Abstract
Protein kinase A (PKA)-independent actions of adenosine 3′,5′-cyclic monophosphate (cAMP) are mediated by Epac, a cAMP sensor expressed in pancreatic β-cells. Evidence that Epac might mediate the cAMP-dependent inhibition of β-cell ATP-sensitive K+ channels (KATP) was provided by one prior study of human β-cells and a rat insulin-secreting cell line (INS-1 cells) in which it was demonstrated that an Epac-selective cAMP analogue (ESCA) inhibited a sulphonylurea-sensitive K+ current measured under conditions of whole-cell recording. Using excised patches of plasma membrane derived from human β-cells and rat INS-1 cells, we now report that 2′-O-Me-cAMP, an ESCA that activates Epac but not PKA, sensitizes single KATP channels to the inhibitory effect of ATP, thereby reducing channel activity. In the presence of 2′-O-Me-cAMP (50 μm), the dose–response relationship describing ATP-dependent inhibition of KATP channel activity (NPo) is left-shifted such that the concentration of ATP producing 50% inhibition (IC50) is reduced from 22 μm to 1 μm for human β-cells, and from 14 μm to 4 μm for rat INS-1 cells. Conversely, when patches are exposed to a fixed concentration of ATP (10 μm), the administration of 2′-O-Me-cAMP inhibits channel activity in a dose-dependent and reversible manner (IC50 12 μm for both cell types). A cyclic nucleotide phosphodiesterase-resistant ESCA (Sp-8-pCPT-2′-O-Me-cAMPS) also inhibits KATP channel activity, thereby demonstrating that the inhibitory actions of ESCAs reported here are unlikely to arise as a consequence of their hydrolysis to bioactive derivatives of adenosine. On the basis of such findings it is concluded that there exists in human β-cells and rat INS-1 cells a novel form of ion channel modulation in which the ATP sensitivity of KATP channels is regulated by Epac.
Although the second messenger adenosine 3′,5′-cyclic monophosphate (cAMP) activates protein kinase A (PKA), recent studies provide compelling evidence for an alternative mechanism of cAMP signal transduction, one that involves cAMP-regulated guanine nucleotide exchange factors (cAMP-GEF) known as Epacs (the Exchange Protein directly Activated by Cyclic AMP; de Rooij et al. 1998; Kawasaki et al. 1998). The analysis of Epac-mediated signal transduction has been facilitated by the development of Epac-selective cyclic AMP analogues (ESCAs). These synthetic analogues of cAMP incorporate a 2′-O-alkyl substitution on the ribose ring of cAMP, as in 2′-O-Me-cAMP, a modification that impairs their ability to activate PKA, while leaving intact their ability to activate Epac (Christensen et al. 2003). An ESCA that also incorporates a hydrophobic parachlorophenylthio (pCPT) substitution on the adenine moiety of cAMP is 8-pCPT-2′-O-Me-cAMP (Enserink et al. 2002). It is cell permeant and when it is applied to living cells, it stimulates Ca2+ signalling and exocytosis in multiple cell types (Holz et al. 2006, 2008a). We recently reported that 2′-O-Me-cAMP and 8-pCPT-2′-O-Me-cAMP inhibit a sulphonylurea-sensitive K+ current in pancreatic β-cells through an as yet to be determined mechanism (Kang et al. 2006). In the present study we applied the methods of single channel analysis to human pancreatic β-cells and a rat β-cell line (INS-1 cells; a cell line that expresses Epac and KATP channels; Leech et al. 2000; Moritz et al. 2001) in order to investigate the nature of this effect.
ATP-sensitive K+ channels (KATP channels) expressed in β-cells are hetero-octamers comprising Kir6.2 and sulphonylurea receptor-1 (SUR1) subunits (Ashcroft, 2005). Kir6.2 constitutes the K+-selective pore-forming subunit, whereas SUR1 acts as an allosteric regulator of Kir6.2 gating (Nichols, 2006). Although ATP interacts directly with Kir6.2 to close the channel, the binding of Mg-ADP to SUR1 opens the channel. Intriguingly, new studies demonstrate direct interactions of SUR1 with Epac proteins, a finding first reported by Seino and coworkers (Ozaki et al. 2000). For example, when expressed in HEK cells, both Epac1 and Epac2 interact with full-length SUR1 (Kang et al. 2006), and for Epac2 this interaction appears to be mediated by nucleotide-binding fold-1 (NBF-1) of SUR1 (Shibasaki et al. 2004a,b; Bryan et al. 2007). Since cAMP-elevating agents inhibit β-cell KATP channel activity (Holz & Habener, 1992; Holz et al. 1993; Barnett et al. 1994; Gromada et al. 1998; He et al. 1998; Suga et al. 2000; Ding et al. 2001; Light et al. 2002; Kang et al. 2006), we have proposed that the interaction of Epac with SUR1 confers cAMP-dependent regulation of this channel's function (Holz et al. 2006; Kang et al. 2006).
In the present study we sought to determine if the interaction of cAMP with Epac might regulate the sensitivity of KATP channels to ATP. Consistent with this concept, we now demonstrate that in human β-cells and rat INS-1 cells, 2′-O-Me-cAMP sensitizes KATP channels to the inhibitory effect of ATP, thereby producing a left-shift of the concentration–response relationship describing ATP-dependent inhibition of KATP channel activity. We also report that Sp-8-pCPT-2′-O-Me-cAMPS, a cyclic nucleotide phosphodiesterase (PDE) resistant ESCA, produces a decrease of KATP channel activity, thereby demonstrating that the inhibitory actions of ESCAs reported here are unlikely to be a consequence of their hydrolysis to bioactive derivatives of adenosine, a confounding effect observed in other cell types (Laxman et al. 2006). In summary, the new findings presented here demonstrate that there exists in β-cells a novel form of ion channel modulation in which the ATP sensitivity of KATP channels is regulated by Epac.
