Abstract
Flow-mediated dilatation (FMD) of the brachial and radial arteries is an important research tool for assessment of endothelial function in vivo, and is nitric oxide (NO) dependent. The leg skeletal muscle vascular bed is an important territory for studies in exercise physiology. However, the role of endothelial NO in the FMD response of lower limb arteries has never been investigated. The purpose of this study was to examine the contribution of NO to FMD in the superficial femoral artery in healthy subjects. Since physical inactivity may affect endothelial function, and therefore NO availability, spinal cord-injured (SCI) individuals were included as a model of extreme deconditioning. In eight healthy men (34 ± 13 years) and six SCI individuals (37 ± 10 years), the 5 min FMD response in the superficial femoral artery was assessed by echo-Doppler, both during infusion of saline and during infusion of the NO synthase blocker NG-monomethyl-l-arginine (l-NMMA). In a subset of the controls (n = 6), the 10 min FMD response was also examined using the same procedure. The 5 min FMD response in controls (4.2 ± 0.3%) was significantly diminished during l-NMMA infusion (1.0 ± 0.2%, P < 0.001). In SCI, l-NMMA also significantly decreased the FMD response (from 8.2 ± 0.4% during saline to 2.4 ± 0.5% during l-NMMA infusion). The hyperaemic flow response during the first 45 s after cuff deflation was lower in both groups during infusion of l-NMMA, but the effect of l-NMMA on FMD persisted in both groups after correction for the shear stress stimulus. The 10 min FMD was not affected by l-NMMA (saline: 5.4 ± 1.6%, l-NMMA: 5.6 ± 1.5%). Superficial femoral artery FMD in response to distal arterial occlusion for a period of 5 min is predominantly mediated by NO in healthy men and in the extremely deconditioned legs of SCI individuals.
Flow-mediated dilatation (FMD) refers to the vasodilatation observed in a conduit artery in response to elevations in shear stress during reactive hyperaemia. FMD is a valuable non-invasive tool to evaluate endothelial function in humans (Green, 2005). It is reduced in patients with risk factors for cardiovascular diseases such as hypercholesterolaemia, hypertension, diabetes and smoking (Celermajer et al. 1992; Steinberg et al. 1997; Cosentino & Luscher, 1998), as well as in the elderly (Celermajer et al. 1994; Jensen-Urstad & Johansson, 2001). Decreased endo-thelial function, characterized by a reduced bioactivity of nitric oxide (NO), has been proposed as an important early event in the pathogenesis of atherosclerosis (Widlansky et al. 2003), since NO is an important anti-atherogenic molecule. Indeed, decreased FMD is an independent predictor for cardiovascular morbidity and mortality (Neunteufl et al. 2000; Gokce et al. 2002; 2003; Modena et al. 2002).
It has been shown that the FMD response in the brachial and radial arteries, under specific conditions, is mediated by endothelial NO, since selective blockade of NO production with NG-monomethyl-l-arginine (l-NMMA) largely abolishes the response (Joannides et al. 1995; Doshi et al. 2001; Mullen et al. 2001). NO dependency of FMD in other arteries has not been examined.
The effect of regular aerobic exercise on FMD in healthy men (Clarkson et al. 1999; Allen et al. 2003; Green et al. 2003) and in subjects with cardiovascular disease (Maiorana et al. 2001; Fuchsjager-Mayrl et al. 2002) has been examined primarily in the brachial artery. Since exercise-induced vascular adaptations mainly occur in the trained areas (Thijssen et al. 2005), assessment of lower limb FMD may provide more direct and sensitive information about local vascular endothelial function changes. This is particularly relevant since we know that leg and arm vascular beds respond differently to endothelium-dependent and -independent vasodilator agents (Newcomer et al. 2004). In parallel to this, heterogeneity in the conduit vessels in the upper and lower limbs is present regarding vessel dilatation for a given change in shear rate (Wray et al. 2005; Parker et al. 2006). Consequently, FMD responses observed in the brachial artery cannot necessarily be extrapolated to the leg vascular bed. Therefore, the aim of this study was to examine whether the FMD observed in the superficial femoral artery in healthy subjects is NO mediated.
