Abstract
The kinetics of pulmonary O2 uptake is known to be substantially slower when exercise is initiated from a baseline of lower-intensity exercise rather than from rest. However, it is not known whether putative intracellular regulators of mitochondrial respiration (and in particular the phosphocreatine concentration, [PCr]) show similar non-linearities in their response dynamics. The purpose of this study was therefore to investigate the influence of baseline metabolic rate on muscle [PCr] kinetics (as assessed using 31P-magnetic resonance spectroscopy) following the onset of exercise. Seven male subjects completed ‘step’ tests to heavy-intensity exercise (80% of peak work-rate) from a resting baseline and also from a baseline of moderate-intensity exercise (40% of peak work-rate) using a single-leg knee-extensor ergometer situated inside the bore of a 1.5 T super-conducting magnet. The time constant describing the kinetics of the initial exponential-like fall in [PCr] was significantly different between rest-to-moderate (25 ± 14 s), rest-to-heavy (48 ± 11 s) and moderate-to-heavy exercise (95 ± 40 s) (P < 0.05 for all comparisons). A delayed-onset ‘slow component’ in the [PCr] response was observed in all subjects during rest-to-heavy exercise, but was attenuated in the moderate-to-heavy exercise condition. These data indicate that muscle [PCr] kinetics does not conform to ‘linear, first-order’ behaviour during dynamic exercise, and thus have implications for understanding the regulation of muscle oxidative metabolism.
It has been proposed that mitochondrial respiration is intimately linked to the rate of muscle ATP hydrolysis, with one or more of the reactants of this process (e.g. [ADP], [Pi], the phosphorylation potential, and/or [PCr] and [Cr]) activating oxidative phosphorylation through feedback control (Chance et al. 1985; Mahler, 1985; Meyer, 1988). Consistent with this, Rossiter et al. (1999, 2002b) have demonstrated close agreement between muscle [PCr] kinetics (as estimated using 31P-magnetic resonance spectroscopy (MRS) techniques) and pulmonary V̇O2 (p V̇O2) kinetics during both moderate (i.e. below the lactate threshold (LT)) and heavy (> LT) intensity exercise. Moreover, Kindig et al. (2005) reported that acute inhibition of creatine kinase (CK) in isolated Xenopus myocytes led to significantly faster intracellular PO2 kinetics (equivalent to faster V̇O2 kinetics in this model). These data have been interpreted to suggest that the CK reaction buffers changes in [ADP] across a metabolic transient, thus attenuating one of the principal signals responsible for an acceleration of oxidative phosphorylation.
Whether or not the kinetics of muscle [PCr] (and, by implication, V̇O2) shows ‘linear, first-order’ behaviour (Fujihara et al. 1973a,b) following the onset of exercise of different intensities is controversial. During constant-work-rate exercise of moderate intensity, both muscle [PCr] and the phase II pV̇O2 kinetics (which closely reflect the kinetics of O2 consumption in the contracting skeletal muscles; Grassi et al. 1996) change with an apparently exponential time course to reach a steady-state within 2–3 min (Whipp & Wasserman, 1972; Poole et al. 1991; Binzoni et al. 1992; Barstow et al. 1994; McCreary et al. 1996; Rossiter et al. 1999, 2002b). During heavy exercise, however, both muscle [PCr] and pV̇O2 kinetics show a ‘slow’ continued change with time beyond approximately 3 min (Whipp & Wasserman, 1972; Roston et al. 1987; Barstow & Molé, 1991; Poole et al. 1991; Rossiter et al. 2002a,b; Haseler et al. 2004). The limited available evidence indicates that the time constant (τ) describing the decline in muscle [PCr] in the fundamental phase of the response is similar between moderate and heavy intensity exercise (Rossiter et al. 2002b) but whether the phase II pV̇O2 τ is similar or longer during exercise performed above compared to below the LT is more controversial (Poole & Jones, 2005).
It has been repeatedly demonstrated that the phase II pV̇O2 kinetics is markedly slower when exercise is initiated from an elevated baseline metabolic rate, for example, when a step to a higher work-rate is initiated from a pre-existing lower work-rate (Hughson & Morrissey, 1982, 1983; Brittain et al. 2001; MacPhee et al. 2005; Wilkerson & Jones, 2006; Wilkerson & Jones, 2007). There is also evidence that the Gain (that is, the increase in pV̇O2 per unit increase in work-rate) of the fundamental response to moderate (Brittain et al. 2001; MacPhee et al. 2005) and heavy (Wilkerson & Jones, 2006; Wilkerson & Jones, 2007) exercise can be altered during such transitions. These results suggest that oxidative phosphorylation is not exclusively under linear first-order control but that other control mediators or constraints are operative when higher-intensity exercise is initiated from a lower-intensity exercise baseline (Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Wilkerson & Jones, 2007).
To our knowledge, no previous studies have investigated the muscle metabolic responses (including the dynamics of [PCr]) to exercise when the baseline work-rate has been manipulated. Initiating a certain work-rate from a higher compared to a lower baseline work-rate would be expected to facilitate the expression of the energetic features of the higher-threshold muscle fibres (such as more total creatine and fewer mitochondria) which would be recruited to meet the augmented force requirement (Henneman et al. 1965). In turn, this might be predicted to be associated with slower [PCr] splitting kinetics (Meyer, 1988, 1989; Paganini et al. 1997).
