Abstract
Mechanisms of regulatory cell volume increase following cell shrinkage include accumulation of organic osmolytes such as betaine, taurine, sorbitol, glycerophosphorylcholine (GPC) and myo-inositol. Myo-inositol is taken up by the sodium-myo-inositol-transporter SMIT1 (SLC5A3) expressed in a wide variety of cell types. Hypertonicity induces the transcription of the SMIT1 gene upon binding of the transcription factor tonicity enhancer binding protein (TonEBP) to tonicity responsive enhancers (TonE) in the SMIT1 promoter region. However, little is known about post-translational regulation of the carrier protein. In this study we show that SMIT1 is modulated by the serum- and glucocorticoid-inducible kinase SGK1, a protein genomically up-regulated by hypertonicity. As demonstrated by two-electrode voltage-clamp in the Xenopus oocyte expression system, SMIT1-mediated myo-inositol-induced currents are up-regulated by coexpression of wild type SGK1 and constitutively active S422DSGK1 but not by inactive K127NSGK1. The increase in SMIT1 activity is due to an elevated cell surface expression of the carrier while its kinetic properties remain unaffected. According to the decay of SMIT1 activity in the presence of brefeldin A, SGK1 stabilizes the SMIT1 protein in the plasma membrane. The SGK isoforms SGK2, SGK3 and the closely related protein kinase B (PKB) are similarly capable of activating SMIT1 activity. SMIT1-mediated currents are decreased by coexpression of the ubiquitin-ligase Nedd4-2, an effect counteracted by additional coexpression of SGK1. In conclusion, the present observations disclose SGK isoforms and protein kinase B as novel regulators of SMIT1 activity.
Cell volume constancy and cytosolic ion homoeostasis are prerequisites for proper cell function. They are challenged by exposure to extracellular anisotonicity or by alterations of cytosolic osmolarity (Lang et al. 1991, 1998). Rapid equilibration of osmotic gradients serves to drive water into or out of the cell thereby re-establishing initial cell shape and volume (Macknight, 1988; Macknight et al. 1994; Waldegger et al. 1998b). Regulatory volume decrease (RVD) after cell swelling and regulatory volume increase (RVI) after cell shrinkage are achieved acutely by a variety of membrane transport systems and ion channels importing or exporting primarily small ions including sodium, potassium and chloride (Waldegger et al. 1998b; Lang et al. 1998).
Cell volume regulatory uptake of inorganic ions during cell shrinkage may compromise protein stability and affect neuronal electrical excitability (Somero, 1986). To avoid untoward effects of cell volume regulation with inorganic ions, cells additionally make use of non-pertubing uncharged osmolytes (Yancey et al. 1982; Somero, 1986). These include betaine, taurine, sorbitol, glycerophosphorylcholine (GPC) and myo-inositol that passively diffuse through selective ion channels and/or are cotransported by specific membrane carriers (Lang et al. 1991).
Transport of myo-inositol across the outer cell membrane is facilitated by the sodium myo-inositol transporter SMIT1 (SLC5A3) (Berry et al. 1995). SMIT1 is expressed in various tissues investigated and couples concentrative myo-inositol uptake to the sodium gradient (Kwon et al. 1992; Hager et al. 1995). At hypertonicity, SMIT1 expression is rapidly stimulated leading to increased myo-inositol uptake from the extracellular space (Kwon et al. 1992). The significance of SMIT1 in cell protection is illustrated by the observation that pharmacological SMIT1 inhibition leads to severe renal failure in rats (Kitamura et al. 1998).
The genomic regulation of SMIT1 and other organic osmolyte transporters involves the transcription factor tonicity enhancer binding protein (TonEBP) (Miyakawa et al. 1999). TonEBP is activated by hypertonicity and binds to tonicity responsive enhancers (TonE) on the SMIT1 promoter region thereby inducing transcription of the carrier protein (Rim et al. 1997; Miyakawa et al. 1999). Tonicity responsive elements were also identified in the promotor region of other osmoprotective carriers including the betaine transporter BGT1 (SLC6A12) (Takenaka et al. 1994) and the taurine transporter TAUT (SLC6A6) (Ito et al. 2004). These elements are also present in the aldose reductase gene (Ferraris et al. 1996). Binding of TonEBP to the promotor region of osmosensitive genes upon hypertonic conditions was therefore considered to be a general mechanism to protect cells from shrinkage. However, a recent study on osmoprotective gene regulation in the brain demonstrated that in various non-neuronal cells, i.e. astrocytes, oligodendrocytes, ependymocytes, microglial and endothelial cells, TonEBP is neither expressed at basal conditions nor at prolonged hypertonicity (Maallem et al. 2006a,b) despite the fact that robust expression and hypertonic up-regulation of osmoprotective genes was reported to occur in most of those cell types. These include astrocytes (Strange et al. 1994; Bitoun & Tappaz, 2000), endothelial cells (Wiese et al. 1996) and macrophages (Zhang et al. 1996; Denkert et al. 1998). Beyond that, little is known about post-translational regulation of the carrier. It is tempting to speculate that the carrier protein is modulated by phosphorylation. A candidate protein kinase is the serum- and glucocorticoid-inducible kinase SGK1 that belongs to the AGC family of serine and threonine kinases. SGK1 is a downstream kinase in the phosphatidylinositol 3-kinase (PI3K) signalling pathway (Kobayashi et al. 1999) which has been shown to modulate a variety of ion channels and transporters (reviewed in Lang et al. 2006). SGK1 was initially cloned from a rat mammary tumour line (Webster et al. 1993) and later identified to be an early gene up-regulated by hypertonicity (Waldegger et al. 1997, 1998a). The kinase has been detected in a wide variety of species including shark and Caenorhabditis elegans. In mammals, SGK1 is expressed in virtually all tissues tested. However, transcript levels vary profoundly among different cell types of any given tissue (reviewed in Lang et al. 2006).
