Abstract
Metabotropic glutamate receptors (mGluRs) have been implicated in a diverse variety of neuronal functions. Studies reviewed here indicate that exaggerated signalling through mGluR5 can account for multiple cognitive and syndromic features of fragile X syndrome, the most common inherited form of mental retardation and autism. Since a reduction of mGluR5 signalling can reverse fragile X phenotypes, these studies provide a compelling rationale for the use of mGluR5 antagonists for the treatment of fragile X and related disorders.
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. In addition to ligand-gated ion channels, glutamate signals through G-protein-coupled metabotropic receptors (mGluRs). Signalling by group 1 (Gp1) mGluRs occurs postsynaptically via Gq second messenger cascades, and it is now clear that one consequence at many synapses is stimulation of local protein synthesis (Weiler & Greenough, 1993; Weiler et al. 1997). The machinery for protein synthesis is localized to the base of dendritic spines, and protein synthesis is thought to be a requirement for stable, enduring modification of the synapse (Steward & Schuman, 2003). It is perhaps not surprising then, that activation of GpI mGluRs can have lasting functional and even structural consequences, and that many of these consequences have been found to be dependent on the translation of new proteins (Merlin et al. 1998; Huber et al. 2000; Raymond et al. 2000; Snyder et al. 2001; Vanderklish & Edelman, 2002; Naie & Manahan-Vaughan, 2005).
Gp1 mGluRs have recently been implicated in the pathogenesis of fragile X syndrome (FXS), the leading inherited cause of mental retardation and an identified cause of autism (Dolen et al. 2007). FXS is caused by a mutation that leads to transcriptional silencing of the FMR1 gene, which encodes the fragile X mental retardation protein (FMRP) (Pieretti et al. 1991). FMRP functions as a negative regulator of protein synthesis (Li et al. 2001; Qin et al. 2005a; Dolen et al. 2007). In the absence of the FMR protein, human patients with FXS have both cognitive (moderate to severe mental retardation), as well as syndromic impairments (behavioural problems, dysmorphic features and seizure disorder) which characterize the disease (Hagerman & Hagerman, 2002).
Since synaptic plasticity is the foundation of most theories of cognitive function in the brain, early studies examined a possible deficit in hippocampal synaptic plasticity in the Fmr1 knock-out (KO) mouse model of the disease. However, these initial results were disappointing, since both protein synthesis-independent and -dependent forms of hippocampal long-term potentiation (LTP) were normal in the Fmr1 KO (Godfraind et al. 1996; Paradee et al. 1999). It was later discovered, however, that a novel form of long-term depression (LTD) in the hippocampus (Huber et al. 2000) was exaggerated in the Fmr1 KO (Huber et al. 2002). Unlike the NMDA receptor-dependent forms of plasticity examined previously (Godfraind et al. 1996; Paradee et al. 1999), this synaptic depression is induced by activation of GpI mGluRs (mGluR-LTD), and is normally protein synthesis dependent. Moreover, this LTD phenotype in the Fmr1 KO does not represent a general increase in synaptic depression, since the classical NMDA receptor-dependent form of LTD is normal in these mice. These results inspired both excitement and controversy.
Previous studies had shown that stimulation of synaptic GpI mGluRs with dihydroxyphenylglycine (DHPG) leads to translation of FMRP at synapses in vitro (Weiler et al. 1997), raising the possibility that FMRP might be necessary for effecting down-stream consequences of GpI mGluR activation. In contrast, the finding that mGluR-LTD is exaggerated in Fmr1 KO mice suggested that instead, FMRP and GpI mGluRs might work in functional opposition, where GpI mGluRs activate protein synthesis and FMRP suppresses it.
