Abstract
This report considers serotonergic (5-HT) effects on spinal motoneurons, reviewing previous data and presenting a new study showing distinct effects of two 5-HT receptor subtypes. We previously investigated the effects of 5-HT on motoneurons in a slice preparation from the spinal cord of the adult turtle. In agreement with previous studies, we had found that 5-HT applied to the extracellular medium promoted a voltage sensitive plateau potential. However, we also reported that this effect was only observed in half of the motoneurons; 5-HT inhibited the firing of the other half of the motoneurons recorded from. To investigate the reasons for this, we applied 5-HT focally by means of the microiontophoresis technique. Facilitation of plateau potentials was observed when 5-HT was released at sites throughout the somatodendritic region. However, motoneurons were inhibited by 5-HT when selectively applied in the perisomatic region. These two effects could be induced in the same motoneuron. With pharmacological tools, we demonstrate here that the facilitation of plateau potentials is mediated by 5-HT2 receptors and the inhibitory effect is due to the activation of 5-HT1A/7 receptors.
Review and introduction
Serotonin (5-HT) is one of the main neuromodulators in the central nervous system. In the adult spinal cord, 5-HT primarily originates from neurons belonging to the raphe spinal pathway. 5-HT is released on different types of neurons, and these include motoneurons, suggesting a role in motor function. Modulation of motoneurons by 5-HT has been widely studied and it is commonly agreed that it induces a depolarization associated with an increase in input resistance (Vandermaelen & Aghajanian, 1980; White & Fung, 1989; Wang & Dun, 1990; Elliott & Wallis, 1992; Hsiao et al. 1997). This effect has been ascribed to inhibition of a potassium leak conductance (VanderMaelen & Aghajanian, 1980; Wang & Dun, 1990; Elliott & Wallis, 1992; Hsiao et al. 1997; Perrier et al. 2003), facilitation of an inward rectifying conductance (Takahashi & Berger, 1990; Hsiao et al. 1997) or facilitation of a low threshold calcium current (Berger & Takahashi, 1990). 5-HT also increases the excitability of motoneurons by inhibiting the medium afterhyperpolarization (mAHP) following action potentials (Hounsgaard et al. 1988a; White & Fung, 1989; Berger et al. 1992; Bayliss et al. 1995; Wikstrom et al. 1995; Hsiao et al. 1997; Grunnet et al. 2004), by decreasing the threshold for sodium action potentials (Fedirchuk & Dai, 2004) and by facilitating a plateau potential either mediated by CaV1.3 calcium channels (Hounsgaard & Kiehn, 1989; Simon et al. 2003) or by a persistent sodium current (Harvey et al. 2006). However, in addition to these excitatory effects, a few studies also notes that 5-HT induces a hyperpolarization of motoneurons (Phillis et al. 1968; Holohean et al. 1990; Zhang, 1991) associated with a decrease in input resistance (Wang & Dun, 1990). This discrepancy suggested that 5-HT either regulates the firing of distinct motoneurons differently (Schmidt & Jordan, 2000) or that it differentially modulates different compartments of the same motoneurons.
To investigate this, we tested the effects of 5-HT on a slice preparation made from the lumbar enlargement of the spinal cord of the adult turtle (Chrysemys scripta elegans). We monitored the excitability of motoneurons by injecting intracellular depolarizing current pulses, and to discard possible presynaptic effects, we blocked fast synaptic potentials. Figure 1, adapted from Perrier & Hounsgaard (2003), summarizes the effects of 5-HT. In agreement with previous studies (Hounsgaard & Kiehn, 1989; Delgado-Lezama et al. 1997), we found that 5-HT promoted a voltage sensitive plateau potential (Fig. 1Ab). However, this result was only obtained in 55% (12/22) of the motoneurons tested. In the remaining 45% (10/22), 5-HT had a powerful inhibitory effect that could be sufficient to annihilate a plateau present in control conditions (Fig. 1Ac). To find out if these two different effects could occur on the same motoneurons, we selectively depolarized the lateral dendrites of the motoneurons by means of an electric field applied through the slice (Hounsgaard & Kiehn, 1993; Delgado-Lezama et al. 1999). Under these conditions, 5-HT added to the bath promoted a plateau potential in all motoneurons tested (14/14; Perrier & Hounsgaard, 2003). These results suggested that the serotonergic receptors located on the lateral dendrites selectively promote plateau potentials.
