Abstract
The primary control of spinal motoneurone excitability is mediated by descending monoaminergic systems, which have diffuse effects on multiple motor pools. Much of the sensory input evoked by movement is also distributed broadly to multiple joints. The muscle spindle Ia afferent system, however, is sharply focused, with Ia excitation restricted to close synergists and Ia reciprocal inhibition only shared between antagonists acting at a single joint. We studied the interaction of neuromodulatory and sensory inputs in determining the movement-related receptive field (MRRF) of motoneurones during passive joint movements of the cat hindlimb. In a decerebrate preparation with tonic monoaminergic input to the cord, the MRRFs tended to be focused for the ankle and knee extensor motor pools studied. Ankle rotation produced larger synaptic currents in ankle extensors than knee or hip rotations and knee rotation dominated input to the knee extensors. The persistent inward current (PIC) in motoneurone dendrites, which is facilitated by monoaminergic input, amplified the MRRF about 2-fold, consistent with its effects on other inputs. Acute spinal transaction markedly broadened MRRFs, with hip rotation generating large currents in both ankle and knee extensors. Spinalization also eliminated amplification of MRRFs, as expected from elimination of descending monoaminergic input. Ia reciprocal inhibition is very effective in suppressing dendritic PICs and thus provides a local and specific PIC control system to oppose the diffuse PIC facilitation from descending monoaminergic systems. The focused MRRF seen in the intact cord state would allow reciprocal inhibition to fulfil this role without undue interference from multijoint input from other afferent systems.
A neuron's output is generated by the interaction between its electrical properties and its synaptic inputs. Synaptic inputs with neuromodulatory actions, however, alter the electrical properties of neurons and thus change the relation between neuronal input and output. The effect of neuromodulatory input on synaptic processing is especially important in spinal motoneurones (Alaburda et al. 2002; Heckman et al. 2003; Hultborn et al. 2004). These cells receive synaptic inputs from the cortex, brainstem and sensory afferents, most of which are processed by spinal interneurons before reaching motoneurones (Fyffe, 2001; Powers & Binder, 2001). Multiple neuromodulatory inputs influence motoneurones (Rekling et al. 2000) but the axons that release either serotonin or noradrenaline have especially profound effects (Powers & Binder, 2001; Heckman et al. 2003; Hultborn et al. 2004). These monoamines hyperpolarize spike threshold (Krawitz et al. 2001; Fedirchuk & Dai, 2004), depolarize the resting membrane potential, reduce the AHP and facilitate persistent inward currents (PICs) (Rekling et al. 2000; Powers & Binder, 2001). PICs largely originate in dendritic regions (Hounsgaard & Kiehn, 1993; Lee & Heckman, 1996; Bennett et al. 1998a), where they have a potent and direct action on synaptic inputs from motor commands and sensory afferents. Functionally relevant, PICs amplify excitatory synaptic current as much as 5-fold (Lee & Heckman, 2000; Hultborn et al. 2003) and facilitate self-sustained firing (Hounsgaard & Kiehn, 1985; Hounsgaard et al. 1988). PIC amplitude is directly proportional to the level of monoaminergic drive (Lee & Heckman, 2000), potentially allowing descending motor commands to adjust the gain of synaptic processing for different motor tasks (Heckman et al. 2005).
The monoaminergic systems project in a diffuse fashion throughout the cord (Björklund & Skagerberg, 1982), and thus are likely to simultaneously influence excitability in many motor pools. Yet PICs are highly sensitive to synaptic inhibition (Hultborn et al. 2003; Kuo et al. 2003). The reciprocal inhibition shared between antagonists has a particularly strong effect on the PIC (Hyngstrom et al. 2007b). This reciprocal inhibition is generated by muscle spindle Ia afferents, which are highly sensitive to changes in joint angle (Matthews, 1972). As a result, the link between reciprocal inhibition and the PIC amplitude tends to allow independent joint rotations to be ‘sculpted’ from a diffuse background of monoaminergic facilitation of multiple motor pools (Heckman et al. 2007; Hyngstrom et al. 2007b). In contrast, other muscle afferents (Ib, II, III and IV), as well as the cutaneous and joint afferents, utilize polysynaptic pathways with wider patterns of convergence onto motoneurones, spanning multiple spinal segments and multiple joints (Jankowska, 1992, 2001). All of these sensory inputs are activated by movements.