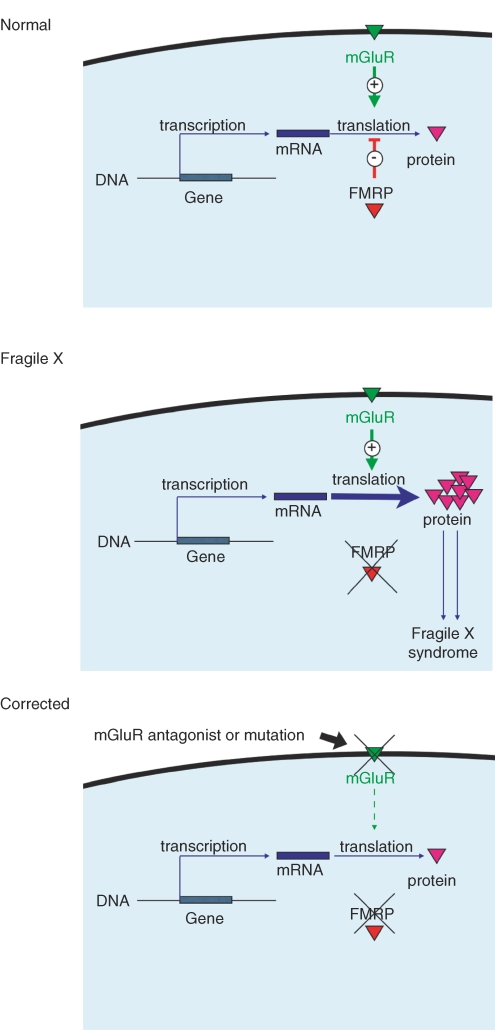
Excitement for the latter model grew with (1) the recognition of a number of parallels between phenotypic features of the disease and predicted or known consequences of (over) activation of GpI mGluRs and (2) the proposed mechanistic link between FMRP and GpI mGluRs at the level of protein synthesis regulation. These ideas were outlined in what is now known as ‘the mGluR theory of fragile X’ (Bear et al. 2004) which made the prediction that widespread phenotypic features of FXS could be corrected by down-regulation of GpI mGluRs (see Fig. 1).
Figure 1. Model for the pathogenesis and correction of FXS.
The mGluR theory of fragile X posits that mGluR5 and FMRP regulate translation of mRNA at the synapse in a functionally opponent manner – mGluR5 activation initiates protein synthesis and FMRP suppresses it. In the absence of FMRP, as is the case in FXS, mGluR5-dependent protein synthesis proceeds unchecked, and consequent excessive translation leads to the diversity of clinical features that make up the syndrome. Our results demonstrate that this progression can be corrected by genetic reduction of mGluR5 activity.
Tests of the mGluR theory
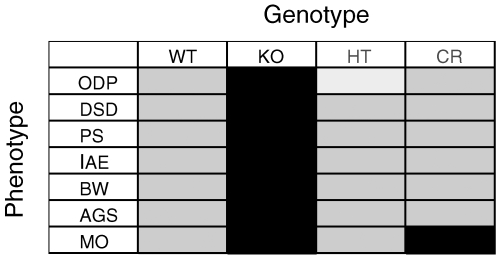
In order to test the mGluR theory, we recently made double mutant mice by crossing Grm5 (encodes mGluR5) mutant mice (Lu et al. 1997) with Fmr1 mutant mice (Consortium, 1994), which yielded male littermates of four different genotypes: wild-type (WT) [Fmr1 (+/Y) Grm5 (+/+)], Fmr1 knockout [Fmr1 (−/Y) Grm5 (+/+)], Grm5 heterozygote [Fmr1 (+/Y) Grm5 (+/−)], and the double heterozygote cross [Fmr1 (−/Y) Grm5 (+/−)]; these animals are termed WT, KO, HT and CR, respectively. Using these mice we established a number of different Fmr1 KO phenotypes relevant to the disorder and examined these in the context of mGluR5 knockdown (Dolen et al. 2007). The phenotypes examined can be broadly categorized into either cognitive or syndromic features of the disease; cognitive phenotypes include ocular dominance plasticity (ODP), dendritic spine density (DSD), and inhibitory avoidance extinction (IAE). Phenotypes of syndromic features include audiogenic seizure (AGS), body weight (BW), and macroorchidism (MO). In addition, these studies examined the role of FMRP and mGluR5 in the regulation of protein synthesis (PS). As summarized in Fig. 2, in all cases but one (MO), genetic reduction of mGluR5 signalling by 50% returned Fmr1 KO phenotypes significantly closer to WT, substantiating the opponent regulatory role for mGluR5 and FMRP (Dolen et al. 2007).
Figure 2. Genetic rescue of FXS phenotypes.
Seven measures of cognitive and syndromic function were assayed in animals of four genotypes as a test of the mGluR theory of FXS. In each of the seven measures (ocular dominance plasticity (ODP), dendritic spine density (DSD), inhibitory avoidance extinction (IAE), audiogenic seizure (AGS), body weight (BW) and macroorchidism (MO)) Fmr1 KO mice showed exaggerated responses (dark grey). Six out of 7 fragile X phenotypes were brought significantly closer to wild type levels (light grey) by Grm5 knockdown in the CR mice. Mice with a 50% reduction in mGluR5 signalling were not significantly different from WT in all cases, except for ODP where they showed reduced levels of plasticity (white). These results suggest that fragile X is a syndrome of excess, which can be corrected by decreasing mGluR5 signalling.
