Abstract
The influence of motor preparation on human motoneuron activity was studied by combining single motor unit recording techniques with reaction-time (RT) methods. The tonic activity of wrist extensor motor units associated with voluntary isometric contractions was analysed during preparation for a ballistic wrist extensor muscle contraction, using a time preparation procedure. Two durations of the preparatory period elapsing between the warning signal and the response signal were used in separate blocks of trials: a short preparatory period (1 s) allowing optimum time preparation, and a longer, non-optimum one (3 s). Changes in motoneuron tonic discharge patterns not associated with any changes in the force output were observed during the preparatory period, which suggests that these changes were subtle enough to prevent any changes in muscle contraction from occurring before the forthcoming movement. The changes observed were a lengthening of the mean interspike interval (ISI) and a decrease in the ISI variability. These data confirm that inhibitory mechanisms are activated during motor preparation and suggest that spinal inhibitory mechanisms are involved in the preparatory processes. The mechanisms possibly involved, such as presynaptic inhibition, dysfacilitation processes or AHP conductance changes, are discussed. The fact that the preparation-induced effects on motoneuron activity were particularly prominent during the last part of the 3 s preparatory period suggests that they were probably related to the neural processes underlying temporal estimation. The anticipatory changes in motoneuron activity observed here during preparation for action provide evidence that central influences act on spinal motoneurons well before it is time to act.
Motor preparation contributes to improving motor performances. It has been clearly established in studies using reaction-time tasks that advance information about the action to be performed at the occurrence of the response signal (RS) shortens the reaction time (RT). When the warning signal (WS) introduced provides prior information about the timing of the forthcoming response signal, it is thought to remove time uncertainty. When it provides information about the movement parameters, it is thought to help the subject to prepare for a specific response. The terms ‘time preparation’ and ‘event preparation’ have been used to refer to the former and latter processes, respectively (Requin et al. 1991; Hasbroucq et al. 1999). In time preparation procedures, the subject/s readiness-to-respond can be controlled via the duration of the preparatory period (or foreperiod), which is the interval between WS and RS (Woodrow, 1914). When a constant duration is used within a block of trials, it has been established that the RTs are shorter in the case of short-lasting compared with long-lasting preparatory periods (Hasbroucq et al. 1997; Tandonnet et al. 2003).
After Jasper/s pioneering research (Jasper et al. 1958), many studies were carried out on the central processes specifically involved in preparation for movement (see Evarts et al. 1984, for a review). Authors using single neuron recording techniques on awake monkeys trained to perform pre-cuing RT motor tasks reported that changes in the neural activity of many supraspinal motor structures occurred up to several hundreds of milliseconds before the onset of the response signal (Riehle & Requin, 1995; see Riehle, 2005, for a review). Anticipatory changes in neuronal activity have been observed in particular in the primary motor cortical areas (M1) involved in shaping the motor command to be transmitted to the spinal motor networks (Tanji & Evarts, 1976; Riehle & Requin, 1989; see Riehle, 2005, for a review).
Surprisingly, anticipatory changes in motor cortical neural activity, such as that of the pyramidal neurons which exert monosynaptic excitatory effects on motoneurons, have been reported to occur without any consistent associated changes in EMG activity. Studies on muscle activity during motor preparation have yielded contradictory results and have not succeeded in establishing the existence of anticipatory changes in motor output (Brunia & Vingerhoets, 1980; Haagh & Brunia, 1985; Lecas et al. 1986; Tanji et al. 1988). Changes in the excitability of spinal motor networks have been detected, however, by assessing the responsiveness of these networks to peripheral and central afferent inputs. The responsiveness of human motoneurons to muscle spindle afferent volleys was found to decrease during the preparatory period, just prior to the onset of the response signal (Bonnet et al. 1981). The amplitude of the monosynaptic reflexes triggered in the muscle involved in the motor task decreased only when the duration of the foreperiod was constant within a block of trials. This pattern has been consistently observed with both Hoffmann (H) and Tendon (T) reflexes with short foreperiods lasting up to 1 s (Brunia, 1983; Hasbroucq et al. 1999). A decrease in the amplitude of the T-reflex has also been thought to occur in the case of longer foreperiods (Brunia et al. 1982). Changes in the responsiveness of the spinal neural networks to cortico-spinal volleys have also been reported to occur during the preparatory period. In studies in which RT methods were combined with transcranial magnetic stimulation (TMS) applied to the motor cortex, the motor potential (MEP) evoked in the muscle involved was depressed prior to the onset of the response signal (Hasbroucq et al. 1997; Touge et al. 1998). The decrease in cortico-spinal excitability reflected here was observed in the case of time but not event preparation, and only during short foreperiods (lasting up to 1 s) (Hasbroucq et al. 1999).
Stronger support for the idea that spinal processes are involved in motor preparation was provided by data obtained on monkeys: the firing rates of spinal interneurons showed changes which were specifically associated with prior information about the movement parameters (Prut & Fetz, 1999; see Fetz et al. 2002, for review). The activity of spinal interneurons characterized on the basis of their facilitatory or inhibitory effects on muscle activity was found in these studies to be modulated several hundreds of milliseconds prior to movement onset. The question therefore arises as to whether or not these changes in premotoneuronal activity occurring during motor preparation may affect the motoneuronal activity without inducing any anticipatory changes in the net joint torque. To our knowledge, the only study in which the effects of motor preparation on single motor unit activity have been investigated in humans focused solely on the period elapsing between the response signal and the onset of the voluntary motor response, i.e. on the reaction time (Kimm & Sutton, 1973). Here it was proposed to investigate whether motoneuron tonic activity may undergo any changes during the preparatory period. For this purpose, we used an approach in which single motor unit recordings were combined with a time preparation protocol. In order to vary the subject/s readiness-to-respond, the changes in motor unit tonic activity occurring during the preparatory period were tested using two different foreperiods. The subtle changes in the motoneuron discharge patterns detected indicate that some central influences do act on spinal motoneurons during preparation for action.
