothermia versus heat stress within sites). The rate of rise was 55 ± 7%max CVC °C−1 at untreated sites and 33 ± 6%max CVC °C−1 at 7-NI-treated sites. The rate of rise was significantly attenuated at the 7-NI-treated sites (P < 0.01 between sites). At the peak of heat stress, CVC had risen to 66 ± 4%max CVC at the untreated site, but only to 51 ± 4%max CVC at the 7-NI-treated site (P < 0.05 untreated heat stress versus 7-NI). The overall CVC results are summarized in Fig. 3.
Protocol 2
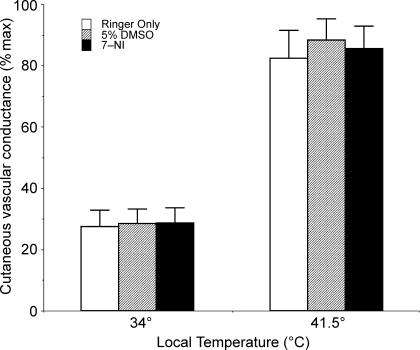
With the skin warmed to a Tloc of 34°C, CVC at three microdialysis sites averaged 27 ± 5%max CVC at sites perfused with Ringer only, 28 ± 5%max CVC at sites perfused with 5% DMSO in Ringer, and 28 ± 5%max CVC at sites that received 2 mm 7-NI in 5% DMSO and Ringer. There were no significant differences between these sites at a Tloc of 34°C (P > 0.05 between sites).
During local warming of the skin to 41.5°C, CVC increased significantly to plateaus at all sites (P < 0.05, 34°C versus 41.5°C for all sites). At a Tloc of 41.5°C, CVC averaged 83 ± 9%max at sites perfused with Ringer only, 88 ± 7%max CVC at sites perfused with 5% DMSO in Ringer, and 85 ± 5%max CVC at sites that received 2 mm 7-NI in 5% DMSO in Ringer. There were no significant differences between these sites with Tloc values of 41.5°C (P > 0.05 between sites). These results are summarized in Fig. 4.
Figure 4. Cutaneous vascular conductance (CVC) responses to local heating of the skin with and without nNOS antagonism.
With a Tloc of 34°C, CVC did not differ between sites (P > 0.05). With local warming to 41.5°C, CVC increased significantly at all sites (P < 0.05, 34°C versus 41.5°C). At a Tloc of 41.5°C, CVC did not differ between sites (P > 0.05), thus nNOS antagonism failed to alter the vasodilatation induced by local warming of the skin.
Discussion
Selective blockade of the nNOS isoform attenuated cutaneous active vasodilatation induced by whole-body heat stress, but did not alter the vasodilatation induced by local warming of the skin. These results clarify how different constituitively expressed NOS isoforms are used to control specific vasomotor responses in humans, in vivo: the nNOS isoform is responsible for NO generation during centrally mediated, reflex cutaneous active vasodilatation in whole-body heat stress, but is not involved in increasing SkBF in response to axon reflex-mediated, local warming of the skin.
The results of protocol 1 show that the administration of 7-NI by intradermal microdialysis did not alter the cutaneous vasoconstriction induced by whole-body cold stress. This demonstrates that 7-NI did not measurably alter the function of noradrenergic vasoconstrictor nerves. The preservation of this response also demonstrates that 7-NI did not alter the responsiveness of skin vessels to neural inputs, thus obviating any concerns that reduced vascular responsiveness could explain attenuation of cutaneous active vasodilatation during whole-body heat stress.