Despite the potential for broad sensory convergence onto motoneurones during movement, our hypothesis is that the basic receptive field of a motoneurone in response to passive joint rotation is focused, being restricted to its own muscle, close synergists and direct antagonists. To test this hypothesis, we applied passive movements to the hindlimb and investigated the resulting movement-related receptive fields (MRRFs) of motoneurones in two conditions: the decerebrate, a preparation with substantial descending monoaminergic drive, and following acute spinal transection to eliminate descending monoaminergic drive along with all other descending inputs. Our results tended to support the hypothesis but revealed that loss of descending input resulted in broadening of receptive fields.
Methods
Ethical approval
All procedures were fully approved by Northwestern University Institutional Animal Use and Care Committee. All 23 experiments were done using the decerebrate feline preparation (for details see Lee & Heckman, 1998a,b).
Surgical procedures
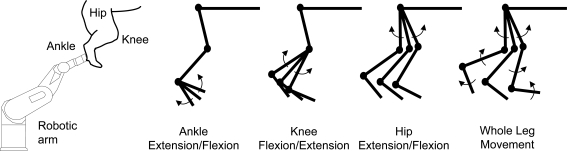
Surgical procedures were completed under deep anaesthesia (1.5–3% isofluorane in a 1: 3 mixture of O2 and NO2 administered through a ventilator). Anaesthetic level was adjusted in response to constantly monitoring changes in blood pressure and heart rate as well as by responses to paw pinch. Keeping the hindlimb skin as intact as possible, nerves to the medial and lateral gastrocnemius muscles (16 animals) and vastus medialus (5 animals) were identified and a cuff electrode was applied for antidromic stimulation of motoneurones. A laminectomy was done from L3 to L7 to expose the spinal cord and the cord was bathed in mineral oil. A precollicular decerebration was performed, anaesthesia discontinued, and paralysis induced with gallamine triethiodide (Sigma-Aldrich). In 10 experiments, the cord was transected at T10 prior to the decerebration. Intracellular recording began at least 45 min after the decerebration. To diminish movement-related cutaneous input, in one experiment, keeping musculature intact, the hindlimb was denervated to the pad of the paw using blunt dissection following spinalization. The paw was attached to a robotic arm with six degrees of freedom (Staubli, AG Robotics). The robot was adjusted so that the hindlimb was positioned into a start position: the ankle at 88 deg relative to the tibia, the knee at 130 deg, and the hip at 105 deg (Fig. 1). This position is well within documented maximum and minimum passive ranges for the cat hindlimb (Goslow et al. 1973). At the end of the experiment, the animals were euthanized by injection of potassium citrate followed by bilateral pneumothorax.
Figure 1. Schematic of the robot and hindlimb configuration.
The plantar surface of the paw is attached to the robot and positioned to allow the full range of motion of the ankle. The distance from the centre of rotation of the ankle, knee and hip joints to the end point of the robotic arm is calculated to allow precise (± 2 mm) and reliable rotations around each joint as well as around all three joints simultaneously for a whole leg movement. The computer controlled robotic arm flexed and extended the ankle (± 15 deg), knee (± 15 deg) and hip (± 10 deg).
Intracellular recordings
Intracellular recordings of lumbar spinal motoneurones were done using sharp electrodes (3–5 MΩ) filled with 2 m potassium citrate. Single electrode discontinuous voltage clamp (switching frequency 8–10 kHz) was applied using an Axoclamp 2A amplifier. Data with inadequate settling were rejected. To enhance the low frequency gain in the negative feedback loop, an external gain circuit was used which allowed for gains of 100–300 nA mV−1 (for details see Lee & Heckman, 2000).