SGK1 and SMIT1 are coexpressed in various organ systems including kidney (Kwon et al. 1992; Hou et al. 2002; McCormick et al. 2005) and brain (Kwon et al. 1992; Warntges et al. 2002). To investigate a putative regulation of the myo-inositol transporter SMIT1 by the hypertonicity-induced protein kinase SGK1, both proteins were coexpressed in the Xenopus oocyte system. Functional studies were performed by two-electrode voltage-clamp and tracer flux measurements and the myo-inositol-induced inward current and [3H]myo-inositol uptake were taken as a measure for SMIT1 activity. The study provides new insights into the signalling of cell volume regulatory events upon shrinkage of cells by increased extracellular osmolarity.
Methods
Expression in Xenopus laevis oocytes
cRNA encoding wild type or haemagglutinin (HA)-tagged SMIT1 (Hager et al. 1995), wild type human Nedd4-2 (Debonneville et al. 2001), the respective SGK protein kinase constructs (Waldegger et al. 1997; Kobayashi et al. 1999) and protein kinase B (Alessi et al. 1996) have been synthesized as described (Wagner et al. 2000). Dissection of Xenopus laevis ovaries, collection and handling of the oocytes has been described in detail elsewhere (Wagner et al. 2000). Briefly, female Xenopus laevis frogs were anaesthetized by submersion in an aqueous ice-cold 0.1% 3-aminobenzoic acid ethyl ester solution (Sigma, St Louis, MO, USA) and placed on ice for surgery. A small abdominal incision was carried out (0.5–1 cm) and small pieces of the ovaries were removed. Afterwards the cut was sewn with a reabsorbable suture. Frogs were kept in drug-free water until reflexes were fully recovered. After two surgeries, frogs were killed humanely at the end of the experiments by cervical dislocation. All experiments were carried out according to guidelines of the animal welfare committee of the University of Tuebingen, Germany.
Oocytes were injected with 7.5 ng of the respective kinase cRNA and/or 5 ng Nedd4-2 cRNA or water on the first day after preparation of the oocytes and subsequently with 25 ng SMIT1 cRNA.
Myo-inositol-evoked currents were highly variable between different batches of oocytes. For data display, currents were normalized to the arithmetic mean of the control group (cells solely injected with SMIT1 cRNA) of each preparation and modulation of SMIT1 activity was reported as percentage changes.
Electrophysiological studies
All experiments were performed at room temperature 5 days after injection. Two-electrode voltage-clamp recordings were performed at a holding potential of −60 mV. The data were filtered at 10 Hz, and recorded with a GeneClamp 500 amplifier, a DigiData 1300 A/D–D/A converter and the pCLAMP 9.0 software package for data acquisition and analysis (Axon Instruments, USA). The control solution (superfusate-ND96) contained 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2 and 5 mm Hepes, pH 7.4. Myo-inositol was added to the solutions at the indicated concentrations. The final solutions were titrated to the pH indicated using HCl or NaOH. The flow rate of the superfusion was 20 ml min−1 and a complete exchange of the bath solution was reached within about 10 s.
Tracer flux studies
SMIT activity was also assessed by [3H]myo-inositol uptake. Oocytes were microinjected with 25 ng of SMIT cRNA alone or together with S422DSGK1 (7.5 ng) or with H2O (control oocytes). After 5–6 days, measurements of Na+-dependent [3H]myo-inositol uptake were performed. [3H]myo-inositol uptake was initiated by transferring oocytes to a ND96 solution containing 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 5 mm Hepes (pH 7.4), 96 mm NaCl (ND96-Na) or 96 mm ChCl (ND96-Ch), 10 μCi ml−1[3H]myo-inositol (14 Ci mmol−1, Amersham, Freiburg, Germany) and 300 μm of unlabelled myo-inositol. After incubation for 30 min at room temperature with [3H]myo-inositol (linear range of uptake), uptake was terminated by washing the oocytes four times with ice-cold ND96-Ch. Oocytes were individually transferred into scintillation vials and dissolved by adding 200 μl of 10% SDS. Radioactivity incorporated into the cells was measured with a liquid scintillation counter.
