Abstract
Recently, we have shown that specific, transient carotid chemoreceptor (CC) inhibition in exercising dogs causes vasodilatation in limb muscle. The purpose of the present investigation was to determine if CC suppression reduces muscle sympathetic nerve activity (MSNA) in exercising humans. Healthy subjects (N = 7) breathed hyperoxic gas (FIO2∼1.0) for 60 s at rest and during rhythmic handgrip exercise (50% maximal voluntary contraction, 20 r.p.m.). Microneurography was used to record MSNA in the peroneal nerve. End-tidal PCO2 was maintained at resting eupnoeic levels throughout and breathing rate was voluntarily fixed. Exercise increased heart rate (67 versus 77 beats min−1), mean blood pressure (81 versus 97 mmHg), MSNA burst frequency (28 versus 37 bursts min−1) and MSNA total minute activity (5.7 versus 9.3 units), but did not change blood lactate (0.7 versus 0.7 mm). Transient hyperoxia had no significant effect on MSNA at rest. In contrast, during exercise both MSNA burst frequency and total minute activity were significantly reduced with hyperoxia. MSNA burst frequency was reduced within 9–23 s of end-tidal PO2 exceeding 250 mmHg. The average nadir in MSNA burst frequency and total minute activity was −28 ± 2% and −39 ± 7%, respectively, below steady state normoxic values. Blood pressure was unchanged with hyperoxia at rest or during exercise. CC stimulation with transient hypoxia increased MSNA with a similar time delay to that obtained with CC inhibition via hyperoxia. Consistent with previous animal work, these data indicate that the CC contributes to exercise-induced increases in sympathetic vasoconstrictor outflow.
The carotid chemoreceptors (CCs) are the major oxygen sensor in the body and CC stimulation causes a reflex-mediated increase in ventilation (Olson et al. 1988; Curran et al. 2000). Importantly, however, CC stimulation also elicits increases in sympathetic vasoconstrictor outflow to the skeletal muscle, renal and mesenteric vascular beds (Rutherford & Vatner, 1978; Balkowiec et al. 1993; Sun & Reis, 1994a; Guyenet, 2000). Previous investigators have demonstrated that carotid chemosensitivity is enhanced with exercise. Forster et al. (1974) found, in humans, that the ventilatory response to the CC stimulant doxapram was greater during exercise as compared to rest, and exercise has been shown to greatly potentiate the ventilatory response to hypoxia (Weil et al. 1972). Biscoe & Purves (1967) demonstrated, in anaesthetized animals, that passive exercise increased CC activity via feedback from the exercised limb, although subsequent studies failed to confirm this effect (Davies & Lahiri, 1973; Aggarwal et al. 1976). Although the steady-state ventilatory response to exercise seems to be unaffected by CC inhibition (Boetger & Ward, 1986; Henson et al. 1992), the ventilatory kinetics to incremental exercise are slowed when chemoreceptors are inhibited with dopamine (Boetger & Ward, 1986). Thus, previous research suggests that the CCs are sensitized during exercise.
Exercise significantly increases sympathetic vasoconstrictor outflow, which reduces blood flow to non-contracting muscle and other inactive vascular beds, thereby redistributing the cardiac output to contracting muscle (Buckwalter & Clifford, 1999). Increased sympathetic vasoconstrictor activity also constrains the increase in blood flow in contracting muscle during exercise (Joyner et al. 1992; Buckwalter & Clifford, 1999) in order to maintain blood pressure (Rowell & O'Leary, 1990). It is generally assumed that the increased sympathetic nervous system activity during exercise is due to feedforward mechanisms such as central command, feedback from muscle metaboreceptors, muscle mechanoreceptors and/or a resetting of systemic baroreceptors (Rowell & O'Leary, 1990).
Recently we have shown in dogs that specific, transient inhibition of the CC during exercise caused peripheral vasodilatation as demonstrated by increases in hindlimb flow and conductance (Stickland et al. 2007). Vasodilatation was not observed when the CCs were inhibited at rest, indicating that tonic CC activity does not influence resting vasoconstrictor tone. In addition, the vasodilatation following CC inhibition during exercise was abolished with α-adrenergic blockade, suggesting that vasodilatation was due to a reduction in sympathetic outflow. These results demonstrated an important role for the CC in exercise cardiovascular control and indicate that the CC contributes to the increased sympathetic nerve activity observed with exercise.
It remains to be determined whether the reflex vasodilatation that was observed after CC inhibition in the exercising dog (Stickland et al. 2007) was, indeed, the result of withdrawal of chemoreceptor-induced sympathoexcitation. Moreover, it is unclear whether this reflex is functional in exercising humans. Hyperoxia has been shown to rapidly inhibit the CC (Nye et al. 1981). Therefore the purpose of the present investigation was to determine if CC inhibition with hyperoxia affects muscle sympathetic nerve activity (MSNA) during exercise in humans. Consistent with our previous animal studies, we hypothesized that the CCs are sensitized with exercise and therefore inhibiting the CCs with transient hyperoxia will reduce MSNA during exercise, while little effect will be observed with CC inhibition at rest.
Methods
Ethical approval
Seven healthy men aged 33 ± 1 years (range, 28–35 years), of normal weight (85 ± 6 kg) and height (180 ± 2 cm), served as subjects after providing written, informed consent. All subjects were normotensive and free from cardiovascular and pulmonary disease. Experimental procedures and protocols were approved by the University of Wisconsin Center for Health Sciences Institutional Review Board and conformed with the Declaration of Helsinki.
Cardio-respiratory measures
Subjects breathed through a mouthpiece with the nose occluded. Airflow was measured by a heated pneumotachograph (model 5719, 0–100 l min−1; Hans Rudolph, Kansas City, MO, USA), tidal volume and breathing frequency were calculated, and end-tidal PO2 (PET O2) and PCO2 (PET CO2) were measured using Applied Electrochemistry (Pittsburgh, PA, USA) O2 and CO2 sensors. A single lead electrocardiogram was continuously recorded. Blood pressure was monitored using beat-by-beat photoplethysmography obtained on a toe (Finapres model 2300; Ohmeda, Englewood, CO, USA). In addition, arterial oxygen saturation was obtained from an ear oximetry probe (Biox 3740; Ohmeda). Force output data from two handgrip dynamometers were likewise recorded. Earlobe capillary blood was obtained at rest and immediately following exercise and analysed for blood lactate using an electrochemical analyser (YSI 1500 Sport, OH, USA).