Experimental protocols and data analysis
With the hindlimb held in the start position, motoneurones (MG, LGS, or VM) were identified by antidromic stimulation. In current clamp mode, spike heights were assessed and cells with spike heights less than 60 mV were rejected. Holding the cell 5–10 mV below the estimated resting membrane potential, a slow voltage ramp was applied (30–40 mV/5 s). The voltage onset of the PIC was estimated at the threshold for the negative slope region (Fig. 2). The cell was then stepped to the onset of the PIC (5–10 mV depolarized to the resting membrane potential) and current was measured over 11 s. To prevent the interaction of any ‘warm up’ effects on the PIC from the previous voltage step command (Bennett et al. 1998b; Hyngstrom et al. 2007), we waited ∼30 s and the step was then repeated while the robot passively flexed and extended the ankle (± 15 deg), the knee (± 15 deg), the hip (± 10 deg) and then generated combined ankle (± 15 deg), knee (± 15 deg) and hip (± 10 deg) rotations to produce a whole limb movement (see Fig. 3). Each movement was repeated two times at a speed of ∼1 Hz with a delay of 0.3–0.5 s between each set of movements. In this way, we were able to measure at the soma the current generated by both the synaptic input and the PIC. In two animals, motion video analysis was done to confirm that the robot could move joints separately without influence at other joints. Movements at joints not involved in the primary rotation were found to vary from 0 deg to 2 deg from the starting position. This was also confirmed using the endpoint signal generated by the robot, sampled at 100 Hz.
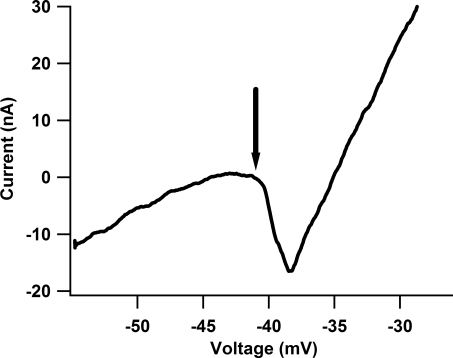
Figure 2. Current–voltage (I–V) relationship for one cell from the cord-intact condition.
During voltage clamp, the PIC manifests as a downward deflection which results in a negative slope region. As the cell is further depolarized, outward conductances are activated which restore the positive slope of the current trace. Since the cell is effectively clamped at the soma, changes in the measured PIC due to synaptic activity must be due to activation or inhibition of voltage-dependent channels on the dendrites. To assess the impact of the PIC on the MRRF, a voltage step to the onset of the PIC (arrow) is applied. For this cell, a 15 mV step was applied (see Fig. 3).
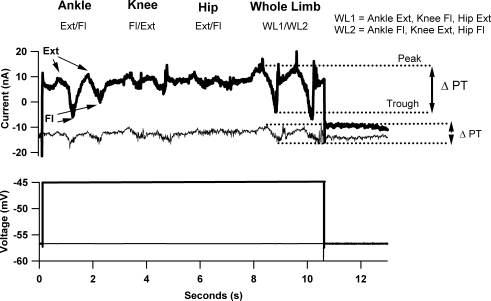
Figure 3. Intracellular response of an ankle extensor motoneuron to the movement and voltage clamp protocols from the decerebrate, cord-intact condition.
The movement-related IN was recorded while the ankle, knee and hip were flexed and extended (2 repetitions, see Fig. 1 and Methods) individually and then in a combination whole limb movement. To examine the effects of the PIC, the movement was repeated at a voltage where the PIC is activated (bottom panel, dark trace, see Fig. 2 for I–V function of this cell) and hyperpolarized to PIC onset (bottom panel, thin trace). To assess the movement-related IN, for each movement the peak to trough difference in IN was calculated (ΔPT, see arrows) for each movement repetition and averaged. Note for each movement, the ΔPT is larger in the depolarized condition. As was generally seen in cells from the decerebrate, cord-intact condition, the order of ΔPT amplitude was whole limb, ankle, knee and hip. This indicates a focus MRRF that is dominated by Ia muscle afferent pathways.