Methods
Cell culture
With informed consent for tissue use for research, human islets of Langerhans were obtained from cadaver donors. They were provided, with safeguards for donor anonymity, by the Islet Cell Resource Service of the National Institutes of Health, National Center for Research Resources. (http://icr.coh.org/). Approval for the use of human islets in the laboratory of G. G. Holz was granted by the Institutional Review Board of New York University School of Medicine. Single cell suspensions of human islet cells were prepared by digestion of islets with trypsin-EDTA, and single cells were plated onto glass coverslips (25CIR-1; Fisher Scientific) coated with 1 mg ml−1 concanavalin A (type V; Sigma-Aldrich, St Louis, MO, USA). Cell cultures were maintained in a humidified incubator (95% air, 5% CO2) at 37°C in CMRL-1066 modified culture medium (Mediatech, Inc., Herndon, VA, USA; cat. no. 99–603-CV) containing 10% (v/v) fetal bovine serum (FBS). β-Cells were identified by fluorescence microscopy after infection of the cultures with adenovirus directing expression of enhanced yellow fluorescent protein (EYFP) under the control of the rat insulin 2 gene promoter (Kang et al. 2003). INS-1 cells (passages 70–90) were obtained from Dr C. B. Wollheim (University Medical Center, Geneva, Switzerland). INS-1 cells are an immortalized cell line derived from a radiation-induced insulinoma of rat pancreas (Asfari et al. 1992). INS-1 cells were maintained in RPMI 1640 medium containing 10 mm Hepes, 11.1 mm glucose, 10% FBS, 100 U ml−1 penicillin G, 100 μg ml−1 streptomycin, 2.0 mm l-glutamine, 1.0 mm sodium pyruvate, and 50 μm 2-mercaptoethanol (Asfari et al. 1992; Chepurny & Holz, 2007). INS-1 cells were passaged by trypsinization and subcultured once a week. Reagents for INS-1 cell culture were obtained from Invitrogen Life Technologies (Rockville, MD, USA).
Measurement of single channel KATP currents
For the detection of single KATP channel currents in inside-out patches, pipettes pulled from borosilicate glass were fire-polished and back-filled with a solution containing (mm): 140 KCl, 1.0 MgCl2, 2.0 CaCl2, 5 Hepes (300 mosmol l−1; pH 7.3). Patches were excised into a bath solution containing (mm): 70 K2SO4, 2.0 MgCl2, 0.1 CaCl2, 1.1 EGTA, 0.2 GTP, 5 Hepes, and the indicated [ATP]. The free [Ca2+] of this solution was 160 nm, the pH was 7.3, and the osmolarity was 300 mosmol l−1. The patch potential was maintained at −100 mV in order to measure inward K+ currents through KATP channels. Data were acquired using an Axopatch 200B amplifier under the control of pCLAMP v.9.2 (Molecular Devices, Sunnyvale, CA, USA). Channel currents were low-pass filtered at 1 kHz (8-pole Bessel filter) and digitized at 5 or 10 kHz. All experiments were performed at 22–26°C on the stage of an inverted microscope.
Analysis of single channel KATP currents
KATP channel activity was determined prior to, during and following recovery from the application of test substances. Channel activity was calculated as the value NPo from 20 to 40 s intervals of data, where N is the number of channels active in the patch, and P is the probability that a channel is open. Values of NPo were determined by constructing all-points amplitude histograms of channel activity (bin width 0.5 pA) using pCLAMP v.9.2. Gaussian curves were superimposed on the histograms using Origin 7.5 (OriginLab Corp., Northampton, MA, USA). For studies in which values of NPo were compared amongst groups of patches exposed to different test solutions, statistical significance of the results was evaluated using SigmaStat software (Systat Software, San Jose, CA, USA) in order to perform one-way analysis of variance (ANOVA) with Dunnett's post hoc test for comparing control and test groups. Differences amongst the groups were considered significant at P < 0.05. Best-fits of the dose–response relationships describing the actions of 2′-O-Me-cAMP or ATP to inhibit KATP channel activity were obtained using the nonlinear regression curve-fitting module of SigmaPlot v.10.0 (Systat Software). The data were fitted to the Hill equation:
| (1) |
where IC50 is the concentration of 2′-O-Me-cAMP or ATP producing 50% inhibition, h is the Hill coefficient, X is the is the value of NPo in the presence of 2′-O-Me-cAMP or ATP, and Xc is the NPo value in the absence of 2′-O-Me-cAMP or ATP.
Application of test solutions and sources of reagents
cAMP analogues were applied to the inner surfaces of inside-out patches using a RSC-160 rapid solution changer (Molecular Kinetics Inc., Indianapolis, IN, USA) or a Dynaflow Pro II microfluidics drug delivery system using 8- or 16-well chambers (Cellectricon Inc., Gaithersburg, MD, USA). Rapid exchange of test solutions was performed under computer control. 2′-O-Me-cAMP, Sp-8-pCPT-2′-O-Me-cAMPS, Rp-cAMPS, 6-Bnz-cAMP, cAMP and 2′-O-Me-cGMP were obtained from BIOLOG (Bremen, Germany). ATP and GTP were from Sigma-Aldrich.
Results
2′-O-Me-cAMP inhibits KATP channel activity in INS-1 cells
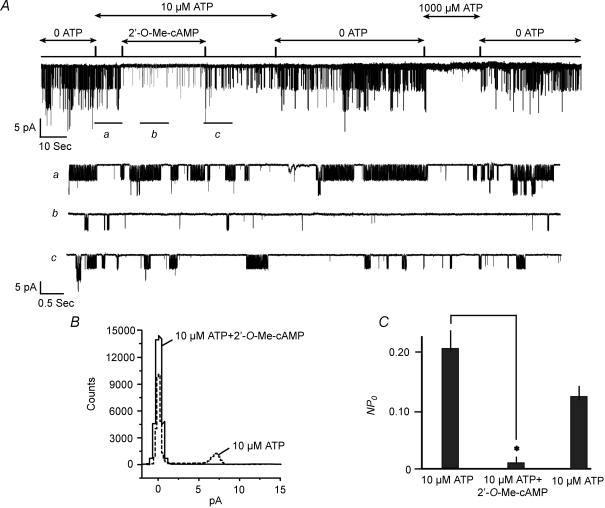
When the inner surfaces of excised patches were exposed to a fixed concentration of ATP (10 μm), the Epac-selective cAMP analogue 2′-O-Me-cAMP (50 μm) inhibited INS-1 cell KATP channel activity in 16 of 20 patches (Fig. 1A). Measurement of NPo values deduced from an all-points amplitude histogram (Fig. 1B) demonstrated that 2′-O-Me-cAMP reduced channel activity by 94 ± 5% in patches in which an effect of the ESCA was observed (Fig. 1C). This action of the ESCA was unlikely to be a consequence of a direct channel block because in 4 of 20 patches exhibiting KATP channel activity, 2′-O-Me-cAMP was without action whereas 1 mm ATP remained effective (data not shown). The inhibitory action of 2′-O-Me-cAMP developed over 5–10 s, longer than that measured for the onset of inhibition by ATP, despite the fact that the test solution containing 2′-O-Me-cAMP was delivered to the patch within 200 ms. With continual application of 2′-O-Me-cAMP, little or no desensitization was evident. Upon washout of 2′-O-Me-cAMP, partial recovery of channel activity was observed within 10–30 s, after which the effect of 2′-O-Me-cAMP was repeatable.