Physical inactivity is a common risk factor for cardiovascular diseases and for atherosclerosis. It has recently been demonstrated that conduit artery function in the extremely inactive legs deteriorates at a greater rate than in the arms of spinal cord-injured (SCI) individuals (Stoner et al. 2006). To examine the applicability of superficial femoral artery FMD as a marker of NO-mediated vasodilatation in a muscle vascular bed subject to extreme inactivity, SCI individuals were included. Endothelial NO production during reactive hyperaemia was blocked by infusion of l-NMMA into the common femoral artery. We hypothesize that the superficial femoral artery FMD in normal (healthy subjects) as well as in abnormal conditions (SCI individuals) is mediated through endothelial NO production.
Methods
Subjects
Eight healthy male controls (C, 34 ± 4 years, 185 ± 2 cm, 82 ± 7 kg) and six male spinal cord-injured individuals (SCI, 37 ± 4 years, 179 ± 1 cm, 72 ± 3 kg) participated in this study. Subjects with a history of cardiovascular diseases, diabetes, hypercholesterolaemia or hypertension were excluded from the study. Control subjects were non-smokers who used no medication. The SCI subjects continued their medication, which did not interfere with the cardiovascular system, throughout the study. Two spinal cord-injured individuals smoked, but refrained from smoking 3 days prior to testing. The spinal cord-injured individuals had complete motor and sensory spinal cord lesions of traumatic origin varying from thoracic 1–12 (American Spinal Injury Association A). The level of the spinal lesion was assessed by clinical examination. The Hospital Ethics Committee approved the study. All subjects gave their written informed consent prior to the study. The study conformed to the principles outlined in the Declaration of Helsinki.
Protocol
Measurements were carried out in the morning after an overnight fast. Subjects were asked to empty their bladder before examination and they refrained from alcohol, caffeine, vitamin C and exercise at least 18 h prior to the test. Room temperature was controlled at 23–24°C.
FMD with NO blockade (5 min)
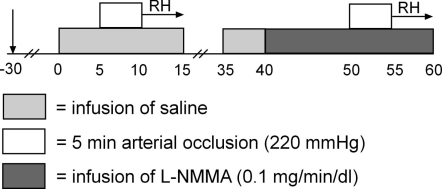
A cannula (Angiocath 16 gauge, Becton Dickinson, Sandy, UT, USA) was introduced into the femoral artery under local anaesthesia (4 ml lidocaine 20 mg ml−1) using a modified Seldinger technique. The intra-arterial cannula was used for drug administration and for continuous blood pressure monitoring. Heart rate was also continuously monitored using lead II from an electrocardiogram. The measurements started at least 30 min after cannulation of the femoral artery (Fig. 1). First, measurements of vessel diameter and red blood cell velocity of the superficial femoral artery (SFA) were performed during saline (0.9% NaCl) infusion. FMD was assessed after a cuff was inflated around the upper right for 5 min to suprasystolic pressure of 220 mmHg. The 5 min occlusion period was based on the current knowledge of NO dependency of the brachial artery FMD (Mullen et al. 2001), and on the frequent use of this protocol in previous studies examining the lower leg conduit artery FMD (Parker et al. 2006; Stoner et al. 2006; Thijssen et al. 2007). The cuff placement was distal to the arterial cannulation site, with the artery insonated between the site of the cannulation and the placement of the cuff. After cuff deflation, hyperaemic blood flow velocity in the SFA was recorded on videotape for the first 45 s, followed by registration of the vessel diameter for 5 min (Corretti et al. 2002; Pyke & Tschakovsky, 2005).
Figure 1. Schematic overview of the invasive study protocol.
RH, reactive hyperaemia.
Twenty minutes after cuff release, baseline measurements were repeated during infusion of saline in order to verify that haemodynamic parameters had returned to pre-occlusion levels. Subsequently, l-NMMA was infused into the femoral artery at a dose of 0.1 mg min−1 per decilitre (dl) of leg volume. Using a similar dose in the forearm, Mullen (Mullen et al. 2001) abolished FMD of the brachial artery. In addition, a previous study that infused this dose into the femoral artery showed minimal systemic effects (Bleeker et al. 2005c). Resting echo Doppler measurements of SFA were performed between 8 and 10 min during the 10 min pre-infusion period of l-NMMA. Infusion of l-NMMA was continued during the 5 min arterial occlusion and 5 min after cuff release. During the whole protocol, infusion rate was kept constant at a volume rate of 10 μl min−1 dl−1. In a subgroup of the healthy controls (n = 3, 34 ± 9 years), no effect of the infusate was found on blood flow measurement or blood flow of the superficial femoral artery (without infusate, 64 ± 15 ml min−1; with infusate, 61 ± 12 ml min−1).
