Abstract
Survival requires adequate pulmonary ventilation which, in turn, depends on adequate contraction of muscles acting on the chest wall in the presence of a patent upper airway. Bulbospinal outputs projecting directly and indirectly to ‘obligatory’ respiratory motoneurone pools generate the required muscle contractions. Recent studies of the phasic inspiratory output of populations of single motor units to five muscles acting on the chest wall (including the diaphragm) reveal that the time of onset, the progressive recruitment, and the amount of motoneuronal drive (expressed as firing frequency) differ among the muscles. Tonic firing with an inspiratory modulation of firing rate is common in low intercostal spaces of the parasternal and external intercostal muscles but rare in the diaphragm. A new time and frequency plot has been developed to depict the behaviour of the motoneurone populations. The magnitude of inspiratory firing of motor unit populations is linearly correlated to the mechanical advantage of the intercostal muscle region at which the motor unit activity is recorded. This represents a ‘neuromechanical’ principle by which the CNS controls motoneuronal output according to mechanical advantage, presumably in addition to the Henneman's size principle of motoneurone recruitment. Studies of the genioglossus, an obligatory upper airway muscle that helps maintain airway patency, reveal that it receives simultaneous inspiratory, expiratory and tonic drives even during quiet breathing. There is much to be learned about the neural drive to pools of human inspiratory and expiratory muscles, not only during respiratory tasks but also in automatic and volitional tasks, and in diseases that alter the required drive.
Background
Neural control of the mammalian respiratory system must ensure adequate ventilation to provide continuous oxygenation, carbon dioxide removal and hydrogen ion regulation for survival. The system must function during sleep, in the absence of many behavioural drives, and also during wakefulness. The repertoire of the system must allow a range of non-ventilatory functions, some of which are involved with ventilation directly, such as coughing and vocalization, and some which are involved indirectly, such as movement and postural control of the axial skeleton. Added to this, parts of the upper airway must share the same space for the passage of air, for the ingestion of food and drink, and sometimes for vomiting. So while quiet breathing may be simple, the coordination of the multiplicity of respiratory-related tasks is not. No comparable multitasking exists for movements of the limbs.
One region of the medulla, the pre-Bötzinger complex, can act as a pacemaking rhythm generator (Smith et al. 1991), perhaps as a coupled oscillator with the nearby parafacial respiratory group (Onimaru & Homma, 2003). When these two regions remain intact after transection of the brainstem, rhythm generation persists in vivo (Feldman & Del Negro, 2006). This reductionist approach has focused attention on medullary generators rather than suprapontine, pontine and spinal contributors to the patterned output from the many motor nuclei that produce ventilatory movements.
Compared with the developments from studies in experimental animals including reduced neonatal preparations, views about human respiratory output seem somewhat primitive – muscles are ‘obligatory’ inspiratory muscles if they contract during tidal inspiration, and ‘accessory’ if they contract when inspiratory drive is increased during exercise or loading. Recent studies have revealed that if an inspiratory muscle has a major mechanical action to expand the rib cage, it is likely to be recruited during quiet breathing (De Troyer et al. 2005; see below). However, we know comparatively little about how a particular drive generated in the medulla is distributed to the many respiratory muscles. It must emanate from bulbospinal projections within so-called dorsal and ventral respiratory groups of medullary respiratory neurones, as has been confirmed using electrophysiological (e.g. Hilaire & Monteau, 1976; Davies et al. 1985; Merrill & Lipski, 1987; Segers et al. 1987) and anatomical tracing methods (e.g. Rikard-Bell et al. 1984; Feldman et al. 1985). The consensus is that the strength of the direct monosynaptic projections to the phrenic and thoracic motor nuclei is relatively weak, particularly for the external intercostal motor nuclei (see De Troyer et al. 2005). Estimates of the direct input to intercostal motoneurones suggest that it provides less than 10% of the depolarization observed in central respiratory drive potentials, and therefore it is likely that the bulk of the phasic input arrives via excitatory spinal interneurones (cf. Saywell et al. 2007). Populations of propriospinal interneurones which receive medullary inspiratory drive have been described in the cervical (e.g. Bellingham & Lipski, 1990) and thoracic cord (Kirkwood et al. 1988).