To evaluate the effective synaptic current (IN) with diminished PICs, we repeated this sequence with the cell held at a steady voltage level of 5–10 mV hyperpolarized to the resting membrane potential (same voltage from which the initial voltage ramp was initially applied). Current–voltage plots (I–V) were constructed for each cell. Input conductance was calculated as the slope of a line fitted through the I–V function over a range of 10 mV at a voltage 10–15 mV hyperpolarized to the onset of the PIC. PIC amplitudes were determined by extending the I–V regression line and subtracting the I–V function from it. Modulation in IN due to joint flexion/extension was calculated by determining the difference between the amplitudes of the peaks and troughs (ΔPT, see Fig. 3). To differentiate between the excitatory or inhibitory contribution to IN, we calculated the amplitude from a baseline current level (no movements) to the associated peak, representing the net outward current (−ΔPT), and trough, net inward current (+ΔPT). Estimation of change in muscle length for each end range of movement was calculated using a muscle model developed by Burkholder & Nichols (2004). Data were processed and analysed using Microsoft Excel and SPSS software. For statistics, Student's t test was used to evaluate significant differences with α set at 0.05. Multiple comparisons were made using a repeated measures ANOVA, and post hoc analysis was done with Bonferroni's correction.
Results
PICs enhance multijoint movement-related synaptic input to spinal motoneurones
Data for this study were collected from spinal motoneurones innervating muscles generating either ankle extension (medial gastrocnemius (MG) and lateral gastrocnemius/soleus (LGS) or knee extension (vastus medialus). MG and LG also cross the knee and generate knee flexion, but S does not (LG and S motoneurones were studied as a single group because the nerve to S is a branch passing through LG and extensive surgical disruption of sensory input would have been required to isolate the S nerve). To examine each cell's movement-related receptive field (MRRF) in the sagittal plane, a six degree of freedom computer controlled robotic arm flexed and extended the ankle, knee, and hip joint separately and then simultaneously for a whole limb movement (Fig. 1 and also see Methods).
Previously, we have shown that reciprocal inhibition generated by moderate changes in joint position modulates PIC amplitude by ∼50% (Hyngstrom et al. 2007). In this study, our six-degree-of-freedom robotic arm was used to rotate joints throughout the hindlimb to determine whether these passive movements evoked strong input in motoneurones from polysynaptic multimodal pathways that tend to link motor pools acting at different joints (e.g. group II and cutaneous afferents; Ib afferents were unlikely to be strongly activated as the preparation was neuromuscularly blocked). Cells were voltage clamped and the effects of movement were quantified as changes in the net synaptic current generated at the soma (i.e. the effective synaptic current, IN; Heckman & Binder, 1988; Binder et al. 1998). The pattern of the resulting IN defined each motoneurone's movement-related receptive field (MRRF). In the preparation used here (decerebrate cat), there is tonic monoaminergic drive from the brainstem resulting in relatively constant levels of monoamines to the spinal cord throughout the experiment (Baldissera et al. 1981).
In the first set of experiments, except for the minor surgery required to place small nerve cuffs for antidromic identification of motoneurones (see Methods), the hindlimb was left intact, leaving movement-related sensory input as undisturbed as possible. To determine the effects of the PIC on the MRRF, we first assessed the voltage onset of the PIC by applying a slow linear voltage ramp (30–40 mV/5 s). The PIC manifests as a downward deflection in the current trace with a negative slope region (see Fig. 2). Since we have previously shown that our voltage clamp method (Heckman & Lee, 2001) is extremely effective at controlling voltage at the soma (the presumed site of electrode penetration) (Lee & Heckman, 2000; Kuo et al. 2003; Lee et al. 2003; Hyngstrom et al. 2007), any amplification of the MRRF by the PIC occurs in the dendritic regions of the cell (outside of the clamp). The PIC voltage onset was estimated to coincide with the onset of the negative slope region apparent in the current trace (Fig. 2, arrow). Holding the cell 5–10 mV hyperpolarized to the resting membrane potential, we then applied a depolarizing voltage step corresponding to the approximate voltage onset of the PIC. From a mid-point position, the robot then flexed (Fl) and extended (Ext) the ankle (± 15 deg), knee (± 15 deg), and hip (± 15 deg) (see Figs 2 and 3; note 2 repetitions per movement; and see also Methods). For the whole limb movement, each joint was rotated the same amount as in the single joint movements and moved from the starting position to (1) ankle extension, knee flexion and hip extension and then to (2) ankle flexion, knee extension and hip flexion. The joint excursion during the whole limb movement is within the range seen during functional activities such as ambulation and crouching (Goslow et al. 1973). We then repeated the same movements holding the cell at a hyperpolarized voltage (equal to the voltage level before application of the step) to prevent PIC activation by the input (Lee & Heckman, 2000).