Site directed mutagenesis
The haemagglutinin (HA)-tagged SMIT1 was generated by two-stage PCR site-directed mutagenesis using the primers: SMIT1-HA,s: 5′-GAA TGG GCA GCA GGC CTA CGA CGTA CCA GAT TAC GCT TGT CCC TAG CCA TCC-3′; SMIT1-HA,as: 5′GGA TGG CTA GGG ACA TAG CGT AAT CTG GTA CGT CGT AGC CTG CTG CCC ATTC-3′. The HA-tagged SMIT1 mutant was sequenced to verify the presence of the desired mutation.
Detection of cell surface expression by chemiluminescence
Defolliculated oocytes were first injected with SGK1 cRNA or water and 1 day later with SMIT1 (HA), which contains an HA epitope inserted extracellularly. Oocytes were incubated with 1 μg ml−1 primary rat monoclonal anti-HA antibody (clone 3F10, Boehringer, Biberach, Germany), and 2 μg ml−1 secondary, peroxidase-conjugated affinity-purified F(ab′)2 goat anti-rat IgG antibody (Jackson ImmunoResearch, West Grove, USA). Individual oocytes were placed in 100 μl of ND96 and 20 μl of SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce, Rockford, USA), and chemiluminescence was quantified in a luminometer (Victor, Perkin Elmer, Wellesley, USA) integrating the signal over a period of 1 s. Results display normalized relative light units (RLU).
Statistical analysis
Data are provided as means ± s.e.m., n represents the number of oocytes investigated. All data were tested for significance using ANOVA, and only results with P < 0.05 were considered as statistically significant.
Results
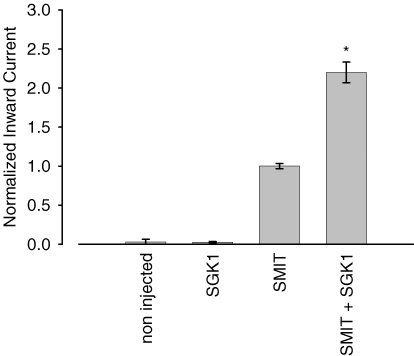
In order to investigate whether the SGK1 protein kinase plays a role in the regulation of the sodium-myo-inositol-transporter SMIT1, the carrier was expressed in Xenopus laevis oocytes and myo-inositol-induced currents were taken as a measure for SMIT1 activity in the presence and absence of the respective protein kinase. Xenopus oocytes injected with SMIT1 showed an inward current of 16 ± 2 nA (n = 136) when superfused with 2 mm myo-inositol (Fig. 1). Coexpression of SGK1 increased the current to 220 ± 13% (n = 153) of the control value. As shown in Fig. 1, myo-inositol-induced currents were low in oocytes solely injected with water (0.1 ± 0.2 nA, n = 29) or in cells injected with SGK1 in the absence of SMIT1 (0.4 ± 0.1 nA, n = 3).
Figure 1. Stimulation of myo-inositol-evoked currents by the serum- and glucocorticoid-inducible kinase SGK1.
Non-injected oocytes or oocytes solely injected with the SGK1 protein kinase did not display any myo-inositol-sensitive current. Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 alone.
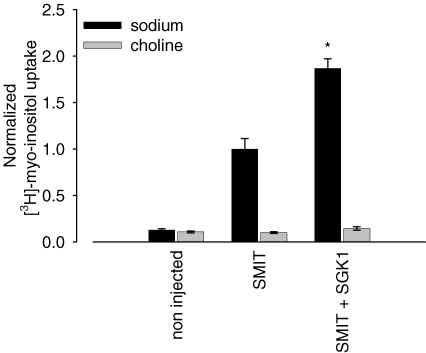
To demonstrate that the observed increase of myo-inositol-evoked currents in SMIT1-expressing cells by SGK1 is mediated by sodium-dependent transport, we performed isotope uptake studies with these cells. Uptake of radiolabelled [3H]myo-inositol was activated upon coexpression of SGK1 to 186 ± 6%, n = 39, compared to oocytes solely expressing the SMIT1 transporter. In Na+-free ND96 (ND96-Ch) solution, [3H]myo-inositol uptake in SMIT1-expressing oocytes was not significantly different from the transport measured in non-injected oocytes (Fig. 2).
Figure 2. Stimulation of myo-inositol uptake by the serum- and glucocorticoid-inducible kinase SGK1.
Uptake of radiolabelled [3H]myo-inositol is activated upon coexpression of SGK1 in SMIT1-injected oocytes. Arithmetic means ± s.e.m. of [3H]myo-inositol tracer fluxes. * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 alone.