Figure 1. Inhibition of KATP channel activity by 2′-O-Me-cAMP in INS-1 cells.
A, 2′-O-Me-cAMP was applied at a concentration of 50 μm to an inside-out patch. The inhibition of channel activity by 2′-O-Me-cAMP is illustrated on a compressed time scale (top trace) or on an expanded time scale (traces a, b, and c) in order to depict channel activity prior to (a), during (b), and following washout (c) of 2′-O-Me-cAMP. Unitary currents measured in this patch resulted from the activity of KATP channels because they were abolished during application of 1 mm ATP (top trace). Arrows at the top indicate when the patch was exposed to each test solution. Patch potential was −100 mV here and in subsequent figures. B, all-points amplitude histogram depicting KATP channel activity prior to (dotted line) or during (continuous line) application of 50 μm 2′-O-Me-cAMP to the same patch depicted in panel A. C, population study summarizing the action of 50 μm 2′-O-Me-cAMP to decrease values of NPo in excised patches under conditions in which the ATP concentration was fixed at 10 μm. Channel activity prior to and following washout of 2′-O-Me-cAMP is depicted by the left-most and right-most bars, respectively. Values of NPo for each bar are the mean ±s.e.m. for 16 patches and were not subjected to normalization. *The value of NPo was different (P < 0.05 here and in subsequent figures).
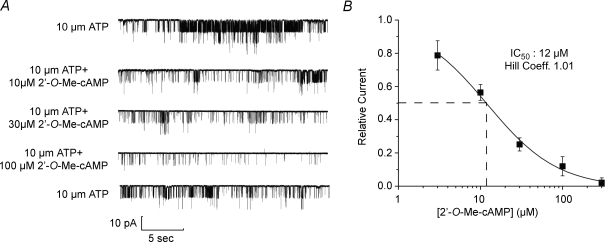
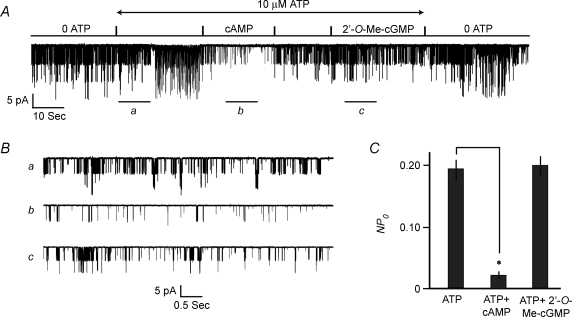
Determination of the cumulative dose–response relationship demonstrated the potency of 2′-O-Me-cAMP in this assay (Fig. 2A and B). In the presence of 10 μm ATP, the concentration of 2′-O-Me-cAMP producing a 50% inhibition of channel activity (IC50) was 12 μm, a value in agreement with the reported affinity of 2′-O-Me-cAMP for Epac1 (Christensen et al. 2003). The action of 2′-O-Me-cAMP was mimicked by a high concentration (300 μm) of cAMP (Fig. 3A–C), whereas channel activity was unaffected by 100 μm 2′-O-Me-cGMP (Fig. 3A and C; note that 2′-O-Me-cGMP fails to activate Epac; Kang et al. 2006). It may be concluded that the inhibitory action of 2′-O-Me-cAMP reported here results from a selective interaction of this ESCA with a cAMP-binding protein that most likely corresponds to Epac.
Figure 2. Dose–response relationship for INS-1 cell KATP channel inhibition by 2′-O-Me-cAMP.
A, KATP channel activity was measured under conditions in which a progressively higher concentration of 2′-O-Me-cAMP was administered while maintaining the ATP concentration at 10 μm. Illustrated are continuous data subdivided into five traces. The top trace indicates control activity in the absence of 2′-O-Me-cAMP. The bottom trace indicates recovery of channel activity after wash out of 2′-O-Me-cAMP. B, cumulative dose–response relationship describing the inhibition of KATP channel activity, as generated using the experimental design illustrated in panel A. For each concentration of 2′-O-Me-cAMP the value of NPo was normalized relative to a value of 1.0, which represents the relative current measured when patches were exposed to 10 μm ATP in the absence of 2′-O-Me-cAMP. Each data point (squares) is the mean ±s.e.m. for 5 patches. Dashed lines indicate the IC50 concentration of 2′-O-Me-cAMP.
Figure 3. INS-1 cell KATP channel activity is inhibited by cAMP but not 2′-O-Me-cGMP.
A, cAMP (300 μm) or 2′-O-Me-cGMP (100 μm) was applied under conditions in which an excised patch was continuously exposed to 10 μm ATP. B, the same experiment illustrated in A but depicted on an expanded time scale. Traces a, b and c correspond to the time periods indicated by the horizontal bars in the trace illustrated in A. C, population study summarizing the action of cAMP (300 μm) and the lack of action of 2′-O-Me-cGMP (100 μm) to alter values of NPo in excised patches exposed to 10 μm ATP. Values of NPo for each bar are the mean ±s.e.m. for 6 patches and were not normalized.