FMD with NO blockade (10 min)
To examine whether the period of arterial occlusion influences the nature of the FMD response in the superficial femoral artery, we repeated the above-stated experiments after an arterial occlusion period of 10 min. The results of the 5 min occlusion were compared with the 10 min arterial occlusion.
Measurements and analyses
Red blood cell velocities and vessel diameter of superficial femoral artery were measured with an echo Doppler device (Megas Esaote, Firenze, Italy) with a 5–7.5 MHz broadband linear array transducer. The sample volume was placed in the centre of the artery and images were made approximately 3 cm distal to the bifurcation into the deep and the superficial femoral artery. The angle of insonation during velocity assessments was consistently below 60 deg. Arterial diameter measures were assessed from arterial B-mode images with the vessel parallel to the transducer. For baseline diameter measurements, two consecutive images in the longitudinal view were frozen at the peak systolic (Ds) and end-diastolic phase (Dd). Off line, three measurements were performed per diameter image, and the mean diameter (D) was calculated by using the formula: 1/3(systolic diameter) + 2/3(diastolic diameter). For baseline velocity measurements, four images with a total of 10–12 velocity profiles were obtained and manually traced afterwards by a single investigator. The average of these 10–12 Doppler spectra waveforms was used to calculate mean velocity (Vmean). Subsequently, mean blood flow in ml min−1 was calculated as π(radius)2×Vmean (cm s−1) × 60 and mean wall shear rate (MWSR) was calculated as 4Vmean/D (s−1).
Hyperaemic velocity was recorded on videotape for the first 45 s after cuff release. After 45 s, superficial femoral artery diameter was recorded continuously until 5 min after cuff release for assessment of the FMD. Reactive hyperaemic blood velocity was calculated from the flow velocity integral (FVI) every 5 s, from 15 to 45 s after cuff release, which was manually traced by a single investigator. Subsequently, mean blood flow in ml min−1 was calculated as π(radius)2×Vmean (cm s−1) × 60 and mean wall shear rate (MWSR) was calculated as 4Vmean/D (s−1).
Vessel diameters of the SFA after reactive hyperaemia were measured off-line from videotape at 50, 60, 90, 120, 240 and 300 s after cuff release to measure FMD. All diameters were measured at the end-diastolic phase of the cardiac cycle, corresponding to the R wave of a simultaneous ECG signal. FMD in the SFA was expressed as both the maximal absolute and relative diameter change in end-diastolic baseline diameter. MWSR area-under-the-curve (MWSR-AUC) was calculated from 15 to 45 s after cuff release (Pyke & Tschakovsky, 2007). To correct for the shear stress stimulus, the ratio between the relative FMD response (%FMD) and the primary stimulus for vessel dilatation (MWSR-AUC) was calculated. Reproducibility of the measurements in the superficial femoral artery in our lab is reported to be 1.5% for baseline diameter, 14% for blood flow and 15% for FMD (de Groot et al. 2004).
Statistical analysis
The primary end point of this study was the superficial femoral artery FMD. We decided that with an estimated s.d. of 35% of the baseline value, a mean relevant effect of the l-NMMA infusion should be at least 50% (for the control and SCI comparison) and calculated that with an alpha of 0.05, six subjects per group would be needed to achieve a power of 90%. Statistical analyses were performed using SPSS 12.0 computer software (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov test indicated a normal (Gaussian) distribution of data. Results are expressed as mean ±s.e.m. Differences during the whole period of reactive hyperaemia in blood flow, mean wall shear rate and diameter between the two experimental conditions (saline versusl-NMMA) were examined using a 2-way repeated measures ANOVA. To assess differences in FMD response during saline and l-NMMA infusion in controls and SCI individuals (for the 5 min as well as 10 min ischaemic stimulus), a Student/s t test for paired groups was used. To examine differences between groups, an unpaired Student/s t test was used. Differences were considered to be statistically significant at a 2-sided probability value of ≤ 0.05.
Results
Baseline vascular characteristics
Resting and maximal superficial femoral artery diameters were significantly lower in spinal cord-injured (SCI) individuals compared with controls (0.55 ± 0.04 and 78 ± 0.02 cm, respectively), while mean wall shear rate (MWSR) was higher in SCI than in controls (50 ± 4 and 13 ± 1 s−1, respectively). Resting blood flow through the superficial femoral artery (SFA) did not differ between controls and SCI (75 ± 12 and 98 ± 18 ml min−1, respectively). Controls showed a significantly larger leg volume than SCI (11.1 ± 0.7 and 7.9 ± 0.3 l, respectively). After correction for leg volume, SCI individuals demonstrated a higher blood flow compared with controls (13 ± 2 and 7 ± 1 ml min−1 l−1, respectively).