Ocular dominance plasticity
It has been known for many years that Gp1 mGluR signalling is highest in visual cortex during the period of maximal ODP (Dudek & Bear, 1989); these studies suggested that postnatal down-regulation of mGluR signalling might be important for the normal maturation and synaptic stabilization of the brain. However, experiments investigating the role of mGluRs in monocular deprivation (MD)-induced plasticity using drug treatments were inconclusive (cf. Hensch & Stryker, 1996; Huber et al. 1998).
Recently, using the molecular genetic approach, we have shown an important role for mGluRs, as well as FMRP, in the regulation of ODP during development (Dolen et al. 2007). A 50% reduction in mGluR5 expression prevents ocular dominance plasticity induced by 3 days of MD (which is sufficient to trigger depression of deprived-eye responses in WT mice), suggesting that this receptor normally serves to enable plasticity in the visual cortex. In contrast, in the absence of FMRP, Fmr1 KO mice show altered ODP that we interpret as ‘hyperplasticity.’ The response to MD is characterized by both deprived-eye response depression and open-eye response potentiation (which is seen in WT mice only after longer deprivation durations), suggesting that this protein normally serves to restrict plasticity in the visual cortex. This exaggerated plasticity phenotype seen in Fmr1 KO mice is reversed in CR mice, consistent with opponent regulation of this process by FMRP and mGluR5. Interestingly, ODP is known to be protein synthesis dependent (Taha & Stryker, 2002); it is tempting to speculate that like mGluR-LTD in the hippocampus (Nosyreva & Huber, 2006), ODP in the Fmr1 KO is protein synthesis independent, due to the mGluR5-mediated overproduction of proteins that support plasticity, and that a 50% reduction in mGluR5 expression rescues the KO phenotype by preventing the overproduction of such proteins (Dolen et al. 2007).
Dendritic spine morphology
Changes in dendritic spine shape and number are thought to be the morphological expression of physiological and biochemical synaptic modifications (Harris & Stevens, 1989; Wallace & Bear, 2004). Consistent with this idea, stimulation of Gp1 mGluRs on hippocampal neurons leads to parallel modifications of the synapse including: internalization of AMPA and NMDA receptors (Snyder et al. 2001), synaptic depression (Huber et al. 2000), and increased density of long thin spines (Vanderklish & Edelman, 2002). Significantly, each of these responses to mGluR activation requires protein synthesis.
Dendritic spine abnormalities have long been associated with mental retardation (Marin-Padilla, 1972). FXS is associated with an increase in the density of long, thin spines (Irwin et al. 2000), a phenotype that has been recapitulated in mouse models of the disease (Comery et al. 1997). Interestingly, this phenotype exactly parallels dendritic spine changes seen in response to activation of Gp1 mGluRs (Vanderklish & Edelman, 2002). We hypothesized that spine morphology is opponently regulated by FMRP and mGluR5.
To test this hypothesis, visual cortical pyramidal neurons were visualized using the Golgi-Cox staining method. This analysis confirmed that spine density is significantly increased in the KO mice and, further, that the mutant phenotype is rescued by the 50% reduction in mGluR5 expression in CR mice (Dolen et al. 2007). Interestingly, the HT mice showed no difference from WT in spine density, suggesting that while the decrease in Grm5 gene dosage was not sufficient to cause an alteration of spine density by itself, it is sufficient to rescue the increased spine density phenotype seen in the Fmr1 KO.
Behaviour: learning and memory
Ultimately, changes in the machinery of synaptic plasticity must be related to behavioural output. A role for mGluR5 in behavioural measures of learning and memory has been established using the Grm5 KO mice. These mice show impairments in spatial learning, contextual fear conditioning, and reward based learning (Lu et al. 1997; Chiamulera et al. 2001), consistent with mechanistic studies implicating these receptors in various forms of synaptic plasticity. Furthermore, studies using the mGluR5 receptor antagonist, MPEP, have shown that mGluR5 is necessary for reference and working memory performance as well as hippocampal LTP (Naie & Manahan-Vaughan, 2004), and that this effect is protein synthesis dependent (Naie & Manahan-Vaughan, 2005).
Establishing behavioural learning phenotypes in the Fmr1 KO mouse has been surprisingly difficult, particularly in light of the profound cognitive impairments seen in human patients with the disease. For example, strain-specific variation has confounded attempts to identify learning and memory phenotypes in the Fmr1 KO, since phenotypes established on the FVB clonal background have been absent in C57Bl6/J strains (Bernardet & Crusio, 2006), raising the possibility that in the FVB clonal strain, background polymorphisms aggravate Fmr1 KO phenotypes.