2′-O-Me-cAMP acts independently of PKA to inhibit KATP channel activity in INS-1 cells
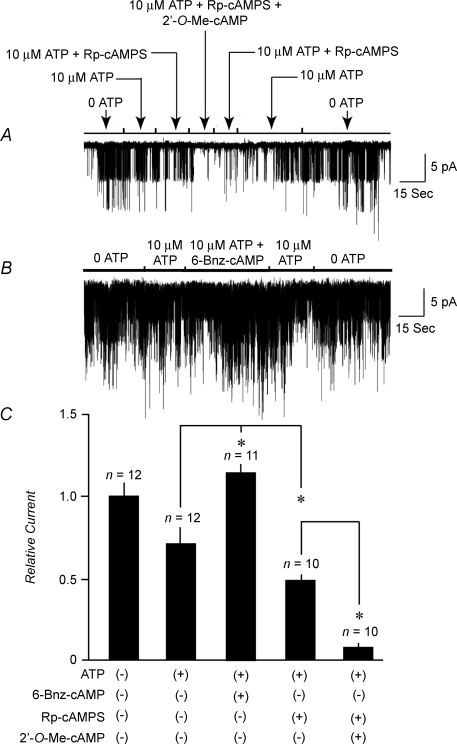
Although ESCAs activate Epac in a selective manner, they have a limited ability to activate PKA when tested at concentrations in excess of 100 μm (Enserink et al. 2002). However, such a PKA-mediated action of 2′-O-Me-cAMP is unlikely to explain the findings reported here. This conclusion is reached because 50 μm 2′-O-Me-cAMP inhibited KATP channel activity under conditions in which the inner surfaces of excised patches were exposed to 100 μm Rp-cAMPS (Fig. 4A and C). Rp-cAMPS is a cAMP antagonist that blocks the activation of PKA by cAMP (Dostmann et al. 1990), but which fails to prevent the activation of Epac by ESCAs (Rangarajan et al. 2003; Holz et al. 2008a). Interestingly, Rp-cAMPS, alone, inhibited INS-1 cell KATP channel activity when patches were exposed to a fixed concentration (10 μm) of ATP (Fig. 4A and C). In contrast, 6-Bnz-cAMP (100 μm), a cAMP analogue that activates PKA but not Epac (Christensen et al. 2003), stimulated channel activity (Fig. 4B and C). Such findings suggest a dual action of cAMP, one in which channel activity is stimulated in a PKA-mediated manner, whereas channel activity is inhibited in an Epac-mediated manner. Consistent with this concept, we found that when patches were exposed to 10 μm ATP, a low concentration of cAMP (1 μm) increased the value of NPo by 20 ± 5% (mean ±s.e.m., n = 5 patches), whereas a high concentration of cAMP (300 μm) decreased the value of NPo by 88 ± 4% (n = 6 patches).
Figure 4. Contrasting actions of Rp-cAMPS and 6-Bnz-cAMP in INS-1 cells.
A, pretreatment of patches with 100 μm Rp-cAMPS failed to prevent the inhibition of KATP channel activity by 50 μm 2′-O-Me-cAMP. Arrows indicate the time intervals during which test substances were applied. B, treatment of patches with 100 μm 6-Bnz-cAMP stimulated KATP channel activity in a reversible manner. C, population study summarizing findings presented in A and B. The actions of ATP (10 μm), 6-Bnz-cAMP (100 μm), Rp-cAMPS (100 μm), and 2′-O-Me-cAMP (50 μm) to affect KATP channel activity in INS-1 cells are illustrated. Values of NPo were normalized relative to the channel activity measured in an ATP-free solution which was assigned a relative current value of 1.0. Relative current values for each bar are the mean ±s.e.m. for the number of patches indicated.
2′-O-Me-cAMP interacts cooperatively with ATP to inhibit KATP channels in INS-1 cells
We next sought to determine to what extent 2′-O-Me-cAMP and ATP interact to inhibit KATP channel activity. To this end, the action of 2′-O-Me-cAMP was assessed under conditions in which test solutions contained either no ATP or a fixed concentration of ATP. When patches were exposed to a test solution containing no ATP, 2′-O-Me-cAMP (50 μm) inhibited KATP channel activity, but this action of the ESCA was slow in onset (Fig. 5A). Thus, in the absence of 2′-O-Me-cAMP, the value of NPo measured in an ATP-free solution was 0.18 ± 0.03 and this value decreased to 0.06 ± 0.02 during a 40 s application of 2′-O-Me-cAMP. Subsequently, the value of NPo recovered to 0.13 ± 0.02 after washout of 2′-O-Me-cAMP (mean ±s.e.m., n = 5 patches). It may be concluded that the inhibitory action of 2′-O-Me-cAMP reported here is not strictly ATP-dependent. Despite this fact, when excised patches were exposed to a test solution containing both 2′-O-Me-cAMP and 30 μm ATP, a faster and stronger inhibition of KATP channel activity was measured (Fig. 5B). This interaction of 2′-O-Me-cAMP and ATP to inhibit KATP channels resulted in a statistically significant difference when comparing values of NPo measured after a 10 s application of ATP, or after a 10 s application of ATP in the presence of 2′-O-Me-cAMP (Fig. 5C).
Figure 5. Interaction of ATP and 2′-O-Me-cAMP to inhibit KATP channels in INS-1 cells.
A, KATP channel activity was inhibited by 2′-O-Me-cAMP (50 μm) in the absence of ATP. Note that the inhibition of channel activity developed slowly. Channel activity recovered upon wash out of 2′-O-Me-cAMP. B, in the presence of ATP (30 μm), the inhibitory action of 2′-O-Me-cAMP (50 μm) was faster in onset and of greater magnitude. C, population study summarizing the interaction of ATP and 2′-O-Me-cAMP to decrease values of NPo. For each patch, ATP was tested at a concentration of 0, 1 and 30 μm in the absence or presence of 50 μm 2′-O-Me-cAMP. Values of NPo for each bar in C are the mean ±s.e.m. for 7 patches and were normalized relative to the channel activity measured in an ATP-free solution, which was assigned a value of 1.0.
2′-O-Me-cAMP sensitizes INS-1 cell KATP channels to the inhibitory effect of ATP
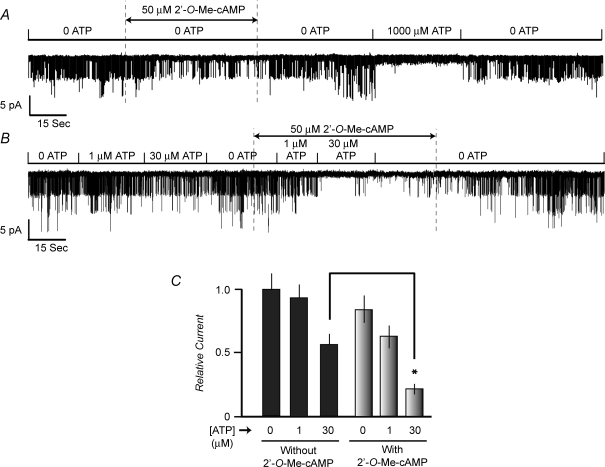
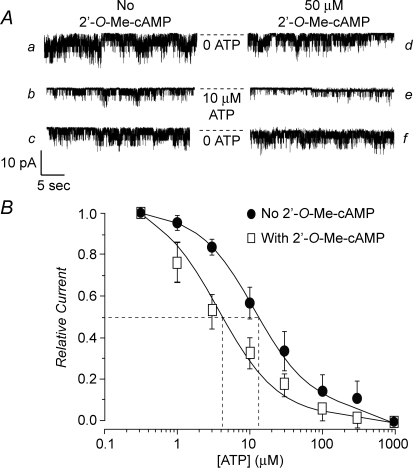
Exposure of excised patches to 2′-O-Me-cAMP produced a left-shift of the dose–response relationship describing the inhibitory effect of ATP at the KATP channels of INS-1 cells (Fig. 6A and B). For this analysis, values of NPo measured at different concentrations of ATP (1–300 μm) were normalized relative to the baseline channel activity measured in the presence of 0.3 μm ATP. Thus, for patches not exposed to 2′-O-Me-cAMP, a relative current value of 1.0 represents the channel activity measured in the presence of 0.3 μm ATP alone. For patches exposed to 2′-O-Me-cAMP, a relative current value of 1.0 represents the channel activity measured in the combined presence of 0.3 μm ATP and 50 μm 2′-O-Me-cAMP. Using this analysis, the concentration of ATP producing a 50% decrease of channel activity (IC50) was determined to be 14 μm in the absence of 2′-O-Me-cAMP (Fig. 6B). Remarkably, the IC50 value for inhibition of KATP channel activity by ATP decreased to 4 μm when patches were exposed to 50 μm 2′-O-Me-cAMP (Fig. 6B).