Before administration of l-NMMA, the superficial femoral artery blood flow and diameter had returned to baseline values in both groups (Table 1). Superficial femoral artery blood flow decreased significantly in both groups during l-NMMA infusion (P < 0.05). Infusion of l-NMMA did not have any effect on baseline diameter of the superficial femoral artery nor did it affect mean arterial blood pressure or heart rate in either group (Table 1).
Table 1.
Baseline characteristics
| Baseline 1 (Saline) | Baseline 2 (Saline) | l-NMMA | |
|---|---|---|---|
| Diameter (cm) | |||
| Controls | 0.78 ± 0.02 | 0.79 ± 0.02 | 0.77 ± 0.02 |
| SCI | 0.55 ± 0.02 | 0.55 ± 0.02 | 0.55 ± 0.02 |
| Superficial femoral artery blood flow (ml min−1) | |||
| Controls | 75 ± 12 | 82 ± 12 | 46 ± 7* |
| SCI | 98 ± 18 | 85 ± 14 | 59 ± 14* |
| Heart rate (beats min−1) | |||
| Controls (n = 7) | 55 ± 2 | 57 ± 3 | 55 ± 3 |
| SCI | 58 ± 4 | 57 ± 3 | 58 ± 3 |
| Mean arterial pressure (mmHg) | |||
| Controls (n = 7) | 90 ± 3 | 93 ± 3 | 95 ± 3 |
| SCI | 98 ± 3 | 101 ± 3 | 103 ± 3 |
P < 0.05, significantly different from baseline 2. Due to technical problems, no registration is present of the heart rate and mean arterial pressure of one control subject.
FMD and hyperaemic responses
Controls
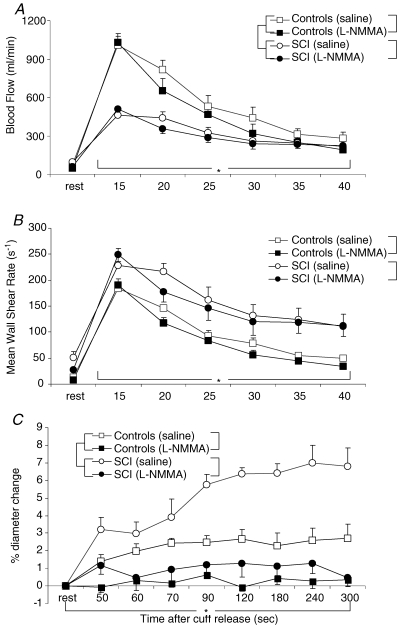
After the 5 min period of distal leg ischaemia, peak blood flow was maximal within 15 s with a subsequent decay in blood flow thereafter (Fig. 2A). At 45 s post-cuff deflation, blood flow had decreased but not fully returned to baseline (saline, 281 ± 133 versus 75 ± 34 ml min−1; l-NMMA, 190 ± 74 versus 46 ± 21 ml min−1). Post-ischaemic flow and MWSR were lower during l-NMMA infusion than during saline infusion (Fig. 2A and B, P = 0.023 for post-ischaemic blood flow, and P = 0.024 for MWSR). During reactive hyperaemia, the area-under-the-curve for MWSR during saline infusion (488 ± 33) was larger than during l-NMMA infusion (413 ± 40, P = 0.02). After cuff release, FMD in the SFA of controls was 4.2 ± 0.3% during saline infusion (Fig. 3). Infusion of l-NMMA almost completely abolished the FMD response (1.0 ± 0.2%, P < 0.001) (Fig. 3). Analysing the overall course of the FMD, the change in diameter during reactive hyperaemia was completely abolished during l-NMMA infusion (Fig. 2C). After correction for its stimulus (area-under-the-curve for MWSR), a marked difference in FMD response between saline (0.0089 ± 0.0009 AU) and l-NMMA infusion (0.0026 ± 0.0006 AU, P < 0.001) was still observed (Fig. 3).
Figure 2. Baseline and post-ischaemic blood flow, mean wall shear rate and superficial femoral diameter.
