Abstract
Ageing reduces endothelium-dependent vasodilatation through an endothelial nitric oxide synthase (NOS) signalling pathway. The purpose of this study was to determine whether arginase activity diminishes endothelium-dependent vasodilatation in skeletal muscle arterioles from old rats, and whether NOS substrate (l-arginine) and cofactor (tetrahydrobiopterin; BH4) concentrations are reduced. First-order arterioles were isolated from the soleus muscle of young (6 months old) and old (24 months old) male Fischer 344 rats. In vitro changes in luminal diameter in response to stepwise increases in flow were determined in the presence of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10−5 mol l−1), the arginase inhibitor Nω-hydroxy-nor-l-arginine (NOHA, 5 × 10−4 mol l−1), exogenous l-arginine (3 × 10−3 mol l−1) or the precursor for BH4 synthesis sepiapterin (1 μmol l−1). Arteriolar l-arginine and BH4 content were determined via HPLC. Ageing decreased flow-mediated vasodilatation by 52%, and this difference was abolished with NOS inhibition. Neither inhibition of arginase activity nor addition of exogenous l-arginine had any effect on flow-mediated vasodilatation; arteriolar l-arginine content was also not different between age groups. BH4 content was lower in arterioles from old rats (94 ± 8 fmol (mg tissue)−1) relative to controls (234 ± 21 fmol (mg tissue)−1), and sepiapterin elevated flow-mediated vasodilatation in arterioles from old rats. These results demonstrate that the impairment of endothelium-dependent vasodilatation induced by old age is due to an altered nitric oxide signalling mechanism in skeletal muscle arterioles, but is not the result of increased arginase activity and limited l-arginine substrate. Rather, the age-related deficit in flow-mediated vasodilatation appears to be the result, in part, of limited BH4 bioavailability.
Advanced age is associated with a reduction in skeletal muscle vascular conductance (Martin et al. 1991; Cook et al. 1992; Lawrenson et al. 2003) and impaired endothelium-dependent vasodilatation (Taddei et al. 1995; Gerhard et al. 1996; DeSouza et al. 2000; Muller-Delp et al. 2002). Muller-Delp et al. (2002) and Spier et al. (2004) have further shown that the reductions in flow- and agonist-mediated endothelium-dependent vasodilatation of arterioles from skeletal muscle that are associated with old age occur via a nitric oxide (NO) signalling pathway. Several possible mechanisms may underlie this deficit in NO signalling, including limited substrate (l-arginine) or cofactor (e.g. tetrahydrobiopterin; BH4) bioavailability, reduced abundance or activity of endothelial NO synthase (eNOS), and increased degradation of NO. Indeed, it has been reported that an up-regulation of arginase expression and activity occurs in large conduit arteries from old rats, which could diminish eNOS activity by limiting intracellular l-arginine availability (Berkowitz et al. 2003; White et al. 2006). Because the local chemical milieu differs in conduit arteries and resistance arteries within skeletal muscle, the primary purpose of this study was to test the hypothesis that arginase activity decreases endothelium-dependent flow-induced vasodilatation in the skeletal muscle microcirculation (i.e. arterioles) from old rats and, consequently, that arteriolar l-arginine levels are lower. A secondary purpose was to determine whether ageing decreases the arteriolar concentration of BH4, a cofactor essential for eNOS production of NO (Shi et al. 2004).
Methods
This study was approved by the Institutional Animal Care and Use Committees at West Virginia and Texas A&M Universities, and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animals
Six-month-old (n = 41) and 24-month-old (n = 39) male Fischer 344 rats were obtained from Harlan (Indianapolis, IN, USA). The animals were housed in a temperature-controlled (23 ± 2°C) room with a 12–12 h light–dark cycle. Water and rat chow were provided ad libitum.