Figure 6. 2′-O-Me-cAMP increases the ATP sensitivity of INS-1 cell KATP channels.
A, experimental design for establishment of the dose–response relationship describing the interaction of ATP and 2′-O-Me-cAMP to inhibit KATP channels. The KATP channel activity is illustrated for a single excised patch under conditions in which no 2′-O-Me-cAMP was present (traces labelled as a, b and c) or during administration of 50 μm 2′-O-Me-cAMP (right series of traces labelled as d, e and f). Traces a and c as well as d and f illustrate channel activity in an ATP-free solution. Traces b and e illustrate channel activity during exposure of the patch to 10 μm ATP. Each test solution was administered in the order a through e and the duration of exposure to each test solution was 45 s. Note that channel activity was inhibited by ATP in a reversible manner, and that the inhibitory effect of ATP was stronger under conditions in which the patch was also exposed to 2′-O-Me-cAMP. Dashed lines indicate the pipette current corresponding to the closed state of the channels. B, dose–response relationship describing the action of ATP to inhibit KATP channel activity under conditions in which excised patches were not exposed to 2′-O-Me-cAMP (•) or when patches were exposed to 50 μm 2′-O-Me-cAMP (□). • indicate values of NPo normalized relative to a value of 1.0, which represents the relative current measured in the presence of 0.3 μm ATP alone. □ indicate values of NPo normalized relative to a value of 1.0, which represents the relative current measured in the combined presence of 0.3 μm ATP and 50 μm 2′-O-Me-cAMP. Each data point is the mean ±s.e.m. for 5 patches. Dashed lines indicate the method by which the IC50 concentration of ATP was estimated. For patches exposed to 2′-O-Me-cAMP, it was confirmed that similar values of channel activity existed prior to and following washout of the ESCA.
Inhibition of KATP channel activity by 2′-O-Me-cAMP in human pancreatic β-cells
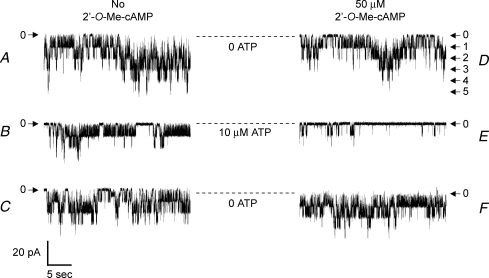
The physiological relevance of Epac-mediated KATP channel regulation was evaluated using human pancreatic β-cells maintained in primary cell culture. β-Cells were identified on the basis of rat insulin 2 gene promoter-directed expression of EYFP (Kang et al. 2003) and KATP channel activity was monitored in excised inside-out patches. Similar to what was observed for INS-1 cells, 2′-O-Me-cAMP exerted a dose-dependent inhibitory action (IC50 12 μm; data not shown) to reduce channel activity under conditions in which the concentration of ATP was maintained at 10 μm. Furthermore, a fixed concentration of 2′-O-Me-cAMP (50 μm) facilitated the inhibitory action of ATP at the channels (Fig. 7). To evaluate the action of 2′-O-Me-cAMP in greater detail, the dose–response relationship describing ATP-dependent inhibition of KATP channel activity was determined in the absence or presence of 2′-O-Me-cAMP. In the absence of 2′-O-Me-cAMP, the concentration of ATP producing a 50% inhibition of channel activity was 21 μm. However, in the presence of 50 μm 2′-O-Me-cAMP, a left-shift of the ATP dose–response relationship was observed, and under these conditions the concentration of ATP producing a 50% inhibition of channel activity was 1 μm (n = 6 patches).
Figure 7. Interaction of ATP and 2′-O-Me-cAMP to inhibit KATP channels in human pancreatic β-cells.
KATP channel activity is illustrated for an excised patch under conditions in which no 2′-O-Me-cAMP was present (traces a, b and c) or during administration of 50 μm 2′-O-Me-cAMP (right series of traces d, e and f). Arrows indicate the current levels at which channels are closed (0) or when one or more channels are open (1–5). Dashed lines indicate the pipette current corresponding to the closed state of the channels. Traces a and c as well as d and f illustrate channel activity in an ATP-free solution. Traces b and e illustrate channel activity during exposure of the patch to 10 μm ATP. Each solution was administered in the order a through e and the duration of exposure to each solution was 45 s. Note that channel activity was inhibited by ATP in a reversible manner, and that the inhibitory effect of ATP was stronger under conditions in which the patch was exposed to 2′-O-Me-cAMP.