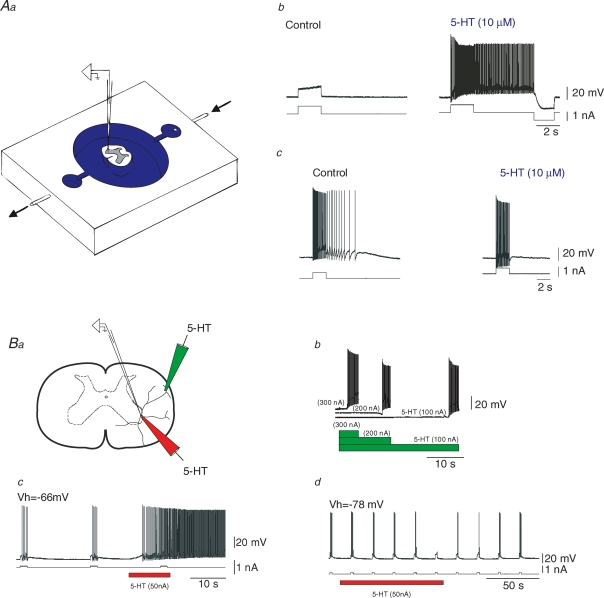
Figure 1. Serotonin induces heterogeneous effects on motoneurons.
Aa, scheme of the set-up. A slice in a continuously perfused recording chamber (arrows). Drugs were applied to the extracellular medium. Intracellular recordings from motoneurons. b and c, responses to depolarizing current pulses in 2 motoneurons. b, extracellular addition of 5-HT promoted a plateau potential characterized by a bistable firing that was abolished by a hyperpolarizing current pulse. c, another motoneuron had plateau properties characterized by an afterdischarge in control condition. Addition of serotonin inhibited the firing of the motoneuron. Ba, scheme of the iontophoresis set-up. A glass pipette filled with serotonin was either positioned close to a distal dendrite or close to the soma of the recorded cell. b, release of 5-HT close to a dendrite promoted a plateau potential with a latency that decrease with the amount of serotonin released. Traces separated for the clarity of the figure. c and d, the excitability of the motoneuron was tested by injecting depolarizing current pulses. c, release of 5-HT close to the soma promoted a plateau potential. d, when the motoneuron was hyperpolarized by means of a negative bias current, release of 5-HT at same intensity and same position as in Bc had an inhibitory effect characterized by a gradual decrease in the number of action potentials generated by the current pulse. All the recordings in B are from the same motoneuron. Fast synaptic potentials were blocked by a mixture of CNQX, AP5, Bicuculline and strychnine. Figure adapted from Perrier & Hounsgaard (2003); used with permission of the American Physiological Society.
To investigate the spatial segregation of the effects induced by 5-HT further, we used the microiontophoresis technique (Fig. 1Ba). We positioned a glass pipette filled with 5-HT, either close to the distal dendrite or close to the soma of a recorded motoneuron. 5-HT was released by passing a current through the pipette. When released close to a distal dendrite, 5-HT always induced a plateau potential (n = 5; Figs 1 and 2). When released close to the cell body, 5-HT could also facilitate a plateau potential (n = 10; Fig. 1Bc). However, when the plateau potential was inhibited by hyperpolarizing the neuron with a negative bias current, the release of serotonin induced an inhibitory effect sufficient to prevent the firing of the motoneuron (n = 15; Fig. 1Bd). This suggests that 5-HT receptors located in different cellular compartments of the same motoneuron are coupled to distinct functional pathways. The 5-HT receptors expressed in the dendritic tree of motoneurons specifically facilitate plateau potentials while the 5-HT receptors present in the perisomatic region facilitate plateau potentials and have an inhibitory effect.
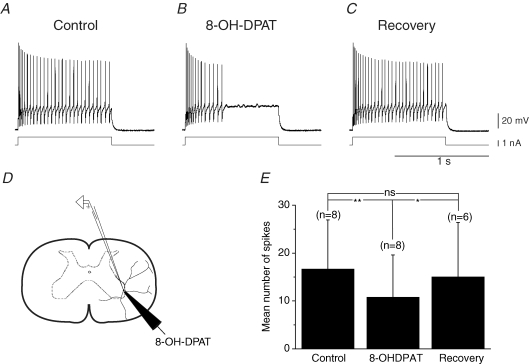
Figure 2. 5-HT1A receptors located close to the soma inhibit the firing of motoneurons.