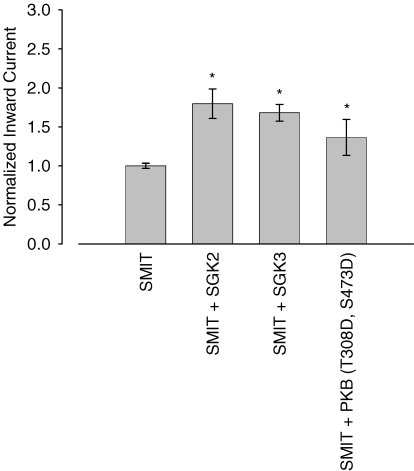
SGK1 shares ∼80% homology with its isoforms SGK2 and SGK3 and the PKB. When coexpressed with SMIT1 in Xenopus oocytes, the respective kinases were similarly capable of activating myo-inositol-evoked currents (Fig. 3). Compared to control cells solely injected with SMIT1, SGK2 activated the current to 180 ± 19% (n = 44) and SGK3 to 168 ± 11% (n = 42). Likewise the constitutively active PKB (T308D, S473D) stimulated SMIT1 activity to 136 ± 23% (n = 24).
Figure 3. Stimulation of the sodium myo-inositol transporter SMIT1 by SGK isoforms and PKB.
The SGK isoforms 2 and 3 and to a lesser extent the protein kinase B were similarly capable of up-regulating SMIT1 currents in Xenopus oocytes. Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 alone.
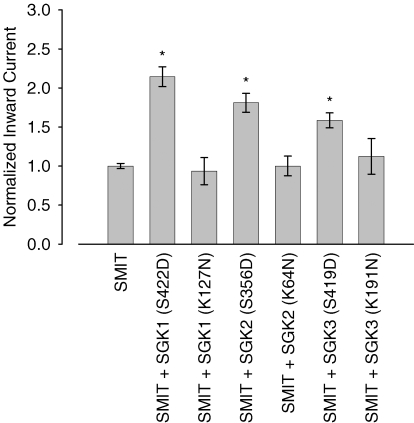
To explore whether modulation of SMIT1 currents depends on SGK1–3 kinase catalytical activity and is not due to non-phosphorylation-dependent protein–protein interaction, constitutively active and inactive mutants of the respective kinases were coexpressed with SMIT1 in Xenopus oocytes. While the constitutively active SGK1 (S422DSGK1) increased myo-inositol-induced currents to 215 ± 13% (n = 116) of the control value, no increase of current was observed upon coinjection of the kinase-dead SGK1 (K127NSGK1). In K127NSGK1 coexpressing oocytes, the SMIT1-mediated currents were not significantly different to the currents in cells solely injected with the SMIT1 transporter (94 ± 17%, n = 15). Similarly, the constitutively active S356DSGK2 (181 ± 12% of control, n = 52) and S419DSGK3 (159 ± 10% of control, n = 50) significantly increased the SMIT1-mediated current. In contrast, coexpression of the inactive K64NSGK2 (100 ± 13% of control, n = 9) and K191NSGK3 (112 ± 23% of control, n = 8) did not significantly affect SMIT1 currents (Fig. 4).
Figure 4. Up-regulation of SMIT1 by the SGK isoforms critically depends on the catalytical activity of the respective protein kinases.
While coexpression of constitutively active SGK1 (S422D), SGK2 (S356D) and SGK3 (S419D) activated SMIT1, coexpression of inactive SGK1 (K127N), SGK2 (K64N) and SGK3 (K191N) did not affect SMIT1-mediated currents in Xenopus oocytes. Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 alone.
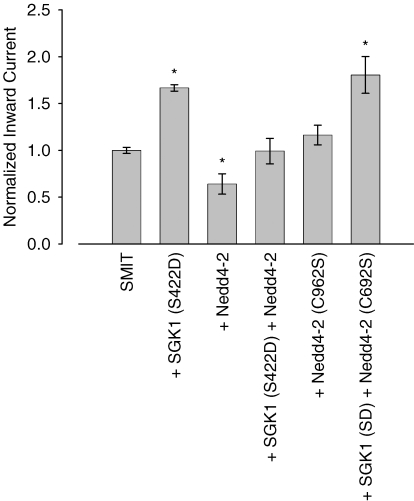
The molecular mechanism of SGK action on the SMIT1 transporter may either be due to a direct phosphorylation of its target protein or by phosphorylation and thereby inactivation of the ubiquitin ligase Nedd4-2, an effect described in previous reports, i.e. for the epithelial sodium channel ENaC (Debonneville et al. 2001) or the excitatory amino acid transporter EAAT1 (Boehmer et al. 2003). To test whether SMIT1 activity is modulated by the ubiquitin ligase Nedd4-2 both proteins were coexpressed in Xenopus oocytes. Indeed, Nedd4-2 coinjection reduced myo-inositol currents to 64 ± 11% (n = 19) of control while expression of the inactive Nedd4-2 (C962S) mutant did not affect the currents significantly (116 ± 11%, n = 18). The inhibiting effect of the ubiquitin ligase was partially reversed by further coexpression of S422DSGK1 (99 ± 14% of control, n = 9). S422DSGK1 expression in this series of experiments stimulated the SMIT1 currents to 167 ± 3% (n = 6) of control, a value comparable to the coexpression of S422DSGK1 together with the inactive Nedd4-2 (C962S) and SMIT1 (181 ± 20%, n = 9) (Fig. 5).