The present review focuses on the detailed behaviour of populations of single motor units which are active during quiet breathing in awake human volunteers and patients. The motoneurones innervate obligatory inspiratory muscles. The advantage is that these recordings provide insights into how the descending inspiratory drive is sculpted in vivo during wakefulness in a way which is not known for other experimental preparations. The limitations are that the mechanisms driving motoneuronal discharge cannot be determined precisely. Despite this, the data lead to new questions about the brainstem output to respiratory muscles.
Studies of human respiratory muscle output
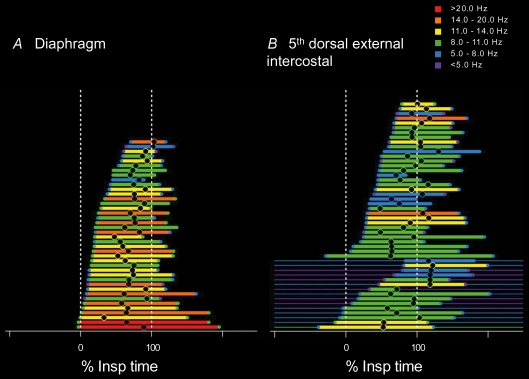
We can study the final output of the central nervous system to muscles by recording the electromyographic activity of single motor units. The timing of firing and the firing rates of a population of single motor units within a muscle indicate the descending and reflex neural drive to that motoneurone pool. We have studied the motor unit firing properties of five obligatory inspiratory muscles in humans to determine how neural drive is distributed across and within the pools during normal breathing (Saboisky et al. 2007b). Electromyographic activity was recorded using selective intramuscular electrodes from the diaphragm, scalene muscle, 2nd space parasternal intercostal, and both the 3rd and 5th space dorsal external intercostal muscles. The electrodes are moved to about 10 sites within each muscle and the electromyographic activity is decomposed to provide the spike trains from single motor units. Recordings are usually made during quiet breathing and there is little or no discomfort while they are made. The timing and amount of drive to each of the different muscles vary systematically during normal breathing (Saboisky et al. 2007b). Figure 1 illustrates the profiles of activation of populations of motor units recorded from the diaphragm and the 5th space dorsal external intercostal muscle. These muscles showed the most extreme patterns of inspiratory activity. The figure shows the times of recruitment and derecruitment, relative to inspiratory time, of each of the motor units during normal breathing. Activity of the motor units is ordered according to their onset times. The colours indicate the onset, peak and end firing frequencies of each unit. While both muscles were activated during inspiration, the motor units recorded from the diaphragm were recruited earlier, on average after 26% inspiratory time (Tinsp), and reached higher firing frequencies (mean 12.6 Hz), while units in the 5th space dorsal external intercostal were recruited significantly later in the breath, on average after 43%Tinsp, and reached lower firing frequencies (mean 10.1 Hz). The remaining three muscles were recruited on average at distinct times falling between the diaphragm and 5th space dorsal external intercostal muscles. The 3rd space dorsal external intercostal muscle, scalene muscle and 2nd space parasternal intercostal muscles were recruited at 29%, 32%, 34%Tinsp (respectively), and reached mean peak frequencies of 11.9 Hz, 9.3 Hz, 10.8 Hz, respectively. The distinct patterns of recruitment observed during normal quiet breathing in these five inspiratory muscles suggest a non-uniform activation of the different motoneurone pools that may be required to produce efficient ventilation.
Figure 1. Time and frequency plot (TAFPLOT) of the discharge of motor units in two human inspiratory pump muscles.