While the extent of cutaneous active vasodilatation was attenuated by 7-NI treatment, the internal temperature threshold at which that active vasodilator system was first activated was unaltered by nNOS antagonism. The Tor at which CVC began to rise during whole-body heating did not differ between sites treated with 7-NI and untreated control sites. This finding shows that nNOS activation coincides with the initiation of cutaneous active vasodilatation. This finding is consistent with prior studies that used non-isoform-specific NOS antagonists, such as NG.-nitro-l-arginine-methyl ester (l-NAME) and NG.-monomethyl-l-arginine (l-NMMA), that found the initiation of cutaneous active vasodilatation was not altered by NOS blockade (Dietz et al. 1994; Kellogg et al. 1998; Shastry et al. 1998; Wilkins et al. 2003). While non-isoform-specific antagonists also attenuated cutaneous active vasodilatation during prolonged heat stress, it was unclear whether nNOS, eNOS or both isoforms were involved in the process. Given that 7-NI attenuated cutaneous active vasodilatation during prolonged heat stress, but did not alter the initiation of cutaneous active vasodilatation, our results show that the nNOS isoform generates NO from the very initial phase of cutaneous active vasodilatation and continues during prolonged whole-body heat stress.
Protocol 2 tested the hypothesis that nNOS is involved in increasing SkBF in response to local skin warming. In contrast to protocol 1, the results of protocol 2 show that nNOS antagonism did not alter the vasodilatation induced by local skin warming and leads us to conclude that the nNOS isoform is not involved in the generation of the NO required for increasing SkBF during local warming. Our finding in protocol 2 contrasts directly with prior studies that used the non-isoform-specific NOS antagonist l-NAME and found non-isoform-specific NOS antagonism greatly attenuated the extent of local, axon reflex vasodilatation during local skin warming (Kellogg et al. 1999; Minson et al. 2001, 2002; Houghton et al. 2007). Given that iNOS is not constituitively expressed in skin and that nNOS antagonism was without effect, our results strongly suggest that eNOS is the isoform that mediates peripheral, axon reflex vasodilatation during local skin heating.
The increase in CVC by 5% DSMO in normothemia for protocol 1 was not observed in protocol 2 with Tloc maintained at 34°C. This difference is attributable to Tloc being slightly lower in protocol 1 (where local LDF/heater probe holders were not used) than in protocol 2. The slightly warmer Tloc in protocol 2 masked the increase in CVC that was observed with 5% DMSO in protocol 1. Whole-body Tsk may also have differed slightly between the normothermic period of protocol 1 and the initial period of protocol 2. This difference is unlikely to confound the separate results of the two protocols because the vasodilator effect of increased Tloc is known to be unaltered by whole-body cooling or warming (Wenger et al. 1985; Pérgola et al. 1993).
Our conclusions regarding the physiological roles of the nNOS isoform in vasomotor control mechanisms in humans, in vivo, are predicated on the specificity of nNOS isoform blockade as administered by intradermal microdialysis. Since pharmacological agents such as acetylcholine can activate both nNOS and eNOS, there is no pharmacological method to definitively characterize the extent to which selective nNOS blockade was achieved (Moore et al. 1993b). The selectivity of 7-NI for the nNOS isoform lies in the differential uptake of this inhibitor into neurons, but not endothelial cells (Babbage et al. 1993; Moore et al. 1993a,b; Yoshida et al. 1994; Southan & Szabo, 1996; Jiang et al. 1999). Although in vitro studies on the isolated enzymes have demonstrated that 7-NI can inhibit both the eNOS and nNOS, in vivo studies have consistently shown no evidence of an effect of 7-NI on eNOS (Babbage et al. 1993; Moore et al. 1993a,b; Yoshida et al. 1994; Southan & Szabo, 1996; Jiang et al. 1999). For example, in vivo, 7-NI has no effects on blood pressure or on endothelium-dependent relaxation of blood vessels (Babbage et al. 1993; Moore et al. 1993a,b; Yoshida et al. 1994; Southan & Szabo, 1996; Jiang et al. 1999).