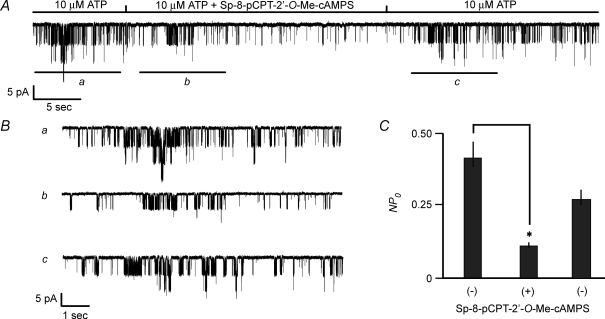
PDE-resistant Sp-8-pCPT-2′-O-Me-cAMPS inhibits KATP channel activity
PDE-catalysed hydrolysis of ESCAs can generate bioactive derivatives of adenosine, substances that alter cellular function independently of Epac (Laxman et al. 2006). Such a confounding effect of cyclic nucleotide metabolism can complicate the interpretation of studies using ESCAs (Holz et al. 2008a). This complication may be circumvented by validating that a PDE-resistant ESCA such as Sp-8-pCPT-2′-O-Me-cAMPS mimics the action of a PDE-sensitive ESCA (Laxman et al. 2006). To validate that the inhibitory action of 2′-O-Me-cAMP reported here did not result from its hydrolysis to bioactive derivatives of adenosine, it was confirmed that Sp-8-pCPT-2′-O-Me-cAMPS inhibited KATP channel activity in INS-1 cells under conditions in which excised patches were exposed to a fixed concentration (10 μm) of ATP (Fig. 8A and B). Values of NPo deduced from an all-points amplitude histogram (not shown) demonstrated that on average, 100 μm Sp-8-pCPT-2′-O-Me-cAMPS decreased KATP channel activity by 72 ± 3% (Fig. 8C).
Figure 8. Inhibition of KATP channels by PDE-resistant Sp-8-pCPT-2′-O-Me-cAMPS.
A, Sp-8-pCPT-2′-O-Me-cAMPS (100 μm) was applied under conditions in which a patch was continuously exposed to 10 μm ATP. B, the same experiment illustrated in A but illustrated on an expanded time scale. Traces a, b and c correspond to the time periods indicated by the horizontal bars in the trace illustrated in A. C, population study summarizing the action of Sp-8-pCPT-2′-O-Me-cAMPS (100 μm) to decrease values of NPo in excised patches exposed to 10 μm ATP. Values of NPo for each histogram bar are the mean ±s.e.m. for 8 patches and were not normalized.
Discussion
Evidence for Epac-mediated regulation of KATP channel activity
Here the novel pharmacological properties of Epac-selective cAMP analogues (ESCAs) have been exploited in order to assess the potential role of cAMP sensor Epac as a determinant of KATP channel activity in human pancreatic β-cells and rat INS-1 cells. Using two structurally related ESCAs, 2′-O-Me-cAMP and Sp-8-pCPT-2′-O-Me-cAMPS, we document an inhibitory effect of ESCAs on the activity of KATP channels at the single channel level. Such findings extend on our prior study of human β-cells and rat INS-1 cells in which the ESCA 8-pCPT-2′-O-Me-cAMP was demonstrated to inhibit a sulphonylurea-sensitive K+ current measured under conditions of whole-cell recording (Kang et al. 2006). The main finding of the present study is that 2′-O-Me-cAMP sensitized single KATP channels to the inhibitory effect of ATP, thereby reducing channel activity. This sensitizing action of the ESCA produced a left-shift of the dose–response relationship describing ATP-dependent inhibition of channel activity so that the concentration of ATP producing a 50% inhibition (IC50) was reduced from approximately 22 μm to 1 μm for human β-cells, and from 14 to 4 μm for INS-1 cells. Conversely, under conditions in which the ATP concentration was held constant at 10 μm, 2′-O-Me-cAMP exerted a dose-dependent action to inhibit KATP channel activity, an effect that was both reversible and repeatable. On the basis of such findings it is concluded that there exists a previously unrecognized form of ion channel modulation in which the ATP sensitivity of β-cell KATP channels is regulated by Epac.
Interpretation of findings obtained using Sp-8-pCPT-2′-O-Me-cAMPS
Findings presented here are the first to demonstrate the efficacy of Sp-8-pCPT-2′-O-Me-cAMPS as an inhibitor of KATP channel function. An assessment of the action of Sp-8-pCPT-2′-O-Me-cAMPS was deemed necessary because in studies of the protozoan Trypanosoma, Laxman et al. (2006) reported that 8-pCPT-2′-O-Me-cAMP (a PDE-sensitive ESCA) exerted an antiproliferative effect not mediated by Epac. Evidently, PDE converts 8-pCPT-2′-O-Me-cAMP to 8-pCPT-2′-O-Me-adenosine, a bioactive metabolite that exerts Epac-independent actions to alter cellular function. Thus, if the PDEs of β-cells or INS-1 cells were to also hydrolyse ESCAs, a PDE-sensitive ESCA such as 2′-O-Me-cAMP might act independently of Epac to inhibit KATP channel function. However, such an Epac-independent mechanism of ESCA action is unlikely to explain the findings presented here. This conclusion is reached because we found that in INS-1 cells, PDE-resistant Sp-8-pCPT-2′-O-Me-cAMPS inhibited KATP channel activity in a manner analogous to that of 2′-O-Me-cAMP.
Laxman et al. (2006) also reported the surprising finding that the PDEs of Trypanosoma are inhibited by 8-pCPT-2′-O-Me-cAMP. Thus, if ESCAs were to inhibit the PDEs expressed in β-cells or INS-1 cells, the net effect might be increased levels of cAMP, activation of PKA, and the Epac-independent inhibition of KATP channel function. This confounding scenario is unlikely for three reasons. First, Rp-cAMPS, a selective inhibitor of PKA activation, failed to antagonize the inhibitory action of 2′-O-Me-cAMP at the KATP channels of INS-1 cells. Secondly, 6-Bnz-cAMP, a selective activator of PKA, failed to reproduce the action of 2′-O-Me-cAMP, and in fact 6-Bnz-cAMP stimulated rather than inhibited KATP channel function. Third, in our prior study (Kang et al. 2006) we reported that 8-pCPT-2′-O-Me-cAMP failed to inhibit the macroscopic KATP current measured under conditions in which INS-1 cells were transfected with a dominant negative Epac1 that fails to bind cAMP. Thus, available evidence indicates that it is Epac rather than PKA that mediates the inhibitory action of ESCAs at KATP channels.