We discovered a learning and memory phenotype that is robustly expressed in Fmr1 KO mice on the C57Bl6/J background. Previous studies had examined inhibitory avoidance (IA) learning in Fmr1 KO mice on the FVB background (Qin et al. 2005b), but consistent with earlier reports using the Morris water maze (Dobkin et al. 2000), the IA phenotype is absent on the C57Bl6/J background (Consortium, 1994; Dolen et al. 2007). However, we went on to examine IA extinction, since this form of hippocampal learning and memory is also protein synthesis dependent (Power et al. 2006). IA extinction is exaggerated in Fmr1 KO mice, and restored to WT levels in CR mice, consistent with an opponent role for mGluR5 and FMRP in regulating learning and memory at the behavioural level (Dolen et al. 2007).
A recent study showed that IA induces long-term potentiation (LTP) of Schaffer collateral synapses in area CA1 of the hippocampus (Whitlock et al. 2006). Although neither NMDA-dependent LTP in CA1 (Godfraind et al. 1996; Paradee et al. 1999) nor IA learning (Consortium, 1994; Dolen et al. 2007) is altered in the Fmr1 KO, the increased IA extinction in Fmr1 KO mice could be due, at least in part, to excessive mGluR-dependent synaptic weakening. Indeed, like IA extinction, mGluR-LTD in the fragile X mice was reduced by reducing mGluR5 expression by 50%.
It is noteworthy that no behavioural phenotype was detected in the mGluR HT mice. Thus, while the 50% reduction of mGluR5 expression is below the threshold to disrupt IA extinction or dendritic spine density, it is sufficient to correct the fragile X phenotype. This finding suggests that partial down-regulation of mGluR5 can have therapeutic benefit without disrupting normal function (see below).
Syndromic features: epilepsy, growth, macroorchidism
In addition to synaptic plasticity, Gp1 mGluRs have been implicated in a variety of other functions. For example, agonists of Gp1 mGluRs act as convulsants in rodents (Conn & Pin, 1997) and selective Gp1 mGluR antagonists block seizures in a range of rodent models of epilepsy (Chapman et al. 2000; Yan et al. 2005). In addition, mGluR5 is a regulator of feeding behaviour, and mGluR5 antagonists are known to be appetite suppressants (Bradbury et al. 2005). Finally, GpI mGluR RNAs are abundantly expressed in the testicles, with high levels of both mGluR5 and mGluR1 expression in the seminiferous tubuli and germ cells (Storto et al. 2001); their function there is not known.
The most common neurological abnormality in FXS is epilepsy, occurring in approximately 20% of children with the disease, and presenting as seizure and EEG abnormalities (Musumeci et al. 1988). This phenotype has been robustly replicated in Fmr1 KO mice using the audiogenic seizure paradigm (Musumeci et al. 2000; Yan et al. 2005; Dolen et al. 2007). In the Fmr1 KO mice, increased seizure susceptibility is attenuated by reducing expression of mGluR5 (Dolen et al. 2007) and by receptor antagonism with MPEP (Yan et al. 2005). Interestingly, studies have shown that the generation of epileptiform bursts is both mGluR and protein synthesis dependent (Merlin et al. 1998), consistent with the opponent regulation of translation-dependent epileptogenesis by FMRP and mGluR5.
Children with FXS show accelerated prepubescent growth (Loesch et al. 1995). We were able to recapitulate this phenotype in the Fmr1 KO mouse, and showed that this phenotype is also rescued by selective reduction in mGluR5 expression (HT mice did not have altered growth compared to WT) (Dolen et al. 2007). Although it is currently not known how Gp1 mGluRs regulate growth, it is interesting to note that these receptors are highly expressed in the lateral and ventromedial hypothalamus (van den Pol et al. 1995), brain regions that are thought to be important for the endocrine regulation of growth. While it is not clear how the reduction in mGluR5 gene dosage leads to a rescue of the Fmr1 KO growth phenotype, it is clear that at the cellular level, FMRP and mGluR5 work in an opponent fashion to regulate body weight.
Finally the macroorchidism phenotype (large testicles) has been recognized for over 20 years, and occurs in over 80% of adult males with FXS (Nielsen et al. 1982). This phenotype is also robustly recapitulated in the Fmr1 KO model of the disease (Consortium, 1994; Dolen et al. 2007); however, the pathogenesis of this phenotype is unlikely to be related to mGluR5, since we were not able to correct this phenotype by either mGluR5 knockdown or knockout strategies (Dolen et al. 2007). Still the possibility remains that FMRP interacts with the other GpI mGluR, mGluR1, in the pathogenesis of this phenotype.