Sympathetic nerve activity
Postganglionic MSNA in the right peroneal nerve was recorded directly using the microneurography technique (Vallbo et al. 1979). The neural signals were passed to a differential preamplifier, an amplifier (total gain, 100 000), a band-pass filter (700–2000 Hz) and an integrator (time constant, 100 ms). Placement of the recording electrode within a muscle nerve fascicle was confirmed by: (1) the presence of muscle twitches, but not paresthesias, in response to electrical stimulation; (2) the pulse-synchronous nature of the nerve activity; (3) the appearance of afferent activity in response to tapping or stretching of muscle, but not gentle stroking of skin, in the appropriate receptive fields; and (4) the absence of neural activation in response to a startle stimulus. Once an acceptable neural recording (signal-to-noise ratio > 3: 1) was obtained, the subject was instructed to maintain the leg in a relaxed position for the duration of the study. Sympathetic bursts were identified by computer-assisted inspection of the mean voltage neurogram. For purposes of quantification, MSNA was expressed as burst frequency (bursts min−1), burst amplitude (arbitrary units) and total minute activity (burst frequency × mean burst amplitude). MSNA total minute activity during the hyperoxic and hypoxic interventions was also expressed as a percentage of the baseline steady-state level.
Experimental protocols
Prior to the experimental session, a practice was conducted on a separate day to familiarize each subject with the protocol. The set-up and exercise protocol was similar to the full experimental session; however, MSNA data were not obtained.
Following instrumentation, subjects breathed freely on the mouthpiece for 10 min so that resting eupnoeic data could be obtained. Interventions at rest and during exercise were then performed at a breathing frequency of 20 breaths per minute, with inspiratory time/total breath time ≈ 0.3, and PET CO2 was maintained at resting eupnoeic levels by adding CO2 to the inhaled gas. Hyperoxia interventions (FIO2∼1.0) were conducted at rest and during steady-state exercise. For exercise interventions, subjects performed rhythmic bilateral handgrip exercise at 50% maximal voluntary contraction at a frequency of 20 contractions per minute, which was coordinated with breathing such that contractions occurred during inspiration (i.e. duty cycle ∼0.3). Target handgrip force output was displayed on an oscilloscope to provide visual feedback to the subject. Reported data are of the mean individual response from two to five interventions during each condition (i.e. rest and during exercise). At least two hyperoxia interventions at rest were conducted prior to exercise and additional interventions were conducted at rest several minutes following termination of exercise. No differences in response to hyperoxia were noted between interventions performed prior to versus following exercise and therefore resting data were combined.
At the beginning of each condition (i.e. rest or exercise), each subject was permitted to find a comfortable tidal volume at the paced breathing frequency and once a steady state in ventilation and MSNA had been determined, the subject was instructed to maintain this tidal volume during each intervention. Target tidal volume was displayed on an oscilloscope to provide visual feedback. For each intervention, data were collected for 3 min: 1 min of steady-state room air breathing, 1 min of hyperoxia or hypoxia, 1 min of return to control. Interventions were separated by 3–4 min to allow P_ET O2 to normalize and a steady state to be established. Between interventions subjects were permitted breaks from paced breathing.
To further characterize the time-course of the MSNA response to varying FIO2 values, transient hypoxic interventions (target PET O2∼45 mmHg, FIO2∼0.1) were also conducted for 1 min, both at rest and during exercise. These interventions were preceded by a normoxic control period and data collection continued during return to normoxia following hypoxia. Hypoxic interventions were performed after the hyperoxia trials so as to avoid any possible hypoxia-induced sensitization of the CC which could confound the hyperoxic trials.
Data analysis
All signals were digitized and stored on the hard drive of a personal computer for subsequent analysis and on a polygraph as previously described (TA-4000; Gould, Cleveland, OH, USA) (Taha et al. 1995). Bursts of MSNA were detected by custom-written computer program (St Croix et al. 1999) and confirmed by visual inspection of the integrated neurogram by one investigator (M.K.S.). The amplitude of each burst of MSNA and the beat-to-beat levels of arterial blood pressure were determined by computer.
For all inferential analyses, the probability of type I error was set at 0.05. Group data for each variable are expressed as means ± s.e.m. One minute mean steady-state values for each condition (free breathing, paced breathing, exercise) were compared using a repeated measures ANOVA. The mean 15 s changes from baseline with inhaled gas (hyperoxia or hypoxia) were compared between rest and exercise using a two-way repeated measures ANOVA. Upon detection of an effect, paired t test comparisons were made and a Bonferroni correction factor was applied to maintain family wise error rate at 0.05. The nadir 15 s value for burst frequency and MSNA total activity within each hyperoxic intervention was also determined and the nadir at rest was compared to the nadir during exercise using a paired t test.
Results
Effects of hyperoxia at rest
With paced breathing, breathing frequency was increased (14 versus 20 breaths min−1), while tidal volume decreased (728 versus 511 ml), resulting in small increases in minute ventilation (8.3 versus 10.2 l min−1) as compared to eupnoeic breathing (P < 0.05 for all). End-tidal PCO2 was maintained at spontaneous eupnoeic levels (42 ± 2 mmHg) throughout paced resting and exercise trials via CO2 supplementation of the inspired air. As compared to spontaneous eupnoeic breathing, paced breathing at rest did not change heart rate (68 versus 67 beats min−1), mean arterial pressure (83 versus 81 mmHg), MSNA burst frequency (28 versus 28 bursts min−1), or MSNA total minute activity (5.2 versus 5.7 units, P > 0.05 for all).
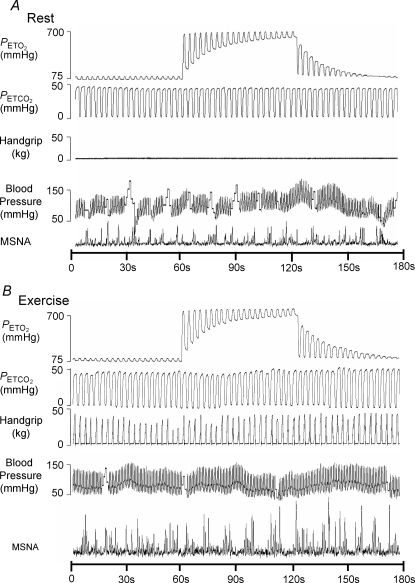
A representative hyperoxia trial at rest in a subject is illustrated in Fig. 1 (top panel). Mean data are reported in Table 1. Hyperoxia resulted in a rapid increase in PET O2 such that PET O2 exceeded 250 mmHg within 2.6 ± 0.4 breaths or 8.2 ± 1.4 s. Hyperoxia did not result in a significant change in burst frequency or MSNA total minute activity from baseline. Blood pressure, heart rate, tidal volume, breathing frequency and PET CO2 were unchanged with hyperoxia from baseline normoxic steady-state values.