Figure 3 shows the modulation of IN by hindlimb movement for an ankle extensor motoneurone (MG) in the depolarized (top panel, dark trace) and hyperpolarized voltage conditions (top panel, thin trace) as a series of peaks and troughs. Differences between the peak (P) and trough (T) amplitudes (ΔPT) of IN for each voltage command condition were then calculated (Fig. 3). Note that ΔPT is larger for each movement in the depolarized condition, where PICs are present, as compared to the hyperpolarized condition. Each cell's MRRF was then defined by its set of ΔPT values generated by the ankle, knee, hip and whole limb movements. For this cell, for both hyperpolarized and depolarized conditions, ΔPT was largest during the whole leg movement and ankle ΔPT was greater than knee and hip, giving a focused MRRF.
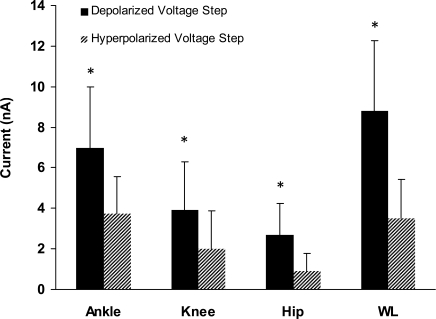
The MRRFs were calculated for 15 ankle extensor motoneurones in both the depolarized (Fig. 4A) and hyperpolarized voltage conditions (not shown). For 12 of these cells, whole limb movement data were collected as well (Fig. 4A, continuous lines). For this sample of cells, mean PIC amplitude was 15.5 ± 9.2 nA. As shown in Fig. 5, mean ΔPT amplitudes were significantly larger (paired t test, P = 0.0005) in the depolarized condition as compared to the hyperpolarized condition for each respective movement (mean ΔPT depolarized, hyperpolarized ± s.d., ankle: 6.9 ± 2.9 nA, 3.7 ± 1.8 nA; knee: 3.9 ± 2.4 nA, 2.0 ± 1.85 nA; hip: 2.6 ± 1.5 nA, 0.9 ± 0.9 nA; whole leg (WL): 8.7 ± 3.5 nA, 3.5 ± 1.9 nA). Thus, dendritic PICs can enhance the IN from movement-related input from the whole limb. This consistency of PIC actions on MRRFs could be explained by the generally global distribution of synaptic inputs to motoneurones (Fyffe, 2001; but see Grande et al. 2005; Grande et al. 2007a) and the recent computational work showing the highest density of Ca2+ channels to occur midway out on the dendrites (Elbasiouny et al. 2005; Grande et al. 2007b).
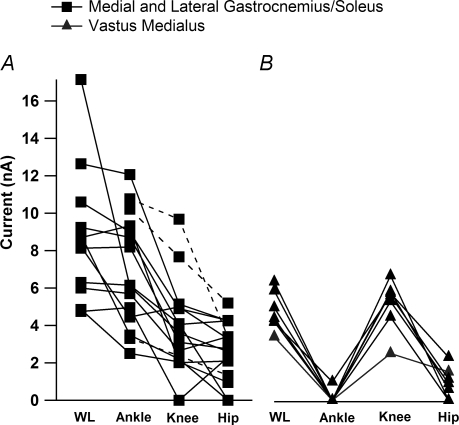
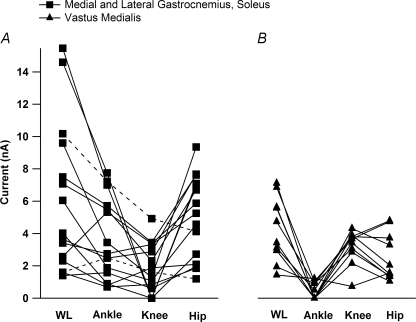
Figure 4. The average ΔPT amplitudes for each movement for all MG/LGS and vastus medialus (VM) motoneurones in the decerebrate, cord-intact condition.