The firing time for each single motor unit recorded from the diaphragm (A) and the 5th dorsal external intercostal muscle (B) during quiet breathing, relative to the time of inspiration. For each unit, the thick horizontal line represents the time that the firing frequency increases in the inspiratory or expiratory phase of respiration. The thin horizontal line indicates tonic firing of the motor unit at other times. The units are ordered relative to their onset time. The colour of the thick horizontal line denotes the peak firing frequency (range 5–20 Hz; see colour scale). The onset and end firing frequencies are denoted by the coloured circles at the ends of the line. The time of the peak firing is indicated as a black circle. Data modified from Saboisky et al. (2007b) with permission of the American Physiological Society.
The variation in the patterns of activation of inspiratory motor units across muscles begs the question: how is it organized? The simplest explanation would be that it may be organized at the level of the motoneurone. Henneman's size principle (Henneman, 1957: Henneman et al. 1965) states that motoneurone recruitment order depends largely on cell size as this is related to cell ‘excitability’. Small-sized motoneurones are therefore recruited before large ones. This principle holds for most limb motoneurone pools (Cope & Pinter, 1995). It is possible that the different inspiratory motoneurone pools comprise different-sized motoneurones that might determine their recruitment profiles during inspiration. However, this is unlikely and there is some evidence from our data that this is not the case.
The muscles active in inspiration act on the chest wall in different proportions to create negative pressure and the inflow of air into the lungs. However, these muscles are also active in voluntary movements involving the trunk, and postural tasks requiring control of spinal stability while standing or sitting upright (e.g. Gandevia et al. 2002). In some cases, we recorded inspiratory motor units that continued to discharge throughout expiration (see also Butler et al. 2001; Gandevia et al. 2006; Saboisky et al. 2007b). These ‘tonically firing’ units presumably have an inspiratory and postural function. The proportion of these units in each of the five inspiratory muscles differs. Although the diaphragm was activated earliest and to the greatest extent during inspiration, it had no tonically active units. Conversely, the largest numbers of tonically firing units were in the 5th dorsal external intercostal muscles (see Fig. 1, tonic firing indicated by thin horizontal lines). Even though these tonic units in the 5th dorsal external intercostal muscle are active and therefore already at threshold, the inspiratory increase in firing can begin throughout inspiration. This late increase in activation of already-active units means that the differences in recruitment patterns of motor units across inspiratory motoneurone pools does not depend simply on differences in recruitment thresholds across pools (i.e. the size principle). Indeed, the distribution of inspiratory drive must be organized, even within a motoneurone pool, at a premotoneuronal level (Saboisky et al. 2007b).
Gradients of neural drive across intercostal muscles
Further evidence that the inspiratory drive is non-uniform comes from studies of the neural drive to intercostal muscles in humans and in dogs (De Troyer et al. 2003; Gandevia et al. 2006; for review see De Troyer et al. 2005). Drive is directed preferentially to those muscles or portions of muscles with the greatest mechanical advantage for inspiration. Inspiratory mechanical advantage (and respiratory effect) has been defined for respiratory muscles (Wilson & De Troyer, 1992, 1993) and measured directly for most inspiratory muscles in the dog (De Troyer & Legrand, 1995; De Troyer et al. 1996b; Legrand et al. 1996, 1997; Wilson et al. 1998). It has also been measured indirectly in the human using computed tomography imaging to derive the fractional shortening in muscle length over a standard lung volume (from functional residual capacity to total lung capacity, De Troyer et al. 1998; Wilson et al. 2001; Legrand et al. 2003).