The purpose of the present investigation was therefore to examine the influence of baseline work-rate on muscle [PCr] dynamics following the onset of heavy-intensity single-leg knee-extension exercise. Subjects completed step transitions to heavy-intensity exercise both from a resting baseline and from a baseline of moderate-intensity exercise while inside the bore of a 1.5 T super-conducting magnet. We hypothesized that muscle [PCr] dynamics would be slower and that [PCr] would fall proportionally more per unit change in work-rate when heavy-intensity exercise was initiated from a baseline of moderate-intensity exercise compared to rest.
Methods
Subjects
Seven healthy male subjects (mean ±s.d.: age 26 ± 5 years; stature 1.82 ± 0.03 m; mass 80.7 ± 7.3 kg) volunteered and gave written informed consent to participate in the study which had been approved by the local Research Ethics Committee and which conformed with the Declaration of Helsinki. None of the subjects had participated in structured exercise activities for at least 6 months prior to their involvement in the study and could therefore be considered to be untrained. The subjects were instructed to arrive at the laboratory for each of their tests in a well-hydrated state, and having consumed no food, caffeine or alcohol during the previous 3 h.
Experimental protocol
The single-legged knee-extension exercise tests were conducted in the prone position with the subjects positioned inside a whole body MRI system. A 6 cm 31P transmit/receive surface coil was placed within the subject bed and the subject asked to lie upon it such that the coil was centred over the quadriceps muscle of the right leg. Subjects were then secured to the ergometer bed with Velcro straps at the thigh, buttocks and lower back to minimize extraneous movement during the protocol. The right foot was connected to a pulley system which permitted a non-magnetic weight to be lifted and lowered. Exercise was performed at a rate of 40 repetitions per minute with the subjects lifting and lowering the mass over a distance of ∼0.22 m in accordance with a visual cue projected onto the front wall of the scanner room. The contraction phase of the knee extensors and the interrogation of the quadriceps by 31P-MRS were synchronized.
Initially, each subject completed an incremental exercise test to volitional exhaustion. Following a 2 min period of rest, the subjects commenced knee-extension exercise against an initial basket load of 0.5 kg. Thereafter, the basket load was increased by 0.5 kg at the end of each minute until the subjects were no longer able to maintain the required frequency of 40 repetitions per minute. The subjects received strong verbal encouragement to continue for as long as possible.
Subsequently, the subjects completed a series of ‘step’ exercise tests to work-rates corresponding to 40% and 80% of the highest work-rate attained in the initial incremental exercise test. The tests consisted of 2 min rest followed by a step increase to either: (1) a ‘heavy’ work-rate (80% of the peak value attained in the incremental test) which was maintained for 6 min; or (2) a ‘moderate’ work-rate (40% of the peak value attained in the incremental test) which was maintained for 6 min and which was then immediately increased to 80% of the incremental test peak value for a further 6 min. ‘Heavy’ exercise was therefore performed from both a resting baseline and from a pre-existing lower-intensity exercise baseline. In total, the subjects completed each of the protocols four times in order to enhance the signal-to-noise ratio (Lamarra et al. 1987; Rossiter et al. 2000). The tests were completed in random order and on separate days within a period of 21 days.
MRS measurements
MRS was performed in the Peninsula Magnetic Resonance Research Centre, Exeter, using a 1.5 T superconducting MR scanner (Intera, Philips, the Netherlands). Initially, fast field echo images were acquired in order to determine that the muscle was positioned correctly relative to the coil. This was aided by placing cod liver oil capsules, which yield high intensity signal points within the image, adjacent to the coil allowing its orientation relative to the muscle volume under examination to be assessed. A number of pre-acquisition steps were carried out in order to optimize the signal from the muscle under investigation. An automatic shimming protocol was then undertaken within a volume that defined the quadriceps muscle and matching and tuning of the coil was then performed. To ensure that the examined muscle was consistently at the same point relative to the coil during exercise, the subject was visually queued via a display consisting of two vertical bars, one which moved at a constant rate with a frequency of 0.67 Hz and one which monitored their foot movement via a sensor present within the pulley to which they were connected. Thus, the subject endeavoured to match the movements of these two bars. The work done by the subject was recorded via a non-magnetic strain gauge present within the pulley mechanism, enabling work-rate to be calculated.
Prior to and during exercise, data were acquired every 1.5 s, with a spectral width of 1500 Hz, and 1000 data points. Phase cycling with four phase cycles was employed, leading to a spectra being acquired every 6 s. The subsequent spectra were quantified via peak fitting, assuming prior knowledge, using the jMRUI (version 2) software package employing the AMARES fitting algorithm (Vanhamme et al. 1997). Spectra were fitted assuming the presence of the following peaks: inorganic phosphate (Pi), phosphodiester, phosphocreatine (PCr), α-ATP (2 peaks, amplitude ratio 1 : 1), γ-ATP (2 peaks, amplitude ratio 1 : 1) and β-ATP (3 peaks, amplitude ratio 1 : 2 : 1). In all cases, relative amplitudes were corrected for partial saturation due to the repetition time relative to longitudinal relaxation time (T1). Intracellular pH was calculated using the chemical shift of the Pi spectral peak relative to the PCr peak (Taylor et al. 1983). Resting and end-exercise values of PCr, Pi and pH were calculated over the last 60 s of the rest period and the last 30 s of exercise period.