Therapeutic implications
Current medications used for FXS are aimed at treating symptoms of the disease without regard to underlying pathophysiology. This treatment regimen includes the usual battery of neuropsychiatric pharmocopia, including mood stabilizers, antipsychotics, anticonvulsants, antidepressants, anxiolytics and psychostimulatants. While this symptomatic approach can ameliorate certain features, often medication used for one symptom can exacerbate others. Most importantly, none of these treatments is effective in correcting cognitive impairment, arguably the most debilitating feature of the disease.
The data reviewed here provide the first real hope of global therapy for FXS – increased mGluR5 signalling provides a thread that connects diverse manifestations of the disease. Of course this model awaits validation in forthcoming clinical trials in humans. As the signalling cascade between mGluR5 and FMRP is delineated, and the theory is broadened to include other relevant neurotransmitter systems, additional targets may be identified. For now, it is important to note that metabotropic glutamate receptors are particularly amenable to pharmacological manipulation (Marino & Conn, 2006), and these studies provide compelling evidence that these receptors if targeted appropriately (i.e. with antagonists), will have significant therapeutic value for the treatment of FXS and related disorders.
Acknowledgments
This work was supported by NIMH, NICHD, FRAXA, Simons Foundation, and NRSA Fellowship (to G.D.).
References
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. ScientificWorldJournal. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford ND, Anderson J, Varney MA, Strack AM. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Nanan K, Williams M, Meldrum BS. Anticonvulsant activity of two metabotropic glutamate group I antagonists selective for the mGlu5 receptor: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), and (E)-6-methyl-2-styryl-pyridine (SIB 1893) Neuropharmacology. 2000;39:1567–1574. doi: 10.1016/s0028-3908(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. The Dutch-Belgian Fragile X Consortium. [PubMed] [Google Scholar]
- Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of Fragile X Syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. A biochemical correlate of the critical period for synaptic modification in the visual cortex. Science. 1989;246:673–675. doi: 10.1126/science.2573152. [DOI] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X Syndrome: Diagnosis, Treatment, and Research. The Johns. Hopkins University Press; 2002. [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Ocular dominance plasticity under metabotropic glutamate receptor blockade. Science. 1996;272:554–557. doi: 10.1126/science.272.5261.554. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Sawtell NB, Bear MF. Effects of the metabotropic glutamate receptor antagonist MCPG on phosphoinositide turnover and synaptic plasticity in visual cortex. J Neurosci. 1998;18:1–9. doi: 10.1523/JNEUROSCI.18-01-00001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucl Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hoang NH. Growth in stature in fragile X families: a mixed longitudinal study. Am J Med Genet. 1995;58:249–256. doi: 10.1002/ajmg.1320580311. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Structural abnormalities of the cerebral cortex in human chromosomal aberrations: a Golgi study. Brain Res. 1972;44:625–629. doi: 10.1016/0006-8993(72)90324-1. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RK. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Colognola RM, Ferri R, Gigli GL, Petrella MA, Sanfilippo S, Bergonzi P, Tassinari CA. Fragile-X syndrome: a particular epileptogenic EEG pattern. Epilepsia. 1988;29:41–47. doi: 10.1111/j.1528-1157.1988.tb05096.x. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Investigations of the protein synthesis dependency of mGluR-induced long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology. 2005;49:35–44. doi: 10.1016/j.neuropharm.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Nielsen KB, Tommerup N, Dyggve HV, Schou C. Macroorchidism and fragile X in mentally retarded males. Clinical, cytogenetic, and some hormonal investigations in mentally retarded males, including two with the fragile site at Xq28, fra(X)(q28) Hum Genet. 1982;61:113–117. doi: 10.1007/BF00274199. [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block ‘reconsolidation’ but impairs extinction: the role of re-exposure duration. Learn Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005a;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005b;135:999–1009. doi: 10.1016/j.neuroscience.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- Storto M, Sallese M, Salvatore L, Poulet R, Condorelli DF, Dell'Albani P, Marcello MF, Romeo R, Piomboni P, Barone N, Nicoletti F, De Blasi A. Expression of metabotropic glutamate receptors in the rat and human testis. J Endocrinol. 2001;170:71–78. doi: 10.1677/joe.0.1700071. [DOI] [PubMed] [Google Scholar]
- Taha S, Stryker MP. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron. 2002;34:425–436. doi: 10.1016/s0896-6273(02)00673-6. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995;362:134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. J Neurosci. 2004;24:6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci U S A. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]