Abstract
The dendrites of spinal motoneurones are highly active, generating a strong persistent inward current (PIC) that has an enormous impact on processing of synaptic input. The PIC is subject to regulation by descending neuromodulatory systems releasing the monoamines serotonin and noradrenaline. At high monoaminergic drive levels, the PIC dominates synaptic integration, generating an intrinsic dendritic current that is as much as 5-fold larger than the current entering via synapses. Without the PIC, motoneurone excitability is very low. Presumably, this descending control of the synaptic integration via the PIC is used to adjust the excitability (gain) of motoneurones for different motor tasks. A problem with this gain control is that monoaminergic input to the cord is very diffuse, affecting many motor pools simultaneously, probably including both agonists and antagonists. The PIC is, however, exquisitely sensitive to the reciprocal inhibition mediated by length sensitive muscle spindle Ia afferents and Ia interneurones. Reciprocal inhibition is tightly focused, shared only between strict mechanical antagonists, and thus can act to ‘sculpt’ specific movement patterns out of a background of diffuse neuromodulation. Thus it is likely that motoneurone gain is set by the interaction between diffuse descending neuromodulation and specific and focused local synaptic inhibitory circuits.
Although once considered the classic example of passive synaptic processing, motoneurone dendrites are now know to be highly active, with a strong complement of voltage-sensitive channels. This active integration is not unusual; probably every type of neurone has active dendrites. The study of motoneurones, however, provides unusual insights into CNS function in two important respects. Firstly, the active properties of motoneurone dendrites are subject to particularly strong neuromodulatory control (Heckman et al. 2003). This neuromodulatory control is dominated by systems that descend from brainstem nuclei and release the monoamines serotonin (5HT) and noradrenaline (NA). The activity of these brainstem nuclei is markedly state dependent, raising the exciting possibility that active synaptic integration in motoneurones is adjusted to meet the demands of different motor tasks. Secondly, the motoneurone is unique in being the only CNS neurone whose firing pattern can be readily measured in human subjects, because it normally drives the muscle fibres that it innervates in a one-to-one fashion (Binder et al. 1996). The motoneurone thus provides a unique window on neuroneal function and, in particular, on how neuromodulatory input influences synaptic integration in dendrites. Recent studies have begun to extend our understanding of this issue into abnormal function in neurotrauma and neurodegeneration.
In this review, we focus on persistent inward currents (PICs) in motoneurones. Dendritic PICs have an enormous impact on synaptic integration in motoneurones and it is largely via the PIC that descending neuromodulatory systems control this integration. It should, however, be kept in mind that the monoamines influence several other important motoneurone properties (Rekling et al. 2000; Powers & Binder, 2001), hyperpolarizing spike voltage threshold (Krawitz et al. 2001; Fedirchuk & Dai, 2004), decreasing the spike afterhyperpolarization amplitude (Berger et al. 1992; Manuel et al. 2006) and generating subthreshold depolarization (Wang & Dun, 1990). Together with the actions on the PIC, these monoaminergic effects induce a very potent increase in the excitability of motoneurones.
The ionic mechanisms of the PIC have been reviewed elsewhere (Powers & Binder, 2001; Alaburda et al. 2002; Hultborn, 2002; Heckman et al. 2003), but an important point for synaptic integration is that, in mammals, both a slow activating L-type Ca2+ current and a fast activating persistent Na+ current contribute approximately equally to the PIC. Several lines of evidence support a dendritic location for much of the PIC (Hounsgaard & Kiehn, 1993; Lee & Heckman, 1996; Bennett et al. 1998; Carlin et al. 2000; Heckman et al. 2000). Although a recent study has called into question some of these methods for identifying dendritic origin (Moritz et al. 2007), there is little doubt the PIC is largely dendritic. Studies based on channel immunohistochemistry and computer simulations further support this conclusion (Simon et al. 2003; Ballou et al. 2006; Zhang et al. 2006) as do modelling studies (Bui et al. 2006; Elbasiouny et al. 2006; Grande et al. 2007). At present the evidence for a dendritic origin of the NaPIC rests on voltage clamp methods (Jones & Lee, 2006), but hopefully immunohistochemical analyses will soon become available. Estimates by indirect methods based on voltage clamp of synaptic input suggest at least 70% of the total PIC is dendritic (Lee & Heckman, 2000).