Modelling procedures
For analysis of the [PCr] kinetics during step exercise, the [PCr] data were first expressed as the percentage change relative to the resting baseline which was assumed to represent 100%. For each subject, the four like-transitions were time-aligned and averaged together to increase the signal-to-noise ratio and to improve confidence in the parameter estimates derived from the subsequent model fits (Lamarra et al. 1987; Rossiter et al. 2000). The [PCr] responses were then modelled using non-linear least-squares regression techniques similar to those described by Rossiter et al. (2001; Model 1) and Barstow et al. (1996; Model 2):
| (1) |
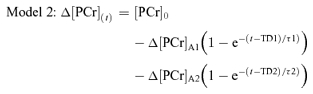 |
(2) |
where [PCr]0 is the value of [PCr] at time zero (onset of exercise), Δ[PCr]ss is the projected asymptotic value, and τ is the time constant of the response (Model 1), and where [PCr]0 is the value of [PCr] at time zero, Δ[PCr]A1 and Δ[PCr]A2 are the asymptotic values to which [PCr] projects for the faster and slower components of the [PCr] response, respectively, TD1 and TD2 are the time delays and τ1 and τ2 are the time constants associated with those processes (Model 2). For Model 1, the magnitude of any possible [PCr] slow component (which might be expected at 80% but not 40% of peak work-rate) was calculated as the difference between the asymptotic amplitude of the fundamental [PCr] response and the average [PCr] measured over the last 30 s of exercise at that work-rate. The ‘Gain’ of the [PCr] response, calculated as the percentage change in [PCr] divided by the work-rate (%/W), was determined for the fundamental phase and, where appropriate, for the entire response (i.e. with the inclusion of any slow component phase of the response).
Statistics
Differences in [PCr] kinetics between the three conditions (moderate exercise, heavy exercise initiated from rest and heavy exercise initiated from moderate exercise) were tested using a one-way analysis of variance with repeated measures and subsequently with a Newman–Keuls post hoc test to locate differences. Significance was accepted when P < 0.05. Unless otherwise indicated, values are expressed as mean ±s.d.
Results
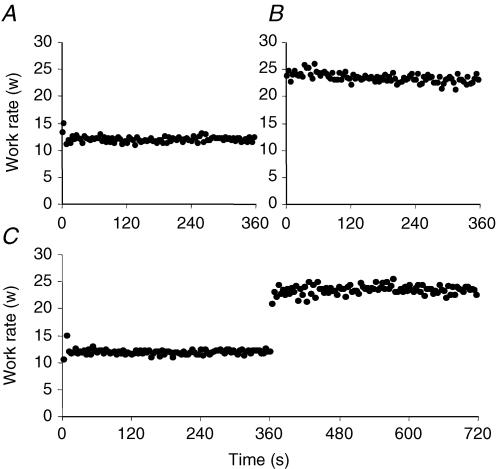
The peak work-rate attained in the incremental test was 28 ± 4 W. The work-rates applied during moderate and heavy exercise were therefore 11 ± 2 W and 22 ± 4 W, respectively, and the change in work-rate in the moderate-to-heavy exercise condition was 11 ± 2 W. The subjects were able to maintain the work-rate with minimal variability throughout the exercise protocols (Fig. 1). The principal results for the analysis of [PCr] kinetics are reported in Table 1 (for Model 1) and Table 2 (for Model 2). The [PCr] responses of a typical subject are illustrated in Fig. 2.
Figure 1. Figure illustrating the relative constancy of the measured work rate throughout exercise in a representative subject.
A, B and C show the responses to rest-to-moderate, rest-to-heavy and rest-to-moderate-to-heavy exercise, respectively.
Table 1.
[PCr] kinetics during rest-to-moderate, rest-to-heavy and moderate-to-heavy exercise transitions as established using Model 1
| Rest-to-moderate | Rest-to-heavy | Moderate-to-heavy | |
|---|---|---|---|
| [PCr] at baseline (%) | 100 ± 0 | 100 ± 0 | 85 ± 3*† |
| Time constant (s) | 25 ± 14 | 48 ± 11* | 95 ± 40*† |
| Fundamental amplitude (%) | 15 ± 3 | 50 ± 11* | 45 ± 11* |
| Fundamental Gain (% W−1) | 1.4 ± 0.3 | 2.3 ± 0.7* | 4.1 ± 0.8*† |
| Slow phase time delay (s) | N/A | 152 ± 51 | 142 ± 31 |
| Slow phase amplitude (%) | N/A | 15 ± 8 | 6 ± 14 |
| [PCr] at end-exercise (%) | 85 ± 3 | 35 ± 9* | 34 ± 10* |
| Total Gain (% W−1) | 1.4 ± 0.3 | 3.0 ± 0.6* | 4.9 ± 1.0*† |
Significantly different from rest-to-moderate exercise condition (P < 0.01)
Significantly different from rest-to-heavy exercise condition (P < 0.01).
Table 2.
[PCr] kinetics during rest-to-moderate, rest-to-heavy and moderate-to-heavy exercise transitions as established using Model 2
| Rest-to-moderate | Rest-to-heavy | Moderate-to-heavy | |
|---|---|---|---|
| [PCr] at baseline (%) | 100 ± 0 | 100 ± 0 | 85 ± 3*† |
| Time constant (s) | 25 ± 14 | 63 ± 21* | 111 ± 57*† |
| Fundamental amplitude (%) | 15 ± 3 | 56 ± 7 * | 50 ± 12* |
| Slow phase time delay (s) | N/A | 167 ± 60 | 157 ± 20 |
| Slow phase amplitude (%) | N/A | 11 ± 3 | 3 ± 9† |
| [PCr] at end-exercise (%) | 85 ± 3 | 33 ± 7 * | 32 ± 10* |
Significantly different from rest-to-moderate exercise condition (P < 0.05)
significantly different from rest-to-heavy exercise condition (P < 0.05).