Average baseline and post-ischaemic blood flow (A) and mean wall shear rate (B) during the first 45 s of reactive hyperaemia, and (C) percentage change in superficial femoral diameter during the post-ischaemic period from minute 1 to minute 5 in controls (squares) and SCI individuals (circles) during infusion of saline (open) or l-NMMA (filled). Data are presented as mean +s.e.m. The brackets represent the ANOVA results. *Significant time effect for both groups (P < 0.05, 2-way ANOVA). †Significant interaction for condition (saline or l-NMMA) × time for both groups separately. ‡Significant between group effect (P < 0.05, 2-way ANOVA). During infusion of l-NMMA no time effect in superficial femoral artery diameter was observed in both groups (1-way ANOVA).
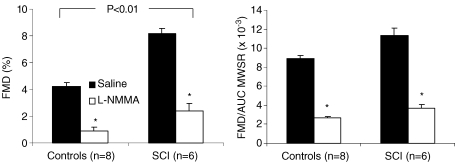
Figure 3. FMD.
FMD presented as: A, maximal relative change in superficial femoral artery diameter, and B, the corrected FMD for the area-under-the-curve of the post-ischaemic mean wall shear rate (AUC-MWSR) in controls and SCI individuals during infusion of saline (filled bars) or l-NMMA (open bars). Results are presented as mean +s.e.m.*Significantly different between saline and l-NMMA at P < 0.05 (Student/s t test).
SCI individuals
Post-ischaemic flow and MWSR were lower during l-NMMA infusion than during saline infusion (Fig. 2, P = 0.006 for post-ischaemic blood flow and P = 0.003 for MWSR). At 45 s post-cuff deflation, blood flow had decreased but was not yet returned to baseline (saline, 216 ± 91 versus 98 ± 43 ml min−1; l-NMMA, 223 ± 91 versus 59 ± 33 ml min−1). During reactive hyperaemia, the area-under-the-curve for MWSR during saline infusion (803 ± 100) was larger compared with the area-under-the-curve for MWSR during l-NMMA infusion (744 ± 99, P = 0.047). FMD response in the SFA in SCI was 8.2 ± 0.4% under saline infusion, while infusion of l-NMMA significantly reduced the FMD response (2.4 ± 0.5%, P < 0.001, Fig. 3). By correcting the FMD response for its stimulus (area-under-the-curve for MWSR), a reduced FMD was still observed (saline, 0.0113 ± 0.0020 AU; l-NMMA, 0.0037 ± 0.0009 AU).
FMD response in SCI was significantly larger than in controls during saline (SCI: 8.2 ± 0.4%; controls: 4.2 ± 0.3%; P < 0.001) as well as during infusion of l-NMMA (SCI: 2.4 ± 0.5%, controls: 1.0 ± 0.2, P = 0.03). However, after correction for the eliciting stimulus (area-under-the-curve for MWSR), no difference was observed between SCI and controls, either during saline (SCI, 0.0113 ± 0.0020 AU; controls, 0.0089 ± 0.0009 AU; P = 0.35) or during l-NMMA infusion (SCI, 0.0037 ± 0.0009 AU; controls, 0.0026 ± 0.0006 AU, P = 0.31) (Fig. 3).
FMD with NO blockade (10 min)
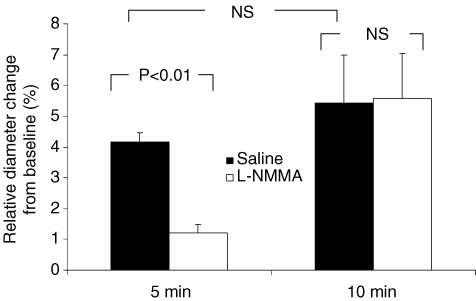
These experiments were performed in a subset of the original group of healthy controls (n = 6). The subpopulation of healthy controls is representative for the original population regarding age (40 ± 4 versus 34 ± 4 years, respectively), body weight (86 ± 6 versus 82 ± 7 kg, respectively), systolic blood pressure (124 ± 3 versus 128 ± 3 mmHg, respectively) and diastolic blood pressure (70 ± 4 versus 76 ± 4 mmHg, respectively). Baseline femoral artery did not differ between both experiments (5 min, 7.9 ± 0.2 mm; 10 min, 7.9 ± 0.2 mm). The 5 min as well as the 10 min FMD procedure resulted in a dilatation of the superficial femoral artery (Fig. 4). After 5 min occlusion, the superficial femoral artery dilatation was significantly reduced during infusion of l-NMMA, whereas no difference in artery dilatation is observed during the 10 min FMD procedure between saline and l-NMMA (Fig. 4). During reactive hyperaemia after the 10 min occlusion, the area-under-the-curve for MWSR during saline infusion (1270 ± 345) was not different from l-NMMA infusion (1447 ± 318, P = 0.42). Also correcting the superficial femoral artery dilatation after 10 min ischaemia for the area-under-the-curve for MWSR, no difference was observed between saline (0.0071 ± 0.0028 AU) and l-NMMA infusion (0.0047 ± 0.0009 AU; P = 0.34).