A, the ΔPT amplitudes for the MG/LGS motoneurones (n = 15). MG and LG are bi-articular muscles, crossing the ankle and knee joints. Their primary action is to extend the ankle, but they also have a flexor moment at the knee. In three cells (dashed lines), data were only collected for the single joint movements. As noted in Fig. 3, the order of ΔPT amplitude was whole limb, ankle, knee and hip. B, the ΔPT amplitudes for vastus medialus motoneurones (n = 7). Vastus medialus is a uni-articular knee extensor. The order of average ΔPT amplitudes was WL > knee > hip > ankle.
Figure 5. The mean ΔPT amplitudes of ankle extensor motoneurons for the depolarized and hyperpolarized voltage conditions.
For each joint movement the ΔPT was significantly greater in the depolarized voltage condition than in the hyperpolarized voltage condition for all joint movements. This suggests that PICs enhance all movement-related synaptic input.
The MRRFs clearly include both net outward currents (the peaks, representing either synaptic inhibition or perhaps disfacilitation), and net inward currents (the troughs, due to synaptic excitation or perhaps disinhibition) with respect to the average baseline. On average, both the net inward component of the ΔPT (+ΔPT) and net outward component (−ΔPT) were amplified by the dendritic PIC (see Table 1 for +ΔPT and −ΔPT values, paired t test between voltage conditions for each movement, P≤ 0.04; only the knee +ΔPT comparison failed to reach statistical significance (paired t test, P = 0.13).
Table 1.
The −ΔPT and +ΔPT of ankle extensor motoneurons in the cord-intact and acutely spinalized conditions
| Depolarized cord intact | Hyperpolarized cord intact | Spinalized | ||||
|---|---|---|---|---|---|---|
| Joint movement | −ΔPT (n = 15) | +ΔPT (n = 15) | −ΔPT (n = 15) | +ΔPT (n = 15) | −ΔPT (n = 15) | +ΔPT (n = 15) |
| Ankle Extension/Flexion | ||||||
| Mean ± s.d. (nA) | 2.7 ± 2.3 | 4.8 ± 2.1 | 1.2 ± 2.1 | 2.8 ± 1.7 | 1.8 ± 2.1 | 2.3 ± 1.8 |
| Min (nA) | 0.5 | 1.1 | 0.2 | 1.25 | 0.0 | 0.4 |
| Max (nA) | 9.1 | 8.1 | 7.2 | 5.8 | 7.9 | 6.0 |
| Knee Flexion/Extension | ||||||
| Mean ± s.d. (nA) | 1.8 ± 1.2 | 2.0 ± 1.2 | 0.2 ± 0.85 | 1.6 ± 1.5 | 1.3 ± 1.7 | 1.72 ± 2.1 |
| Min (nA) | 0.22 | 0.22 | 0.0 | 0.6 | 0.0 | 0.0 |
| Max (nA) | 3.9 | 3.9 | 2.1 | 5.2 | 5.2 | 7.2 |
| Hip Extension/Flexion | ||||||
| Mean ± s.d. (nA) | 1.7 ± 0.9 | 1.2 ± 0.9 | 0.6 ± 0.8 | 0.6 ± 0.7 | 2.3 ± 2.5 | 4.0 ± 2.6 |
| Min (nA) | 0.7 | 0.2 | 0.3 | 0.2 | 0.0 | 0.3 |
| Max (nA) | 3.9 | 2.8 | 2.8 | 1.7 | 9.9 | 9.3 |
| WL1/WL2 | ||||||
| Mean ± s.d. (nA) | 2.8 ± 1.8 | 6.1 ± 2.6 | 1.6 ± 2.2 | 1.8 ± 2.5 | 4.4 ± 2.3 | 5.8 ± 3.1 |
| Min (nA) | 0.9 | 2.9 | 0.5 | 0.9 | 1.7 | 0.1 |
| Max (nA) | 6.1 | 9.3 | 2.6 | 4.6 | 8.7 | 11.2 |
MRRFs of ankle extensor motoneurones are focused