Beyond the above evidence for the selectivity of 7-NI as an in vivo nNOS antagonist, the dichotomous results of protocols 1 and 2 verify the specificity of nNOS antagonism in our studies. We found the same concentration of 7-NI that significantly attenuated cutaneous active vasodilatation during whole-body heat stress failed to have any effect on skin vasodilatation induced by local skin warming. Both of these responses are known to be dependent on NO generation by NOS based on studies with non-isoform-specific antagonists (Kellogg et al. 1998, 1999, 2003; Shastry et al. 1998, 2000; Minson et al. 2001, 2002; Houghton et al. 2007). The divergent findings of an attenuating effect of 2 mm 7-NI in whole-body heat stress, but yet no effect of 2 mm 7-NI in local skin warming shows that selective nNOS had been achieved. If this were not the case, and only non-selective NOS blockade had been achieved, the vasodilatations induced by both whole-body heat stress and local skin warming would have been attenuated.
Immunohistochemical studies of human skin have found nNOS in cutaneous nerves around dermal microvessels (Ibba-Manneschi et al. 2006). In addition, nNOS has been found to be co-localized with VIP in cholinergic nerves (Ventura et al. 1997) such as those that mediate cutaneous active vasodilatation through a co-transmitter system (Kellogg et al. 1995). Considering the results of the present study, these observations suggest that nNOS located in cutaneous cholinergic nerves is activated to generate NO during cutaneous active vasodilatation.
Another possibility is that nNOS located in sweat glands is activated to generate NO as an adjunct to sweat production. The nNOS isoform has been found in myoepithelial cells in eccrine sweat glands in the dermis (Zancanaro et al. 1999). Activation of nNOS in this location could also contribute to NO generation during heat stress. Increases in NO near sweat glands could effect periglandular hyperaemia and thus contribute not only to cutaneous vasodilatation, but also to increased fluid delivery to the sweat glands to maintain sweat production. This possibility is consistent with the longstanding idea that cutaneous active vasodilatation and sweat gland activation are mechanistically linked (Grant & Holling, 1938; Brengelmann et al. 1981; Sugenoya et al. 1995, 1998; Shibasaki et al. 2002; Kamijo et al. 2005).
When combined with findings of prior studies, the finding that nNOS is mechanistically involved in cutaneous active vasodilatation has a number of implications. The observation that treatment of skin with botulinum toxin abolishes cutaneous active vasodilatation shows that the neurotransmitters required for the initiation and maintenance of active vasodilatation must be stored in small and large vesicles within cholinergic nerve terminals (Kellogg et al. 1995; Morris et al. 2001). NO cannot be stored in vesicles in nerve terminals and hence, nitrergic transmission by NO is insensitive to botulinum toxin. These observations mean that the generation and release of NO is not the primary neurotransmitter mechanism for initiating or maintaining cutaneous active vasodilatation; rather, one or more neurotransmitters stored in vesicles within cutaneous cholinergic nerve terminals must be released to initiate and maintain the process. If nitrergic transmission by NO directly initiated and maintained cutaneous active vasodilatation, cutaneous active vasodilatation would be preserved after botulinum treatment, which it is not (Kellogg et al. 1995).
Given the foregoing findings, one or more neurotransmitters stored within the vesicles of cholinergic nerve terminals must be released to initiate and maintain cutaneous active vasodilatation. One such stored cholinergic neurotransmitter is ACh. ACh might cause NO generation through nNOS activation via pre-junctional muscarinic receptors on skin nerves or on sweat gland myoepithelial cells; however, prior observations obviate such a role for ACh. In the present study, we found that although nNOS antagonism attenuated the extent of cutaneous active vasodilatation during whole-body heating, NOS blockade did not delay the initiation of the process; this was also found in studies using non-isoform-specific antagonists (Dietz et al. 1994; Kellogg et al. 1998; Shastry et al. 1998; Wilkins et al. 2003). In addition, bioavailable NO measurements increase simultaneously with the onset of active vasodilatation during whole-body heating (Kellogg et al. 2003). In contrast, blockade of muscarinic receptors with atropine before whole-body heating delays the initiation of active vasodilatation (Roddie et al. 1957; Fox & Hilton, 1958; Kellogg et al. 1995), but has no effect once cutaneous active vasodilatation is established and SkBF has risen to high levels (Shastry et al. 2000). If ACh release and muscarinic receptor activation were involved in NO generation, both atropine and NOS antagonism should delay the onset of active vasodilatation and attenuate established active vasodilatation. Instead, the effects of atropine and NOS antagonists are dichotomous, therefore ACh activation of NOS isoforms via muscarinic receptors cannot be involved in cutaneous active vasodilatation; ACh-dependent mechanisms and NO-dependent mechanisms clearly must be separate. This suggests that either one or more co-transmitters released from cholinergic nerves activates nNOS with consequent NO generation during whole-body heat stress and raises the possibility that ACh contributes to cutaneous active vasodilatation through other mechanisms, that perhaps include prostaglandin production (McCord et al. 2005).