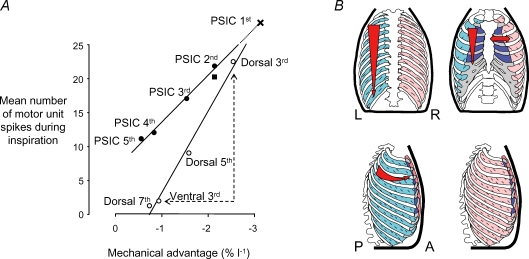
Figure 2 illustrates the close relationship between mechanical advantage and neural drive for the inspiratory intercostal muscles in humans. The neural drive is given as a number of inspiratory motor unit spikes derived from the peak firing frequencies and the duration of inspiratory discharge. During inspiration, the external intercostal muscles in humans and dogs are activated earlier in each breath, and to a larger extent in the regions and spaces where inspiratory mechanical advantage is largest (for review see De Troyer et al. 2005). In humans, the motor units fire at higher frequencies in the rostral spaces (11.9 Hz in the 3rd dorsal space) than in the more caudal spaces (6.7 Hz in the 7th dorsal space). There are even differences in activation within a space; activation is greater dorsally, and less ventrally (6.0 Hz in the 3rd ventral space), which corresponds to the reduction in inspiratory mechanical advantage in the ventral portions of these muscles (Wilson et al. 2001; see Fig. 2).
Figure 2. Neuromechanical matching in the human intercostal muscles.
A, the mechanical advantage is plotted against the mean number of motor unit spikes during inspiration (i.e. neural drive) for different regions of the parasternal intercostals (•) and external intercostals (○). There is a strong linear relation between the mechanical advantage and neural drive for each muscle (r2= 0.99). The line for parasternal intercostals has been extrapolated to predict the mechanical advantage of the muscle fibres in the first space (marked as a cross). Note that the medial and lateral (marked as a square) portions of the 2nd parasternal intercostal muscle have a similar mechanical advantage. Note also that there is an appropriately low neural drive to the ventral portion of the 3rd external intercostals, which has a low mechanical advantage compared to the dorsal portion. This figure is modified from Gandevia et al. (2006). B, the gradients of mechanical advantage and inspiratory drive are shown schematically for the parasternal intercostals and the external intercostals. The letters L, R, P and A refer to left, right, posterior and anterior, respectively.
Although the parasternal intercostal muscles are continuous with the internal layer of intercostal muscles, they are active in inspiration. For these muscles, there are similar rostrocaudal gradients of neural drive for both humans and dogs (Fig. 2; Gandevia et al. 2006; see also De Troyer et al. 2005). The average firing rate for parasternal intercostal motor units in the 1st space was 13.4 Hz compared with just 8.0 Hz in the 5th space. Although the mechanical advantage could not be derived for the 1st parasternal intercostal muscle, it can be estimated from the linear relationship between mechanical advantage and neural drive for the other four spaces. Along the parasternal intercostal space, for the dog, the neural drive is greatest medially and reduced more laterally (Legrand et al. 1996). However, in the human there is no difference in activation (Fig. 2; Gandevia et al. 2006), probably reflecting the differences in rib curvature and muscle fibre orientation between the human and the dog (De Troyer et al. 2005).
As argued above, across the intercostal muscles there is a recruitment principle related to ‘neuromechanical’ matching of central respiratory drive (Butler et al. 2007). It has been argued on theoretical grounds that the timing and degree of activation of these muscles by the neuromechanical matching principle may be metabolically efficient (De Troyer et al. 2005). But what is the mechanism for the control? The gradients of neural activity across the intercostal muscles are preserved in dogs even when all respiratory muscle, thoracic joint and skin feedback is removed by phrenic nerve section or dorsal rhizotomy (De Troyer & Legrand, 1995; De Troyer et al. 1996a). The lack of change in activation pattern despite disruption of these inputs suggests that the pattern is preset. Although equivalent experiments cannot be done as in experimental animals, it is likely that the pattern is also preset in humans. This is supported by the observation that the pattern of activation of inspiratory muscles in the neck is constant across a wide range of lung volumes and muscle lengths during voluntary inspiratory efforts (Hudson et al. 2007). Studies in patients with limited respiratory output would be consistent with this view (Estenne et al. 1998). A preset activation of different motoneurone pools or parts of a motoneurone pool may be organized partly at a spinal level via different-sized motoneurones, through a network of inhibitory interneurones, or at a higher level, but this is not yet known. Propriospinal interneurones at the upper cervical level (C1–C3) (Lipski & Duffin, 1986; Lipski et al. 1993) as well as Renshaw cells may be involved (Lipski et al. 1985). If we think of the different portions of intercostal muscles, with different mechanical advantages for different tasks, as separate muscles (even within the same intercostal space), then the neuromechanical principle of motoneurone recruitment is superimposed on the Henneman's size principle of recruitment. This is not likely to be a feature unique to respiratory muscles. There are some examples of task-dependent changes in motoneurone recruitment within the biceps brachii motoneurone pool which would be consistent with this proposal (ter Haar Romeny et al. 1982; Tax et al. 1989). Due to wide variation in mechanical advantage within many axial respiratory muscles, the ability to deliver drive differentially across the motoneurone pool may be especially important. This would also apply to muscles with a wide origin which can produce different directions of movement (e.g. the deltoid and trapezius; see Herrmann & Flanders, 1998).