The basic features of integration of excitatory synaptic input and its neuromodulatory control are illustrated in Fig. 1. The PIC, which is activated near threshold for spiking, has two primary effects: amplification and prolongation of synaptic input (Lee & Heckman, 1996; Lee & Heckman, 2000). Initial studies focused on the prolongation, i.e. the long tail current persisting after the input ceases (Fig. 1A). This prolonged current is responsible for the so-called ‘bistable’ behaviour, in which self-sustained firing of the cells can be initiated by a relatively brief pulse of excitatory input and then turned off by a pulse of inhibition (Schwindt & Crill, 1980, 1981; Hounsgaard et al. 1988). This PIC behaviour is largely confined to low input conductance motoneurones, which are likely to be part of type S motor units (Lee & Heckman, 1998a,b). As these are the motor units involved in posture, it is very likely that self-sustained firing due to PICs provides the baseline tone for this fundamental behaviour (Hounsgaard et al. 1988; Lee & Heckman, 1998b). In recent years, the focus has shifted to amplification of synaptic input by the PIC while the input is on (Fig. 1A, depolarized state) (Lee & Heckman, 1996; Bennett et al. 1998; Delgado-Lezama et al. 1999; Lee & Heckman, 2000; Prather et al. 2001; Hultborn et al. 2003; Lee et al. 2003).
Figure 1. Basic effects of the dendritic PIC on effective synaptic currents in spinal motoneurones.
A, during voltage clamp, a brief (1.5 s) period of excitatory, synaptic input was delivered to the cell via activation of muscle spindle Ia afferents. When the cell was held hyperpolarized, this produced an effective synaptic current with a sharp onset and offset matching input duration. At a depolarized holding potential at approximately spike voltage threshold in unclamped conditions, a repetition of the very same input had a much different result. Current during the input was much larger (amplification) and a prolonged tail current was generated, both due to activation of the dendritic (PIC). As this input does not act via either NMDA receptors or metabotropic glutamate receptors, the amplification can be attributed to the PIC (Lee & Heckman, 2000). Inward (depolarizing) currents are downward, the usual voltage clamp convention. B, the interaction between amplification of effective synaptic current and level of descending neuromodulatory input (labelled as weak, medium and strong). In the weak state, there is no significant PIC and synaptic current steadily decreases as holding potential is slowly depolarized. At medium and high levels, activation of the PIC causes a large increase in current, at a voltage level where spikes would be generated in unclamped conditions. As the cell is depolarized further, current sharply declines. This saturation is likely to be due both to loss of driving force in depolarized dendritic regions and to activation of dendritic voltage-sensitive outward currents. Inward (depolarizing) currents are downward. Based on data from Lee & Heckman (2000).
The higher the monoaminergic input to motoneurones, the stronger this amplification (Fig. 1B) (Lee & Heckman, 2000). In our studies we have observed amplification factors as large as 10-fold in some cells; in the standard decerebrate preparation, amplification factors are typically about 3-fold. The discovery of synaptic amplification fundamentally changed our view of motoneurone synaptic integration (Binder, 2002; Heckman et al. 2003; Hultborn et al. 2003): most of the current needed to drive motoneurone firing is now understood to come from the motoneurone dendritic tree and thus the synapses on the dendrites act primarily to control PIC activation. In short, motoneurone dendrites are highly active processors of their synaptic inputs. The PIC has been shown to amplify several different sources of sensory input: muscle spindle Ia excitation (Lee & Heckman, 2000) and inhibition (Kuo et al. 2003), cutaneous excitation (Prather et al. 2002) and recurrent inhibition via Renshaw cells (Hultborn et al. 2003). Descending inputs are also likely to interact with the PIC but studies of this issue are needed.