While it is clear from the foregoing discussion that NO does not directly initiate or maintain cutaneous active vasodilatation, our findings strongly suggest that rather than acting as a direct mediator of vasodilatation, NO acts to increase synergistically the vasodilating effects of other neurotransmitters. NO generated by nNOS in cholinergic nerves and/or sweat glands could increase pre-junctional neurotransmitter release or magnify the post-junctional effects of released neurotransmitters as has been found with VIP and other peptide transmitters (Grider et al. 1992; Jia & Stamler, 1999; Toda & Herman, 2005). The existence of synergistic effects between NO and the neurotransmitters that cause cutaneous active vasodilatation during heat stress in humans is consistent with the foregoing model of nNOS mechanisms.
Finally, could eNOS have a role in centrally mediated reflex cutaneous active vasodilatation? The non-selective NOS antagonist, l-NAME, attenuates the increase in SkBF induced by whole-body heat stress by about 30–40% (Kellogg et al. 1998; Shastry et al. 1998, 2000). With the selective nNOS antagonist 7-NI, we were able to attenuate SkBF increases by approximately 30%. The similarity of attenuation reported for l-NAME and our finding with 7-NI suggests the possibility that nNOS is the sole NOS isoform involved in NO generation during centrally mediated reflex vasodilatation as effected by whole-body heat stress. However, in contrast to the nNOS isoform, no specific inhibitor of the eNOS isoform is available for in vivo studies at present. We therefore cannot exclude a role for eNOS in thermoregulatory control of the cutaneous circulation at this time.
In summary, we found that nNOS antagonism with 7-NI significantly attenuated cutaneous active vasodilatation during heat stress, but did not attenuate the vasodilatation induced by local warming of the skin. Collectively, these results show that NO generation required for cutaneous active vasodilatation in heat stress is mediated by the nNOS isoform. This result demonstrates a specific role for nNOS activation in centrally mediated thermoregulatory reflex cutaneous vasodilatation in humans. Whether only this isoform effects NO increases during cutaneous active vasodilatation remains to be proven. In contrast, generation of NO to effect the vasodilatation induced by local skin warming is not through the nNOS isoform. Whether the eNOS isoform effects NO increases during local skin warming remains to be proven. These results demonstrate a specific mechanistic role for nNOS activation in the vasomotor control of the human vasculature.