Figure 4. Relative change in the superficial femoral artery.
Relative change in the superficial femoral artery during a 5 and 10 min FMD procedure, under infusion of saline (filled bars) or l-NMMA (open bars) in a subpopulation of the original group of healthy able-bodied control subjects (n = 6). A paired t test is used to compare the diameter change to the situation with saline and l-NMMA infusion, but also between the 5 and 10 min FMD. Error bars represent s.e.m.
Discussion
In the present study we demonstrate that the FMD response of the superficial femoral artery in healthy men and in spinal cord-injured (SCI) individuals predominantly reflects NO-mediated endothelium-dependent vasodilatation. FMD of the superficial femoral artery in response to a 5 min period of ischaemia induced by a cuff placed distal to the site of artery insonation therefore represents a suitable method to examine NO bioavailability and endothelial function in the lower limbs.
Contribution of NO to FMD in controls
Previous reports utilizing l-NMMA as a competitive antagonist have demonstrated a crucial role for NO in radial (Joannides et al. 1995; Mullen et al. 2001) and brachial artery (Doshi et al. 2001) dilatation in response to brief periods of reactive hyperaemia. In the present study the increase in superficial femoral artery diameter in healthy men, after a brief episode of reactive hyperaemia, was almost completely abolished during infusion of l-NMMA. Thus, in accordance with data in the upper limbs, our data demonstrate that the FMD response of the superficial femoral artery in healthy men reflects NO-mediated endothelium-dependent vasodilatation. One may argue that the impact of l-NMMA on arterial diameter may be due, in part, to a diminished flow stimulus. Indeed, blood flow, MWSR and area-under-the-curve for MWSR during reactive hyperaemia were slightly lower during infusion of l-NMMA than during saline infusion. Nevertheless, when the FMD response was corrected for its stimulus (area-under-the-curve for MWSR), a marked difference between saline and l-NMMA infusion was still observed.
The FMD of the superficial femoral artery after blockade of NO production was ∼1% in the controls in the present study. Lieberman et al. (Lieberman et al. 1996) observed a 7% FMD of the brachial artery during administration of l-NMMA. The discrepancy with the present results can be explained by placement of the occlusion cuff relative to the ultrasound probe. In the present study the ultrasound probe was proximal to the cuff, whereas Lieberman et al. (1996) placed the cuff on the upper arm with the site of measurement in the ischaemic area distal to the cuff. Under these circumstances FMD is affected by vasodilators other than NO which are released in response to ischaemia, and may also be complicated by myogenic responses as a result of the pressure fall inside the artery during occlusion (Doshi et al. 2001). In other studies where the arterial measuring site was proximal to the cuff, l-NMMA reduced radial artery vasodilatation to 0.7–2% (Mullen et al. 2001) or converted radial dilatation to vasoconstriction (Joannides et al. 1995), whilst brachial artery FMD was completely abolished (Doshi et al. 2001). In all studies the FMD response was investigated after a 5 min occlusion period causing a brief shear stress stimulus. The nature of the shear stress stimulus is critical to the NO dependency, since the FMD response to more sustained stimuli (caused by release of 15 min ischaemia, skin warming or distal infusion of acetylcholine) is unaffected by l-NMMA (Mullen et al. 2001). Parallel to this, we could demonstrated in a subset of the control subjects that a 10 min period of ischaemia results in a dilatation of the superficial femoral artery that is unlikely to be the result of an endothelium-dependent NO-mediated mechanism only. This suggests that, similar to the brachial artery, the duration of the ischaemic period is critical for the NO dependency of the superficial femoral artery.