Figure 1.
Representative trace of a subject receiving hyperoxia at rest (top) and during exercise (bottom).
Table 1.
Cardiorespiratory data during hyperoxia trials at rest (N = 7)
| Normoxia | Hyperoxia | Return to normoxia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–15 s | 16–30 s | 31–45 s | 46–60 s | 0–15 s | 16–30 s | 31–45 s | 46–60 s | ||
| Heart rate | 67 | 67 | 66 | 66 | 66 | 64 | 64 | 64 | 66 |
| (beats min−1) | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 4 | 4 |
| Mean arterial pressure | 81 | 80 | 78 | 79 | 78 | 80 | 79 | 79 | 78 |
| (mmHg) | 11 | 10 | 12 | 10 | 11 | 11 | 11 | 11 | 10 |
| Tidal volume | 511 | 524 | 514 | 494 | 517 | 514 | 515 | 484 | 511 |
| (ml) | 40 | 33 | 35 | 35 | 34 | 34 | 39 | 38 | 43 |
| Breathing frequency | 20 | 20 | 20 | 21 | 20 | 20 | 20 | 21 | 20 |
| (breaths min−1) | 0.2 | 0.6 | 0.2 | 0.7 | 0.2 | 0.2 | 0.3 | 1.5 | 0.2 |
| Minute ventilation | 10.2 | 10.3 | 10.3 | 9.9 | 10.3 | 10.3 | 10.2 | 9.9 | 10.3 |
| (l min−1) | 0.8 | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 | 0.9 | 0.9 | 0.9 |
| End-tidal PO2 | 108 | 290* | 446* | 523* | 556* | 208* | 155* | 153* | 148* |
| (mmHg) | 2 | 17 | 24 | 26 | 22 | 22 | 2 | 1 | 3 |
| End-tidal PCO2 | 41 | 41 | 41 | 41 | 41 | 41 | 41 | 41 | 41 |
| (mmHg) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| SpO2 | 98 | 98 | 99* | 99* | 100* | 99* | 99* | 99* | 99* |
| (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Burst frequency | 28 | 29 | 27 | 30 | 29 | 25 | 27 | 27 | 27 |
| (bursts min−1) | 3 | 5 | 4 | 4 | 4 | 4 | 3 | 4 | 3 |
| Total minute actvity | 5.7 | 5.6 | 6.1 | 6.6 | 6.7 | 5.8 | 5.4 | 6.0 | 5.3 |
| (units) | 1.8 | 1.8 | 1.9 | 2.4 | 2.3 | 2.3 | 2.2 | 2.1 | 1.9 |
Values are mean (± s.e.m., value below).
Note: P < 0.05 versus baseline. SpO2= arterial O2 saturation.
Effects of exercise and of hyperoxia during exercise
A representative exercise hyperoxic trial in the same subject is illustrated in Fig. 1 (lower panel). Group mean responses are detailed in Table 2. Exercise in normoxia significantly increased heart rate (67 versus 77 beats min−1), mean blood pressure (81 versus 97 mmHg), tidal volume (511 versus 719 ml), MSNA burst frequency (28 versus 37 bursts min−1) and MSNA total activity (5.2 versus 9.3 units) above resting values (P < 0.05 for all, see Tables 1 and 2 for baseline resting and exercise steady-state data). Blood lactate, however, was not different between rest (0.7 ± 0.1 mm) and exercise (0.7 ± 0.1 mm).
Table 2.
Cardiorespiratory data during hyperoxia trials during exercise (N = 7)
| Rest Normoxia | Exercise Normoxia | Exercise Hyperoxia | Exercise Return to normoxia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–15 s | 16–30 s | 31–45 s | 46–60 s | 0–15 s | 16–30 s | 31–45 s | 46–60 s | |||
| Heart rate | 67 | 77† | 77 | 75 | 74* | 74* | 73* | 73* | 74* | 75 |
| (beats min−1) | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 4 |
| Mean arterial pressure | 81 | 97† | 97 | 99 | 100 | 99 | 98 | 100 | 99 | 98 |
| (mmHg) | 11 | 12 | 12 | 11 | 11 | 11 | 12 | 11 | 11 | 11 |
| Tidal volume | 511 | 719† | 731 | 728 | 742 | 748 | 740 | 754 | 749 | 739 |
| (ml) | 40 | 79 | 83 | 88 | 75 | 60 | 84 | 92 | 85 | 91 |
| Breathing frequency | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| (breaths min−1) | 0.2 | 0.0 | 0.1 | 0.1 | 0.1 | 0.5 | 0.1 | 0.3 | 0.2 | 0.2 |
| Minute ventilation | 10.2 | 14.5† | 14.6 | 14.7 | 14.4 | 15.0 | 15.0 | 15.0 | 15.0 | 14.9 |
| (l min−1) | 0.8 | 1.6 | 1.6 | 1.7 | 1.6 | 1.3 | 1.7 | 1.8 | 1.8 | 1.8 |
| End-tidal PO2 | 108 | 114 | 357* | 523* | 593* | 635* | 271* | 157* | 149* | 145* |
| (mmHg) | 2 | 5 | 26 | 25 | 20 | 8 | 31 | 3 | 2 | 5 |
| End-tidal PCO2 | 41 | 41 | 41 | 41 | 40 | 40 | 41 | 41 | 41 | 42 |
| (mmHg) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| SpO2 | 98 | 98 | 98 | 99* | 99* | 99* | 99* | 99* | 99* | 99* |
| (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Burst frequency | 28 | 37† | 35 | 31* | 29* | 30* | 33 | 32 | 34 | 32 |
| (bursts min−1) | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 2 | 3 | 2 |
| Total minute actvity | 5.7 | 9.3† | 8.7 | 8.6 | 7.3* | 7.2* | 8.7 | 8.7 | 8.9 | 9.0 |
| (units) | 1.8 | 2.0 | 1.8 | 2.3 | 1.8 | 1.6 | 2.6 | 2.1 | 1.9 | 2.1 |
Values are mean (± s.e.m., value below).
Note: P < 0.05 versus steady-state baseline at rest.
P < 0.05 versus baseline during exercise.