A potential mechanistic explanation for KATP channel regulation by Epac
The exact molecular mechanism by which cAMP, acting through Epac, alters KATP channel ATP sensitivity remains to be determined. Epac is a cAMP-GEF, and it activates the Ras-related GTPases Rap1 and Rap2, thereby influencing multiple cellular functions (reviewed by Bos, 2003, 2006; Holz, 2004a, 2006, 2008a). Notably, Epac is reported to act via Rap GTPase to stimulate a novel membrane-associated phospholipase C-ɛ that is expressed in INS-1 cells and which was first discovered by Kelley and coworkers (Kelley et al. 2001; G. G. Kelley, personal communication). Phospholipase C-ɛ catalyses the breakdown of polyphosphoinositides and it has been implicated in the cAMP-dependent regulation of intracellular Ca2+ handling in cardiac myocytes (Oestreich et al. 2007; Pereira et al. 2007) and in mammalian cell lines (Schmidt et al. 2001; Evellin et al. 2002). Given the established role of phosphatidylinositol bisphosphate (PIP2) as a regulator of KATP channel function (Nichols, 2006), and given that PIP2 supports KATP channel activity at least in part by reducing the channel's sensitivity to ATP (Baukrowitz et al. 1998; Shyng & Nichols, 1998), a cAMP signalling mechanism that uses Epac, Rap, and phospholipase C-ɛ to reduce levels of PIP2 in the plasma membrane would be expected to increase the sensitivity of KATP channels to ATP. Thus, the SUR1 subunit of KATP channels might be viewed as a scaffold protein at which a signalling complex comprising Rap and phospholipase C-ɛ forms as a consequence of the interaction of Epac with NBF-1 of SUR1. It will be of particular interest to ascertain in future studies to what extent the targeted gene deletion of phospholipase C-ɛ alters cAMP signalling and KATP channel function in β-cells.
Physiological significance of Epac-mediated KATP channel regulation
Numerous reports have recently appeared in which Epac is proposed to mediate stimulatory actions of cAMP on insulin secretion from β-cells (Seino & Shibasaki, 2005; Holz et al. 2006; Shibasaki et al. 2007). This Epac-mediated action of cAMP does not exist in isolation, but is instead complemented by a stimulatory effect of cAMP that is mediated by PKA (Holz, 2004a). Importantly, both the Epac and PKA-mediated pro-secretagogue actions of cAMP are known to be contingent on exposure of β-cells to elevated concentrations of glucose. This observation has led to the conclusion that cAMP exerts its secretagogue action by potentiating glucose-stimulated insulin secretion (GSIS). From a theoretical standpoint, cAMP could potentiate GSIS at either a late or an early step in stimulus–secretion coupling. In fact, a late step does exist in which cAMP, acting through Epac and PKA, facilitates the fusion of secretory granules with the plasma membrane (Renstrom et al. 1997; Takahashi et al. 1999; Kashima et al. 2001; Fujimoto et al. 2002; Eliasson et al. 2003; Hatakeyama et al. 2006, 2007). In marked contrast, evidence also exists that cAMP potentiates GSIS at an earlier step, one that involves the regulated closure of KATP channels. In one proposed model, cAMP acts through Epac and PKA to render KATP channels more sensitive to the increase of cytosolic ATP/ADP concentration ratio that occurs when β-cells are exposed to high levels of glucose (Holz, 2004b). This effect is achieved because cAMP promotes KATP channel closure by modulating the ATP and/or ADP sensitivity of KATP channels. The model is supported by published findings demonstrating inhibitory actions of cAMP-elevating agents at KATP channels, effects that are reinforced by glucose metabolism, and which are either ATP or ADP dependent (Holz & Habener, 1992; Holz et al. 1993; Barnett et al. 1994; Gromada et al. 1998; He et al. 1998; Suga et al. 2000; Ding et al. 2001; Light et al. 2002; Kang et al. 2006).
With these points in mind, the primary physiological significance of the findings presented here is that an Epac-mediated mechanism is shown to exist by which cAMP regulates the ATP sensitivity of KATP channels in human β-cells and rat INS-1 cells. Whether such findings are applicable to rat or mouse β-cells remains to be determined. This might be the case because in a prior study of rat β-cells, Suga et al. (2000) found that the cAMP-elevating hormone glucagon-like peptide-1 (GLP-1) increased the sensitivity of single KATP channels to ATP, an effect analogous to that reported here for 2′-O-Me-cAMP. This action of GLP-1, which resulted in a decrease of membrane conductance accompanied by membrane depolarization, was not blocked by Rp-cAMPS, as expected if it is Epac rather than PKA that regulates KATP channel ATP sensitivity.
In the study of Suga and coworkers the IC50 value for inhibition of KATP channel activity by ATP was 11.6 μm in the absence of GLP-1, and 5.6 μm in the presence of GLP-1 (Suga et al. 2000). Thus, in the presence of GLP-1, the IC50 value describing the inhibitory action of ATP at rat β-cell KATP channels is nearly identical to the IC50 value (4 μm) we measured under conditions in which the KATP channels of rat INS-1 cells were exposed to 2′-O-Me-cAMP. Although these IC50 concentrations of ATP are well below the millimolar concentrations of ATP known to exist in the cytosol of intact cells, it is noteworthy that our findings, as well as those of Suga and coworkers, were obtained under conditions in which the inner surfaces of excised patches were exposed to a bath solution to which no Mg-ADP was added. Since Mg-ADP stimulates KATP channel function, and since Mg-ADP is present in the cytosol of intact cells, it might be that in the presence of Mg-ADP, agents such as GLP-1 or 2′-O-Me-cAMP increase KATP channel ATP sensitivity at concentrations of ATP that are within the millimolar range and which are considered to be physiological.
Although not examined here, cAMP-elevating agents may also regulate the ADP sensitivity of KATP channels. In a mammalian cell line expressing recombinant Kir6.2 and SUR1, Light and coworkers reported that application of the catalytic subunit of PKA (cPKA) to inside-out patches bathed in a low concentration of Mg-ADP (0.2 mm) resulted in a decrease of KATP channel activity (Light et al. 2002). In that study, the ATP sensitivity of KATP channels was not affected by cPKA, whereas the stimulatory effect of 0.2 mm Mg-ADP at the channel was diminished by cPKA. It was suggested that cAMP-elevating agents such as GLP-1 close KATP channels by reducing the channel's sensitivity to Mg-ADP. Taken together, the findings of Light and coworkers, as well as the findings presented here, provide evidence for an action of cAMP to decrease KATP channel activation by ADP while simultaneously increasing the channel's inhibition by ATP. Such an effect would be expected to render KATP channels highly sensitive to the increase of cytosolic ATP/ADP concentration ratio that occurs upon exposure of β-cells to elevated levels of glucose. In this manner, GLP-1 and glucose metabolism would interact to promote membrane depolarization and Ca2+ influx, two key events in β-cell stimulus–secretion coupling (Holz et al. 2008b).