The control of active dendritic processing via descending monoaminergic inputs is likely to be fundamentally important in normal motor behaviour. The key issue is that the brainstem nuclei that are the origin of the 5HT and NA inputs to the cord are highly state dependent. In fact, the requirement for a reasonable degree of excitability in the brainstem is the reason PICs were overlooked in motoneurones in the classic studies, which relied on deep anaesthesia. The unanaesthetized decerebrate preparation was thus essential to discovery of the interaction between PICs and monoamines (Hounsgaard et al. 1988). The 5HT system projecting to the cord varies its activity in proportion to intensity of motor outflow – for example, the faster the speed of locomotion the higher the firing rate of neurones in the caudal raphe nuclei that are the primary source of spinal 5HT projections (Jacobs et al. 2002). The NA system appears to primarily vary its activity with state of arousal (Aston-Jones et al. 2001). Both systems are inactive in the sleeping state. Potentially, the state-dependent nature of monoaminergic input would allow the mode of synaptic integration in motoneurones to be adjusted for different motor tasks. Because raphe output increases with increasing speed of locomotion, one possibility is that motoneurone excitability grows in proportion to the level of force. Much further work, however, needs to be done to define the relation between motoneurone excitability and motor tasks.
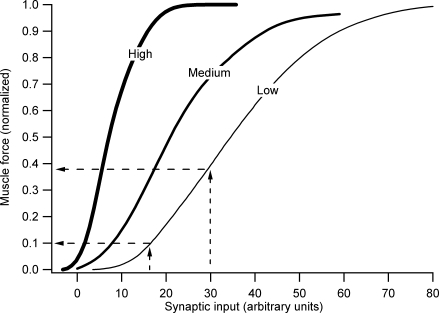
One point, however, requires emphasis: the effect of monoamines on motoneurone excitability is so strong that even motor behaviours requiring small to moderate forces would be virtually impossible without substantial monoaminergic drive. This point is illustrated in the computer simulations shown in Fig. 2 (which are based on the cat medial gastrocnemius pool and muscle; Heckman & Binder, 1991). Systematic studies by Powers, Binder and colleagues (reviewed by Powers & Binder, 2001) show that maximal and simultaneous activation of several different descending and sensory systems only generates a total of about 25–30 nA of current in motoneurones (cf. Cushing et al. 2005). Figure 2 shows that in the absence of monoaminergic input, this ‘maximal effort’ would scarcely produce 40% of maximal force. Even generating the force required for posture in this particular muscle (i.e. ∼5–10% of maximum; Walmsley et al. 1978) would require effective synaptic currents reaching 15 nA. This level is much more than the ∼5 nA generated by combined maximal stimulation of the vestibulospinal tract and muscle spindle Ia afferents. A moderate level of monoaminergic input, perhaps consistent with the tonic level occurring in the waking state, allows the force required for posture to be generated by only a few nanoamps, a reasonable amount for vestibulospinal and Ia inputs. Further increases in monoaminergic input would allow maximum force to be attained at the 20–30 nA maximum input level that descending motor systems are capable of producing.
Figure 2. Computer simulations of the effect of increasing descending monoaminergic drive to motoneurones on the net input–output gain of a motor pool and the muscle that is innervates.
The transformation is from average synaptic current to the whole motor pool (x-axis) to total force generated by the entire muscle (y-axis). The simulations are based on the extensive data for the cat medial gastrocnemius motor pool and muscle. The right vertical dashed line indicates the input level produced by activation of many sensory and descending systems. The associated horizontal dashed line indicates that this ‘maximal’ activation generates less than 40% of maximum force. The lower horizontal dashed line and its associated vertical line indicate the maximum input output levels for postural activity in this motor pool and muscle. Simulations based on studies by Heckman & Binder (1991).