References
- Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser-Doppler perfusion imaging. J Invest Dermatol. 1994;102:808–811. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- Aoki K, Stephens DP, Johnson JM. Diurnal variation in cutaneous vasodilator and vasoconstrictor systems during heat stress. Am J Physiol Regul Integr Comp Physiol. 2001;281:R591–R595. doi: 10.1152/ajpregu.2001.281.2.R591. [DOI] [PubMed] [Google Scholar]
- Babbage RC, Bland-Ward PA, Hart SL, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substances. Br J Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg J. Evidence for a role for vasoactive intestinal peptide in active vasodilation in the cutaneous vasculature in humans. J Physiol. 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brengelmann GL, Freund PR, Rowell LB, Olerud JE, Kraning KK. Absence of active vasodilation associated with congenital absence of sweat glands in humans. Am J Physiol Heart Circ Physiol. 1981;240:H571–H575. doi: 10.1152/ajpheart.1981.240.4.H571. [DOI] [PubMed] [Google Scholar]
- Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol. 1998;110:1–7. doi: 10.1046/j.1523-1747.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- Cas-Grierson M-M, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–193. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Warner DO, Joyner MJ. Is nitric oxide involved in cutaneous vasodilation during body heating in humans? J Appl Physiol. 1994;76:2047–2053. doi: 10.1152/jappl.1994.76.5.2047. [DOI] [PubMed] [Google Scholar]
- Fox RH, Edholm OG. Nervous control of the cutaneous circulation. Br Med Bull. 1963;19:110–114. doi: 10.1093/oxfordjournals.bmb.a070027. [DOI] [PubMed] [Google Scholar]
- Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilation. J Physiol. 1958;142:219–232. doi: 10.1113/jphysiol.1958.sp006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming; evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci. 1938;3:273–285. [Google Scholar]
- Grider JR, Murthy KS, Jin J-G, Makhlouf GM. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol Gastrointest Liver Physiol. 1992;262:G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2007;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibba-Manneschi L, Niissalo S, Milia AF, Allanore Y, Del Rosso A, Pacini A, Manetti M, Toscano A, Cipriani P, Liakouli V, Giacomelli R, Kahan A, Konttinen YT, Mattucci-Cerinic M. Variations on neuronal nitric oxide synthase in systemic sclerosis skin. Arthritis Rheumat. 2006;54:202–213. doi: 10.1002/art.21543. [DOI] [PubMed] [Google Scholar]
- Jia L, Stamler JS. Dual actions of S-nitrosylated derivative of vasoactive intestinal peptide as a vasoactive intestinal peptide-like mediator and nitric oxide carrier. Eur J Pharmacol. 1999;366:79–86. doi: 10.1016/s0014-2999(98)00921-2. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Kaku T, Hada J, Hayashi Y. 7-Nitroindazole reduces nitric oxide concentration in rat hippocampus after forebrain ischemia. Eur J Pharmacol. 1999;380:117–121. doi: 10.1016/s0014-2999(99)00555-5. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol. 1981;50:814–818. doi: 10.1152/jappl.1981.50.4.814. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly M, Blatteis C, editors. Handbook of Physiology – Environmental Physiology. New York: Oxford University Press; 1996. pp. 215–243. [Google Scholar]
- Kamijo Y-I, Lee K, Mack GW. Active cutaneous vasodilation in resting humans during mild heat stress. J Appl Physiol. 2005;98:829–837. doi: 10.1152/japplphysiol.00235.2004. [DOI] [PubMed] [Google Scholar]
- Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol. 1989;257:H1599–H1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pérgola PE, Kosiba WA, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 2003;94:1971–1977. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Kleinhart H, Pautz A, Linker K, Scharz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–226. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Kreidstein ML, Pang CY, Carlsen LN, Xu N. Evidence for endothelium-dependent and endothelium-independent vasodilation in human skin flaps. Can J Physiol Pharmacol. 1992;70:1208–1216. doi: 10.1139/y92-168. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Alfie ME, Sigmon DH, Rahaleb N, Sehesely EG, Carretero OA. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32:856–861. doi: 10.1161/01.hyp.32.5.856. [DOI] [PubMed] [Google Scholar]
- Levin NW, Morris AT, Lavarias VA, Wang Y, Glabman MB, Leung JP, Yusurf SA, LeVoci AL, Polaschegg HD, Kaufman AM. Effects of body core temperature reduction on haemodynamic stability and haemodialysis efficacy at constant ultrafiltration. Nephrol Dial Transplant. 1996;11:31–34. doi: 10.1093/ndt/11.supp2.31. [DOI] [PubMed] [Google Scholar]