Methods
This study was performed on 10 neurologically sound right-handed subjects (4 females and 6 males) aged 20–27 years. The experimental procedure was approved by the Ethics Committees of the local University of Medicine (CCPPRB-Marseille 2) and the CNRS (approval no. 03006). Prior to the experiments, all the subjects gave their informed written consent to the experimental procedure as required by the Declaration of Helsinki (1964).
Procedure
The subjects were seated in an adjustable armchair. Their right forearm was placed in a groove and the distal end was immobilized. The hand was placed in a semiprone position, with the wrist flexed at an angle of 10 deg. The back of the hand was permanently in contact with an isometric force transducer device. The subjects had to selectively contract their wrist extensor muscles by pushing on the force transducer device with the back of their hand while keeping their finger muscles relaxed. They were asked to voluntarily recruit a single motor unit and to maintain an isometric extension force slightly above the motor unit recruitment threshold so that a 10–12 Hz tonic firing rate was recorded. This meant that a specific force level was associated with each of the single motor units (SMUs) tested. The force was displayed on an oscilloscope screen (0.2 V per division) placed in front of the subjects. At each SMU recording session, the corresponding force signal was adjusted by the experimenter to make it coincide with the middle of a target zone delimited by two 20 mm long, 6 mm wide plastic ribbons, each fixed to one side of the oscilloscope screen. Variations of ±0.15 N were allowed within this target zone. This visual force feedback system therefore helped the subjects to maintain fairly steady force levels and to concentrate on the motor preparation procedure without having to obey any heavy accuracy constraints. The subsequent data analysis showed that the force levels produced ranged from 1 to 3 N, and the extensor muscle activity from 5 to 10% of the maximum EMG, which indicates that low-threshold motor units were mainly recorded.
Pre-cued reaction time (RT) task
All the signals used in this study were auditory signals. The preparatory period began with the warning signal (WP) (frequency, 500 Hz; duration, 100 ms) and ended with the response signal (RS) 1 kHz, 100 ms. The RS was triggered either 1 s or 3 s after the WS. To start a trial, the subject had to perform a wrist isometric extension at the force level required to recruit the motor unit under investigation by tracking the target on the oscilloscope with the visual force feedback provided. The auditory signals were triggered when the force and the motor unit discharge rate were stabilized, from 3 to 8 s after the onset of isometric contraction of the wrist extensor muscle. The trial ended with the motor response, i.e. a ballistic wrist extension performed as fast as possible after the RS. No specific instructions about the amplitude of the ballistic contraction were given to the subjects, apart from the fact that they were asked to perform not a strong but a fast motor response. Off-line data analysis showed the force peaks to be equal to 1 or 2 times the isometric force level maintained before the RS. The subjects returned to 0 force level at the end of each trial. After a minimum rest period of 2 s, they launched the next trial at their own pace. Blocks of 20 trials with fixed short or long foreperiods were run alternately as long as the motor unit recordings remained stable. Before each block was run, the subjects were informed about the duration of the preparatory period (short or long) used in the next 20 trials.
To prevent the occurrence of premature or automatic motor responses, the RS was sometimes unpredictably replaced by a no-response signal (NoRS) (6 kHz, 25 ms). Two to seven NoRSs were included in each block. The motor responses were assessed by performing on-line force signal analysis. An error signal (ES) (50 Hz, 300 ms) was emitted whenever the motor response to the RS failed to be detected or was premature or was not appropriate (see the section on Data analysis).
Prior to each experiment, the subjects were familiarized with the various auditory signals and with the task. They then performed one block of 20 trials under each of the two foreperiod conditions. These two recording sequences were analysed on-line to assess the subject/s ability and to determine their shortest RT, which was used later by the experimenters to continually encourage the subjects to produce their best task performance levels.
Data recordings
Due to the one-to-one relationship between the action potentials occurring in motoneurons and in the muscle fibres they innervate, the pattern of activity of single human motoneurons can be deduced from single motor unit activity recordings. The activity of single motor units (SMUs) was recorded here in the extensor carpi radialis muscle (ECR) using tungsten microelectrodes (12 MΩ, tested at 1 kHz, Frederick Haer and Co., USA). The microelectrode was moved around until a stable recording of clearly identifiable SMU potentials was obtained. The motor unit spike trains were amplified, filtered (300 Hz to 3 kHz) and discriminated on-line using a dual window discriminator (BAK Electronics, USA). The overall electromyographic (EMG) activity of the ECR and the flexor carpi radialis (FCR) muscles was recorded using pairs of Ag–AgCl surface electrodes placed 2 cm apart. The amplified EMG signal was filtered (30 Hz to 10 kHz). The surface electrodes and the microelectrode were connected to amplifiers (Grass P511K, USA) via probes, with an isolated earth for optimum subject protection (current leakage amounted to less than 3 μA). The wrist extension force was measured with a highly sensitive strain gauge (Grass FT03, USA, 0.16 V N−1), amplified (Grass 7P122D, USA) and calibrated in newtons.
The force, EMG and microelectrode signals were sampled on-line using the CED 1401 interface and the Spike 2–5 software program (CED, UK) and digitized at sampling rates of 1, 5 and 25 kHz, respectively. The behavioural task was monitored via the CED 1401 interface, which delivered the sequences of auditory signals (WS, RS, NoRS, ES) and recorded them along with the corresponding electromyographic and biomechanical analog signals in the datafiles generated by the Spike2 program.
Data analysis
The Spike2 software program was used off-line to analyse the analog and digital signals.
Reaction time and error rate
The reaction time (RT) was taken as the interval between the RS and the onset of the ballistic extension, defined by an increase in the force level. The reaction time was computed on-line after each wrist extension movement using scripts specially written for the Spike2 software program. For this purpose, the mean force level and the corresponding standard deviation (s.d.) were assessed during the 500 ms period prior to the RS. The response onset was taken to occur when the force level became greater than the pre-cue mean + 10 s.d. When no response onset had been detected 700 ms after the RS, the response was taken to be incorrect and the error signal (ES) sounded. Another performance indicator, the error rate, which was defined as the ratio between non-valid and valid trials, was also used to assess each of the recording sequences.