Arteriolar preparation
The rats were anaesthetized with sodium pentobarbital (60 mg kg−1, i.p.). The gastrocnemius–plantaris–soleus muscle group from each hindlimb was carefully dissected free and placed in cold (4°C) physiological saline solution (PSS) containing (mmol l−1): NaCl 145.0, KCl 4.7, CaCl2 2.0, MgSO4 1.17, NaH2PO4 1.2, glucose 5.0, pyruvate 2.0, EDTA 0.02 and 3-(N-morpholino)propanesulphonic acid (Mops) buffer 3.0, and 1 g (100 ml)−1 bovine serum albumin; pH 7.4. The animals were then killed by decapitation. With the aid of a dissecting microscope (Olympus SVH10), first-order (1A) arterioles from the soleus muscle, which is composed primarily of highly oxidative fibres (Delp & Duan, 1996) and demonstrates decreased vascular conductance during exercise in old animals (Musch et al. 2004), were isolated and removed from the surrounding muscle tissue as previously described (Muller-Delp et al. 2002; Spier et al. 2004). The arterioles (length, 0.5–1.0 mm; inner diameter, 90–150 μm) were transferred to a Lucite chamber containing PSS equilibrated with room air. Each end of the arteriole was cannulated with resistance-matched micropipettes and secured with nylon suture. After cannulation, the microvessel chamber was transferred to the stage of an inverted microscope (Olympus IX70) equipped with a video camera (Panasonic BP310), video caliper (Microcirculation Research Institute) and data-acquisition system (MacLab/Macintosh) for on-line recording of intraluminal diameter. Arterioles were initially pressurized to 60 cmH2O with two independent hydrostatic pressure reservoirs. Leaks were detected by pressurizing the vessel, and then closing the valves to the reservoirs and verifying that intraluminal diameter remained constant. Arterioles that exhibited leaks were discarded. Arterioles that were free from leaks were warmed to 37°C and allowed to develop initial spontaneous tone during a 30–60 min equilibration period.
Evaluation of vasodilator responses
Upon displaying a steady level of spontaneous tone, arterioles were exposed to graded increases in intraluminal flow in the absence of changes in intraluminal pressure. This was accomplished by altering the heights of independent fluid reservoirs in equal and opposite directions so that a pressure difference was created across the vessel without altering mean intraluminal pressure. Diameter measurements were determined in response to incremental pressure differences of 4, 10, 20, 40 and 60 cmH2O. Volumetric flow (Q) was then calculated from inner diameter (d) and mean red cell velocity (Vrbc), which was determined in a subset of arterioles at each of the pressure gradients, according to the following equation (Davis, 1987; Kuo et al. 1990; Muller-Delp et al. 2002):
Vasodilator responses to the cumulative addition of the nitric oxide donor sodium nitroprusside (SNP, 10−10–10−4 mol l−1) were then determined as previously described (Muller-Delp et al. 2002; Spier et al. 2004). At the end of the SNP concentration–response determination, the Mops buffer solution was replaced with Ca2+-free Mops buffer solution for 1 h to obtain the maximal passive diameter (Muller-Delp et al. 2002; Spier et al. 2004).
Effects of NG-nitro-l-arginine methyl ester (l-NAME), Nω-hydroxy-nor-l-arginine (NOHA), exogenous l-arginine and sepiapterin
To determine the role of nitric oxide synthase (NOS), arginase, l-arginine and sepiapterin in the reduction in flow-induced vasodilatation associated with old age, responses to flow were evaluated in the presence of one of the following: (1) the NOS blocker l-NAME (10−5 mol l−1, n = 12–14 per group) (Muller-Delp et al. 2002; Spier et al. 2004); (2) the arginase inhibitor NOHA (5 × 10−4 mol l−1, n = 9 per group) (Berkowitz et al. 2003); (3) exogenous l-arginine (3 × 10−3 mol l−1, n = 9–11 per group) (Delp et al. 1993; Zhang et al. 2004); or (4) the precursor for BH4 synthesis sepiapterin (1 μmol l−1, n = 13 per group) (Bagi et al. 2004). After flow-mediated vasodilatation was determined under each of these conditions, maximal vessel diameter was determined by replacing the Mops buffer solution with Ca2+-free Mops buffer solution for 1 h. All drugs were purchased from Sigma Chemical, LKT Laboratories or Bachem Inc.