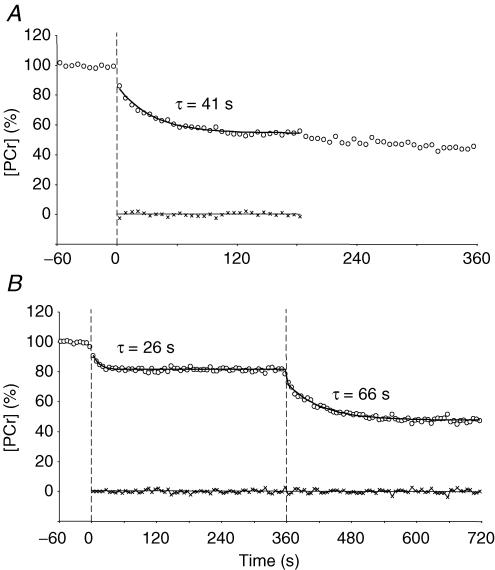
Figure 2. [PCr] responses of a typical subject in the rest-to-heavy exercise condition (A) and the rest-to-moderate exercise and moderate-to-heavy exercise condition (B).
The residuals for the model fits are shown at the foot of each plot. Note the progressive slowing of the time constant (τ) from rest-to-moderate exercise (26 s), rest-to-heavy exercise (41 s), and moderate-to-heavy exercise (66 s). Note also that, in this example, the delayed-onset [PCr] slow component evident in the rest-to-heavy exercise condition is absent in the moderate-to-heavy exercise condition.
Responses during the exercise bouts
The resting [PCr]/[ATP] ratio was 4.89 ± 0.38 and the resting [Pi]/[ATP] ratio was 0.55 ± 0.08. These values are similar to those reported in the literature (Kemp et al. 2007). For example, Praet et al. (2006) recently reported a mean [PCr]/[ATP] ratio of ∼4.50 and a mean [Pi]/[ATP] ratio of ∼0.57 in the quadriceps of young healthy subjects. With the assumption that [ATP] is 8.2 mm at rest (Taylor et al. 1986), the resting [PCr] and [Pi] in our subjects can be estimated to be approximately 40 ± 3 mm and 4.5 ± 0.7 mm, respectively.
During moderate exercise, the [PCr] response was well fitted by a mono-exponential function using both models (i.e. there was no evidence of a ‘slow component’ in the response). [PCr] began to decline in a near-exponential fashion with no discernible delay following the onset of exercise to achieve a steady-state within approximately 120 s (Fig. 2). The metabolic perturbation at this intensity was slight as evidenced by the small changes in [PCr] (6.2 ± 1.3 mm fall; Table 1) and pH (baseline: 7.07 ± 0.02 versus end-exercise: 7.06 ± 0.02; P = 0.59).
When heavy exercise was initiated from a resting baseline, the [PCr] response was clearly not well described by a single exponential function; rather, following the initial near-exponential phase, [PCr] continued to decline with time such that ∼35% of muscle [PCr] was remaining after 6 min of exercise (Table 1 and Fig. 2B). This represented a total fall in [PCr] of 20.2 ± 6.2 mm. This exercise intensity was also associated with a significant reduction in muscle pH (baseline: 7.06 ± 0.03 versus end-exercise: 6.90 ± 0.07; P < 0.01).
When heavy exercise was initiated from a baseline of moderate exercise, [PCr] fell to a lesser extent during both the fundamental and slow phases of the response when compared to the rest-to-heavy exercise condition (Fig. 2). However, the [PCr] at the end of 6 min of heavy exercise was not different between the conditions despite the differences in baseline [PCr] ([PCr] fell 15.3 ± 3.0 mm from a baseline of 34.0 ± 1.2 mm; Table 1). Interestingly, there was some evidence of a ‘trimming out’ of the [PCr] slow component during the moderate-to-heavy exercise condition with the [PCr] slow component being eliminated in two subjects (Fig. 2) and reduced in four of the remaining five subjects. The intramuscular pH at the end of exercise in this condition (6.89 ± 0.06) was similar to that measured when heavy exercise was initiated from rest (6.90 ± 0.07). However, the reduction in pH per unit increment in work-rate was significantly greater when heavy exercise commenced from the moderate exercise baseline (rest-to-heavy exercise: 0.01 ± 0.01 pH units W−1versus moderate-to-heavy exercise: 0.02 ± 0.01 pH units W−1; P < 0.01).
Comparison between the exercise bouts
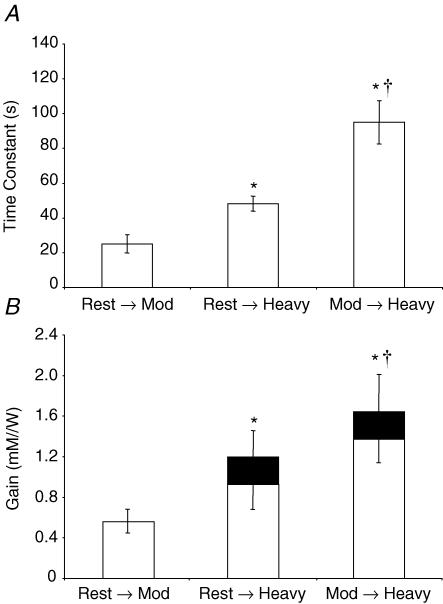
The τ for the fundamental phase of the [PCr] response was significantly different between experimental conditions irrespective of the model used (see Tables 1 and 2, and Fig. 2 for the response of a representative subject). Using Model 1, τ increased from 25 ± 14 s during moderate exercise, to 48 ± 11 s during heavy exercise, and to 95 ± 40 s during moderate-to-heavy exercise (Fig. 3A). Similarly, using Model 2, τ increased from 25 ± 14 s during moderate exercise, to 63 ± 21 s during heavy exercise, and to 111 ± 57 s during moderate-to-heavy exercise.