The profound effect of descending monoaminergic inputs on motoneurone excitability has important implications for injury and disease. Loss of monoaminergic input in spinal injury almost eliminates PICs in motoneurones, yet within 2–3 months the PICs have recovered to normal or greater levels despite the continued absence of monoamines (Bennett et al. 2004; Li et al. 2004; Harvey et al. 2006). PICs may also be very important in neurodegenerative diseases. The NaPIC is up-regulated in motoneurones cultured from embryonic mutant SOD1 mice (Kuo et al. 2005), which is the standard transgenic model of amyotrophic lateral sclerosis (ALS) (Siddique & Deng, 1996). Further changes in motoneurone properties also occur in neonatal SOD1 animals (Bories et al. 2007), perhaps due to increases in cell size to compensate for increased NaPICs. Thus, it appears reasonable to conclude that the PIC is so important to motoneurone excitability that homeostatic mechanisms exist to maintain its amplitude. This suggests homeostatic regulation of PICs may also occur in normal function. Motoneurone electrical properties do adapt significantly during exercise (Gardiner et al. 2006). The profound changes in the PIC during spinal injury suggest that it may also adapt to exercise. The recent development of a rat preparation likely to have significant PICS in motoneurones will allow evaluation of this hypothesis (Button et al. 2006).
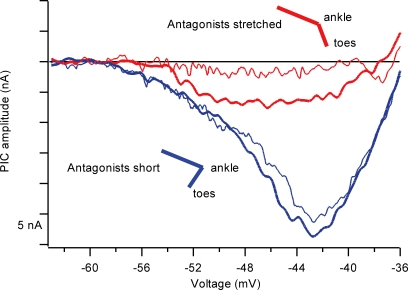
Recently, we have realized that, despite the above advantages, the PIC and its descending monoaminergic control system have significant potential problems for motor control. One key problem is that the descending projections are highly diffuse – the monoaminergic system is not organized in the specific fashion that characterizes, e.g. the corticospinal system (Björklund & Skagerberg, 1982). This same problem has been emphasized for descending neuromodulation of pain circuits in the dorsal horn of the spinal cord (Mason, 2005). Moreover, the concept of diffuse control of spinal neurone excitability has been previously been suggested for the γ-motoneurone system with the concept of ‘fusimotor set’ (Prochazka, 1989). Thus increasing the excitability of one motor pool via monoaminergic input necessarily results in increased excitability within many motor pools, potentially including agonists and antagonists acting at multiple joints. The solution to this problem may well lie within local inhibitory circuits within the cord. The dendritic PIC is highly sensitive to inhibition (Hultborn et al. 2003; Kuo et al. 2003), especially Ia reciprocal inhibition evoked by length changes in antagonist muscles. Rotations of the ankle joint that produce almost undetectable changes in reciprocal inhibition measured at the soma of the cell nonetheless reduce the amplitude of the PIC by about 50% (Hyngstrom et al. 2007) (Fig. 3). Like Ia monosynaptic excitation, Ia reciprocal inhibition is sharply focused, being restricted to strict antagonist pairs (Nichols & Cope, 2001). For example, the PICs in medial gastrocnemius (MG) motoneurones (which extend the ankle) are highly sensitive to joint movement that changes the length of MG's primary antagonists (the pretibial flexors tibialis anterior (TA) and extensor digitorum longus (TA/EDL)), but insensitive to movements at the knee or hip joints. Elimination of length changes in TA and EDL eliminate PIC modulation in MG, despite significant changes in length of MG synergists and other muscles in the pretibial compartment (Hyngstrom et al. 2007). Thus, reciprocal inhibition provides the capacity for sculpting of specific movement patterns from the diffuse background of neuromodulation generated by descending monoaminergic tracts.
Figure 3. Effect of changes in ankle joint position on the PIC in an ankle extensor motoneurone.
The PIC is manifest as a strong upward deflection in current during voltage clamp of a cat medial gastrocnemius (MG) motoneurone (in normal voltage clamp converntion, inward current is negative, but the sign of the current here has been reversed to match Figs 2 and 4). For both blue traces, the ankle joint angle was such that the antagonists to MG were short; for both the red traces, the antagonist was stretched inducing reciprocal Ia inhibition and greatly reducing the amplitude of the PIC. The thick traces indicate the first pair of trials, the thin traces a repeat to show that the PIC could be turned on and off consistently. Data from Hyngstrom et al. (2007).