Conclusion
The emerging role of the cAMP sensor Epac as a regulator of ion channel function is an unexpected and exciting development. The potential significance of this newly recognized mechanism of cAMP signal transduction is emphasized by the fact that Epac influences not only KATP channel function, as reported here, but also the function of voltage-dependent Ca2+ channels, Cl− channels, Na+ channels, and Ca2+-dependent K+ channels in a variety of cell types (Novara et al. 2004; Aromataris et al. 2006; Helms et al. 2006; Ster et al. 2007). There is also evidence for the Epac-mediated regulation of intracellular Ca2+ release channels, including IP3 receptors and ryanodine receptors (Kang et al. 2001, 2003, 2005; Schmidt et al. 2001; Tsuboi et al. 2003; Morel et al. 2005; Yip, 2006; Oestreich et al. 2007; Pereira et al. 2007). Thus, it would seem that a new paradigm of ion channel modulation has been established in which the second messenger cAMP exerts its effects via Epac.
Acknowledgments
The authors acknowledges the support of the NIH (R01 DK045817 and R01 DK069575 to G.G.H.; R01 HL064838 to W.A.C.) and the American Diabetes Association (Research Award to C.A.L.).
References
- Aromataris EC, Roberts ML, Barritt GJ, Rychkov GY. Glucagon activates Ca2+ and Cl− channels in rat hepatocytes. J Physiol. 2006;573:611–625. doi: 10.1113/jphysiol.2006.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett DW, Pressel DM, Chern HT, Scharp DW, Misler S. cAMP-enhancing agents ‘permit’ stimulus-secretion coupling in canine pancreatic islet β-cells. J Membr Biol. 1994;138:113–120. doi: 10.1007/BF00232639. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bryan J, Munoz A, Zhang X, Dufer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007;453:703–718. doi: 10.1007/s00424-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Chepurny OG, Holz GG. A novel cyclic adenosine monophosphate responsive luciferase reporter incorporating a nonpalindromic cyclic adenosine monophosphate response element provides optimal performance for use in G protein coupled receptor drug discovery efforts. J Biomolec Screen. 2007;12:740–746. doi: 10.1177/1087057107301856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP-kinase. Discriminating analogs demonstrate that Epac and cAMP-kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Ding WG, Kitasato H, Matsuura H. Involvement of calmodulin in glucagon-like peptide-1-(7–36)-amide induced inhibition of the ATP-sensitive K+ channel in mouse pancreatic β-cells. Exp Physiol. 2001;86:331–339. doi: 10.1113/eph8602173. [DOI] [PubMed] [Google Scholar]
- Dostmann WRG, Taylor S, Genieser H-G, Jastorff B, Døskeland SO, Øgreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinase I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem. 1990;265:10484–10491. [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, Triest MV, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. A novel Epac-selective cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Evellin S, Nolte J, Tysack K, vom Dorp F, Thiel M, Weernink PA, Jakobs KH, Webb EJ, Lomasney JW, Schmidt M. Stimulation of phospholipase C-ε by the M3 muscarinic acethycholine receptor mediated by cyclic AMP and the GTPase Rap2B. J Biol Chem. 2002;277:16805–16813. doi: 10.1074/jbc.M112024200. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic β-cells. Involvement of cAMP-GEFII·Rim2·Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide-1 stimulates exocytosis in human pancreatic beta cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998;47:57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–282. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse β-cells. J Physiol. 2007;582:1087–1098. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LP, Mears D, Atwater I, Kitasato H. Glucagon induces suppression of ATP-sensitive K+ channel activity through a Ca2+/calmodulin-dependent pathway in mouse pancreatic β-cells. J Membr Biol. 1998;166:237–244. doi: 10.1007/s002329900465. [DOI] [PubMed] [Google Scholar]
- Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L710–L722. doi: 10.1152/ajplung.00486.2004. [DOI] [PubMed] [Google Scholar]
- Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004a;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic β-cells. Horm Metab Res. 2004b;36:787–794. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: New tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cellular Signal. 2008b;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic β-cells and the glucose competence concept. Trends Biochem Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Heart E, Leech CA. Synchronizing Ca2+ and cAMP oscillations in pancreatic β-cells: a role for glucose metabolism and GLP-1 receptors? Am J Physiol Cell Physiol. 2008a;294:C4–C6. doi: 10.1152/ajpcell.00522.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kuhtreiber WM, Habener JF. Pancreatic β-cells are rendered glucose competent by the insulinotropic hormone glucagon-like peptide-1-(7–37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor-II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β-cells. J Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J Physiol. 2006;573:595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII·Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C-ε: a novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxman S, Riechers A, Sadilek M, Schwede F, Beavo JA. Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc Natl Acad Sci U S A. 2006;103:19194–19199. doi: 10.1073/pnas.0608971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Holz GG, Chepurny O, Habener JF. Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic β-cells. Biochem Biophys Res Commun. 2000;278:44–47. doi: 10.1006/bbrc.2000.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol. 2002;16:2135–2144. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompre AM, Vandecasteele G, Lezoualc'h F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- Moritz W, Leech CA, Habener JF. Regulated expression of adenosine triphosphate-sensitive potassium channel subunits in pancreatic β-cells. Endocrinology. 2001;142:129–138. doi: 10.1210/endo.142.1.7885. [DOI] [PubMed] [Google Scholar]
- Nichols CG. K-ATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Novara M, Baldelli P, Cavallari D, Carabelli V, Giancippoli A, Carbone E. Exposure to cAMP and β-adrenergic stimulation recruits CaV3 T-type channels in rat chromaffin cells through Epac cAMP-receptor proteins. J Physiol. 2004;558:433–449. doi: 10.1113/jphysiol.2004.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C-ε plays a critical role in β-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Benitah JP, Lezoualc'h G, Gomez AM. Epac modulates Ca2+ spark by Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C–calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biolol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004a;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004b;53:S59–S62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Ster J, De Bock F, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, Bockaert J, Fagni L. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci U S A. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S, Kanno T, Ogawa Y, Takeo T, Kamimura N, Wakui M. cAMP-independent decrease of ATP-sensitive K+ channel activity by GLP-1 in rat pancreatic β-cells. Pflugers Arch. 2000;440:566–572. doi: 10.1007/s004240000279. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic β-cells. Proc Natl Acad Sci U S A. 1999;96:760–765. doi: 10.1073/pnas.96.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 β-cells. Biochem J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KP. Epac mediated Ca2+ mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291:F882–F890. doi: 10.1152/ajprenal.00411.2005. [DOI] [PubMed] [Google Scholar]