Multiple neural drives in the human genioglossus
The genioglossus is a major upper airway dilator and it provides a dramatic example of a muscle that receives a heterogeneous combination of drives even during eupnoea. In the cat, recordings from the medial branch of the hypoglossal nerve have revealed that some motoneurones are active in inspiration while others are active in expiration (Hwang et al. 1983), although whether these fibres innervated the same muscle was not known. We became interested in the single motor unit activity of the genioglossus in humans during normal breathing, as multiunit EMG recorded by intramuscular electrodes suggests that the muscle is activated throughout respiration (Mezzanotte et al. 1992; Tangel et al. 1992), and hence the pattern of activation may resemble that in the cat. This pattern contrasts with that of the diaphragm and other pump muscles, which are not usually active during expiration, with the exception of a small proportion of tonically firing motor units (Fig. 1). In addition, in humans, the genioglossus contracts ∼100 ms prior to the onset of airflow (Strohl et al. 1980).
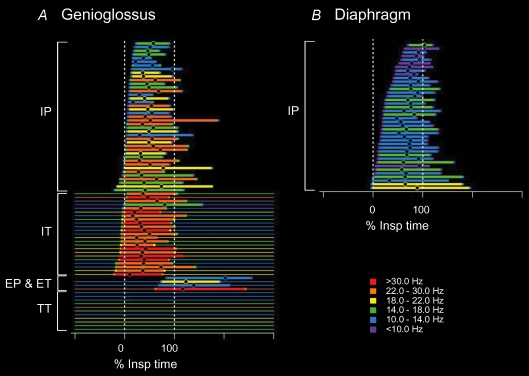
Recordings of single motor unit activity from the genioglossus in healthy human subjects during quiet breathing reveal that its activity differs from that in ‘pump’ muscles in several ways. Figure 3 depicts the firing profiles of the population of genioglossus motor units (adapted from Saboisky et al. 2007a). First, we identified several different classes of motor unit behaviour (Saboisky et al. 2006; see also Tsuiki et al. 2000; Eckert et al. 2007). As in the pump muscles, many units were active either in inspiration only, or throughout respiration but with an increased discharge in inspiration and a tonic discharge throughout expiration. However, we also found motor units that increased their discharge in expiration, and a large number of motor units that fired continuously without any respiratory modulation. The different types of firing could be recorded simultaneously from the same intramuscular electrode, so although units could be active during opposite phases of the respiratory cycle, they are likely to have a complementary role in maintaining airway patency. Second, the firing rates of motor units in the genioglossus are on average twice as high as those of the pump muscles. Firing rates reach up to ∼25 Hz on average for tonically active genioglossus units (Saboisky et al. 2006) compared with 12.6 Hz for the diaphragm and 10.1 Hz for the 5th dorsal external intercostal motor units. Third, genioglossus motor units are recruited prior to or at the beginning of inspiration, significantly earlier (on average ∼5%Tinsp for inspiratory phasic units) than the inspiratory pump muscles (26%Tinsp for the diaphragm) where motor units continue to be recruited throughout the breath.