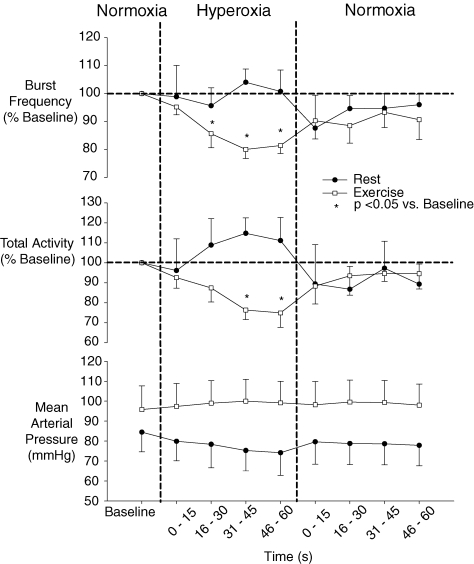
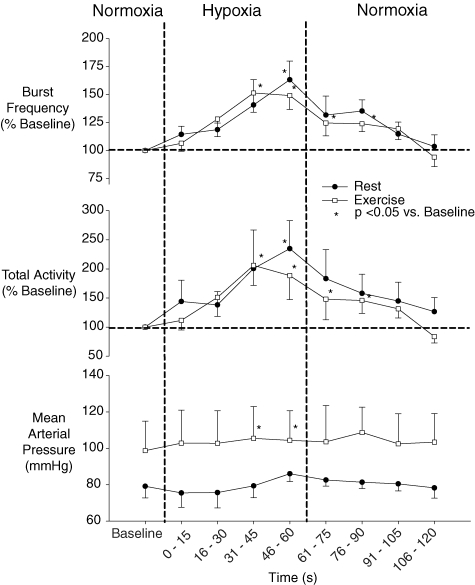
Similar to rest, hyperoxia resulted in a rapid increase in PET O2 such that PET O2 exceeded 250 mmHg within 2.3 ± 0.2 breaths or 6.9 ± 0.7 s. In contrast to rest, transient hyperoxia during exercise resulted in a significant reduction from baseline exercise steady-state values in both burst frequency and MSNA total activity. Mean burst frequency was reduced with hyperoxia beginning at the 16–30 s time-point and continuing through to the 46–60 s time-point. MSNA total activity was significantly reduced at the 31–45 s and 46–60 s time-points (see Fig. 2 and Table 2). Heart rate was reduced with hyperoxia, while blood pressure, tidal volume, breathing frequency and PET CO2 were unchanged from baseline exercise steady-state values with hyperoxia (see Table 2).
Figure 2.
Mean (± s.e.m.) MSNA and mean arterial blood pressure response to hyperoxia at rest and during exercise (n = 7).
In the return back to normoxic breathing following hyperoxia, PET O2 remained elevated above the normoxic baseline for 60 s after hyperoxia onset and thereafter returned to < 120 mmHg. MSNA burst frequency and total activity were not significantly different from control during the normoxic period following hyperoxia.
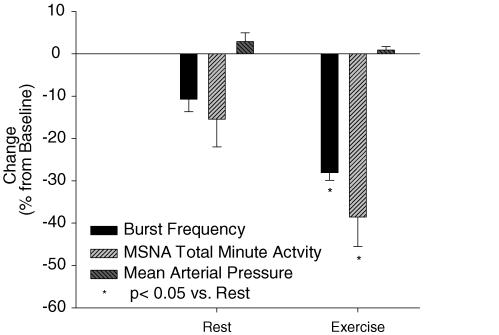
The nadir 15 s burst frequency and total MSNA value within each 60 s period of hyperoxia was also determined for each trial and reported in Fig. 3. At rest, on average, the nadir 15 s value occurred during the 30–45 s time-point of hyperoxia. At rest, the nadir values for burst frequency and MSNA total activity were 11% and 15%, respectively, below baseline. The nadir 15 s MSNA value while breathing hyperoxia during exercise also occurred, on average, during the 30–45 s time-point and averaged −28% below normoxic control for burst frequency and −39% for MSNA total activity. The maximum decrease in MSNA was significantly greater during exercise as compared to rest. Blood pressure recorded during the same period at the lowest burst frequency and MSNA total activity was not different from baseline either at rest or during exercise.
Figure 3.
Mean nadir burst frequency and MSNA total minute activity with hyperoxia, as a percentage of steady-state baseline (N = 7). Note: change in mean arterial pressure is the difference between steady-state baseline and the pressure obtained at the same time as the nadir in MSNA.
Effects of hypoxia at rest and during exercise
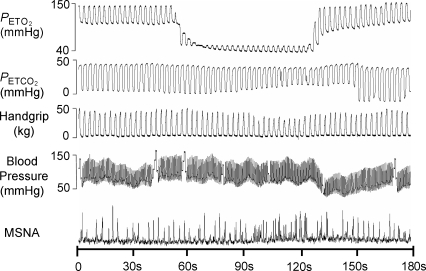
A representative trace of an exercise hypoxia trial is illustrated in Fig. 4 and mean responses at rest and during exercise are detailed in Tables 3 and 4, and Fig. 5. Hypoxia reduced PET O2 below 50 mmHg within 21.7 ± 1.3 s (8.0 ± 0.4 breaths) following the switch from room air to hypoxia at rest and within 26.2 ± 4.3 s (10.1 ± 1.6 breaths) during exercise. Despite feedback to voluntarily maintain tidal volume, tidal volume and consequently minute ventilation increased with hypoxia both at rest and during exercise. Heart rate was increased during both rest and exercise with hypoxia, while blood pressure increased with hypoxia only during exercise.
Figure 4.
Representative trace of a subject breathing a hypoxic gas mixture for ∼60 s during exercise.
Table 3.