A, a depolarizing current pulse generated a train of 28 action potentials. B, when 8-OH-DPAT was iontophoresed close to the cell body, the discharge (14 spikes) stopped shortly after the beginning of the current pulse. C, the firing properties were recovered after stopping the iontophoresis (30 spikes). D, scheme of the set-up. E, synthesis of the results. 8-OH-DPAT significantly decreased the number of action potentials generated by depolarizing current pulses; control: 16.6 ± 10.3 s.d.; iontophoresis: 10.7 ± 8.8; recovery: 15.0 ± 11.4; significant difference between control and iontophoresis (P = 0.02; paired t test; n = 8); significant difference between iontophoresis and recovery (P = 0.08; paired t test; n = 6); non-significant difference between control and recovery (P = 0.52; paired t test; n = 6).
We made two series of experiments that suggest that the serotonin receptors responsible for facilitation of plateau potentials are the 5-HT2 subtype. First of all, addition of the 5-HT2 receptor agonist (±)-1-[2,5]-dimethoxy-4-iodophenyl-2-aminopropane (DOI; 10 μm) to the extracellular medium, promoted a plateau potential in all motoneurons tested (n = 5/5; Perrier & Hounsgaard, 2003). Second, we induced synaptic release of 5-HT by applying an electrical stimulation on the raphé spinal pathway. This promoted a plateau potential that disappeared in the presence of the 5-HT2 receptor antagonist SB-206553 hydrochloride (10 μm; Perrier & Delgado-Lezama, 2005).
In the study reported here, we investigate the reasons why 5-HT can induce an excitatory or an inhibitory effect on the same motoneurons, depending on the experimental conditions. We show that the different effects induced by 5-HT are mediated by different receptor subtypes that are located on different compartments of the motoneuron.
Methods
Experiments were performed on a slice preparation from the lumbar enlargement of the adult turtle (Chrysemys scripta elegans). After intraperitoneal injection of 100 mg sodium pentobarbitone, turtles were killed by decapitation. The surgical procedures comply with the Danish legislation and are approved by the controlling body under The Ministry of Justice. The methods are summarized here. More detail can be found in Perrier & Hounsgaard (2003).
Slices were perfused in a solution containing (mm): 120 NaCl; 5 KCl; 15 NaHCO3; 2 MgCl2; 3 CaCl2 and 20 glucose saturated with 98% O2–2% CO2 to obtain pH 7.6. Intracellular recordings in current clamp and voltage clamp mode were performed using pipettes filled with 1 m potassium acetate or a mixture of 0.9 m potassium acetate 0.1 m KCl. Motoneurons were selected for study if they had a stable membrane potential of more than −60 mV. Fast synaptic inputs mediated by glutamate, GABA and glycine were eliminated by a mixture of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 25 μm; Tocris), dl-2-amino-5-phosphonopentanoic acid (dl-AP5, 50 μm; Tocris) or d(−)-2-amino-7-phosphono-heptanoic acid (AP7, 25 μm; Tocris), (+)-bicuculline (20 μm; Tocris) and strychnine (10 μm) added to the extracellular medium.
For these microiontophoresis experiments, we used micropipettes filled with 150 mm serotonin hydrochloride (pH 4–4.5) or 40 mm 8-OH-DPAT (pH 4–4.5). The pH value was chosen so that the drugs were ejected by a positive current. Diffusion from the pipette was minimized by applying a constant holding current of −40 nA. Data were analysed statistically by using Student's t-test for two populations (paired or independent) (Origin software, OriginLab Corp., Northampton, MA, USA). Significance was accepted when P < 0.05. Data are presented as means ± standard error of the mean.