Figure 5. Regulation of SMIT1 by the ubiquitin ligase Nedd4-2.
Coexpression of wild type Nedd4-2, but not of the catalytically inactive Nedd4-2 (C962S), significantly down-regulated myo-inositol-induced currents. SGK1 counteracted the inhibiting Nedd4-2 effect and activated SMIT1 currents when coexpressed with inactive Nedd4-2 (C962S). Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 alone.
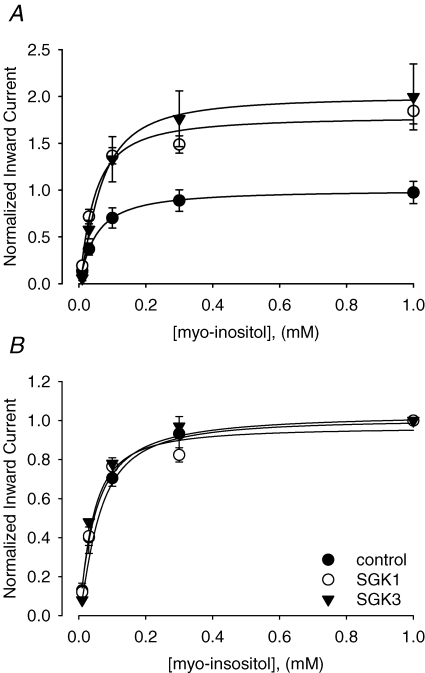
The observed activation of SMIT1 currents may either be due to an increased cell surface expression of the transporter or to a modulation of the carriers substrate affinities. To clarify, whether substrate affinities are modulated upon SGK1 or SGK3 coexpression, dose–response curves were determined upon coinjection of the respective kinases. The apparent half-maximal substrate concentrations were similar upon the coinjection of SMIT1 with the protein kinases SGK1 (40 ± 5 μm, n = 11) or SGK3 (48 ± 11 μm, n = 6) as upon expression of SMIT1 alone (59 ± 5 μm, n = 8). Calculated Vmax values from regression fits with the modified Hill equation equalled 1.8 ± 0.2 (n = 11) for coexpression of SMIT1 with SGK1, 2.0 ± 0.4 (n = 6) for coexpression of SMIT1 with SGK3 as compared to 1.0 ± 0.2 (n = 8) for expression of SMIT1 alone (Fig. 6 and Table 1).
Figure 6. Kinetics of myo-inositol-evoked currents in SMIT1 expressing Xenopus laevis oocytes.
While coexpression of SGK1 and SGK3 increased Vmax of the carrier (see Table 1 and A), SMIT1 substrate affinities for myo-inositol remained unaffected (see Table 1 and B). Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. Data are normalized to the maximum value of the control group (cells solely injected with SMIT) (A) or to maximum value of the respective group injected with SMIT alone, SMIT + SGK1 or SMIT + SGK3 (B).
Table 1.
Kinetics of myo-inositol-evoked currents in SMIT1-expressing Xenopus laevis oocytes
| Km | P | Vmax | P | n | |
|---|---|---|---|---|---|
| SMIT | 59 ± 5 | — | 1.0 ± 0.2 | — | 8 |
| SMIT + SGK1 | 40 ± 5 | n.s. | 1.8 ± 0.2 | < 0.05 | 11 |
| SMIT + SGK3 | 48 ± 11 | n.n. | 2.0 ± 0.4 | < 0.05 | 6 |
While coexpression of SGK1 and SGK3 increased Vmax of the carrier, SMIT1 substrate affinities for myo-inositol were not significantly affected. Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. Data are normalized to the maximum value of the control group (oocytes solely expressing SMIT1). Vmax and Km values were calculated with the modified Hill equation. P values are relative to the control group (oocytes solely expressing SMIT1).
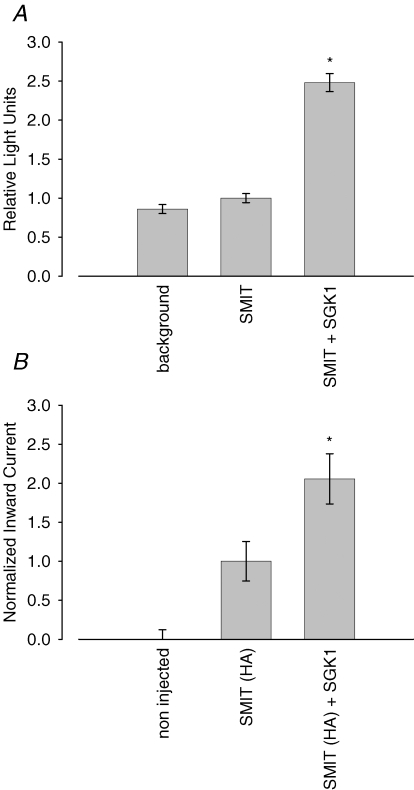
To investigate whether SMIT1 current activation correlates with an increased cell surface abundance of the transporter, a haemagluttinin (HA) tag was inserted into an extracellular loop of the protein. A chemiluminescence assay was used to quantify SMIT1 expression at the outer membrane by a primary HA antibody coupled to peroxidase. The emitted light directly corresponds to the amount of protein at the cell surface. To test for functionality of the newly generated SMIT1 (HA) constructs and their comparative activation by SGK1 relative to the wild type SMIT1, cells used for the chemiluminescence assay were tested for myo-inositol-sensitive currents in parallel. As depicted in Fig. 6, 2 mm substrate generated inward currents in SMIT1 (HA)-expressing oocytes which were significantly higher in oocytes additionally expressing SGK1 (206 ± 32%, n = 13) than in oocytes expressing SMIT1 (HA) alone (100 ± 25%, n = 13). The chemiluminescence assay revealed that the increase in carrier cell surface expression correlates with the observed increase in currents upon SGK1 coinjection. Non-injected control oocytes display a background fluorescence of 0.9 ± 0.1 relative light units (RLU) (n = 11) while the signal from cells expressing SMIT1 alone equals 1.0 ± 0.1 RLU (n = 11). Coexpression of the SGK1 protein kinase significantly increased the light signal to 2.5 ± 0.1 RLU (n = 11) (Fig. 7).