Determination of arteriolar l-arginine and BH4 content
Arteriolar l-arginine and BH4 content (n = 5 per group) were determined using the HPLC method as previously described (Wu & Meininger, 1995; Meininger et al. 2000). Briefly, 1A arterioles (∼10 mg) from soleus muscles of each animal were pooled. For l-arginine analysis, vessels were homogenized with 0.2 ml 1.5 mol l−1 HClO4, then 0.1 ml 2 mol l−1 K2CO3 was added. The homogenates were centrifuged at 10 000 g for 1 min, and an aliquot (0.2 ml) of the supernatant was used for sample determination of l-arginine content (Wu & Meininger, 1995). For BH4 analysis, arterioles were homogenized in 0.1 ml 0.1 m phosphoric acid containing 5 mm dithioerythritol (an antioxidant), to which 17.5 μl 2 m trichloroacetic acid was added. Extracts were oxidized with acidic or basic iodine. Acidic oxidation quantitatively converts BH4 and dihydrobiopterin to biopterin; basic oxidation converts dihydrobiopterin and BH4 to biopterin and pterin, respectively. Samples were incubated in the dark for 1 h. Excess iodine was removed by adding ascorbic acid (final concentration, 0.1 m). The final solution was analysed on a C18 reversed-phase column using fluorescence detection and authentic biopterin as a standard. The amount of BH4 in the arteriolar extracts was determined from the difference between acidic and basic iodine-generated biopterin (Meininger et al. 2000). The sensitivity of l-arginine and BH4 analyses by HPLC, which was assessed using detection limits defined as a signal-to-noise ratio of 3, was 5 and 2 pmol ml−1, respectively. The reliability of the assays was indicated by the precision (agreement between replicate measurements), evaluated by the relative deviation (mean of absolute deviation/mean of replicate measurements × 100%), and by the accuracy (the nearness of an experimental value to the true value), determined with known amounts of standards and expressed as the relative errors ((measurement value – true value)/(true value × 100%)). The precision and accuracy for the l-arginine analysis were 1.4% and 1.6%, respectively, and for the BH4 analysis were 2.0% and 2.3%, respectively. The values in fmol (mg tissue)−1 and pmol (mg tissue)−1 were calculated on the basis of tissue weight.
Data analysis
For statistical analyses, changes in diameter in response to flow were expressed as a percentage of maximal vasodilatation as previously described (Muller-Delp et al. 2002). Flow–diameter curves were evaluated by repeated measures analysis of variance in order to detect differences within (flow rate) and between (animal groups) factors. Pairwise comparisons between specific levels were made through Scheffe/s post hoc analysis when a significant main effect was found. One-way ANOVA was used to determine age-related differences in l-arginine and BH4 content of arterioles. All data are presented as means ±s.e.m. In all statistical analyses, n indicates the number of animals in each group. Significance was defined as P≤ 0.05.
Results
Vessel characteristics
The development of spontaneous tone did not differ between arterioles from young (54 ± 4%) and old (47 ± 6%) rats (P > 0.05). Likewise, the maximum arteriolar diameter was not different between groups (young, 124 ± 4 μm; old, 129 ± 4 μm; P > 0.05); these results are similar to those previously reported for soleus muscle arterioles (Muller-Delp et al. 2002; Spier et al. 2004).
Vasodilator responses to flow
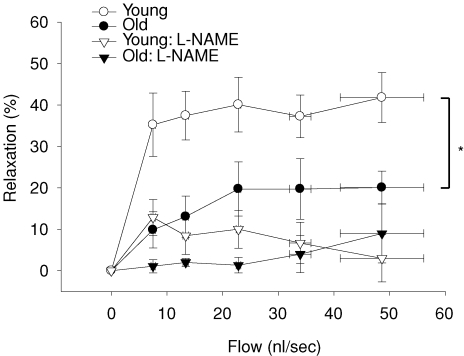
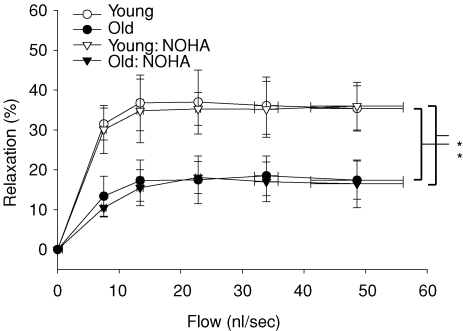
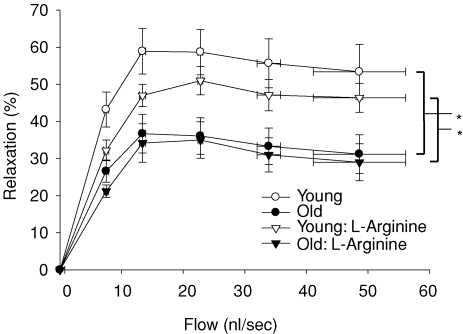
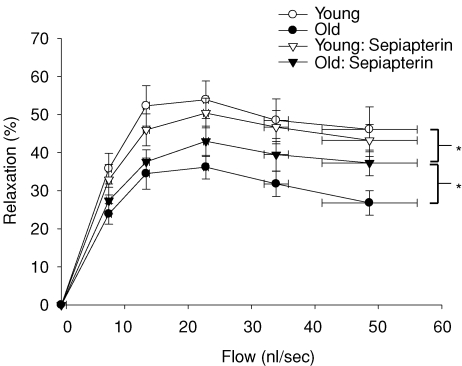