Valid and non-valid trials
Successful trials including no-response signals (NoRS) were pooled with successful trials including RS, since the period of interest occurred prior to these signals. Any trials in which an ES was emitted were excluded from the analysis. Any trials in which the RT was longer than the mean RT + 2 s.d. were taken to be non-valid and were also excluded.
Motor unit activity analysis
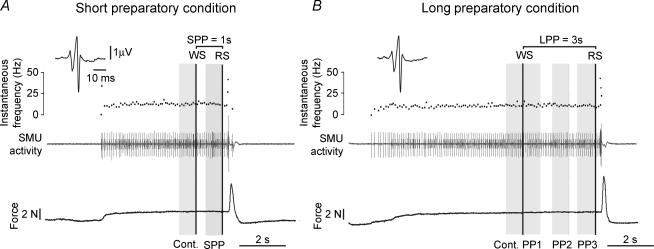
SMU action potentials were discriminated off-line using the Spike2 waveform recognition procedure and each action potential was carefully checked on the computer screen, especially during the preparatory period. SMUs were characterized by the motor unit macro-potential (macro-MUP) obtained by performing spike-triggered averaging on the surface EMG activity of the ECR muscle. The size and shape of the macro-MUP were systematically checked across successive blocks to confirm off-line that the same motor unit had been tested in the various blocks. The motor unit discharge pattern was characterized in terms of the means and standard deviations of the interspike intervals (mISI and s.d.) and by computing the coefficient of variation (CV =s.d./mISI). The values were assessed from eight consecutive ISIs analysed during different periods within each trial, as shown in Fig. 1. These periods included the eight ISIs immediately preceding the WS (control period) and the eight ISIs immediately preceding the RS (preparatory period) under both foreperiod conditions (Fig. 1A and B). In the case of the long foreperiod, the period just prior to the RS was named PP3; the other two periods analysed were that including the eight ISIs immediately following the WS (PP1) and that including the eight ISIs detected during the second third of the preparatory period, i.e. just before the 2 s time point (PP2) (Fig. 1B). The data obtained during the preparatory periods, i.e. during the single period (SPP: short preparatory period) analysed under short preparatory conditions and during each of the three subperiods (PP1, PP2, PP3) analysed under long preparatory conditions, were compared with those obtained during the corresponding control period. With each SMU, the mean ISI, s.d. and CV values were obtained by averaging the values recorded in all the trials performed during the recording sequence. To normalize the data, the values obtained during the preparatory period were also expressed as percentages of the control values, and the percentages obtained at each trial were then averaged to determine the preparation-induced changes undergone by each SMU.
Figure 1. Sequence and timing of events during individual trials illustrating the periods under analysis.
Two recordings performed on the same motor unit under short (A) and long preparatory (B) conditions. Each panel shows the isometric extension force (lower trace), the single motor unit (SMU) activity (middle trace) and the instantaneous discharge frequency curve (top trace). The preparatory period began with the warning signal (WS) and ended with the response signal (RS); both signals are indicated by vertical black lines. The two durations were: 1 s and 3 s. The periods under analysis, each corresponding to about 8 interspike intervals, are shown in grey. The control period (cont.) was the period just prior to the WS. During the short preparatory period, the period just preceding the RS was analysed (SPP), whereas three subperiods (PP1, PP2, PP3) were analysed during the long preparatory period (LPP). The similarity between the motor unit macro-potentials (macro-MUPs) shown in the inset confirm that the same motor unit was actually tested during the whole recording sequence.
Force and surface EMG analysis
The mean force level and the mean rectified EMG activities were measured in 1 s intervals during all the periods analysed (control, SPP, PP1, PP2, PP3) during each trial, and overall averages were then computed for each SMU recording sequence.
Statistical analysis
Non-parametric tests were performed to compare ISI, s.d. and CV between the various periods analysed. The data were therefore expressed as medians ± QD (quartile deviation) averaged across SMUs during each period analysed. The Wilcoxon matched-pairs signed-ranks test was used to determine whether there existed any significant differences between the control and preparatory period in the short foreperiod condition. The Friedman test, i.e. the non-parametric one-way ANOVA for repeated measurements, was used to determine whether there existed any significant differences between the control values and those recorded during the three subperiods analysed under the long foreperiod conditions. When a statistical significance was found to exist on the basis of the Friedman test, Dunn/s post hoc comparisons were performed to test whether any differences existed between control and PP1, PP2 and PP3 periods. The Page test for ordered alternatives (Siegel & Castellan, 1988) was also used to test whether the values obtained during the various periods were ordered in a specific sequence. These same tests were also used to assess the differences between the mean EMGs and mean force levels recorded during the periods analysed. The existence of correlations between the mean ISI and either s.d. or EMG activity was determined using the Spearman rank-order correlation method. Statistical significance level was set throughout at P < 0.05.
Results
The tonic activity of wrist extensor carpi radiali (ECR) motoneurons during a voluntary isometric contraction was analysed while subjects were preparing to perform a ballistic contraction, based on a pre-cueing reaction time (RT) procedure. The main technical difficulty encountered in the present study was maintaining the unitary recordings stable across successive trials because of the changes in the force levels inherent to the motor task, which meant that the SMU under investigation was recruited and de-recruited during alternate tonic and phasic muscle contractions, and keeping these recordings stable for long enough to be able to test the motor unit during a sufficiently large number of trials. Thirty-one motor units were successfully tested under both long and short preparatory conditions, some of them in as many as 181 trials. The population of ECR motor units was analysed as a whole, and the data were pooled, regardless of the number of trials performed in each case. Beforehand, we checked that the discharge pattern of the motor unit analysed during the longest recording sequence was not affected by either the recording time or the number of trials undergone. This was subsequently confirmed by the fact that no significant correlations were found to exist between the extent of the preparation-induced changes in the motor unit discharge patterns and the number of trials undergone.