Figure 3. Mean ±s.e.m time constant for fundamental component [PCr] kinetics (A) and Gain (response amplitude/work-rate) for fundamental component (B, open columns) and slow component (B; filled sections) [PCr] kinetics, as derived from Model 1.
*Significantly different from rest-to-moderate exercise condition and †significantly different from rest-to-heavy exercise condition (P < 0.05).
The functional ‘Gain’ of the [PCr] response (i.e. the change in [PCr] per unit change in work-rate) was also significantly different between the three conditions. The Gain over the fundamental region of the response was 0.56 ± 0.12 mm W−1 for moderate exercise, 0.92 ± 0.28 mm W−1 for heavy exercise, and 1.39 ± 0.27 mm W−1 for moderate-to-heavy exercise (P < 0.05 for all comparisons; Fig. 3B). When the total Gain was considered (i.e. when the [PCr] slow component response is included for the two heavy exercise conditions), the differences were even more pronounced: 0.56 ± 0.12 mm W−1 for moderate exercise, 1.20 ± 0.24 mm W−1 for heavy exercise, and 1.67 ± 0.34 mm W−1 for moderate-to-heavy exercise (P < 0.01 for all comparisons; Fig. 3B).
Discussion
The principal original finding of this investigation was that the τ describing the initial ‘fundamental’ decline in muscle [PCr] was significantly longer (i.e. the kinetics were slower) when heavy exercise was initiated from a baseline of moderate-intensity exercise compared to when it was initiated from rest. Moreover, the ‘Gain’ of the [PCr] response was significantly greater, that is, there was a greater fall in [PCr] for a given increment in work-rate, when heavy exercise commenced from an elevated baseline metabolic rate. These results were consistent with our hypotheses, are coherent with the responses reported previously for pV̇O2 (Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Wilkerson & Jones, 2007), and suggest that the muscle metabolic responses related to oxidative phosphorylation do not consistently show ‘linear, first-order’ behaviour throughout the range of exercise intensities that might typically be studied in humans.
To our knowledge, this is the first study to investigate the effect of differences in baseline work-rate (and hence metabolic rate) on the muscle metabolic responses to exercise as assessed using 31P-MRS techniques. Manipulations of the baseline work-rate are a useful test of the Boltzmann principle of superposition (Fujihara et al. 1973a,b; Lamarra et al. 1983) since, in a system which expresses dynamic linearity, no effect on the response τ or Gain would be expected as a result of such an intervention. The present data, which demonstrate a profound slowing of the kinetics of the initial exponential [PCr] response along with a significantly increased response Gain, therefore indicate that the response dynamics of muscle [PCr] to different work-rate inputs is not dynamically linear. These results are important in that they have implications for our understanding of the regulation of muscle oxidative metabolism (see later discussion).
The ‘size principle’ of motor unit recruitment, as originally demonstrated in cat hind limb muscle by Henneman et al. (1965) and subsequently confirmed in human muscle (e.g. Gollnick et al. 1974; Garnett et al. 1979), states that motor units are recruited in an orderly fashion during voluntary exercise with the smallest motor units (those containing the slow-twitch, fatigue-resistant, type I muscle fibres) having the lowest threshold for activation and being recruited first (Henneman et al. 1965). During low-to-moderate intensity exercise, such as that performed at 40% of peak work-rate in the present study, it would be expected that the majority (if not all) of the required muscle power would be produced by the type I muscle fibres, whereas during higher-intensity exercise, such as that performed at 80% of peak work-rate in the present study, there would be a significant contribution to power production from type II muscle fibres (Gollnick et al. 1974; Vøllestad & Blom, 1985; Ivy et al. 1987; Sahlin et al. 1997; Krustrup et al. 2004). There is evidence that type II muscle fibres have slower V̇O2 kinetics (Crow & Kushmerick, 1982; Kushmerick et al. 1992; Kindig et al. 2003) and are less efficient (i.e. have a greater ATP cost of force production and/or a higher O2 cost of ATP resynthesis; Wendt & Gibbs, 1973; Crow & Kushmerick, 1982; Willis & Jackman, 1994; Reggiani et al. 1997) than type I fibres. Type II fibres also have greater creatine content and a reduced oxidative capacity (lower mitochondrial density and lower oxidative enzyme activity) relative to type I fibres, factors which might be expected to result in slower [PCr] kinetics (Meyer, 1988, 1989; Paganini et al. 1997). On this basis, we believe that a likely explanation for the slower kinetics and greater Gain of the [PCr] response when heavy exercise commences from an elevated work-rate is that this intervention ‘unveils’ the metabolic properties of the type II fibres that will be predominantly recruited across the transition from moderate to heavy intensity exercise.