- Lewis T, Pickering GW. Vasodilation in the limbs in response to warming the body; with evidence for sympathetic vasodilator nerves in man. Heart. 1931;16:33–51. [Google Scholar]
- McCord GR, Cracowski J-L, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2005;291:R596–R602. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Moore PK, Babbage RC, Wallace P, Gaffen ZA, Hart SL. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br J Pharmacol. 1993a;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PK, Wallace P, Gaffen Z, Hart SL, Babbage RC. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br J Pharmacol. 1993b;110:219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Jobling P, Gibbins IL. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am J Physiol Heart Circ Physiol. 2001;281:H2124–H2132. doi: 10.1152/ajpheart.2001.281.5.H2124. [DOI] [PubMed] [Google Scholar]
- Murad F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- Pérgola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilation during body heating. J Physiol. 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69:154–166. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Murray JA, Brengelmann GL, Kraning KK. Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Circulation Res. 1969;24:711–724. doi: 10.1161/01.res.24.5.711. [DOI] [PubMed] [Google Scholar]
- Sears CE, Ashley EA, Casadei B. Nitric oxide control of cardiac function: is neuronal nitric oxide synthase a key component? Philos Trans R Soc Lond B Biol Sci. 2004;359:1021–1044. doi: 10.1098/rstb.2004.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SJ, Choi HG, Chung HJ, Hong CK. Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by international B in keratinocyte cell lines. Br J Dermatol. 2002;147:655–662. doi: 10.1046/j.1365-2133.2002.04849.x. [DOI] [PubMed] [Google Scholar]
- Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol. 2002;282:H232–H236. doi: 10.1152/ajpheart.2002.282.1.H232. [DOI] [PubMed] [Google Scholar]
- Shastry S, Minson CT, Wilson SA, Dietz NK, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- Shastry S, Reed AS, Halliwill JR, Dietz NM, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol. 2002;93:1947–1951. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad A, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1401–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- Sugenoya J, Iwase S, Mano T, Sugiyama Y, Ogawa T, Nishiyama T, Nishimura N, Kimura T. Vasodilator component in sympathetic nerve activity destined for the skin of the dorsal foot of mildly heated humans. J Physiol. 1998;507:603–610. doi: 10.1111/j.1469-7793.1998.603bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugenoya J, Ogawa T, Jmai K, Ohnishi N, Natsume T. Cutaneous vasodilation responses synchronize with sweat expulsions. Eur J Appl Physiol. 1995;71:33–40. doi: 10.1007/BF00511230. [DOI] [PubMed] [Google Scholar]
- Toda N, Herman AG. Gastrointestinal function regulation by nitrergic efferent nerves. Pharmacol Rev. 2005;57:315–338. doi: 10.1124/pr.57.3.4. [DOI] [PubMed] [Google Scholar]
- Ventura S, Bavetta S, Milner P, Ralevic V, Burnstock G. Nitric oxide synthase is co-localized with vasoactive intestinal polypeptide in postganglionic parasympathetic nerves innervating the rat vas deferens. Neuroscience. 1997;83:607–616. doi: 10.1016/s0306-4522(97)00416-8. [DOI] [PubMed] [Google Scholar]
- Wang R, Ghahary A, Shen YJ, Scott PG, Tredget EE. Human dermal fibroblasts produce both nitric oxide and express both constituitive and inducible nitric oxide synthase isoforms. J Invest Dermatol. 1996;106:419–427. doi: 10.1111/1523-1747.ep12343428. [DOI] [PubMed] [Google Scholar]
- Wenger CB, Bailey RB, Roberts MF, Nadel ER. Interaction of local and reflex thermal effects in control of forearm blood flow. J Appl Physiol. 1985;58:251–257. doi: 10.1152/jappl.1985.58.1.251. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilation in humans. J Physiol. 2003;548:963–969. doi: 10.1113/jphysiol.2002.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Limmroth V, Irikura K, Moskowitz MA. The NOS inhibitor, 7-nitroindazole, decreases focal infarct size but not the response to topical acetylcholine in pial vessels. J Cereb Blood Flow Metab. 1994;14:924–929. doi: 10.1038/jcbfm.1994.123. [DOI] [PubMed] [Google Scholar]
- Zancanaro C, Merigo F, Crescimanno C, Orlandini S, Osculati A. Immunohistochemical evidence suggests intrinsic regulatory activity in human eccrine sweat glands. J Anat. 1999;194:433–444. doi: 10.1046/j.1469-7580.1999.19430433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]