Old age resulted in a 52% reduction in flow-induced vasodilatation in muscle arterioles (Fig. 1). There were no differences in arteriolar vasodilator responsiveness to the exogenous NO donor SNP (data not shown), indicating the deficit in flow-mediated vasodilatation occurred within the vascular endothelium. l-NAME significantly reduced flow-induced vasodilatation in soleus muscle arterioles from both groups and abolished age-associated differences in flow-induced vasodilatation between groups (Fig. 1). Flow-mediated vasodilatation in the presence of the arginase inhibitor NOHA was 51% lower in arterioles from old rats compared with arterioles from young animals (Fig. 2), and similar to flow responses without NOHA present. When exogenous l-arginine was added to the bathing solution, the difference in flow-induced vasodilatation due to age was still present (Fig. 3). These results suggest that arginase activity does not limit l-arginine availability in soleus muscle arterioles from old animals. The addition of sepiapterin to the bathing solution resulted in an enhanced flow-induced vasodilatation in arterioles from the old rats, but not in arterioles from the young rats (Fig. 4). However, the enhanced vasodilator response in arterioles from old rats was still lower than the response in arterioles from young animals.
Figure 1. Effects of ageing and l-NAME on flow-induced vasodilatation in soleus muscle arterioles from young and old rats.
*Indicates vasodilatation in response to flow was lower in soleus muscle arterioles from aged rats (P < 0.05). l-NAME reduced flow-induced vasodilatation in arterioles from young and old rats (P < 0.05) and eliminated differences in responsiveness to flow between the young and old. Values are means ±s.e.m. (n = 12–14 per group).
Figure 2. Effects of ageing and arginase inhibition (via Nω-hydroxy-nor-l-arginine; NOHA) on flow-induced vasodilatation in soleus muscle arterioles from young and old rats.
*Indicates vasodilatation in response to flow was lower in soleus muscle arterioles from old rats (P < 0.05). NOHA did not affect the flow-induced vasodilatation in arterioles from either young or old rats. Values are means ±s.e.m. (n = 9 per group).
Figure 3. Effects of ageing and exogenous l-arginine (3 × 10−3 mol l−1) on flow-induced vasodilatation in soleus muscle arterioles from young and old rats.
*Indicates vasodilatation in response to flow was lower in soleus muscle arterioles from old rats (P < 0.05). l-arginine did not affect the flow-induced vasodilatation in arterioles from either young or old rats. Values are means ±s.e.m. (n = 9–11 per group).
Figure 4. Effects of ageing and sepiapterin (1 μmol l−1) on flow-induced vasodilatation in soleus muscle arterioles from young and old rats.
*Indicates vasodilatation in response to flow was different between conditions (P < 0.05). Values are means ±s.e.m. (n = 13 per group).
Arteriolar l-arginine and BH4 content
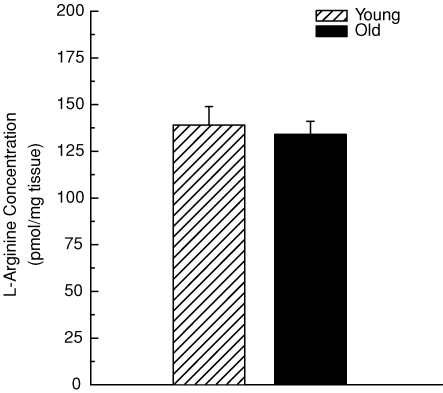
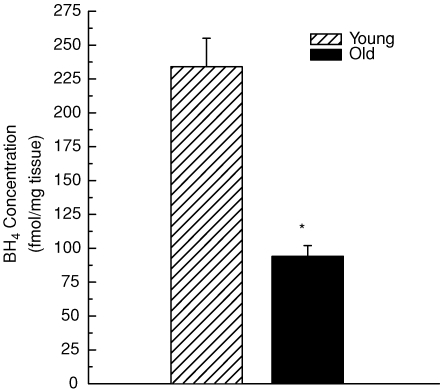
Direct measurements of arteriolar l-arginine content demonstrate that the concentration of the eNOS substrate does not differ between arterioles from young and old rats (Fig. 5); this observation supports the conclusion from the in vitro studies that neither arginase activity nor l-arginine bioavailability adversely affect flow-mediated vasodilatation in skeletal muscle arterioles from old animals. Arteriolar BH4 content, however, was 60% lower in vessels from old rats (Fig. 6). This finding, along with the partial rescue of endothelium-dependent vasodilatation with sepiapterin (Fig. 4), indicates that limited BH4 bioavailability contributes to the impairment of endothelial vasodilator function associated with old age.