During a 5 min occlusion, FMD may be partially dependent upon other vasodilator agents such as EDHF or prostacyclin. In addition, an increase in shear stress can also stimulate endothelin production; blockade of endothelin-A receptors improves FMD in chronic heart failure patients (Berger et al. 2001). Although these NO-independent mechanisms could explain the residual FMD during NO blockade in the present study, we argue that they play a relatively minor role in the superficial femoral artery FMD, since l-NMMA blunted the dilatation up to about 70% and the increase in diameter observed during infusion of saline was inhibited throughout l-NMMA infusion (Fig. 2C). Nonetheless, our finding of NO dependency of FMD in the lower limb is limited to the superficial femoral artery in response to a 5 min distal occlusion.
Contribution of NO to FMD in SCI
In keeping with recent reports after prolonged (spinal cord injury; de Groot et al. 2004) and short-term (unilateral lower limb suspension; Bleeker et al. 2005a) deconditioning or deconditioning by bed rest (Bonnin et al. 2001; Bleeker et al. 2005b), our findings demonstrate an enhanced FMD response in SCI. Recently, Stoner et al. (2006) demonstrated an attenuated posterior tibial artery FMD response in SCI individuals, measuring the artery diameter in the ischaemic area distal to the cuff. As discussed previously, one may speculate whether this response is NO mediated or influenced by other metabolic vasoactive substances. Since deconditioning leads to marked changes in vascular function (Louisy et al. 1997; Watenpaugh et al. 2001; Hopman et al. 2002), we considered the possibility that mechanisms other than NO might mediate FMD under these conditions. Indeed, during blockade of NO production by l-NMMA the FMD response was attenuated but not abolished in SCI and was more preserved than in controls (∼2.4%versus∼1%). However, it has been proposed that the FMD response should be corrected for the eliciting stimulus by taking the post-occlusive area-under-the-curve of the mean wall shear rate (Pyke & Tschakovsky, 2007). After such correction was applied, no differences in FMD response between SCI and controls were evident. Although the contribution of other vasodilating agents cannot be ruled out in explaining the remaining FMD response during l-NMMA infusion in SCI, the FMD of the superficial femoral artery following a 5 min ischaemic stimulus in the deconditioned legs of SCI depends predominantly on NO.
Administration of l-NMMA decreased superficial femoral artery blood flow but did not affect basal superficial femoral artery diameter, and no systemic effects were observed since mean arterial blood pressure and heart rate were not changed during infusion of l-NMMA in both groups. The decrease in blood flow is consistent with the existence of basal release of NO in the leg resistance vessels, which is in agreement with previous observations (Bleeker et al. 2005c). The apparent lack of effect of l-NMMA on basal superficial femoral artery diameter suggests that, consistent with the observations in the radial and brachial artery (Joannides et al. 1995; Doshi et al. 2001; Mullen et al. 2001), NO does not contribute markedly to basal vascular tone in large conduit arteries.
Limitations
A recent study demonstrated that the shear stimulus area-under-the-curve, but not the peak shear stimulus, is the critical determinant of the peak flow-mediated dilatation (Pyke & Tschakovsky, 2007). Although we examined the AUC during a large time window, a limitation in this study is that we could not examine the superficial femoral artery blood flow beyond 45 s after cuff release. After 45 s of cuff release, we found that superficial femoral artery blood flow was markedly reduced, yet did not return to baseline values. In a group of healthy men (n = 6, 33 ± 7 years), we performed additional experiments and examined the flow response for 5 min after cuff release (authors/ unpublished data). We found that blood flow returned to baseline values between 90 and 120 s post-deflation, while blood flow was 2-fold higher than baseline values at 45 s after cuff release. The latter finding was also present in the data set of controls and SCI subjects presented in this paper. In addition, based on previously reported limb differences in vascular control (Newcomer et al. 2004) and conduit vessel responsiveness to a certain change in shear (Wray et al. 2005), one may argue whether correction methods for the brachial artery are equally applicable to superficial femoral artery FMD. Indeed, markedly different time-courses of post-occlusive brachial artery dilator response (Pyke & Tschakovsky, 2007) (a peak within 50–80 s) exist compared with the superficial femoral artery change after cuff release as described in this study (peak between 1 and 4 min). Further studies will be required to fully ascertain the appropriate correction for stimulus for lower limb arteries.