Results
Since 5-HT1a receptors have been reported in spinal motoneurons (Takahashi & Berger, 1990; Talley et al. 1997) we tested if they are responsible for the inhibitory effect induced by 5-HT. When we added 8-OH-DPAT (a 5-HT1a/7 receptor agonist) to the bath, most motoneurons responded by a depolarization associated with an increase in input resistance (n = 9/11). This effect was mediated by the inhibition of a TASK-1-like K+ leak current (Perrier et al. 2003). In a smaller fraction of motoneurons (2/11), however, 8-OH-DPAT had a hyperpolarizing effect concomitant with a decrease in input resistance (Perrier et al. 2003). Since the inhibitory effect induced by 5-HT is restricted to an area located close to the cell body of motoneurons, we used the iontophoresis technique to apply serotonergic agonist directly to the perisomatic region. We found that release of 8-OH-DPAT (40 mm) close to the cell body produced a powerful inhibitory effect. We assessed the excitability of motoneurons by injecting depolarizing current pulses lasting 1 s.
Figure 2A shows an example in which the positive pulse applied in control conditions induced a repetitive firing throughout the whole duration of the depolarization. When 8-OH-DPAT was released close to the cell body, the motoneuron still fired action potentials at the beginning of the current pulse. However, after a few hundreds of milliseconds, the motoneuron stopped to produce action potentials (Fig. 2B). The effect was fully recovered a few seconds after stopping the iontophoresis (Fig. 2C). We tested the effect of 8-OH-DPAT applied to the perisomatic region for eight motoneurons (Fig. 2E). This experiment suggests that 5-HT1A/7 receptors, located in the perisomatic region of motoneurons are responsible for the inhibitory effect induced by 5-HT.
Discussion and conclusions
In agreement with previous studies, we found that 5-HT applied to the extracellular medium can exert either excitatory or inhibitory effects on different motoneurons (see Schmidt & Jordan, 2000 for review). However, when we tested the effects of 5-HT with other techniques such as electrical field stimulation or microiontophoresis, we realized that both effects occur in the same motoneurons, but in different compartments. 5-HT promotes plateau potentials by acting on 5-HT2 receptors expressed in the somato-dendritic membrane while an inhibitory effect of 5HT is restricted to the perisomatic region and mediated by 5-HT1A/7 receptors.
Identity of the inhibitory pathway mediated by 5-HT
We found that the inhibitory effect of 5-HT can be mimicked by iontophoresis of 8-OH-DPAT close to the cell body of motoneurons. This compound is an agonist for the 5-HT1A receptors, but also, to a lesser degree, for the 5-HT7 receptors (Markstein et al. 1999). Expression of 5-HT7 receptors has not been reported in motoneurons. On the other hand, low levels of 5-HT1A receptor mRNA is present in hypoglossal motoneurons in adult rats (Talley et al. 1997. Moreover, using a 5-HT1A receptor antibody, Kheck et al. (1995) demonstrated the presence of 5-HT1A receptors on the axon hillock of cervical motoneurons of primates. It is therefore tempting to speculate that the inhibitory effect of 5-HT is induced by 5-HT1A receptors present on the initial segment of motoneurons.
Recently, Deng et al. (2007) showed that 5-HT inhibits the firing of neurons from the entorhinal cortex of the rat by facilitating a leak current mediated by K+ ions. 5-HT1A receptors specifically activate the TWIK-1 type of the two-pore domain K+ channels. This intracellular pathway is a good candidate for the inhibitory effect observed in motoneurons. Conversely, Pan et al. (2006) reported that the KCNQ2/3 ion channels are specifically expressed on the axon hillock of motoneurons of the mouse. These channels are responsible for the M-current expressed by motoneurons (Alaburda et al. 2002). The matching expressions of 5-HT1A receptors and KCNQ2/3 ion channels is also an interesting alternative. A third option could be the inhibition of sodium channels expressed on the axon hillock. We are currently performing experiments to test these possibilities.
Methodological considerations
Studying the effects of serotonin on a particular type of neuron is not as easy as it might appear. When 5-HT is added to the extracellular medium, it activates all the 5-HT receptors present in the different compartments of the neurons and coupled to different intracellular pathways. The resulting effect may be different from what occurs under physiological conditions where 5-HT is released from synapses. It is therefore essential to combine different techniques such as pharmacology, electric field stimulation, microiontophoresis and finally to test the effect of 5-HT released from synapses.