Motor performance and biomechanical parameters
The reaction times were significantly shorter with the short (253.5 ± 23 ms) compared with the long preparatory period (273.7 ± 32.2 ms) (median ± QD, P = 0.02). The error rate was around 10% in both conditions.
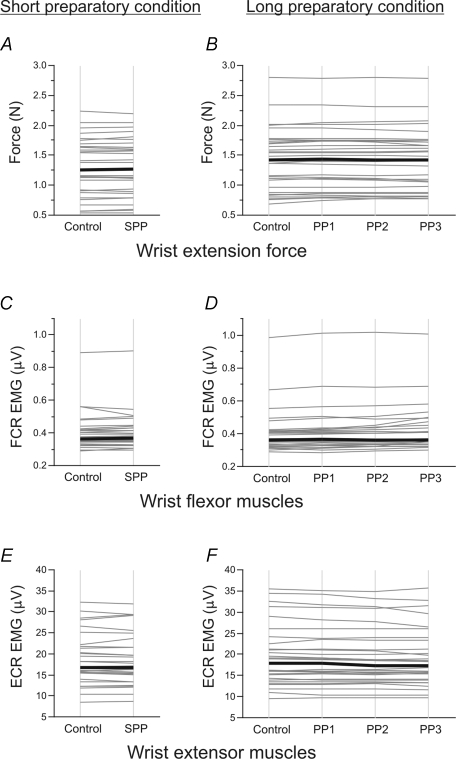
The mean force level and the mean EMG activity obtained in each SMU during the 1 s intervals corresponding to the various periods analysed (control, SPP, PP1, PP2, PP3) under the two foreperiod conditions are illustrated in Fig. 2. Upon pooling the mean force level values (Fig. 2A and B), no differences were found to exist in the force levels between the control period and the short preparatory period (control: 1.26 ± 0.73 N; SPP: 1.26 ± 0.73 N) or between the control and the three subperiods constituting the long preparatory period (control: 1.43 ± 0.73 N; PP1: 1.44 ± 0.75 N; PP2: 1.43 ± 0.74 N; PP3: 1.43 ± 0.71 N).
Figure 2. Influence of motor preparation on motor output.
The mean force (A–B) and mean EMG activity of wrist flexor (FCR; C–D) and extensor (ECR; E–F) muscles are shown under short (graphs on the left) and long (graphs on the right) preparatory conditions. The mean values averaged across trials during the 1 s control and preparatory periods (SPP, PP1, PP2, PP3) for each of the motor units tested are shown by thin grey lines. The black lines give the median values obtained on the whole motor unit population. Note the lack of change in force and muscle activity observed under short and long preparatory conditions, except for the decrease in extensor muscle activity occurring during the last two parts of the long preparatory period (PP2 and PP3).
The overall pattern of muscle activation consistently observed in all the trials showed that the wrist extensor muscles were activated but not the flexor muscles. Upon pooling the data, the FCR rectified surface EMG value was found to be lower than 0.4 μV during each of the periods analysed (Fig. 2C and D). No significant changes were detected in the rectified surface EMG activity of the ECR muscles between the control period (16.80 ± 6.23 μV) and the short preparatory period (16.77 ± 6.45 μV) (Fig. 2E). In the long preparatory condition, the level of ECR activity decreased gradually during the foreperiod (Page test, P < 0.01). Although this decrease was fairly small in comparison with the control period (17.84 ± 7.76 μV), it was significant in the case of PP2 (PP2: 17.32 ± 8.37 μV) and PP3 (17.32 ± 8.35 μV) (Dunn/s test, P < 0.05) (Fig. 2F).
Motor preparation and motor unit discharge pattern
Changes in interspike interval (ISI) duration
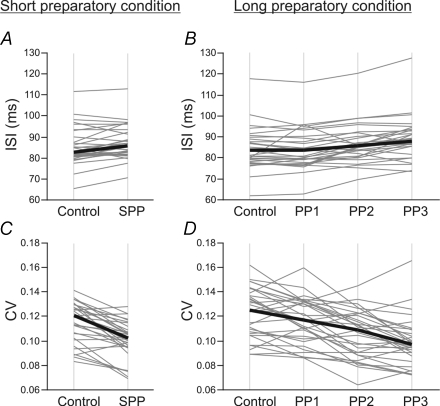
The effects of motor preparation on ISI duration are shown under both foreperiod conditions in Fig. 3A and B. The mean values obtained during the control and preparatory periods in each of the SMUs (grey lines) are shown in this figure, along with the median values obtained on the SMU population as a whole (black lines). Upon pooling the data, the ISI was found to be significantly longer during the short preparatory period (85.8 ± 4.9 ms) than during the control period (82.6 ± 4.4 ms) (P < 0.01). In the long preparatory condition, significant differences were detected between the periods analysed (Friedman test, P < 0.001). A significant ISI lengthening (based on post hoc comparisons using Dunn/s test, P < 0.01) was found to occur during the late parts of the foreperiod, PP2 (86.0 ± 4.5 ms) and PP3 (87.9 ± 3.8 ms), in comparison with the control period (83.6 ± 4.9 ms). No significant differences were detected between the control period and the period immediately following the warning signal (PP1) (83.6 ± 4.8 ms). A Page test confirmed that the ISI increased gradually across the successive periods analysed in the long preparatory condition (control < PP1 < PP2 < PP3) (P < 0.001).
Figure 3. Influence of motor preparation on motor unit discharge patterns under short (graphs on the left) and long (graphs on the right) preparatory conditions.