Figure 5. l-Arginine concentration in arterioles from the soleus muscle of young and old rats.
Ageing had no effect on arteriolar l-arginine content (P > 0.05). n = 5 per group.
Figure 6. Tetrahydrobiopterin (BH4) concentration in arterioles from the soleus muscle of young and old rats.
*Indicates significant difference in BH4 content to that of arterioles from young rats (P < 0.05). n = 5 per group.
Discussion
The purpose of this study was (1) to confirm our previous observations that ageing diminishes flow-mediated vasodilatation in soleus muscle arterioles through an NOS-mediated signalling mechanism (Muller-Delp et al. 2002), and (2) to determine whether an age-dependent decline in the bioavailability of l-arginine or BH4 is associated with the impairment of endothelium-dependent relaxation in the microcirculation. The results demonstrate that flow-mediated vasodilatation is decreased with old age in the skeletal muscle microcirculation, and that this occurs through an NOS-mediated signalling mechanism (Fig. 1), as previously shown (Muller-Delp et al. 2002). This impairment in endothelium-dependent relaxation is not the result of greater arginase activity (Fig. 2) and, correspondingly, decreased eNOS substrate availability (Figs 3 and 5). Rather, a deficit in BH4 cofactor bioavailability is associated with the age-dependent decline in endothelial vasodilator function (Figs 4 and 6). To our knowledge, these are the first data to demonstrate the effects of ageing on l-arginine and BH4 levels in the microcirculation.
The primary purpose of the present study was to investigate the possible role of arginase in the impairment of endothelium-dependent vasodilatation in the skeletal muscle microcirculation associated with old age. The hypothesis that arginase is up-regulated with ageing is based on the work of Berkowitz and colleagues (Berkowitz et al. 2003; White et al. 2006), who demonstrated that endothelial arginase abundance and activity are elevated with ageing in rat conduit arteries. Although l-arginine levels were not directly assessed in these studies, the reported increase in arginase activity would lead to a deficit in the eNOS substrate l-arginine and a consequent decrease in NO production (Morris, 2000). Results from the present study differ from this previous work (Berkowitz et al. 2003; White et al. 2006) in that neither inhibition of arginase activity (Fig. 2) nor supplementation with exogenous l-arginine (Fig. 3) had any effect on endothelium-dependent vasodilatation in arterioles from old animals. Further, direct measures of arteriolar l-arginine content (Fig. 5) demonstrated no deficiency in the eNOS substrate relative to that in arterioles from young animals, and the amount of arginine in the vessels from old rats is sufficient as the nitrogenous substrate for eNOS (Wu & Morris, 1998). Thus, these results do not support a role for up-regulated arginase activity in the impairment of endothelium-dependent vasodilatation in the skeletal muscle microcirculation associated with old age. The disparity regarding the significance of arginase in limiting l-arginine bioavailability and endothelium-dependent vasodilatation between the present study and previously reported findings (Berkowitz et al. 2003; White et al. 2006) are likely to result from the differential effects of ageing on the arterial vasculature. In previous work (Berkowitz et al. 2003; White et al. 2006), endothelium-dependent vasodilatation in the rat aorta, a conduit artery, was examined whereas in the present study we investigated resistance arteries from skeletal muscle. Undoubtedly the local chemical milieu, including exposure to reactive oxygen species (Davies et al. 1982; Reid et al. 1992), differs between arterioles within metabolically active skeletal muscle and conduit arteries.