Cardiorespiratory data during hypoxia trials at rest (N = 6)
| Normoxia | Hypoxia | Return to normoxia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–15 s | 16–30 s | 31–45 s | 46–60 s | 0–15 s | 16–30 s | 31–45 s | 46–60 s | ||
| Heart rate | 66 | 65 | 69 | 74* | 74* | 75* | 71 | 69 | 69 |
| (beats min−1) | 5 | 5 | 6 | 7 | 7 | 7 | 6 | 6 | 6 |
| Mean arterial pressure | 79.1 | 75.5 | 75.7 | 79.4 | 86.0 | 82.6 | 81.3 | 80.5 | 78.2 |
| (mmHg) | 6.5 | 7.9 | 8.4 | 6.5 | 4.2 | 3.4 | 3.5 | 3.9 | 5.6 |
| Tidal volume | 491 | 501 | 581 | 636* | 646* | 659* | 547 | 544 | 542 |
| (ml) | 62 | 54 | 42 | 55 | 58 | 69 | 76 | 51 | 56 |
| Breathing frequency | 20 | 19 | 20 | 20 | 20 | 21 | 20 | 20 | 20 |
| (breaths min−1) | 0.4 | 1.1 | 0.2 | 0.3 | 0.7 | 0.8 | 1.2 | 0.5 | 0.2 |
| Minute ventilation | 9.7 | 9.7 | 11.7 | 12.8* | 12.9* | 13.1* | 10.9 | 10.7 | 11.0 |
| (l min−1) | 1.3 | 1.3 | 0.9 | 1.2 | 1.4 | 1.5 | 1.7 | 1.1 | 1.1 |
| End-tidal PO2 | 101 | 57* | 49* | 44* | 41* | 54* | 74 | 81 | 87 |
| (mmHg) | 6 | 2 | 2 | 2 | 1 | 5 | 4 | 4 | 4 |
| End-tidal PCO2 | 41 | 41 | 40 | 39 | 39 | 40 | 42 | 42 | 42 |
| (mmHg) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 |
| SpO2 | 96 | 96 | 93* | 89* | 85* | 83* | 88* | 93 | 95 |
| (%) | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 |
| Burst frequency | 26 | 29 | 31 | 37 | 42* | 35 | 35 | 29 | 27 |
| (bursts min−1) | 2 | 3 | 3 | 8 | 6 | 7 | 5 | 5 | 5 |
| Total minute actvity | 4.2 | 5.0 | 6.4 | 9.6 | 12.8* | 8.1 | 6.9 | 6.0 | 5.0 |
| (units) | 1.0 | 1.3 | 1.8 | 4.7 | 5.2 | 3.4 | 1.7 | 2.3 | 1.4 |
Values are mean (± s.e.m., value below).
Note: P < 0.05 versus baseline.
Note: blood pressure at rest N = 4.
Table 4.
Cardiorespiratory data during hypoxia trials during exercise (N = 6)
| Rest Normoxia | Exercise Normoxia | Exercise Hyperoxia | Exercise Return to normoxia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–15 s | 16–30 s | 31–45 s | 46–60 s | 0–15 s | 16–30 s | 31–45 s | 46–60 s | |||
| Heart rate | 66 | 78† | 79 | 82 | 86* | 87* | 87* | 82 | 79 | 78 |
| (beats min−1) | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 4 | 4 |
| Mean arterial pressure | 79.1 | 99† | 103 | 103 | 105* | 104* | 104 | 103 | 102 | 103 |
| (mmHg) | 6.5 | 16 | 18 | 18 | 17 | 16 | 20 | 14 | 17 | 16 |
| Tidal volume | 491 | 666† | 692 | 762 | 834* | 866* | 860* | 764 | 709 | 713 |
| (ml) | 62 | 69 | 56 | 36 | 65 | 56 | 74 | 82 | 74 | 63 |
| Breathing frequency | 20 | 20 | 20 | 20 | 21 | 21 | 21 | 20 | 21 | 20 |
| (breaths min−1) | 0.4 | 0.1 | 0.1 | 0.3 | 0.5 | 0.7 | 0.5 | 0.2 | 0.3 | 0.2 |
| Minute ventilation | 9.7 | 13.3† | 14.0 | 15.4* | 16.9* | 17.9* | 17.2* | 15.3 | 14.3 | 14.2 |
| (l min−1) | 1.3 | 1.4 | 1.1 | 0.7 | 1.2 | 1.1 | 1.3 | 1.7 | 1.5 | 1.2 |
| End-tidal PO2 | 101 | 100 | 58* | 48* | 43* | 41* | 60* | 78 | 89 | 94 |
| (mmHg) | 6 | 1 | 1 | 2 | 1 | 1 | 6 | 5 | 3 | 3 |
| End-tidal PCO2 | 41 | 41 | 41 | 40 | 40 | 39 | 40 | 43 | 42 | 42 |
| (mmHg) | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| SpO2 | 96 | 97 | 97 | 93* | 88* | 85* | 85* | 92* | 95 | 97 |
| (%) | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 0 |
| Burst frequency | 26 | 29 | 30 | 37 | 41* | 40* | 36* | 37* | 35 | 26 |
| (bursts min−1) | 2 | 3 | 2 | 5 | 7 | 5 | 3 | 3 | 4 | 2 |
| Total minute actvity | 4.2 | 7.5† | 7.8 | 12.6 | 15.5* | 14.8* | 10.1* | 10.2* | 9.3 | 6.3 |
| (units) | 1.0 | 1.7 | 1.8 | 5.3 | 6.6 | 3.7 | 2.0 | 2.0 | 2.0 | 1.9 |
Values are mean (± s.e.m., value below).
Note: P < 0.05 versus steady-state baseline at rest.
P < 0.05 versus baseline during exercise.
Figure 5.
Mean MSNA and mean arterial pressure response breathing a hypoxic gas mixture at rest and during exercise (N = 6). Note: mean arterial pressure at rest N = 4.
Transient hypoxaemia resulted in an abrupt increase in burst frequency and MSNA total activity both at rest and during exercise. Resting values were significantly increased above baseline by the 46–60 s time-point following hypoxia onset, whereas exercise values were significantly increased above steady-state baseline at the 31–45 s and 46–60 s time-points. As a percentage of baseline, there were no differences between rest and exercise in the increase in MSNA from hypoxia (see Fig. 5). For both conditions, PET O2 returned to baseline after 15 s of breathing room air. At rest, MSNA was not significantly different from control during the normoxic period following hypoxia. However, during exercise MSNA remained elevated above baseline for 30 s during the normoxic period following hypoxia.
Discussion
Inhibition of the carotid chemoreceptors with hyperoxia resulted in a decrease in MSNA burst frequency and total minute activity during exercise, but not at rest. The MSNA response was rapid and had a similar time-course to chemoreceptor stimulation with hypoxia, indicating the reduction in MSNA from hyperoxia during exercise was secondary to CC inhibition. Consistent with our previous animal experiments (Stickland et al. 2007), these results support the hypothesis that the CCs contribute significantly to sympathetic vasoconstrictor outflow during exercise in humans.