All MG/LGS cells in this sample were excited by ankle flexion, knee extension and hip flexion and inhibited by ankle extension, knee flexion, and hip extension (see Fig. 3 for cell example). On average, whole leg ΔPT was the largest followed by ankle, knee and hip ΔPT, respectively (see paragraph above for means, Fig. 4A for ΔPT amplitudes for each cell). For both voltage conditions, ankle ΔPT was significantly larger than knee and hip ΔPT values (ANOVA, Bonferroni's correction, P = 0.003). Although knee ΔPT was on average larger than hip ΔPT, this comparison did not reach statistical significance (ANOVA, Bonferroni's correction, P > 0.05). In the depolarized condition, knee ΔPT was 56% of ankle ΔPT and was 54% of the ankle ΔPT in the hyperpolarized condition. Hip ΔPT was a smaller proportion of the ankle ΔPT for the depolarized and hyperpolarized voltage conditions (37% and 24%, respectively) as compared to modulations made by knee movements. Since the gastrocnemius muscle complex crosses the knee joint, it was not surprising that knee flexion/extension (Fl/Ext) created larger ΔPT values as compared to the hip. The modest IN modulation seen during hip movement was significantly greater than zero (t test, P = 0.0001) and may be generated by muscle spindle group II or cutaneous afferents, which are known in both the cat and humans to project across multiple spinal segments (see Discussion). This evidence indicates that the MRRFs for ankle extensor are focused but include significant input from the knee and the hip (see Discussion).
A focused MRRF is not limited to ankle extensor motoneurones
In a separate set of experiments, the MRRF was evaluated in seven vastus medialus (VM) motoneurones. VM is a uni-articular knee extensor. As with the ankle extensors, the ΔPT values from ankle, knee, hip and whole leg movements were evaluated for each cell (Fig. 4B). The ΔPT was largest during knee and whole leg movements followed by hip and ankle movements (Fig. 4B). The mean ΔPT for knee flexion (depolarized condition 5.1 ± 1.3 nA, hyperpolarized 2.6 ± 0.8 nA) was significantly larger (paired t test, P = 0.00006) than average ΔPT values for ankle (mean depolarized 0.1 ± 0.37 nA, hyperpolarized 0.0 ± 0.15 nA) and hip movements (mean depolarized 0.8 ± 0.02 nA, hyperpolarized 0.3 ± 0.02 nA). As with the ankle extensor motoneurones, the ΔPT values were significantly greater from the depolarized condition as compared to the hyperpolarized condition for the knee, hip and whole limb movements (paired t test, P = 0.03). For the ankle movements, the very small average ΔPT values for the depolarized and hyperpolarized conditions were not significantly different from zero (t test, P = 0.35). The MRRF for VM appears to be very sharply focused: the ΔPT values generated by hip movements average only about 20% of those for the knee, while ankle ΔPT values were not significantly different from zero and averaged less than 2% of those for knee movements.