Clinical relevance
The non-invasive measurement of the FMD in the brachial artery is a widely accepted and frequently used method to examine endothelial function. Based on the results of this study, we advocate the use of superficial femoral artery FMD in disease states that specifically affect the lower extremities (such as peripheral artery disease) or to examine the effects of exercise training programs in the legs. Moreover, changes in leg conduit arteries are clinically relevant since the peripheral atherosclerotic process occurs more often in arteries of the lower limbs than in the upper limbs (Fukudome et al. 1997). Taken together, we believe that the assessment of the superficial femoral artery FMD provides a relatively unexplored and interesting field of cardiovascular physiology in health and (cardiovascular) disease.
In conclusion, we have demonstrated that superficial femoral artery dilatation to a transient period of increased flow after 5 min of arterial occlusion of the leg represents predominantly NO-dependent dilatation in healthy men and in spinal cord-injured individuals. Consequently, superficial femoral artery FMD can be used as a valid and novel explorative tool to examine endothelial function in the lower limbs in humans.
Acknowledgments
This work was partly supported by the Netherlands Organization for Scientific Research (NWO-grant 825.07.010).
References
- Allen JD, Geaghan JP, Greenway F, Welsch MA. Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc. 2003;35:847–853. doi: 10.1249/01.MSS.0000064931.62916.8A. [DOI] [PubMed] [Google Scholar]
- Berger R, Stanek B, Hulsmann M, Frey B, Heher S, Pacher R, Neunteufl T. Effects of endothelin A receptor blockade on endothelial function in patients with chronic heart failure. Circulation. 2001;103:981–986. doi: 10.1161/01.cir.103.7.981. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol Heart Circ Physiol. 2005a;288:H1747–H1755. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, De Groot PC, Rongen GA, Rittweger J, Felsenberg D, Smits P, Hopman MT. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005b;99:1293–1300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, Kooijman M, Rongen GA, Hopman MT, Smits P. Preserved contribution of nitric oxide to baseline vascular tone in deconditioned human skeletal muscle. J Physiol. 2005c;565:685–694. doi: 10.1113/jphysiol.2005.085936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol. 2001;85:420–426. doi: 10.1007/s004210100483. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Luscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl. 3):S54–S61. [PubMed] [Google Scholar]
- de Groot PCE, Poelkens F, Kooijman M, Hopman MTE. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2004;287:H374–H380. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–635. [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L, Wolzt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Fujii K, Abe I, Ohya Y, Fukuhara M, Kaseda S, Onaka U, Tsuchihashi T, Fujishima M. Ultrasonographic assessment of regional differences in atherosclerotic lesions in patients with hypertension, diabetes mellitus, or both. Hypertens Res. 1997;20:175–181. doi: 10.1291/hypres.20.175. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney J, John F, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasivelydetermined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. J Am College Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1233–1234. doi: 10.1152/japplphysiol.00601.2005. discussion 1237–1238. [DOI] [PubMed] [Google Scholar]
- Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O/Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–H2687. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- Hopman MT, Groothuis JT, Flendrie M, Gerrits KH, Houtman S. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol. 2002;93:1966–1972. doi: 10.1152/japplphysiol.00897.2001. [DOI] [PubMed] [Google Scholar]
- Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med. 2001;250:29–36. doi: 10.1046/j.1365-2796.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- Louisy F, Schroiff P, Guell A. Changes in leg vein filling and emptying characteristics and leg volumes during long-term head-down bed rest. J Appl Physiol. 1997;82:1726–1733. doi: 10.1152/jappl.1997.82.6.1726. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O/Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am College Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3043–H3049. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Bayazeed B, Hook G, Johnson A, Cronin J, Baron AD. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation. 1997;96:3287–3293. doi: 10.1161/01.cir.96.10.3287. [DOI] [PubMed] [Google Scholar]
- Stoner L, Sabatier M, VanhHiel L, Groves D, Ripley D, Palardy G, McCully K. Upper vs lower extremity arterial function after spinal cord injury. J Spinal Cord Med. 2006;29:138–146. doi: 10.1080/10790268.2006.11753867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 2007;190:221–228. doi: 10.1111/j.1748-1716.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Heesterbeek P, van Kuppevelt DJ, Duysens J, Hopman MT. Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc. 2005;37:1112–1118. doi: 10.1249/01.mss.0000170126.30868.fb. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Buckey JC, Lane LD, Gaffney FA, Levine BD, Moore WE, Wright SJ, Blomqvist CG. Effects of spaceflight on human calf hemodynamics. J Appl Physiol. 2001;90:1552–1558. doi: 10.1152/jappl.2001.90.4.1552. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney J, John F, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol. 2005;99:81–86. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]