Evidence for carotid chemoreceptor mediation of the MSNA response to transient hyperoxia
The CCs are considered the major oxygen sensor in the body. The aortic chemoreceptors are also sensitive to changes in PO2, although previous work in cats has shown that the aortic chemoreceptor responsiveness to PO2 is substantially smaller than the CC (Lahiri et al. 1981). More importantly, carotid body denervation in patients free of pulmonary disease abolishes the ventilatory response to hypoxia (Timmers et al. 2003). Similarly, carotid body denervation virtually abolishes the ventilatory (Olson et al. 1988; Curran et al. 2000) and sympathetic (Balkowiec et al. 1993) response to hypoxia in animals. These results support our interpretation that the effects of short-term hyperoxia on MSNA occur as a result of CC inhibition.
However, brain hypoxia per se, if sufficiently severe, is known to increase sympathetic outflow (Sun & Reis, 1994a). Therefore it is possible that inhibition of central hypoxic sensors in the rostral ventrolateral medulla could play a role in the observed reduction in MSNA with hyperoxia. However, we think our data strongly support a role for the CC as the primary mediator of our observed reductions in MSNA in response to transient hyperoxia. In the current study, PET O2 exceeded 250 mmHg, a value known to cause substantial suppression of CC activity (Eyzaguirre & Lewin, 1961a), within 7 s of breathing hyperoxic gas during exercise. In turn, MSNA burst frequency was reduced by the 16–30 s time period, indicating a reduction in MSNA within 9–23 s of CC inhibition. These delay times coincide with those from the lung to the CC estimated in the healthy human, based on the time to ventilatory response following a step increase in end-tidal CO2 (Sebert et al. 1990; Solin et al. 2000) or the lung-to-ear circulation time (Xie et al. 2006). Carotid body denervation or isolation will increase the delay time 1.5- to 2-fold beyond those in the control animal (Sun & Reis, 1994b; Nakayama et al. 2003; Smith et al. 2006). Furthermore, we think it highly unlikely that transient central hyperoxia, per se, would inhibit MSNA relative to normoxia because: (a) substantial levels of hypoxaemia are apparently required to stimulate sympathetic outflow (Sun & Reis, 1994a) or ventilation (Curran et al. 2000) via central mechanisms; and (b) central hyperoxia, in isolation, causes substantial increases – not decreases – in ventilation (Dean et al. 2004) (see further discussion on central hyperoxia below).
Hypoxic trials were also performed at rest and during exercise to examine the time-course of the chemoreceptor response. End-tidal O2 decreased below 50 mmHg within 26.2 ± 4.3 s of breathing hypoxia during exercise, and MSNA was increased with hypoxia by the 31–45 s time-point, indicating that MSNA was increased within 21 s of CC stimulation with hypoxic gas. The timing of the sympathetic response to CC stimulation from hypoxia during exercise was virtually identical to the hyperoxic MSNA response, suggesting that both MSNA responses were mediated via CC modulation.
MSNA during exercise can be modulated by muscle metaboreceptors, muscle mechanoreceptors, baroreceptors and/or central command (Rowell & O'Leary, 1990); therefore, an important question is whether these reflexes were altered by hyperoxia. Blood pressure did not change with transient hyperoxia, indicating that baroreceptor feedback was unaltered with hyperoxia. Subjects maintained steady-state exercise at carefully controlled workloads and thus input from muscle metabo- and mechanoreceptors or central command were unlikely to change substantially within each trial. The increased arterial oxygen content during hyperoxia would increase O2 delivery to working muscle and this may have reduced feedback from muscle metaboreceptor afferents. However, findings in anaesthetized animals have shown that during contraction, afferent nerve traffic is either unaffected in most metaboreceptor afferents (Hill et al. 1992), or even demonstrates a decrease in activity (Arbogast et al. 2000) in response to severe hypoxia (Pa,O2= 22–38 mmHg). Furthermore, Sheriff et al. (1987) have shown that the metaboreflex is not triggered in exercising muscle until blood flow was reduced to the point where lactic acid was produced. Therefore it is doubtful that a 2–3% transient increase in arterial content as imposed in our study would cause a rapid metaboreceptor-induced reduction in MSNA. Again, the relatively short latency of the reduction in MSNA in response to hyperoxia speaks against an effect of systemic O2 transport on muscle metabolism.
In summary, the previous studies documenting a rapid CC response to a change in PO2, our similar MSNA time-course of response to hypoxia, combined with the unlikely effect of rapid hyperoxia on aortic or central chemoreceptors, muscle metaboreceptors, mechanoreceptors, baroreceptors and central command, support the interpretation that the reduction in MSNA during exercise with hyperoxia is most probably secondary to CC inhibition.
Effect of hyperoxia on MSNA: previous work
Our finding that inspired hyperoxia applied transiently reduced MSNA during exercise is at odds with previous reports of steady-state hyperoxia (Seals et al. 1991a; Houssiere et al. 2006). We believe the time-dependent effects of hyperoxia are likely explanations for these divergent findings. Houssiere et al. (2006), who demonstrated an increased MSNA response to exercise with hyperoxia, had subjects inspire a hyperoxic gas mixture for 15 min, while Seals et al. (1991a), who showed a reduction in MSNA at rest with hyperoxia, but no effect during exercise, had subjects breathe hyperoxia for 3–4 min. In contrast, we studied the transient effect of oxygen because we were interested in the influence of the CC alone on MSNA. Examining the transient effects helps to minimize the influence of secondary, time-dependent influences on the steady-state cardiovascular response (Britton & Metting, 1999). Therefore, comparisons to previous reports that used prolonged hyperoxic exposure cannot easily be made because these studies only provide data obtained during steady-state hyperoxia. Importantly, prolonged exposure to hyperoxia can act as a central stimulant (Dean et al. 2004), manifested by substantial dose-dependent increases in minute ventilation (Becker et al. 1996) following an initial transient reduction in ventilation. Indeed, previous work in humans has shown that minute ventilation begins to increase above baseline after ∼4 min of hyperoxia and continues to increase with further hyperoxic exposure (Becker et al. 1995). It was for this reason that we administered hyperoxia for only 1 min in the present study and conducted a time-course analysis of the MSNA response. Thus, is it likely that the secondary central stimulatory effect of prolonged hyperoxia explains why previous studies showed either no effect (Seals et al. 1991a) or an exaggerated MSNA response to hyperoxia during exercise (Houssiere et al. 2006).