Several other mechanisms could underlie the deficiency in NO signalling in resistance arteries associated with old age, including decreased abundance or activity of eNOS, limited cofactor availability, and increased degradation of NO. With ageing, eNOS protein content is not decreased in skeletal muscle arterioles, but is actually greater in arterioles from old animals (Spier et al. 2004); this is similar to results reported in several studies of large conduit arteries (Cernadas et al. 1998; van der Loo et al. 2000). These findings suggest that deficiencies in endothelium-dependent vasodilatation that occur with old age may result, at least in part, from inadequate NO production. Production of NO by eNOS may be regulated by the Ca2+–calmodulin complex (Forstermann et al. 1991) and the cofactor BH4 (Cosentino & Katusic, 1995; Cosentino & Lüscher, 1998; Tiefenbacher, 2001; Vasquez-Vivar et al. 2003). Evidence from numerous studies of pathologies characterized by impaired endothelium-dependent nitroxidergic vasodilatation, such as atherosclerosis, hypercholesterolaemia and diabetes, indicates that availability of BH4 limits eNOS activity and NO production (Cosentino & Katusic, 1995; Tiefenbacher et al. 1996, 2000; Stroes et al. 1997; Maier et al. 2000; Meininger et al. 2000; Tiefenbacher, 2001). Under normal conditions, eNOS, in the presence of sufficient BH4, accepts and stores electrons from nicotinamide adenine dinucleotide phosphate (NADPH) to transform cosubstrates O2 and l-arginine into NO and l-citrulline (Knowles & Moncada, 1994; Vasquez-Vivar et al. 2003). However, under conditions of limited BH4 availabilty, eNOS cannot catalyse the oxidation of l-arginine to NO, but rather will accept electrons from NADPH and donate them to its other substrate, O2, thereby reducing it to superoxide anion (O2−) (Knowles & Moncada, 1994; Vasquez-Vivar et al. 2003). In the aorta from pre-hypertensive spontaneously hypertensive rats (SHRs), for example, impaired endothelium-dependent vasodilatation results from excess production of superoxide that is linked to decreased availability of BH4 (Cosentino et al. 1998). Thus, BH4 plays a crucial role in stabilizing eNOS, preventing the formation of the cytotoxic superoxide, and promoting production of vasoactive NO (Shi et al. 2004).
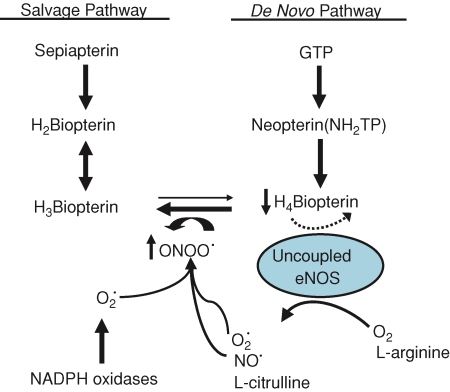
A large body of evidence indicates that oxidative stress increases with age. In the vasculature, both increases in production of reactive oxygen species and decreases in antioxidant enzymes have been reported to occur with advancing age (van der Loo et al. 2000; Woodman et al. 2002). Such age-related increases in reactive oxygen species could diminish BH4 bioavailability as a cofactor for eNOS (Milstien & Katusic, 1999; Laursen et al. 2001) and, in turn, contribute to greater production of superoxide and reduced formation of NO by eNOS (Fig. 7). Results from the present study demonstrate decreased BH4 content in arterioles from old rats (Fig. 6). Furthermore, when exogenous sepiapterin, a precursor for BH4 synthesis (Fig. 7), is added to the bathing solution, there is significant improvement of flow-induced vasodilatation in arterioles from old animals (Fig. 4). Although these results establish a link between impairment of endothelium-dependent vasodilatation and decreased BH4 content associated with old age, they do not determine whether the deficit in vascular BH4 content is related to the BH4 salvage pathway, the BH4de novo synthesis pathway, or oxidative stress (Fig. 7), as the sepiapterin supplementation may simply serve to compensate for BH4 that has been oxidized by peroxynitrite or deficiencies in de novo synthesis of BH4.
Figure 7. The proposed effects of ageing on BH4 bioavailability.
Vascular BH4 content may be limited by reduced synthesis through the de novo or the salvage pathways. Further, because BH4 is susceptible to oxidative degradation, elevations in the production of reactive oxygen species associated with old age could directly decrease vascular BH4 concentration. Peroxynitrite (ONOO·), a product of nitric oxide (NO) and superoxide (O2·) generated through NADPH oxidases and other sources, including xanthine oxidase, cytochrome P-450 enzymes and mitochondria, could further increase catabolism of BH4. With decreased levels of BH4, eNOS becomes uncoupled and leads to generation of O2· rather than NO. Thus, the uncoupling of eNOS decreases the NO available to mediate smooth muscle cell relaxation and increases O2· generation, which could further depress BH4 bioavailability.