Figure 7. Cell surface abundance of SMIT1 is up-regulated by SGK1.
A, arithmetic means ± s.e.m. of protein abundance as reflected by chemiluminescence of SMIT1 (HA). * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 (HA) alone. B, extracellular insertion of haemagluttinin into the SMIT1 sequence does not affect up-regulation of the transporter by SGK1. Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. * indicates statistically significant difference (P < 0.05) to oocytes injected with SMIT1 (HA) alone.
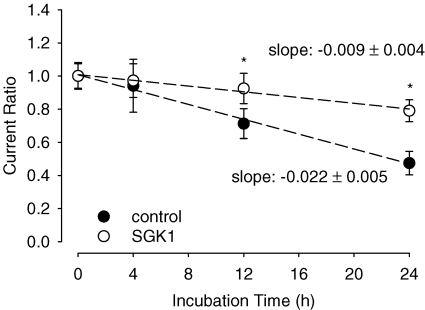
The increase in SMIT1 cell surface expression might be the result of an induced carrier insertion into the membrane or a decreased removal of the carrier protein. To distinguish between these two mechanisms, SMIT1 activity was quantified after different time points of brefeldin A (BFA) administration. This drug inhibits secretory mechanisms by inhibiting vesicle formation at the Golgi apparatus and thus prevents further insertion of carrier protein into the cell membrane. As demonstrated in a previous study from this lab, BFA (5 μm) treatment does not affect viability of the oocytes (Yun et al. 2002). Administration of BFA gradually decreased myo-inositol-evoked currents in SMIT1-expressing oocytes. Compared to control cells solely injected with the SMIT1 carrier, the decrease in currents was less rapid in cells coinjected with SMIT1 and the SGK1 protein kinase. While control oocytes showed a current ratio of 0.7 ± 0.1, n = 12 at t = 12 h, SGK1-coinjected cells displayed a significantly higher current ratio at this time point (0.9 ± 0.1, n = 20). Twenty four hours after BFA incubation the difference in current ratios was even more prominent (0.5 ± 0.1, n = 17, in the control group compared to 0.8 ± 0.1, n = 20, in SGK1-coinjected cells) (Fig. 8).
Figure 8. Coexpression of SGK1 stabilizes the SMIT1 carrier in the oocyte membrane.
Myo-inositol-evoked currents in brefeldin A (5 μm)-treated oocytes at the indicated time points. Arithmetic means ± s.e.m. of myo-inositol (2 mm)-evoked currents. * indicates statistically significant difference (P < 0.05) between oocytes injected with SMIT1 alone and coexpression of SMIT1 together with SGK1.
Discussion
The present study reveals that SMIT1 is activated by the serum- and glucocorticoid-inducible kinase SGK1, its isoforms 2, 3 and the closely related PKB. The present observations thus disclose a novel mechanism of SMIT1 regulation. As SGK1 is strongly up-regulated by both isotonic and hypertonic cell shrinkage (Waldegger et al. 1997, 1998a), the kinase could indeed participate in the up-regulation of SMIT1 activity during regulatory cell volume increase. Indeed, SMIT1 is colocalized with SGK1 in various organs including kidney (Kwon et al. 1992; Hou et al. 2002; McCormick et al. 2005) and brain (Kwon et al. 1992; Warntges et al. 2002). This study investigated regulation of SMIT1 activity in the Xenopus oocyte expression system under isotonic conditions.