Figure 3. Time and frequency plot (TAFPLOT) of the discharge of motor units in the genioglossus and the diaphragm in humans.
The firing time for populations of single motor units recorded in a group of subjects from the genioglossus, an upper airway muscle (A) and the diaphragm, a pump muscle (B) during quiet breathing, relative to the time of inspiration. For each unit, the thick horizontal line represents the time that the firing frequency increases in the inspiratory or expiratory phase of respiration. The thin horizontal line indicates tonic firing of the motor unit at other times. The units are ordered relative to time of the onset of their increased firing. Phasically firing units during inspiration (IP) are shown on top, followed below by tonically firing units that increased their discharge during inspiration (IT). Expiratory phasic and expiratory tonic units (EP and ET) are then shown followed by tonically firing units that did not increase their firing in time with respiration (TT). Data modified from Saboisky et al. (2007a,b) with permission of the American Physiological Society.
Figure 3 highlights the differences in the timing and distribution of inspiratory and expiratory drive across the hypoglossal motoneurone pool, as well as the differences outlined above from the inspiratory pump muscles. The neural output from and within these respiratory motoneurone pools is not uniform and may be controlled spinally or supraspinally, but this has not yet been established.
Conclusions
As measured from the firing of populations of single motor units, the respiratory output from obligatory inspiratory muscles in human subjects breathing quietly when awake is not homogeneous in its respiratory timing, its distribution between and within particular muscles, and its amount. Furthermore, while the activity is usually phasic, especially in the diaphragm, tonic firing with an inspiratory modulation occurs particularly in the lower intercostal muscles, which have an inspiratory action on the rib cage. When the genioglossus, an upper airway muscle, is subjected to the same form of study, a greater number of firing patterns is encountered, including purely tonic firing and even firing with expiratory modulation. The classification of firing patterns observed during quiet breathing is simple, but the magnitude of any respiratory ‘linkage’ of firing patterns can be assessed formally by correlation of firing rate change with lung volume changes (Saboisky et al. 2006). This linkage is likely to change with posture, respiratory drives, and behavioural state (sleep versus wakefulness; see Bailey et al. 2007). Hence, the temptation to link the patterns of firing with the type of motoneurone studied under one condition should be resisted, as the patterns are likely to be labile, dependent on respiratory, postural and other motor demands. Furthermore, the presence of firing in single motor units does not reveal the source of all the extrinsic and intrinsic factors contributing to motoneuronal depolarization. Recent studies in the cat have shown that respiratory activity can appear even in lower limb muscles, but this may not occur through traditional respiratory bulbospinal pathways (Ford & Kirkwood, 2006).
The present studies are limited in several ways. First, so far, they have focused on the motoneuronal output during quiet (presumably non-volitional) breathing, in which there is little detectable activity linked to inspiration in primary and premotor cortical areas (e.g. Macefield & Gandevia, 1991; Raux et al. 2007). During volitional breathing, which does involve the motor cortex (e.g. Colebatch et al. 1991), it remains to be determined whether there is a similar temporal and spatial distribution of motoneuronal output to inspiratory motoneurone pools. If there is, it may suggest the presence of a spinal or subcortical network which is capable of the required coordination of inputs to, and outputs from, the motoneurones. However, it is possible that a mechanism to distribute drive ‘efficiently’ has evolved independently for both corticospinal and respiratory bulbospinal drives to motoneurone pools. Second, drive to expiratory muscles has not been assessed in detail, yet even during eupnoea, rhythmic activity can be recorded from human triangularis sterni muscles during stance (e.g. Estenne et al. 1988) and sitting (unpublished observations).