Results from several previous studies support the observation of decreased vascular BH4 content with ageing. Eskurza et al. (2005) reported that oral supplementation of BH4 improved forearm flow-mediated vasodilatation in older subjects to the level of that observed in young subjects. Likewise, Higashi et al. (2006) found that co-infusion of BH4 with acetylcholine resulted in greater forearm endothelium-dependent vasodilatation in elderly subjects than with acetylcholine alone. Thus, results from these studies and the present study support the hypothesis that decreased vascular BH4 content with ageing impairs vascular endothelial function. However, this notion is not supported by the work of Blackwell et al. (2004), who reported that BH4 content in aortas from old mice was not different from that in young mice. Whether the discrepancy in the results of this study and the present study reflect differences in the rodent model studied (mouse versus rat) or the type of vessel studied (conduit versus resistance) is unknown.
In summary, in the current study we provide the first experimental evidence demonstrating that reductions in BH4 bioavailability in the skeletal muscle microcirculation induced by old age may be a key mechanism underlying the impairment of endothelium-dependent vasodilatation, rather than an increase in arginase activity and limited eNOS substrate l-arginine availability. Given that the impairment of endothelium-dependent vasodilatation is a major risk factor for the development of cardiovascular disease among the elderly, such insight into the mechanisms of endothelial dysfunction may have important clinical implications. However, further experiments will be necessary to determine how reduced availability of BH4 and oxidant stress affect eNOS activity in the microcirculation with age.
Acknowledgments
This work was supported by National Aeronautics and Space Administration grants NAG2-1340 and NCC2-1166, National Institutes of Health Grants R21AG19248, HL 077224–02 and F32 AG25622, and American Heart Association, Texas Affiliate Grant-In-Aid 0255956Y and 0755024Y.
References
- Bagi Z, Toth E, Koller A, Kaley G. Microvascular dysfunction after transient high glucose is caused by superoxide-dependent reduction in the bioavailability of NO and BH4. Am J Physiol Heart Circ Physiol. 2004;287:H626–H633. doi: 10.1152/ajpheart.00074.2004. [DOI] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–H2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sánchez de Miguel L, García-Durán M, González-Fernández F, Millás I, Montón M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Cook JJ, Wailgum TD, Vasthare US, Mayrovitz HN, Tuma RF. Age-related alterations in the arterial microvasculature of skeletal muscle. J Gerontol. 1992;47:B83–B88. doi: 10.1093/geronj/47.3.b83. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Katusic ZS. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Lüscher TF. Tetrahydrobiopterin and endothelial function. Eur Heart J. 1998;19(Suppl. G):G3–G8. [PubMed] [Google Scholar]
- Cosentino F, Patton S, d/Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Lüscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Davis MJ. Determination of volumetric flow in capillary tubes using an optical Doppler velocimeter. Microvasc Res. 1987;34:223–230. doi: 10.1016/0026-2862(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75:1354–1363. doi: 10.1152/jappl.1993.75.3.1354. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Pollock JS, Schmidt HH, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard M, Roddy M-A, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Nimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chavama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, Mccann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Lüscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Martin WH, Ogawa T, Kohrt WM, Malley MT, Korte E, Kieffer PS, Schechtman KB. Effects of aging, gender, and physical training on peripheral vascular function. Circulation. 1991;84:654–664. doi: 10.1161/01.cir.84.2.654. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobioperin deficiency. Biochem J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Morris SM. Regulation of arginine availability and its impact on NO synthesis. In: Ignarro LJ, editor. Nitric Oxide: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 187–197. [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hagemen KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41:415–433. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Lüscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher CP. Tetrahydrobiopterin: a critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction? Am J Physiol Heart Circ Physiol. 2001;280:H2484–H2488. doi: 10.1152/ajpheart.2001.280.6.H2484. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation. 2000;102:2172–2179. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher CP, Chilian WM, Mitchell M, DeFily DV. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation. 1996;94:1423–1429. doi: 10.1161/01.cir.94.6.1423. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1743. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Impaired arginine metabolism and NO synthesis in coronary endothelial cells of the spontaneously diabetic BB rat. Am J Physiol Heart Circ Physiol. 1995;269:H1312–H1318. doi: 10.1152/ajpheart.1995.269.4.H1312. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]