A and B, effects of the preparatory conditions on the mean interspike interval (ISI). The ISI durations during control and preparatory periods (SPP in A; PP1, PP2, PP3 in B) are shown for each of 31 SMUs by thin grey lines. The median values obtained on the whole SMU population are shown by thick black lines. The ISI duration was significantly longer during the short preparatory period (P < 0.01) and the last two parts of the long preparatory period, PP2 and PP3 (P < 0.01), than during the respective control periods. The duration of the ISI increased gradually across the successive subperiods analysed under long preparatory conditions (control < PP1 < PP2 < PP3) (Page test, P < 0.001). C and D, effects on the variability of the discharge patterns. The coefficients of variation (CV) are shown in the same way as above. The CV decreased significantly on average during the short preparatory period (P < 0.001). During the long preparatory period, the mean CV was significantly lower during PP2 and PP3 than during the control period (P < 0.01). A gradual decrease was observed during the successive subperiods (control > PP1 > PP2 > PP3) (Page test, P < 0.001).
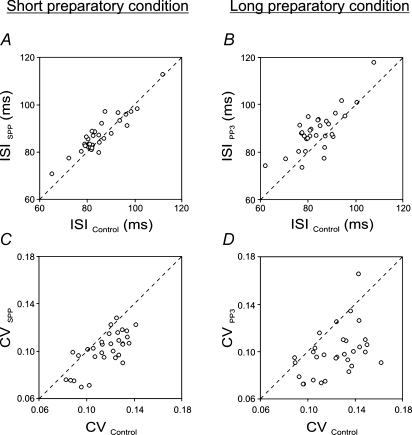
In Fig. 4A and B, the mean ISI durations obtained with each of the SMUs tested during the period just prior to the RS, i.e. SPP in the case of the short foreperiod and PP3 in that of the long foreperiod, are plotted versus the control values. In both conditions, the motor preparation induced a lengthening of the ISI in most of the SMUs, and this effect tended to be stronger under the long compared with the short preparatory conditions.
Figure 4. Preparation-induced changes in motor unit discharge patterns.
The period analysed during the preparatory period was the period just prior to the response signal, i.e. SPP in the short preparatory condition (graphs on the left) and PP3 in the long preparatory condition (graphs on the right). A–B, changes in the duration of the mean interspike interval (ISI). The mean ISI obtained with each of the 31 SMUs during the preparatory periods (ISI SPP in A, ISI PP3 in B; on the ordinate) was plotted versus the mean ISI obtained during the control period (on the abscissa). Under both preparatory conditions, most of the circles were located above the identity line (dashed line), which indicates that ISI tended to lengthen on the whole during the preparatory period. C–D, changes in the variability of the discharge patterns. These graphs were drawn in the same way as above. The coefficient of variation (CV) decreased consistently during the preparatory period, as indicated by the fact that most of the circles were located below the identity line. Note that the effects of motor preparation on ISI and CV were stronger under long than short preparatory conditions.
Changes in CVs id="ss3-2-3" level="3"
The motor preparation-induced changes in the variability of the interspike intervals, as given by the coefficient of variation, are shown in the two foreperiod conditions in Fig. 3C and D. The mean values obtained with each of the SMUs during the control and preparatory periods are shown in this figure (grey lines) along with the median values obtained on the SMU population as a whole (black lines). The CV decreased significantly during the short foreperiod in comparison with the control period (0.121 ± 0.013 and 0.102 ± 0.008, respectively, P < 0.001). During the long foreperiod, the CV decreased significantly during PP2 (0.109 ± 0.017) and PP3 (0.097 ± 0.010) in comparison with the control period (0.125 ± 0.015) (post hoc comparison Dunn/s test, P < 0.01). No significant differences were found to exist between the control and PP1 values (0.117 ± 0.015). The variability of the motor unit discharge pattern decreased gradually across the successive periods analysed in the long foreperiod condition (control > PP1 > PP2 > PP3) (Page test, P < 0.001). This decrease in the variability was observed consistently throughout the SMU population (Fig. 4C and D). As in the case of the ISI changes, the effects of motor preparation on CV during the period just prior to the RS tended to be stronger under the long compared with the short preparatory conditions.
Relationships between the changes in the mean duration and variability of ISIs id="ss3-2-3" level="3"
The expected positive correlation (Matthews, 1996) was found to exist between the duration of the interspike intervals and their variability when the relationships were assessed separately during the control and preparatory periods (Fig. 5). Upon pooling all the data obtained on the whole SMU population, the s.d. of the interspike intervals was found to have increased significantly with the duration of the ISIs during the control periods under both short and long foreperiod conditions (Spearman correlation, ρ= 0.34 and ρ= 0.33, respectively, P < 0.0001) as well as during the preparatory periods under both short and long foreperiod conditions (ρ= 0.26 and ρ= 0.28, respectively, P < 0.0001).
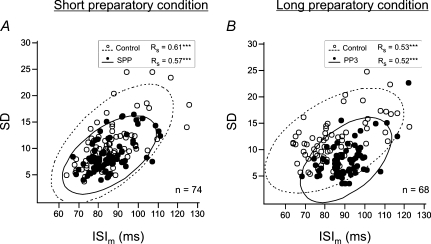
Figure 5. Typical example of correlations between the mean duration and the variability of the interspike intervals analysed in one single motor unit.
The s.d. was plotted versus the mean interspike interval (ISI) during the control period (^) and the preparatory period (•) for each of the trials performed under short preparatory (A, n = 74) and long preparatory (B, n = 68) conditions. In both conditions, a significant positive correlation between ISI and s.d. was observed during the two periods of analysis, i.e. the control period (ellipse with dashed contour) and the preparatory period (ellipse with continuous contour) (Spearman correlation P < 0.001). Note that the s.d. values tended to be lower with equivalent ISI durations during the preparatory than the control period, especially in the case of long foreperiods.