In earlier studies, Hughson and associates argued that the slower pV̇O2 kinetics observed during transitions from an elevated baseline metabolic rate was related to a slower adjustment of cardiac output and hence muscle O2 delivery (Hughson & Morrissey, 1982; Hughson & Morrissey, 1983). More recently, MacPhee et al. (2005) have reported that the slower phase II pV̇O2 kinetics observed in the upper region of the moderate-intensity exercise domain was associated with a slowing of leg blood flow kinetics. Although it might be argued that muscle perfusion is unlikely to be limiting during exercise involving a small muscle mass (less than ∼5 kg) such as that used in the present study (Andersen & Saltin, 1985; see also Haseler et al. 2004), our experimental procedures do not allow us to exclude the possibility that changes in muscle O2 delivery modulated the muscle [PCr] (and V̇O2) kinetics. It remains possible that local differences in muscle blood flow relative to metabolic rate (i.e. Q̇O2/V̇O2) could alter intramyocyte PO2 sufficiently to impact upon [PCr] changes. Interestingly, such heterogeneities of muscle perfusion relative to metabolic rate are particularly apparent in type II muscle fibres (Behnke et al. 2003; McDonough et al. 2005).
Irrespective of the precise mechanism by which muscle [PCr] kinetics is altered by differences in baseline work-rate, what is particularly striking about the present data is that the profile of the [PCr] response is functionally identical to what one might expect to find in the profile of pV̇O2 (Wilkerson & Jones, 2007). It has been suggested that the rate of mitochondrial respiration is linked, either directly or indirectly, to the rate of cytosolic high-energy phosphate splitting (di Prampero & Margaria, 1968; Whipp & Mahler, 1980; Chance et al. 1985; Meyer, 1988; Grassi, 2000). Consistent with this, Rossiter et al. (1999, 2002a,b) have shown that, when the muscle-to-lung transit delay is accounted for, the pV̇O2 kinetics is essentially a mirror image of the muscle [PCr] kinetics during both moderate and heavy exercise (where a slow component is evident in the profiles of both pV̇O2 and muscle [PCr]), and also during subsequent recovery. It is possible that this close correspondence between muscle [PCr] and pV̇O2 is related to the role of PCr in buffering [ADP] across a metabolic transient, thus blunting one of the principal signals responsible for accelerating oxidative phosphorylation (Chance et al. 1981; Kindig et al. 2005).
Although it was not the principal purpose of our study, it is interesting to note that the fundamental component [PCr] kinetics was significantly slower in the rest-to-heavy exercise transition (τ= 48 ± 11 s) than in the rest-to-moderate exercise transition (τ= 25 ± 14 s). To our knowledge, only one previous study has compared muscle [PCr] kinetics between ostensibly moderate-intensity and heavy-intensity exercise (Rossiter et al. 2002b). In that study, the authors reported mean τ values of 33 s for moderate-intensity exercise (in which the [PCr] kinetics were mono-exponential) and 38 s for heavy-intensity exercise (in which a delayed-onset [PCr] slow component was evident), with there being no statistically significant difference between intensities. The explanation for this difference between studies is unclear but might be related to differences in the exercise modality (one-legged concentric/eccentric exercise versus two-legged concentric-only knee extension exercise) or, to the relative exercise intensities at which the subjects were tested. The intensity of the ‘moderate’ exercise condition appears to have been similar between studies ([PCr] fell by ∼11% from rest to the end of exercise in the study of Rossiter et al. and by ∼15% in the present study) but the intensity of the ‘heavy’ exercise condition was greater in our study ([PCr] in the fundamental phase fell by ∼25% in the study of Rossiter et al. and by ∼50% in the present study). It is possible therefore that the exercise intensity was below the so-called ‘critical power’ (CP; Poole et al. 1988) in Rossiter's study, but above it in ours.
Our results confirm the existence of a [PCr]‘slow component’ during heavy-intensity constant-work-rate exercise (Rossiter et al. 2002a; Haseler et al. 2004; Jones et al. 2007). The continued fall in [PCr] with time for the same work-rate is indicative of a progressive loss of muscle efficiency since it represents a greater phosphate cost for the same muscle force generation (Rossiter et al. 2002a). The slow component, both of [PCr] and V̇O2, appears to be associated with the incurrence of a sustained metabolic acidosis and has been suggested to be related in some fashion to the recruitment of type II muscle fibres (Poole et al. 1988, 1991; Whipp, 1994; Barstow et al. 1996; Rossiter et al. 2002a; Krustrup et al. 2004; Jones et al. 2005). An interesting feature of the present study was that the overall [PCr] response in the moderate-to-heavy exercise condition returned towards being first-order (i.e. mono-exponential); the [PCr] slow component was eliminated in 2 of the 7 subjects and was attenuated in 4 of the remaining 5 subjects. One possible interpretation of these data is that the transition from moderate-to-heavy exercise mandates the recruitment of a population of muscle fibres with characteristics (including a long τ and large Gain) which are more homogeneous than is the case during the transition from rest-to-heavy exercise (in which both type I and type II fibres might reasonably be expected to be recruited; Krustrup et al. 2004).
Intracellular pH was not significantly altered across the transition from rest-to-moderate intensity exercise (ΔpH, ∼0.01). This indicates that the energy equivalent to the ‘oxygen deficit’ incurred prior to the establishment of a steady-state in V̇O2 was met almost exclusively by PCr hydrolysis at this work-rate. In contrast, pH fell significantly across the transition from rest-to-heavy intensity exercise (ΔpH, ∼0.16), indicating that this work-rate obligated energy transfer from both ‘anaerobic’ glycolysis and PCr hydrolysis. Intracellular pH and [PCr] were similar at the end of heavy exercise irrespective of the baseline conditions. However, the fall in both pH and [PCr] per unit increase in work-rate was appreciably greater in the moderate-to-heavy exercise condition than the rest-to-heavy exercise condition, suggesting that substrate-level phosphorylation makes a relatively greater contribution to energy turnover across the transition to heavy exercise when it is immediately preceded by moderate-intensity exercise rather than rest. This is presumably consequent, at least in part, to the appreciably slower [PCr] and V̇O2 kinetics which are extant in the former condition. However, it is pertinent to note that our data are generally consistent with the suggestion of Conley et al. (2001) that, according to the CK reaction, [PCr] must decline more when intramuscular pH is low in order to provide a sufficient [ADP] stimulus to maintain the required rate of oxidative phosphorylation.