Hypoxia trials
Hypoxia rapidly increased MSNA burst frequency and total minute activity at rest and during exercise. Our findings are consistent with previous animal work demonstrating rapid sympathoexcitation with systemic hypoxia (Sun & Reis, 1994b) and are also consistent with the < 20 s delay observed in the increases in MSNA induced following apnoea onset in humans (Morgan et al. 1993). These rapid, transient increases in MSNA are probably mediated primarily via hypoxia-induced stimulation of the CCs (Balkowiec et al. 1993; Guyenet, 2000). Despite subjects being encouraged to maintain breathing frequency and tidal volume, similar proportional increases in both tidal volume and minute ventilation were observed at rest and during exercise beginning after 30 s of hypoxia. This hyperpnoea, by itself, would be expected to reduce MSNA slightly via lung stretch (Dempsey et al. 2002); thus, the full effect of hypoxaemia on MSNA was probably underestimated.
We observed that the percentage increase in MSNA with transient hypoxaemia was similar at rest and during exercise. These data are not consistent with the marked increases in ventilatory (Weil et al. 1972) and MSNA (Seals et al. 1991b) responsiveness to hypoxia previously reported during exercise. Our discrepant findings are probably explained by our limited design in which only very transient hypoxia was used at a single dose for the purpose of comparing the time-course of the MSNA excitation with the time-course of inhibition with hyperoxia. To adequately address whether MSNA responsiveness to hypoxia is altered by exercise it would be important to use an adequate dose–response design and to include exposures of sufficient duration to ensure a maximum response to each level of hypoxia.
Exercise-induced chemosensitization
Blood lactate did not increase with exercise, indicating no metabolic acidosis and therefore no apparent increase in circulating CC stimuli. However, CC inhibition resulted in a reduction in MSNA during exercise, but not at rest, which would suggest an exercise-induced sensitization of the CC reflex, i.e. an increased responsiveness (to transient hyperoxia) during exercise in the face of no apparent change in CC stimuli. This hypothesis, based on MSNA changes in exercising humans, is consistent with the effects of specific CC inhibition on limb vascular conductance in dogs during mild-intensity exercise (Stickland et al. 2007). The exact mechanisms for CC sensitization during mild-intensity exercise are unclear, but may occur at the level of the chemoreflex or centrally at the level of integration. For example, exercise-induced sympathoexcitation may increase vasoconstrictor outflow to the CC vasculature, leading to stagnant hypoxia and therefore increased chemoreflex stimulation (Eyzaguirre & Lewin, 1961b; Biscoe & Purves, 1967; Acker & O'Regan, 1981; O'Regan, 1981). Increased circulating levels of angiotensin II may also explain the enhanced CC sensitivity, as angiotensin II is elevated with exercise (Maher et al. 1975) and is a CC stimulant (Allen, 1998; Li et al. 2006). Alternatively, as is the case with exercise-induced resetting of the baroreflex (Raven et al. 2006), the somatic inputs from exercising muscle and/or central command might interact at the level of the nucleus tractus solitarius to enhance the effect of a tonic sensory imput from the CC. Given the increases with incremental exercise in both circulating CC stimuli such as H+, K+, norepinephrine (noradrenaline) and temperature, as well as somatic feedback from locomotor muscles and increased central command, we would anticipate an increasing contribution from CC input to sympathoexcitation with increasing exercise intensity.
Conclusion
When ventilation is tightly controlled, transient CC inhibition with hyperoxia reduces MSNA during exercise. These findings support previous experiments in animals (Stickland et al. 2007) demonstrating an important role for the CC in the sympathetically mediated control of vascular conductance in exercising muscle. The relative importance of the CC to sympathoexcitation and regulation of exercise blood flow in humans both in health and disease remains to be determined, as does the mechanism for the exercise-induced sensitization of the CC.
Acknowledgments
This material is based upon work supported, in part, by the Office of Research and Development, Clinical Science R & D Service, Department of Veterans Affairs. This research was also supported by grants from the NHLBI and the American Heart Association. M.K.S. was supported, in part, by a Natural Sciences and Engineering Council of Canada (NSERC) postdoctoral fellowship. We acknowledge the technical assistance of Dominic Puleo and David Pegelow. We are especially grateful to Paul Rutecki, MD for his support.
References
- Acker H, O'Regan RG. The effects of stimulation of autonomic nerves on carotid body blood flow in the cat. J Physiol. 1981;315:99–110. doi: 10.1113/jphysiol.1981.sp013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal D, Milhorn HT, Jr, Lee LY. Role of the carotid chemoreceptors in the hyperpnea of exercise in the cat. Respir Physiol. 1976;26:147–155. doi: 10.1016/0034-5687(76)90092-x. [DOI] [PubMed] [Google Scholar]
- Allen AM. Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity. J Physiol. 1998;510:773–781. doi: 10.1111/j.1469-7793.1998.773bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast S, Vassilakopoulos T, Darques JL, Duvauchelle JB, Jammes Y. Influence of oxygen supply on activation of group IV muscle afferents after low-frequency muscle stimulation. Muscle Nerve. 2000;23:1187–1193. doi: 10.1002/1097-4598(200008)23:8<1187::aid-mus5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Revenko S, Szulczyk P. Reflex carotid body chemoreceptor control of phrenic sympathetic neurons. Respir Physiol. 1993;92:91–100. doi: 10.1016/0034-5687(93)90122-q. [DOI] [PubMed] [Google Scholar]
- Becker H, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE. Ventilatory response to isocapnic hyperoxia. J Appl Physiol. 1995;78:696–701. doi: 10.1152/jappl.1995.78.2.696. [DOI] [PubMed] [Google Scholar]
- Becker HF, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE. Effect of different levels of hyperoxia on breathing in healthy subjects. J Appl Physiol. 1996;81:1683–1690. doi: 10.1152/jappl.1996.81.4.1683. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Purves MJ. Factors affecting the cat carotid chemoreceptor and cervical sympathetic activity with special reference to passive hind-limb movements. J Physiol. 1967;190:425–441. doi: 10.1113/jphysiol.1967.sp008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetger CL, Ward DS. Effect of dopamine on transient ventilatory response to exercise. J Appl Physiol. 1986;61:2102–2107. doi: 10.1152/jappl.1986.61.6.2102. [DOI] [PubMed] [Google Scholar]