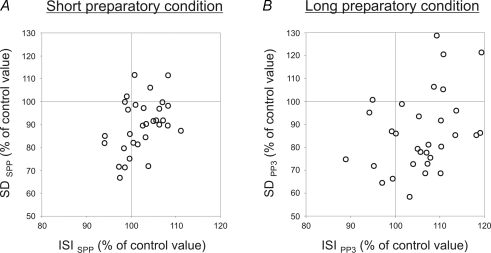
The relationships between the preparation-induced changes in s.d. and those induced in the ISI duration were assessed in each of the SMUs tested (Fig. 6). Under short foreperiod conditions (Fig. 6A), the s.d. decreased in 25 of the 31 SMUs (80.64%) during the preparatory period, as shown by the number of circles present in the two lower frames of the graph. The duration of the ISIs was lengthened in 20 of the 31 SMUs (64.51%) (see the two right-hand frames). When the same population of SMUs was tested under long foreperiod conditions (Fig. 6B), the discharge variability of 25 of the 31 SMUs (80.64%) decreased and the ISI of 23 of the 31 SMUs (79.19%) was lengthened during the late part of the preparatory period (PP3). It can be seen from these two graphs that most of the circles are located in the right-hand lower frame, which was unexpected in view of the positive correlation found to exist between the mean value and the s.d. of the interspike intervals. This finding confirms that the lengthening of the ISIs was associated with a decrease in the variability of the SMU discharge pattern during the preparatory period. Out of the whole SMU population, 51.61% (16 out of 31 units) were affected in this way during the short preparatory period (Fig. 6A) and 58.06% (18 out of 31 units) during the late part of the long preparatory period (PP3) (Fig. 6B).
Figure 6. Relationships between changes in ISI duration and changes in ISI variability in the SMU population.
Preparation-related changes in the standard deviation (s.d.) of the mean interspike interval (on the ordinate) were plotted against the changes in ISI duration (on the abscissa) in the case of each of the 31 SMUs, by expressing the values obtained during the preparatory period as percentages of the control values. Under long preparatory conditions, the data were obtained during the last part (PP3) of the preparatory period. Most of the circles are in the right lower frame of the graphs under both short (A) and long (B) preparatory conditions, which indicates that the decrease in the s.d. was consistently associated with a lengthening of the ISI during the preparatory period.
Correlations between the changes in ISI duration and in EMG activity id="ss4" level="1" type="discussion"
A negative correlation was found to exist between the preparation-induced changes in the mean ISI values recorded in the SMUs and the mean level of EMG activity recorded in the ECR muscles during the corresponding sequences. The level of ECR EMG activity decreased as the duration of the ISI increased. This negative correlation was significant during the short preparatory period (ρ=−0.62, P < 0.001) and during the subperiods, PP1 (ρ=−0.43, P < 0.01), PP2 (ρ=−0.58, P < 0.001) and PP3 (ρ=−0.78, P < 0.0001), constituting the long preparatory period.
Discussion
The results obtained in the present study show the occurrence of anticipatory changes in the tonic activity of ECR motor units during preparation for a ballistic wrist extensor muscle contraction. With the time preparation procedures used in this study, where the duration of the foreperiod is known in advance, the temporal uncertainty is removed and the best motor performances were expected to occur with short foreperiods, given previous findings (Woodrow, 1914; Hasbroucq et al. 1997). The clear-cut decrease in the reaction time observed here under short preparatory conditions provides evidence that the subjects were using the pre-cue to get ready at the right time, and that preparatory processes were actually involved in the motor task.
Anticipatory changes in motoneuron activity
Quantitative trial-by-trial analysis showed that the motor unit interspike intervals increased, whereas the discharge variability decreased during the preparatory period prior to the performance of the motor task. The possibility that these changes in the motor units/ tonic discharge patterns might simply reflect the stabilization of the motor unit activity after the onset of the isometric contraction can be ruled out, since these changes showed different time patterns depending on the preparatory conditions. In the short foreperiod condition, significant changes in the SMU discharge pattern were detected within the first second following the warning signal, whereas they occurred later in the long foreperiod condition, during the second and third seconds. The changes in the motor unit tonic discharge pattern observed were therefore specifically associated with motor preparation. The present data showing that the activity of motoneurons is modulated well before the onset of the response signal provides evidence that anticipatory changes occur in motoneuron activity just as they do in the neural activity of many supraspinal motor structures (see Riehle, 2005, for a review).
These anticipatory changes in the motoneuron activity were not accompanied by any detectable changes in force output. The possibility that force changes were beyond the range of sensitivity of the strain gauge used here is unlikely, as this instrument was sensitive enough to be able to extract single motor unit twitches in previous studies (Schmied et al. 1999). It can therefore be assumed that they are subtle enough to prevent any unwanted changes in muscle contraction from occurring before the forthcoming movement, although a slight decrease in the ECR EMG activity consistent with the ECR motor unit ISI lengthening was found to occur during the long preparatory period. The fact that the changes in the EMG activity of the extensor muscles involved in the motor task were so small may explain why previous authors failed to observe the occurrence of any anticipatory changes in muscle activity prior to the performance of a motor task (Lecas et al. 1986; Tanji et al. 1988).
Involvement of spinal inhibitory mechanisms
The lengthening of the mean interspike interval observed here prior to the response signal demonstrates that inhibitory mechanisms are activated during preparation for movement. This idea contrasts with the data obtained in the only study focusing on this topic so far, in which a specific increase in the firing rate of some motor units was reported to occur during the preparatory period in conditioned monkeys (Mellah et al. 1990). However, the duration of the foreperiods was varied from trial to trial in these monkey experiments. In situations of this kind, conditional probability effects are known to occur, resulting in the involvement of different central processes from those involved here (see Riehle, 2005 for a review). This may explain the discrepancy between the latter data and our own. The present finding that inhibitory processes are activated during the preparatory period is in line with the conclusions of previous surface EMG studies showing that a systematic decrease in the amplitude of both the monosynaptic reflexes (the Hoffmann and Tendon reflexes) and the motor potentials evoked by TMS occurred prior to the onset of the response signal when the duration of the foreperiod remained constant throughout a block of trials (Bonnet et al. 1981; Brunia, 1983; Touge et al. 1998; Hasbroucq et al. 1999). The reflex depression was thought to reflect preparation-induced activation of inhibitory mechanisms acting presynaptically on the Ia afferent terminals and thus controlling the muscle spindle afferent transmission to the motoneurons (Bonnet, 1981; Requin et al. 1991), whereas the MEP depression was thought to reflect changes in the excitability of intracortical and/or spinal inhibitory networks (Hasbroucq et al. 1999; Burle et al. 2004; Davranche et al. 2007).