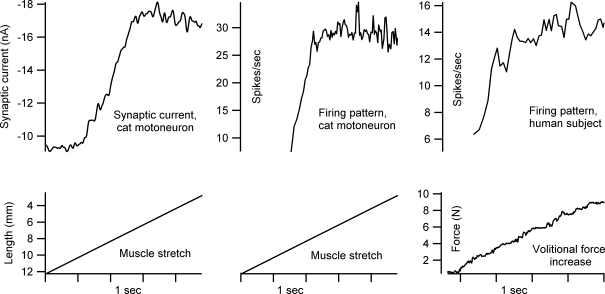
A second problem with dendritic PICs is that, once activated, they tend to greatly reduce the sensitivity to further synaptic input (this saturation is clear in Fig. 1B in the sharp decline in synaptic current at higher voltage levels – note the larger the amplification, the greater the saturation) (Lee & Heckman, 2000; Lee et al. 2003). This saturation is very likely to be due to the dendritic component of the PIC. Figure 4 shows that, during a linearly increasing synaptic input in a cat motoneurone, there is an initial rapid increase in the PIC activation followed by this dendritic PIC saturation. These same phenomena, initial surge and saturation, are also seen in the firing rate modulation in the same motoneurone. Moreover, an initial surge and subsequent saturation are a common feature in human motor unit firing patterns (Binder et al. 1996; Hornby et al. 2002) (see the left most panel of Fig. 4 for an example). This saturation phenomenon has been referred to as the ‘preferred firing range’ (Hornby et al. 2002) or ‘rate limiting’ (Heckman & Binder, 1993). Thus, dendritic PIC properties may play a major role in shaping the trajectory of firing in human motoneurones. Inhibition has the potential to relieve saturation by reducing PIC activation in dendritic regions. Perhaps reciprocal, or push–pull, activation of the PIC is the ideal way to control it – further study of this issue is needed.
Figure 4. Initial surge and saturation in activation of a dendritic PIC corresponds to similar events in motoneurone firing.
The left panel shows the effective synaptic current generated in a cat ankle extensor motoneurone (medial gastrocnemius) during voltage clamp by a slow stretch of the Achilles tendon. The middle panel shows the firing response of the same cell to the same stretch during unclamped conditions. The right panel shows the firing pattern of a human motor unit during a linearly rising volitional command that produced a linearly rising force (biceps muscle; data courtesy of Carol Mottram, at the Rehabilitation Institute of Chicago). Note in all three examples, there is an initial steep increase (surge) followed by a saturation that, during firing, produced rate limitation.
The importance of inhibition in dendritic PIC control suggests the following speculation: descending motor commands that produce specific movement patterns may be coupled to both brainstem monoaminergic nuclei and to reciprocal inhibitory interneurones in the spinal cord. In this way, the diffuse neuromodulation could provide the appropriate overall level of motor pool excitability for a class of motor tasks (e.g. posture, or reaching to a target) and specific spinal inhibitory pathways can be used to focus this excitability to only those motor pools required to actually implement the specific task chosen from this class.
Acknowledgments
This work was supported by NIH/NINDS grant NS034382. We thank Dr Carol Mottram for providing the motor unit firing data for Fig. 4.