- Britton SL, Metting PJ. Reflex regulation of skeletal muscle blood flow. In: Zucker IH, Gilmore JP, editors. Reflex Control of the Circulation. Boca Raton: CRC Press; 1999. pp. 736–764. [Google Scholar]
- Buckwalter JB, Clifford PS. α-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol Heart Circ Physiol. 1999;277:H33–H39. doi: 10.1152/ajpheart.1999.277.1.H33. [DOI] [PubMed] [Google Scholar]
- Curran AK, Rodman JR, Eastwood PR, Henderson KS, Dempsey JA, Smith CA. Ventilatory responses to specific CNS hypoxia in sleeping dogs. J Appl Physiol. 2000;88:1840–1852. doi: 10.1152/jappl.2000.88.5.1840. [DOI] [PubMed] [Google Scholar]
- Davies RO, Lahiri S. Absence of carotid chemoreceptor response during hypoxic exercise in the cat. Respir Physiol. 1973;18:92–100. doi: 10.1016/0034-5687(73)90024-8. [DOI] [PubMed] [Google Scholar]
- Dean JB, Mulkey DK, Henderson RA, 3rd, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol. 2004;96:784–791. doi: 10.1152/japplphysiol.00892.2003. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Lewin J. Chemoreceptor activity of the carotid body of the cat. J Physiol. 1961a;159:222–237. doi: 10.1113/jphysiol.1961.sp006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C, Lewin J. The effect of sympathetic stimulation on carotid nerve activity. J Physiol. 1961b;159:251–267. doi: 10.1113/jphysiol.1961.sp006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Dempsey JA, Vidruk E, Do Pico G. Evidence of altered regulation of ventilation during exposure to hypoxia. Respir Physiol. 1974;20:379–392. doi: 10.1016/0034-5687(74)90034-6. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol. 2000;121:147–162. doi: 10.1016/s0034-5687(00)00125-0. [DOI] [PubMed] [Google Scholar]
- Henson LC, Ward DS, Whipp BJ. Effect of dopamine on ventilatory response to incremental exercise in man. Respir Physiol. 1992;89:209–224. doi: 10.1016/0034-5687(92)90051-w. [DOI] [PubMed] [Google Scholar]
- Hill JM, Pickar JG, Parrish MD, Kaufman MP. Effects of hypoxia on the discharge of group III and IV muscle afferents in cats. J Appl Physiol. 1992;73:2524–2529. doi: 10.1152/jappl.1992.73.6.2524. [DOI] [PubMed] [Google Scholar]
- Houssiere A, Najem B, Cuylits N, Cuypers S, Naeije R, van de Borne P. Hyperoxia enhances metaboreflex sensitivity during static exercise in humans. Am J Physiol Heart Circ Physiol. 2006;291:H210–H215. doi: 10.1152/ajpheart.01168.2005. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol. 1992;263:H1078–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mokashi A, Mulligan E, Nishino T. Comparison of aortic and carotid chemoreceptor responses to hypercapnia and hypoxia. J Appl Physiol. 1981;51:55–61. doi: 10.1152/jappl.1981.51.1.55. [DOI] [PubMed] [Google Scholar]
- Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res. 2006;71:129–138. doi: 10.1016/j.cardiores.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Maher JT, Jones LG, Hartley LH, Williams GH, Rose LI. Aldosterone dynamics during graded exercise at sea level and high altitude. J Appl Physiol. 1975;39:18–22. doi: 10.1152/jappl.1975.39.1.18. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol. 1993;74:2969–2975. doi: 10.1152/jappl.1993.74.6.2969. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Carotid body denervation eliminates apnea in response to transient hypocapnia. J Appl Physiol. 2003;94:155–164. doi: 10.1152/japplphysiol.00722.2002. [DOI] [PubMed] [Google Scholar]
- Nye PC, Hanson MA, Torrance RW. The effect on breathing of abruptly stopping carotid body discharge. Respir Physiol. 1981;46:309–326. doi: 10.1016/0034-5687(81)90129-8. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. J Appl Physiol. 1988;64:666–671. doi: 10.1152/jappl.1988.64.2.666. [DOI] [PubMed] [Google Scholar]
- O'Regan RG. Responses of carotid body chemosensory activity and blood flow to stimulation of sympathetic nerves in the cat. J Physiol. 1981;315:81–98. doi: 10.1113/jphysiol.1981.sp013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Rutherford JD, Vatner SF. Integrated carotid chemoreceptor and pulmonary inflation reflex control of peripheral vasoactivity in conscious dogs. Circ Res. 1978;43:200–208. doi: 10.1161/01.res.43.2.200. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.res.85.5.457. [DOI] [PubMed] [Google Scholar]
- Seals DR, Johnson DG, Fregosi RF. Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol Regul Integr Comp Physiol. 1991a;260:R873–R878. doi: 10.1152/ajpregu.1991.260.5.R873. [DOI] [PubMed] [Google Scholar]
- Seals DR, Johnson DG, Fregosi RF. Hypoxia potentiates exercise-induced sympathetic neural activation in humans. J Appl Physiol. 1991b;71:1032–1040. doi: 10.1152/jappl.1991.71.3.1032. [DOI] [PubMed] [Google Scholar]
- Sebert P, Barthelemy L, Mialon P. CO2 chemoreflex drive of ventilation in man: effects of hyperoxia and sex differences. Respiration. 1990;57:264–267. doi: 10.1159/000195853. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol. 1987;253:H1199–H1207. doi: 10.1152/ajpheart.1987.253.5.H1199. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res. 2007;100:1371–1378. doi: 10.1161/01.RES.0000266974.84590.d2. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol. 1994a;44:197–219. doi: 10.1016/0301-0082(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Hypoxia selectively excites vasomotor neurons of rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol. 1994b;266:R245–R256. doi: 10.1152/ajpregu.1994.266.1.R245. [DOI] [PubMed] [Google Scholar]
- Taha BH, Simon PM, Dempsey JA, Skatrud JB, Iber C. Respiratory sinus arrhythmia in humans: an obligatory role for vagal feedback from the lungs. J Appl Physiol. 1995;78:638–645. doi: 10.1152/jappl.1995.78.2.638. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Wieling W, Marres HA, Folgering HT, Lenders JW. Baroreflex and chemoreflex function after bilateral carotid body tumor resection. J Hypertens. 2003;21:591–599. doi: 10.1097/00004872-200303000-00026. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Weil JV, Byrne-Quinn E, Sodal IE, Kline JS, McCullough RE, Filley GF. Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol. 1972;33:813–819. doi: 10.1152/jappl.1972.33.6.813. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol. 2006;100:171–177. doi: 10.1152/japplphysiol.00440.2005. [DOI] [PubMed] [Google Scholar]