Considerations
In the present study, the measured [PCr] is taken to represent the net balance between the rate of ATP utilization and the rate of ATP resynthesis. Since the work-rates were held constant during the exercise protocol (see Fig. 1), we have assumed that the former was essentially constant and that changes in [PCr] therefore principally reflected changes in ATP resynthesis. However, we were not able to directly measure either ATP utilization or resynthesis rates with the experimental design we employed in the present study. A further complication is that the ATP resynthesis rate cannot be attributed entirely to oxidative phosphorylation. In this respect, further insight into the effects of initiating exercise from different baseline metabolic rates on the control of oxidative phosphorylation might be derived from the measurement of [PCr] dynamics during the recovery from exercise. We were unable to measure [PCr] off-kinetics during all of the conditions in the present study because we were limited to the measurement of 150 spectra and our focus was on assessing the [PCr] responses during exercise with high temporal resolution.
In conclusion, this investigation has demonstrated that the muscle [PCr] response to exercise does not exhibit dynamic linearity: that is, neither the τ nor the Gain of the fundamental exponential-like decrease in [PCr] is constant either when different work-rates are compared (rest-to-moderate versus rest-to-heavy) or when exercise is initiated from different baseline work-rates (rest-to heavy versus moderate-to-heavy). Moreover, a slow component in [PCr] is evident during heavy exercise, although this is ablated when the exercise is initiated from a baseline of moderate-intensity exercise rather than rest. The strikingly longer τ and the greater fundamental and total Gain terms in the [PCr] response to moderate-to-heavy exercise compared to the rest-to-heavy exercise condition parallels the responses in pV̇O2 kinetics that have been reported previously (Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Wilkerson & Jones, 2007). The lack of dynamic linearity in the [PCr] response is indicative of greater complexity in the control of oxidative phosphorylation at higher work-rates.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol. 1994;77:1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Jones AM, Nguyen P, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Molé PA. Linear and nonlinear characteristics of oxygen uptake kinetics during heavy exercise. J Appl Physiol. 1991;71:2099–2106. doi: 10.1152/jappl.1991.71.6.2099. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzoni T, Ferretti G, Schenker K, Cerretelli P. Phosphocreatine hydrolysis by 31P-NMR at the onset of constant-load exercise in humans. J Appl Physiol. 1992;73:1644–1649. doi: 10.1152/jappl.1992.73.4.1644. [DOI] [PubMed] [Google Scholar]
- Brittain CJ, Rossiter HB, Kowalchuk JM, Whipp BJ. Effect of prior metabolic rate on the kinetics of oxygen uptake during moderate-intensity exercise. Eur J Appl Physiol. 2001;86:125–134. doi: 10.1007/s004210100514. [DOI] [PubMed] [Google Scholar]
- Chance B, Eleff S, Leigh JS, Jr, Sokolow D, Sapega A. Mitochondrial regulation of phosphocreatine/inorganic phosphate ratios in exercising human muscle: a gated 31P NMR study. Proc Natl Acad Sci U S A. 1981;78:6714–6718. doi: 10.1073/pnas.78.11.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985;82:8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Kemper WF, Crowther GJ. Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol. 2001;204:3189–3194. doi: 10.1242/jeb.204.18.3189. [DOI] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Prampero PE, Margaria R. Relationship between O2 consumption, high energy phosphates and the kinetics of the O2 debt in exercise. Pflugers Arch. 1968;304:11–19. doi: 10.1007/BF00586714. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Hildebrandt J, Hildebrandt JR. Cardiorespiratory transients in exercising man. I. Tests of superposition. J Appl Physiol. 1973a;35:58–67. doi: 10.1152/jappl.1973.35.1.58. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Hildebrandt J, Hildebrandt JR. Cardiorespiratory transients in exercising man. II. Linear models. J Appl Physiol. 1973b;35:68–76. doi: 10.1152/jappl.1973.35.1.68. [DOI] [PubMed] [Google Scholar]
- Garnett RA, O'Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol. 1979;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B. Skeletal muscle O2 on-kinetics: set by O2 delivery or by O2 utilization? New insights into an old issue. Med Sci Sports Exerc. 2000;32:108–116. doi: 10.1097/00005768-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Kindig CA, Richardson RS, Hogan MC. The role of oxygen in determining phosphocreatine onset kinetics in exercising humans. J Physiol. 2004;558:985–992. doi: 10.1113/jphysiol.2004.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Morrissey M. Delayed kinetics of respiratory gas exchange in the transition from prior exercise. J Appl Physiol. 1982;52:921–929. doi: 10.1152/jappl.1982.52.4.921. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Morrissey M. Delayed kinetics of O2 in the transition from prior exercise. Evidence for O2 transport limitation of O2 kinetics: a review. Int J Sports Med. 1983;4:31–39. doi: 10.1055/s-2008-1026013. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Chi MM, Hintz CS, Sherman WM, Hellendall RP, Lowry OH. Progressive metabolite changes in individual human muscle fibers with increasing work-rates. Am J Physiol Cell Physiol. 1987;252:C630–C639. doi: 10.1152/ajpcell.1987.252.6.C630. [DOI] [PubMed] [Google Scholar]