SMIT1 activation by SGK is critically dependent on the phosphorylation processes as the catalytically inactive kinase isoforms are not capable of stimulating myo-inositol currents. The molecular mechanism of SMIT1 up-regulation may be due to direct phosphorylation of the transporter or by phosphorylation of an intermediate protein by SGK1. One known SGK1 substrate that is involved in membrane transport regulation is the ubiquitin ligase Nedd4-2 (Debonneville et al. 2001; Boehmer et al. 2006; Rajamanickam et al. 2007). SGK1 phosphorylates and abolishes interaction of Nedd4-2 with its target thereby decreasing its removal from the plasma membrane (Debonneville et al. 2001). The ubiquitin ligase is endogenously expressed in Xenopus oocytes and thus expression of SGK1 alone may be capable of activating membrane transport as described for the epithelial sodium channel ENaC (Debonneville et al. 2001) and several excitatory amino acid transporters (Boehmer et al. 2003, 2005, 2006; Rajamanickam et al. 2007). The present study shows that Nedd4-2 down-regulates SMIT1 activity, an effect that is compensated by further expression of SGK1. However, the SMIT1-induced current is significantly larger in oocytes coexpressing SMIT1 with constitutively active S442DSGK1 and inactive C692SNedd4-2 than in oocytes coexpressing SMIT1 with constitutively active S442DSGK1 and wild type Nedd4-2. Thus, S442DSGK1 did not fully inactivate Nedd4-2 and S442DSGK1 may be effective by mechanisms other than Nedd4-2 phosphorylation. Nevertheless, expression of SGK1 in the Xenopus oocyte system leads to phosphorylation of intrinsic Nedd4-2 (Dieter et al. 2004).
The observed increase in SMIT1 activity is due to an increased abundance of the transporter at the cell membrane as revealed by the chemiluminescence assay using the SMIT1 (HA) construct. Upon brefeldin A administration myo-inositol currents in oocytes injected with SGK1 and SMIT1 together decrease less rapidly compared to cells injected solely with the transporter. Obviously, the observed increase in SMIT1 activity is at least partially due to stabilization of SMIT1 in the outer membrane with enhanced dwelling time of the carrier in the cell membrane.
In summary, this study indicates that SGK1 and its isoforms modulate SMIT1 function by increasing its plasma membrane abundance without affecting transport kinetics. SGK1 might partially be effective through phosphorylation and thus inhibition of the intrinsic ubiquitin ligase Nedd4-2. This novel mechanism of SMIT1 regulation at the post-transcriptional level might provide new insights into the signalling of cell volume regulation upon hypertonic stress.
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Berry GT, Mallee JJ, Kwon HM, Rim JS, Mulla WR, Muenke M, Spinner NB. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics. 1995;25:507–513. doi: 10.1016/0888-7543(95)80052-n. [DOI] [PubMed] [Google Scholar]
- Bitoun M, Tappaz M. Gene expression of the transporters and biosynthetic enzymes of the osmolytes in astrocyte primary cultures exposed to hyperosmotic conditions. Glia. 2000;32:165–176. [PubMed] [Google Scholar]
- Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid-inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem. 2003;86:1181–1188. doi: 10.1046/j.1471-4159.2003.01937.x. [DOI] [PubMed] [Google Scholar]
- Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97:911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- Boehmer C, Rajamanickam J, Schniepp R, Kohler K, Wulff P, Kuhl D, Palmada M, Lang F. Regulation of the excitatory amino acid transporter EAAT5 by the serum and glucocorticoid dependent kinases SGK1 and SGK3. Biochem Biophys Res Commun. 2005;329:738–742. doi: 10.1016/j.bbrc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Warskulat U, Hensel F, Haussinger D. Osmolyte strategy in human monocytes and macrophages: involvement of p38MAPK in hyperosmotic induction of betaine and myoinositol transporters. Arch Biochem Biophys. 1998;354:172–180. doi: 10.1006/abbi.1998.0661. [DOI] [PubMed] [Google Scholar]
- Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res. 2004;12:862–870. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- Ferraris JD, Williams CK, Jung KY, Bedford JJ, Burg MB, Garcia-Perez A. ORE, a eukaryotic minimal essential osmotic response element. The aldose reductase gene in hyperosmotic stress. J Biol Chem. 1996;271:18318–18321. doi: 10.1074/jbc.271.31.18318. [DOI] [PubMed] [Google Scholar]
- Hager K, Hazama A, Kwon HM, Loo DD, Handler JS, Wright EM. Kinetics and specificity of the renal Na+/myo-inositol cotransporter expressed in Xenopus oocytes. J Membr Biol. 1995;143:103–113. doi: 10.1007/BF00234656. [DOI] [PubMed] [Google Scholar]
- Hou J, Speirs HJ, Seckl JR, Brown RW. Sgk1 gene expression in kidney and its regulation by aldosterone: spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol. 2002;13:1190–1198. [PubMed] [Google Scholar]