In line with these previous studies on reflex gain modulation, an increase in the presynaptic inhibition acting on somesthesic afferents may account for the ISI lengthening and possibly for the decrease in variability. However, the resulting decrease in sensory synaptic inputs to the motoneurons can be assumed to occur along with a concomitant increase in descending synaptic inputs, given the anticipatory changes observed in many supraspinal structures and in the pyramidal neurons, in particular (Tanji & Evarts, 1976; Evarts et al. 1984; Riehle & Requin, 1989). The decrease in variability observed here may therefore not be entirely attributable to a decrease in synaptic noise. Although presynaptic inhibition may well be triggered by the preparatory processes, the present data based on close analysis of the characteristics of the SMU tonic activity in terms of the firing rate and variability suggests that changes may also occur at the postsynaptic level. As a matter of fact, the relationships between the discharge rate and its variability can be taken as an index to the various processes involved in sustained motoneuron firing (i.e. the net excitatory drive, excitation threshold, after-hyperpolarization and synaptic noise). When a motoneuron is discharging steadily, the variability of the neural discharge rate has been reported to increase as the firing rate decreases (Calvin & Stevens, 1968; Matthews, 1996), whereas the variability of the motor unit discharge rate decreased here during the preparatory period concurrently with a lengthening of the interspike interval. This finding suggests that changes in the integrative properties of motoneurons might occur during motor preparation.
One possible explanation for this finding might be that changes in postsynaptic conductances are induced by the activation of descending neural pathways acting on segmental excitatory and inhibitory interneurons. This possibility is supported by the anticipatory modulation of spinal interneurons observed in trained monkeys (see Fetz et al. 2002, for a review). A dysfacilitation of excitatory interneurons would be in line with the modulation of interneuron activity reported to be most often inhibitory (Prut & Fetz, 1999). If postsynaptic inhibition is involved, hyperpolarizing and shunting inhibition mechanisms might both contribute to the observed modulation of motoneuron firing rate (Monier et al. 2003; Prescott & De Koninck, 2003).
Another possibility might be that motor preparation induces changes in modulatory systems, which results in turn in changes in the intrinsic properties of the motoneurons. Brownstone and colleagues recently established that activation of spinal cholinergic neurons increases motoneuronal excitability by reducing the action potential afterhyperpolarization (AHP) (Miles et al. 2007). Conversely, any dysfacilitation of these cholinergic interneurons induced by the activation of inhibitory processes during motor preparation might lead to a transient AHP enhancement. It has been established that the afterhyperpolarization conductance exerts a similar control over both the gain and the variability of motoneuron activity, i.e. the coefficient of variation of the motoneuron instantaneous firing frequency and the ISI duration are both inversely proportional to the AHP conductance (Manuel et al. 2006). Changes in the AHP conductance would therefore certainly provide a plausible explanation for the increase in ISI duration and the concurrent decrease in ISI variability observed in the present study during the preparatory period. Manuel et al. (2006) suggested that this mechanism may contribute to the efficient control of the force produced by ensuring that the discharge rate is regular when the motoneuron is firing at a low frequency.
The results obtained in the present study therefore argue in favour of the idea that spinal inhibitory mechanisms are involved in motor preparation, which suggests that they might also contribute to the decrease in the MEP amplitude reported to occur during the preparatory period, as recently suggested (van Elswijk et al. 2007).
Methodological and functional considerations
In previous studies using either reflex techniques or TMS methods, no signs of preparation were detected during long foreperiods (Hasbroucq et al. 1997; Touge et al. 1998; Davranche et al. 2007). By contrast, the results of the present study show that the motor unit activity was modulated during the 3 s foreperiod. In surface EMG studies, the effects of motor preparation were assessed indirectly by testing the excitability of the spinal networks to cortical and proprioceptive afferent volleys, and the powerful effects induced by stimulation on motoneurons and premotoneuronal networks may have limited the possibility of detecting preparatory changes in the motor output. Here the effects were assessed directly from the natural tonic activity of motoneurons during a voluntary isometric contraction; under these more natural conditions, it was possible to bring more subtle modulations to light.
The time course of the changes in motoneuron activity found to occur during the long foreperiod suggests that the activation of inhibitory mechanisms is associated with the timing of the response signal. These anticipatory changes are unlikely to reflect attention processes related to movement execution, since other studies have shown that the discharge pattern of wrist motor units was not affected by the level of attention required by a motor task (Schmied et al. 2000; Nafati et al. 2004, 2005). It seems more likely that they might reflect temporal estimation processes used by the subjects to synchronize their preparation with the occurrence of the response signal. As things stand, the functional role of the spinal inhibitory mechanisms operating during preparation for action still remains to be elucidated. The fact that the changes in motoneuron activity were found to be most prominent during the non-optimum 3 s foreperiod, i.e. under conditions where the motor performances were the least efficient, suggests that the activation of inhibitory processes during motor preparation might not contribute to improving the RT, but rather to a general braking mechanism serving to prevent premature motor output from occurring (van Elswijk et al. 2007; Davranche et al. 2007). Experiments are now being conducted to test this assumption.
In conclusion, the anticipatory changes in motoneuron activity observed here provide evidence that central influences act on spinal motoneurons well before it is time to act. The present study therefore shows that advance information may influence the state of the motor system, including even the most peripheral motor neurons in the spinal cord, and supports the idea that motor preparation involves distributed functional processes, in line with the conclusion reached by Fetz and colleagues (Prut & Fetz, 1999; see Prut et al. 2001, for a review).
Acknowledgments
We are grateful to Jessica Blanc for correcting the English manuscript. This research was supported by grants from the CNRS (the French National Scientific Research Centre).