Concluding remarks
Spinal cord injury is characterized by an initial hypotonia lasting several weeks, and followed by a long lasting period of uncontrolled spasms and spasticity. Experiments performed in acute spinal animals suggest that the initial spinal shock is initiated by the down-regulation of plateau potentials in motoneurons caused by the absence of 5-HT. First, an increase in the level of 5-HT in chronic spinal animals restores plateau potentials in motoneurons (Hounsgaard et al. 1988b). Second, a selective activation of 5-HT2 receptors brings back the excitability of extensor motoneurons (Miller et al. 1996) and weight bearing ability (Kim et al. 2001). These observations suggest that, under physiological conditions, the facilitation of plateau potentials induced by the activation of 5-HT2 receptors is critical for the tonus of antigravity muscles. This regulatory mechanism must be finely tuned otherwise it may lead to debilitating spasms and spasticity (Li et al. 2004). In the central nervous system, 5-HT1A receptors have been reported at extrasynaptic and non-synaptic sites (Riad et al. 2000). Our current hypothesis is that the inhibitory effect induced by perisomatic 5-HT1A receptors is triggered by a spillover of 5-HT and acts as a safety mechanism preventing the hyperexcitability of motoneurons.
Acknowledgments
This work was kindly funded by the Lundbeck Foundation, the Novo Nordisk Foundation, the Owensenske Fond, the Foundation Agnes and Poul Friis, the Institut pour la Recherche sur la Moelle épinière et l'Encéphale and the Danish Medical Research Council (Forskningsrådet for Sundhed og Sygdom).
References
- Alaburda A, Perrier JF, Hounsgaard J. An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol. 2002;540:875–881. doi: 10.1113/jphysiol.2001.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Umemiya M, Berger AJ. Inhibition of N- and P-type calcium currents and the after-hyperpolarization in rat motoneurones by serotonin. J Physiol. 1995;485:635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Hounsgaard J. Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. J Physiol. 1999;515:203–207. doi: 10.1111/j.1469-7793.1999.203ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain K+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72:208–218. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and 1-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–361. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Perrier JF. 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J Neurosci Res. 2004;78:845–854. doi: 10.1002/jnr.20318. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC, Davidoff RA. Changes in membrane potential of frog motoneurons induced by activation of serotonin receptor subtypes. Neuroscience. 1990;34:555–564. doi: 10.1016/0306-4522(90)90164-y. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988b;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988a;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Kheck NM, Gannon PJ, Azmitia EC. 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J Comp Neurol. 1995;355:211–220. doi: 10.1002/cne.903550205. [DOI] [PubMed] [Google Scholar]
- Kim D, Murray M, Simansky KJ. The serotonergic 5-HT2C agonist m-chlorophenylpiperazine increases weight-supported locomotion without development of tolerance in rats with spinal transections. Exp Neurol. 2001;169:496–500. doi: 10.1006/exnr.2001.7660. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Markstein R, Matsumoto M, Kohler C, Togashi H, Yoshioka M, Hoyer D. Pharmacological characterisation of 5-HT receptors positively coupled to adenylyl cyclase in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:454–459. doi: 10.1007/pl00005375. [DOI] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–628. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol. 2003;548:485–492. doi: 10.1113/jphysiol.2002.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci. 2005;25:7993–7999. doi: 10.1523/JNEUROSCI.1957-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Tebecis AK, York DH. Depression of spinal motoneurones by noradrenaline, 5-hydroxytryptamine and histamine. Eur J Pharmacol. 1968;4:471–475. doi: 10.1016/0014-2999(68)90037-x. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–266. doi: 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980;287:346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res. 1989;502:205–213. doi: 10.1016/0006-8993(89)90615-x. [DOI] [PubMed] [Google Scholar]
- Wikstrom M, Hill R, Hellgren J, Grillner S. The action of 5-HT on calcium-dependent potassium channels and on the spinal locomotor network in lamprey is mediated by 5-HT1A-like receptors. Brain Res. 1995;678:191–199. doi: 10.1016/0006-8993(95)00183-q. [DOI] [PubMed] [Google Scholar]
- Zhang L. Effects of 5-hydroxytryptamine on cat spinal motoneurons. Can J Physiol Pharmacol. 1991;69:154–163. doi: 10.1139/y91-022. [DOI] [PubMed] [Google Scholar]