- Jones AM, Pringle JS, Carter H. Influence of muscle fibre type and motor unit recruitment on O2 kinetics. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London & New York: Routledge; 2005. pp. 261–293. [Google Scholar]
- Jones AM, Wilkerson DP, Berger NJ, Fulford J. Influence of endurance training on muscle [PCr] kinetics during high-intensity exercise. Am J Physiol Regul Integr Comp Physiol. 2007;293:R392–R401. doi: 10.1152/ajpregu.00056.2007. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed. 2007;20:555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Hogan MC. Effect of extracellular PO2 on the fall in intracellular PO2 in contracting single myocytes. J Appl Physiol. 2003;94:1964–1970. doi: 10.1152/japplphysiol.00893.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Stary CM, Walsh B, Hogan MC. Effects of acute creatine kinase inhibition on metabolism and tension development in isolated single myocytes. J Appl Physiol. 2005;98:541–549. doi: 10.1152/japplphysiol.00354.2004. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. Am J Physiol Cell Physiol. 1992;263:C598–C606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Lamarra N, Whipp BJ, Blumenberg M, Wasserman K. Model-order estimation of cardiorespiratory dynamics during moderate exercise. In: Whipp BJ, Wiberg DM, editors. Modelling and Control of Breathing. Oxford, UK: Elsevier Biomedical; 1983. [Google Scholar]
- Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol. 1987;62:2003–2012. doi: 10.1152/jappl.1987.62.5.2003. [DOI] [PubMed] [Google Scholar]
- McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol. 1996;81:1331–1338. doi: 10.1152/jappl.1996.81.3.1331. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM. Kinetics of O2 uptake, leg blood flow and muscle deoxygenation are slowed in the upper compared to lower regions of the moderate-intensity exercise domain. J Appl Physiol. 2005;99:1822–1834. doi: 10.1152/japplphysiol.01183.2004. [DOI] [PubMed] [Google Scholar]
- Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. J Gen Physiol. 1985;86:135–165. doi: 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol. 1989;257:C1149–C1157. doi: 10.1152/ajpcell.1989.257.6.C1149. [DOI] [PubMed] [Google Scholar]
- Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol Cell Physiol. 1997;272:C501–C510. doi: 10.1152/ajpcell.1997.272.2.C501. [DOI] [PubMed] [Google Scholar]
- Poole DC, Jones AM. Towards an understanding of the mechanistic bases of O2 kinetics. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London & New York: Routledge; 2005. pp. 294–328. [Google Scholar]
- Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–1260. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988;31:1265–1279. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- Praet SF, De Feyter HM, Jonkers RA, Nicolay K, van Pul C, Kuipers H, van Loon LJ, Prompers JJ. 31P MR spectroscopy and in vitro markers of oxidative capacity in type 2 diabetes patients. MAGMA. 2006;19:321–331. doi: 10.1007/s10334-006-0060-0. [DOI] [PubMed] [Google Scholar]
- Reggiani C, Potma EJ, Bottinelli R, Canepari M, Pellegrino MA, Stienen GJ. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J Physiol. 1997;502:449–460. doi: 10.1111/j.1469-7793.1997.449bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Howe FA, Ward SA, Kowalchuk JM, Griffiths JE, Whipp BJ. Intersample fluctuations in phosphocreatine concentration determined by 31P-magnetic resonance spectroscopy and parameter estimation of metabolic responses to exercise in humans. J Physiol. 2000;528:359–369. doi: 10.1111/j.1469-7793.2000.t01-1-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JE, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JE, Whipp BJ. Dynamics of intramuscular 31P-MRS Pi peak splitting and the slow components of PCr and O2 uptake during exercise. J Appl Physiol. 2002a;93:2059–2069. doi: 10.1152/japplphysiol.00446.2002. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JE, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JE, Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol. 2002b;541:991–1002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roston WL, Whipp BJ, Davis JA, Cunningham DA, Effros RM, Wasserman K. Oxygen uptake kinetics and lactate concentration during exercise in humans. Am Rev Respir Dis. 1987;135:1080–1084. doi: 10.1164/arrd.1987.135.5.1080. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Soderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Physiol Cell Physiol. 1997;273:C172–C178. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986;3:44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Wendt IR, Gibbs CL. Energy production of rat extensor digitorum longus muscle. Am J Physiol. 1973;224:1081–1086. doi: 10.1152/ajplegacy.1973.224.5.1081. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. The slow component of O2 uptake kinetics during heavy exercise. Med Sci Sports Exerc. 1994;26:1319–1326. [PubMed] [Google Scholar]
- Whipp BJ, Mahler M. Dynamics of gas exchange during exercise. In: West JB, editor. Pulmonary Gas Exchange. New York: Academic Press; 1980. pp. 33–96. Vol. II. [Google Scholar]
- Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972;33:351–356. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Jones AM. Influence of initial metabolic rate on pulmonary O2 uptake on-kinetics during severe intensity exercise. Respir Physiol Neurobiol. 2006;152:204–219. doi: 10.1016/j.resp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Jones AM. Effects of baseline metabolic rate on pulmonary O2 uptake on-kinetics during heavy-intensity exercise in humans. Respir Physiol Neurobiol. 2007;156:203–211. doi: 10.1016/j.resp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Willis WT, Jackman MR. Mitochondrial function during heavy exercise. Med Sci Sports Exerc. 1994;26:1347–1353. [PubMed] [Google Scholar]