References
- Bonnet M. Comparison of monosynaptic tendon reflexes during preparation for ballistic or ramp movement. Electroencephalogr Clin Neurophysiol. 1981;51:353–362. doi: 10.1016/0013-4694(81)90099-7. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Requin J, Semjen A. Human reflexology and motor preparation. Exerc Sport Sci Rev. 1981;9:119–157. [PubMed] [Google Scholar]
- Brunia CH. Motor preparation: changes in amplitude of Achilles tendon reflexes during a fixed foreperiod of one second. Psychophysiology. 1983;20:658–664. doi: 10.1111/j.1469-8986.1983.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Brunia CH, Scheirs JG, Haagh SA. Changes of Achilles tendon reflex amplitudes during a fixed foreperiod of four seconds. Psychophysiology. 1982;19:63–70. doi: 10.1111/j.1469-8986.1982.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Brunia CH, Vingerhoets AJ. CNV and EMG preceding a plantar flexion of the foot. Biol Psychol. 1980;11:181–191. doi: 10.1016/0301-0511(80)90054-x. [DOI] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Calvin WH, Stevens CF. Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol. 1968;31:574–587. doi: 10.1152/jn.1968.31.4.574. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Shinoda Y, Wise SP. Neurophysiological Approches to Higher Brain Functions. New York: John Wiley and Sons; 1984. [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K, Votaw S. Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev. 2002;40:53–65. doi: 10.1016/s0165-0173(02)00188-1. [DOI] [PubMed] [Google Scholar]
- Haagh SA, Brunia CH. Anticipatory response-relevant muscle activity, CNV amplitude and simple reaction time. Electroencephalogr Clin Neurophysiol. 1985;61:30–39. doi: 10.1016/0013-4694(85)91070-3. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Osman A, Possamai CA, Burle B, Carron S, Depy D, Latour S, Mouret I. Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychol (Amst) 1999;101:243–266. doi: 10.1016/s0001-6918(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Jasper H, Ricci GF, Doane H. Patterns of cortical neuronal discharge during conditioned responses in monkeys. In: Wolstenholme G, O/Connor C, editors. Neurophysiological Basis of Behaviour. Boston: Little Brown; 1958. [Google Scholar]
- Kimm J, Sutton D. Foreperiod effects on human single motor unit reaction times. Physiol Behav. 1973;10:539–542. doi: 10.1016/0031-9384(73)90218-7. [DOI] [PubMed] [Google Scholar]
- Lecas JC, Requin J, Anger C, Vitton N. Changes in neuronal activity of the monkey precentral cortex during preparation for movement. J Neurophysiol. 1986;56:1680–1702. doi: 10.1152/jn.1986.56.6.1680. [DOI] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. The afterhyperpolarization conductance exerts the same control over the gain and variability of motoneurone firing in anaesthetized cats. J Physiol. 2006;576:873–886. doi: 10.1113/jphysiol.2006.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellah S, Rispal-Padel L, Riviere G. Changes in excitability of motor units during preparation for movement. Exp Brain Res. 1990;82:178–186. doi: 10.1007/BF00230849. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Nafati G, Rossi-Durand C, Schmied A. Proprioceptive control of human wrist extensor motor units during an attention-demanding task. Brain Res. 2004;1018:208–220. doi: 10.1016/j.brainres.2004.05.066. [DOI] [PubMed] [Google Scholar]
- Nafati G, Schmied A, Rossi-Durand C. Changes in the inhibitory control exerted by the antagonist Ia afferents on human wrist extensor motor units during an attention-demanding motor task. J Neurophysiol. 2005;93:2350–2353. doi: 10.1152/jn.00996.2004. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc Natl Acad Sci U S A. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre movement instructed delay activity. Nature. 1999;401:590–594. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Prut Y, Perlmutter SI, Fetz EE. Distributed processing in the motor system: spinal cord perspective. Prog Brain Res. 2001;130:267–278. doi: 10.1016/s0079-6123(01)30018-3. [DOI] [PubMed] [Google Scholar]
- Requin J, Brener J, Ring C. Preparation for action. In: Jennings JR, Coles MGH, editors. Handbook of Cognitive Psychophysiology: Central and Autonomic Nervous System Approaches. New York: John Wiley and Sons; 1991. pp. 357–448. [Google Scholar]
- Riehle A. Preparation for action: one of the key functions of motor cortex. In: Riehle A, Vaadia E, editors. Motor Cortex in Voluntary Movements: a Distributed System for Distributed Functions. Boca Raton, FL, USA: CRC Press; 2005. pp. 213–240. [Google Scholar]
- Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. Neuronal correlates of the specification of movement direction and force in four cortical areas of the monkey. Behav Brain Res. 1995;70:1–13. doi: 10.1016/0166-4328(94)00180-n. [DOI] [PubMed] [Google Scholar]
- Schmied A, Pagni S, Sturm H, Vedel JP. Selective enhancement of motoneurone short-term synchrony during an attention-demanding task. Exp Brain Res. 2000;133:377–390. doi: 10.1007/s002210000421. [DOI] [PubMed] [Google Scholar]
- Schmied A, Pouget J, Vedel JP. Electromechanical coupling and synchronous firing of single wrist extensor motor units in sporadic amyotrophic lateral sclerosis. Clin Neurophysiol. 1999;110:960–974. doi: 10.1016/s1388-2457(99)00032-2. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. The case of k related samples. In: Anker JD, editor. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill international edn. Singapore: Fong & Sons Printers; 1988. pp. 168–189. [Google Scholar]
- Tandonnet C, Burle B, Vidal F, Hasbroucq T. The influence of time preparation on motor processes assessed by surface Laplacian estimation. Clin Neurophysiol. 2003;114:2376–2384. doi: 10.1016/s1388-2457(03)00253-0. [DOI] [PubMed] [Google Scholar]
- Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol. 1976;39:1062–1068. doi: 10.1152/jn.1976.39.5.1062. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Woodrow H. Washington: American Psychological Association; 1914. The Measurement of Attention. Psychology monographs Volume 17. [Google Scholar]