Notwithstanding these limitations, our approach has highlighted that conventional views of motor control will need to be expanded to explain the recruitment of axial muscles according to their measured mechanical advantage for inspiratory (and possibly other) tasks. Studies in patients with increased respiratory drive due to chronic obstructive pulmonary disease have shown that drive can be elevated to obligatory inspiratory muscles, especially the diaphragm (De Troyer et al. 1997; Gorman et al. 2005), and that this may form quite a stereotyped output. However, we know little about how the respiratory drives to the axial muscles develop, how they can be modified, and how they are limited during postural and volitional tasks. Such information will help define the neural substrate that ensures respiratory homeostasis and ultimately our survival.
Acknowledgments
The authors' work is supported by the National Health and Medical Research Council of Australia. We are grateful to Ms Anna Hudson and Professor Janusz Lipski for helpful comments on the manuscript.
References
- Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007 doi: 10.1152/jn.00865.2007. in press. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990;533:141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- Butler JE, De Troyer A, Gandevia SC, Gorman RB, Hudson AL. Neuromechanical matching of central respiratory drive: a new principle of motor unit recruitment? Physiol News. 2007;67:22–24. [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge frequencies of single motor units in human diaphragm and parasternal muscles in lying and standing. J Appl Physiol. 2001;90:147–154. doi: 10.1152/jappl.2001.90.1.147. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, Clark JC, Friston KJ, Guz A. Regional cerebral blood flow during volitional breathing in man. J Physiol. 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Pinter MJ. The size principle: still working after all these years. News Physiol Sci. 1995;10:280–286. [Google Scholar]
- Davies JG, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Gorman RB, Gandevia SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol. 2003;546:943–954. doi: 10.1113/jphysiol.2002.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A. Inhomogeneous activation of the parasternal intercostals during breathing. J Appl Physiol. 1995;79:55–62. doi: 10.1152/jappl.1995.79.1.55. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Gayan-Ramirez G, Cappello M, Decramer M. On the mechanism of the mediolateral gradient of parasternal activation. J Appl Physiol. 1996a;80:1490–1494. doi: 10.1152/jappl.1996.80.5.1490. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Gevenois PA, Wilson TA. Mechanical advantage of the human parasternal intercostal and triangularis sterni muscles. J Physiol. 1998;513:915–925. doi: 10.1111/j.1469-7793.1998.915ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Rostrocaudal gradient of mechanical advantage in the parasternal intercostal muscles of the dog. J Physiol. 1996b;495:239–246. doi: 10.1113/jphysiol.1996.sp021588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–1205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estenne M, Derom E, De Troyer A. Neck and abdominal muscle activity in patients with severe thoracic scoliosis. Am J Respir Crit Care Med. 1998;158:452–457. doi: 10.1164/ajrccm.158.2.9710116. [DOI] [PubMed] [Google Scholar]
- Estenne M, Ninane V, De Troyer A. Triangularis sterni muscle use during eupnea in humans: effect of posture. Respir Physiol. 1988;74:151–162. doi: 10.1016/0034-5687(88)90101-6. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford TW, Kirkwood PA. Respiratory drive in hindlimb motoneurones of the anaesthetized female cat. Brain Res Bull. 2006;70:450–456. doi: 10.1016/j.brainresbull.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Butler JE, Hodges PW, Taylor JL. Balancing acts: respiratory sensations, motor control and human posture. Clin Exp Pharmacol Physiol. 2002;29:118–121. doi: 10.1046/j.1440-1681.2002.03611.x. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Hudson AL, Gorman RB, Butler JE, De Troyer A. Spatial distribution of inspiratory drive to the parasternal intercostal muscles in humans. J Physiol. 2006;573:263–275. doi: 10.1113/jphysiol.2005.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman RB, McKenzie DK, Butler JE, Tolman JF, Gandevia SC. Draphragm length and neural drive after lung volume reduction surgery. Am J Respir Crit Care Med. 2006;172:1259–1266. doi: 10.1164/rccm.200412-1695OC. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Herrmann U, Flanders M. Directional tuning of single motor units. J Neurosci. 1998;18:8402–8416. doi: 10.1523/JNEUROSCI.18-20-08402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Monteau R. [Connections between inspiratory medullary neurons and phrenic or intercostal motoneurones (author's transl)] J Physiol (Paris) 1976;72:987–1000. [PubMed] [Google Scholar]