References
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol. 2002;508:219–226. doi: 10.1007/978-1-4615-0713-0_27. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Ballou EW, Smith WB, Anelli R, Heckman CJ. Measuring dendritic distribution of membrane proteins. J Neurosci Methods. 2006;156:257–266. doi: 10.1016/j.jneumeth.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Binder MD. Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Brain Res Rev. 2002;40:1–8. doi: 10.1016/s0165-0173(02)00183-2. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology section 12 Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 1–53. [Google Scholar]
- Björklund A, Skagerberg G. Descending monoaminergic projections to the spinal cord. In: Sjolund B, Bjorklund A, editors. Brain Stem Control of Spinal Mechanisms. Amsterdam: Elsevier Biomedical Press; 1982. pp. 55–88. [Google Scholar]
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. doi: 10.1111/j.1460-9568.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca2+ channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol. 2006;95:225–241. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Button DC, Gardiner K, Marqueste T, Gardiner PF. Frequency-current relationships of rat hindlimb α-motoneurones. J Physiol. 2006;573:663–677. doi: 10.1113/jphysiol.2006.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J Neurophysiol. 2005;94:3465–3478. doi: 10.1152/jn.00439.2005. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Hounsgaard J. Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. J Physiol. 1999;515:203–207. doi: 10.1111/j.1469-7793.1999.203ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol. 2006;570:355–374. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–361. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P, Dai Y, Heckman CJ. Effects of exercise training on α-motoneurons. J Appl Physiol. 2006;101:1228–1236. doi: 10.1152/japplphysiol.00482.2006. [DOI] [PubMed] [Google Scholar]
- Grande G, Bui TV, Rose PK. Estimates of the location of L-type Ca2+ channels in motoneurons of different sizes: a computational study. J Neurophysiol. 2007;97:4023–4035. doi: 10.1152/jn.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulation of the steady-state input-output function of the cat medial gastrocnemius motoneuron pool. J Neurophysiol. 1991;65:952–967. doi: 10.1152/jn.1991.65.4.952. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol. 1993;69:1005–1008. doi: 10.1152/jn.1993.69.4.1005. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Theiss RD, Johnson MD, Lee RH. Active dendrites markedly enhance input-output gain in motoneurons. Soc Neurosci Abs. 2000;26:257.212. [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: a preferred firing range across vertebrate species? Muscle Nerve. 2002;25:632–648. doi: 10.1002/mus.10105. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Adv Exp Med Biol. 2002;508:213–218. doi: 10.1007/978-1-4615-0713-0_26. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol. 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci. 2007;10:363–369. doi: 10.1038/nn1852. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jones SM, Lee RH. Fast amplification of dynamic synaptic inputs in spinal motoneurons in vivo. J Neurophysiol. 2006;96:2200–2206. doi: 10.1152/jn.00537.2006. [DOI] [PubMed] [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol. 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ. Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol. 2003;90:3617–3624. doi: 10.1152/jn.00521.2003. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na+ current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol. 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998a;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998b;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol. 2003;89:27–39. doi: 10.1152/jn.00137.2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol. 2004;91:2236–2246. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. The afterhyperpolarization conductance exerts the same control over the gain and variability of motoneurone firing in anaesthetized cats. J Physiol. 2006;576:873–886. doi: 10.1113/jphysiol.2006.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- Moritz AT, Newkirk GS, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol. 2007;98:1042–1047. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC. The organization of distributed proprioceptive feedback in the chronic spinal cat. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. London: CRC Press; 2001. pp. 305–326. [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Prather JF, Clark BD, Cope TC. Firing rate modulation of motoneurons activated by cutaneous and muscle receptor afferents in the decerebrate cat. J Neurophysiol. 2002;88:1867–1879. doi: 10.1152/jn.2002.88.4.1867. [DOI] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol. 2001;85:43–53. doi: 10.1152/jn.2001.85.1.43. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Negative slope conductance at large depolarizations in cat spinal motoneurons. Brain Res. 1981;207:471—475. doi: 10.1016/0006-8993(81)90381-4. [DOI] [PubMed] [Google Scholar]
- Siddique T, Deng HX. Genetics of amyotrophic lateral sclerosis. Hum Mol Genet. 1996;5:1465–1470. doi: 10.1093/hmg/5.supplement_1.1465. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–266. doi: 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Sukiasyan N, Moller M, Bezprozvanny I, Zhang H, Wienecke J, Hultborn H. Localization of L-type calcium channel CaV1.3 in cat lumbar spinal cord – with emphasis on motoneurons. Neurosci Lett. 2006;407:42–47. doi: 10.1016/j.neulet.2006.07.073. [DOI] [PubMed] [Google Scholar]