- Ito T, Fujio Y, Hirata M, Takatani T, Matsuda T, Muraoka S, Takahashi K, Azuma J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem J. 2004;382:177–182. doi: 10.1042/BJ20031838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Yamauchi A, Sugiura T, Matsuoka Y, Horio M, Tohyama M, Shimada S, Imai E, Hori M. Inhibition of myo-inositol transport causes acute renal failure with selective medullary injury in the rat. Kidney Int. 1998;53:146–153. doi: 10.1046/j.1523-1755.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, Burg MB, Handler JS. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem. 1992;267:6297–6301. [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- Lang F, Volkl H, Woll E, Haussinger D. Mechanisms of cell volume regulation in kidney and liver. Contrib Nephrol. 1991;95:237–245. [PubMed] [Google Scholar]
- Maallem S, Berod A, Mutin M, Kwon HM, Tappaz ML. Large discrepancies in cellular distribution of the tonicity-induced expression of osmoprotective genes and their regulatory transcription factor TonEBP in rat brain. Neuroscience. 2006a;142:355–368. doi: 10.1016/j.neuroscience.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Maallem S, Mutin M, Kwon HM, Tappaz ML. Differential cellular distribution of tonicity-induced expression of transcription factor TonEBP in the rat brain following prolonged systemic hypertonicity. Neuroscience. 2006b;137:51–71. doi: 10.1016/j.neuroscience.2005.07.037. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Bhalla V, Pao AC, Pearce D. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology (Bethesda) 2005;20:134–139. doi: 10.1152/physiol.00053.2004. [DOI] [PubMed] [Google Scholar]
- Macknight AD. Principles of cell volume regulation. Ren Physiol Biochem. 1988;11:114–141. doi: 10.1159/000173158. [DOI] [PubMed] [Google Scholar]
- Macknight AD, Gordon LG, Purves RD. Problems in the understanding of cell volume regulation. J Exp Zool. 1994;268:80–89. doi: 10.1002/jez.1402680203. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamanickam J, Palmada M, Lang F, Boehmer C. EAAT4 phosphorylation at the SGK1 consensus site is required for transport modulation by the kinase. J Neurochem. 2007;102:858–866. doi: 10.1111/j.1471-4159.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- Rim JS, Tanawattanacharoen S, Takenaka M, Handler JS, Kwon HM. The canine sodium/myo-inositol cotransporter gene: structural organization and characterization of the promoter. Arch Biochem Biophys. 1997;341:193–199. doi: 10.1006/abbi.1997.9950. [DOI] [PubMed] [Google Scholar]
- Somero GN. Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol Regul Integr Comp Physiol. 1986;251:R197–R213. doi: 10.1152/ajpregu.1986.251.2.R197. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Paredes A, Morrison R. Osmoregulatory changes in myo-inositol content and Na+/myo-inositol cotransport in rat cortical astrocytes. Glia. 1994;12:35–43. doi: 10.1002/glia.440120105. [DOI] [PubMed] [Google Scholar]
- Takenaka M, Preston AS, Kwon HM, Handler JS. The tonicity-sensitive element that mediates increased transcription of the betaine transporter gene in response to hypertonic stress. J Biol Chem. 1994;269:29379–29381. [PubMed] [Google Scholar]
- Wagner CA, Friedrich B, Setiawan I, Lang F, Broer S. The use of Xenopus laevis oocytes for the functional characterization of heterologously expressed membrane proteins. Cell Physiol Biochem. 2000;10:1–12. doi: 10.1159/000016341. [DOI] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Forrest JN, Jr, Greger R, Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland – a gene induced by hypertonicity and secretagogues. Pflugers Arch. 1998a;436:575–580. doi: 10.1007/s004240050674. [DOI] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldegger S, Steuer S, Risler T, Heidland A, Capasso G, Massry S, Lang F. Mechanisms and clinical significance of cell volume regulation. Nephrol Dial Transplant. 1998b;13:867–874. doi: 10.1093/ndt/13.4.867. [DOI] [PubMed] [Google Scholar]
- Warntges S, Friedrich B, Henke G, Duranton C, Lang PA, Waldegger S, Meyermann R, Kuhl D, Speckmann EJ, Obermuller N, Witzgall R, Mack AF, Wagner HJ, Wagner A, Broer S, Lang F. Cerebral localization and regulation of the cell volume-sensitive serum- and glucocorticoid-dependent kinase SGK1. Pflugers Arch. 2002;443:617–624. doi: 10.1007/s00424-001-0737-1. [DOI] [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese TJ, Dunlap JA, Conner CE, Grzybowski JA, Lowe WL, Jr, Yorek MA. Osmotic regulation of Na-myo-inositol cotransporter mRNA level and activity in endothelial and neural cells. Am J Physiol Cell Physiol. 1996;270:C990–C997. doi: 10.1152/ajpcell.1996.270.4.C990. [DOI] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yun CC, Palmada M, Embark HM, Fedorenko O, Feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F. The serum and glucocorticoid-inducible kinase SGK1 and the Na+/H+ exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K+ channel ROMK1. J Am Soc Nephrol. 2002;13:2823–2830. doi: 10.1097/01.asn.0000035085.54451.81. [DOI] [PubMed] [Google Scholar]
- Zhang F, Warskulat U, Wettstein M, Haussinger D. Identification of betaine as an osmolyte in rat liver macrophages (Kupffer cells) Gastroenterology. 1996;110:1543–1552. doi: 10.1053/gast.1996.v110.pm8613062. [DOI] [PubMed] [Google Scholar]