- Hudson AL, Gandevia SC, Butler JE. The effect of lung volume on the co-ordinated recruitment of scalene and sternomastoid muscles in humans. J Physiol. 2007;584:261–270. doi: 10.1113/jphysiol.2007.137240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, Ninane V, De Troyer A. Mechanical advantage of sternomastoid and scalene muscles in dogs. J Appl Physiol. 1997;82:1517–1522. doi: 10.1152/jappl.1997.82.5.1517. [DOI] [PubMed] [Google Scholar]
- Legrand A, Schneider E, Gevenois PA, De Troyer A. Respiratory effects of the scalene and sternomastoid muscles in humans. J Appl Physiol. 2003;94:1467–1472. doi: 10.1152/japplphysiol.00869.2002. [DOI] [PubMed] [Google Scholar]
- Legrand A, Wilson TA, De Troyer A. Mediolateral gradient of mechanical advantage in the canine parasternal intercostals. J Appl Physiol. 1996;80:2097–2101. doi: 10.1152/jappl.1996.80.6.2097. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61:625–637. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res. 1993;95:477–487. doi: 10.1007/BF00227141. [DOI] [PubMed] [Google Scholar]
- Lipski J, Fyffe RE, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci. 1985;5:1545–1555. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol. 1991;439:545–558. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J Neurophysiol. 1987;57:1837–1853. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux M, Straus C, Redolfi S, Morelot-Panzini C, Couturier A, Hug F, Similowski T. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol. 2007;578:569–578. doi: 10.1113/jphysiol.2006.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK, Nail BS. Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Res Bull. 1984;12:469–477. doi: 10.1016/0361-9230(84)90162-x. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol. 2007a;582:135–146. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007b;102:772–780. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurones. J Physiol. 2007;579:765–782. doi: 10.1113/jphysiol.2006.122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol. 1987;57:1078–1100. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RH., Jr Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol. 1980;49:638–642. doi: 10.1152/jappl.1980.49.4.638. [DOI] [PubMed] [Google Scholar]
- Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Gielen CCAM, van den Tempel CM. Differences in the activation of m. biceps brachii in the control of slow isotonic movements and isometric contractions. Exp Brain Res. 1989;76:55–63. doi: 10.1007/BF00253623. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny BM, Denier van der Gon JJ, Gielen CC. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol. 1982;78:360–368. doi: 10.1016/0014-4886(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Tsuiki S, Ono T, Ishiwata Y, Kuroda T. Functional divergence of human genioglossus motor units with respiratory-related activity. Eur Respir J. 2000;15:906–910. doi: 10.1034/j.1399-3003.2000.15e16.x. [DOI] [PubMed] [Google Scholar]
- Wilson TA, Boriek AM, Rodarte JR. Mechanical advantage of the canine diaphragm. J Appl Physiol. 1998;85:2284–2290. doi: 10.1152/jappl.1998.85.6.2284. [DOI] [PubMed] [Google Scholar]
- Wilson TA, De Troyer A. Effect of respiratory muscle tension on lung volume. J Appl Physiol. 1992;73:2283–2288. doi: 10.1152/jappl.1992.73.6.2283. [DOI] [PubMed] [Google Scholar]
- Wilson TA, De Troyer A. Respiratory effect of the intercostal muscles in the dog. J Appl Physiol. 1993;75:2636–2645. doi: 10.1152/jappl.1993.75.6.2636. [DOI] [PubMed] [Google Scholar]
- Wilson TA, Legrand A, Gevenois PA, De Troyer A. Respiratory effects of the external and internal intercostal muscles in humans. J Physiol. 2001;530:319–330. doi: 10.1111/j.1469-7793.2001.0319l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]