Whole limb movement current modulations are less than the linear sum of individual joint movement current modulations
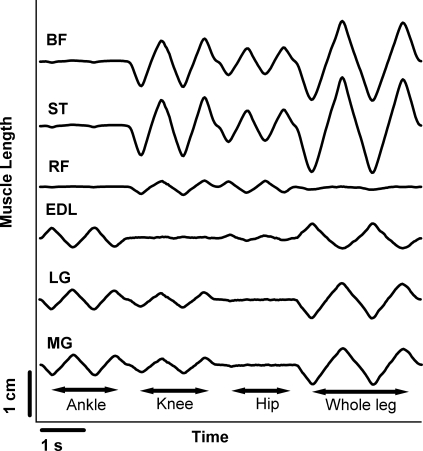
The whole limb movement was generated by simultaneously applying the ankle, knee and hip rotations that were applied individually. Thus, in a simple system, the whole leg ΔPT (WLΔPT) would be a linear summation of the ankle, knee and hip ΔPT values. In 13 of the ankle extensor motoneurones, the mean whole leg ΔPT (8.78 ± 3.5 nA) was significantly less (paired t test, P = 0.012) than the algebraic sum of each cell's ankle, knee and hip ΔPT (12.46 ± 4.9 nA). This represents a nonlinearity of ∼30%. To determine if this nonlinearity was in part due to biomechanical factors, we adapted a muscle length model developed by Burkholder & Nichols (2004) to estimate changes in muscle length due to our movement protocol (see Fig. 6 for selected muscles). The whole limb ΔPT was generated by the transition from peak ankle extension, knee flexion and hip extension to peak ankle flexion, knee extension and hip flexion. For most muscles that cross more than one joint, these movements generated changes in length that were nearly equal (within ∼10%) to the sum of the muscle length changes seen during individual ankle and knee movements (uni-articular muscles of course had the same length change for the whole leg movement as for the individual rotation of the joint that they crossed). For example, the total change in muscle lengths in LG was 0.87 cm and the sum of the individual movements was 0.84. For some bi-articular muscles, however, length remained fairly constant because the combination of joint rotations compensated for each other. For example, rectus femorus (RF) (Fig. 6, 4th trace from the bottom) did not undergo a large length change during the whole leg movement because changes in both knee and hip position compensated for each other in their effects on RF. Other multiarticular muscles such as sartorius (ST) and biceps femorus (BF) with the cural fascia are likely to behave similarly to RF. Because recent work in our lab (Hyngstrom et al. 2008) shows that summation of excitatory and inhibitory synaptic currents exhibit nonlinearities of < ∼10% even when motoneurones have large dendritic PICs, it is likely the large, ∼30% sublinearity seen here is not due to nonlinear summation in motoneurone dendrites but instead to biomechanical effects such as the compensation in RF.
Figure 6. Model showing estimated changes in muscle length for various muscles during the movement protocol.
Adapting a muscle model developed by Burkholder & Nichols (2004), we were able to estimate the change in muscle length (cm) at the ankle (MG, LGS, and EDL), knee and hip joints (RF and BF) during our movement protocol. In almost all muscles examined, changes in muscle length seen during individual joint movements summed linearly when compared to the change in muscle length for the whole limb movement. One exception was RF, a knee extensor and hip flexor, which showed a decreased variation in muscle length during the whole limb movement as compared to the variation in muscle length due to the hip movements.
Spinalization eliminates voltage-dependent enhancement of movement-related IN
Acute spinalization, following decerebration, interrupts descending brainstem inputs to the cord. Along with other effects on the cord, tonic monoaminergic drive is eliminated and the PIC generating capacity of motoneurones is dramatically reduced (Conway et al. 1988; Hounsgaard et al. 1988; Hyngstrom et al. 2007). To examine the effects of diminished PICs, movement-related IN modulation was evaluated in MG/LGS (n = 15) and VM (n = 10) motoneurones following acute spinalization and decerebration. These experiments were separate from the intact cord experiments.
As in the intact cord experiments, the cell was held 5–10 mV hyperpolarized to the resting membrane potential and a slow voltage ramp was applied to assess the voltage onset of any apparent PIC. On average, the PIC amplitude was very small (3.5 ± 3.5 nA). In most cases, no negative slope region was apparent and the voltage was stepped 10–15 mV depolarized from the resting membrane potential. Depolarizing step sizes of this magnitude approximate the average voltage for onset of the PIC (Lee & Heckman, 1998a). The ΔPT values were then calculated for each movement in both voltage conditions (Table 2). With limited PICs present, the ΔPT values were not significantly different between voltage conditions (paired t test, P = 0.06) for each motoneurone sample. Consequently, ΔPT values were averaged to quantify the MRRF for each motoneurone pool sample (see next section).
Table 2.
ΔPT values of ankle extensor motoneurones for each single and multijoint movement for both the cord-intact and acutely spinalized condition
| Joint movement | Depolarized cord intact (n = 15) | Hyperpolarized cord intact (n = 15) | Spinalized (n = 15) |
|---|---|---|---|
| Ankle Extension/Flexion | |||
| Mean ± s.d. (nA) | 6.9 ± 2.9 | 3.7 ± 1.8 | 3.7 ± 2.5 |
| Min (nA) | 2.5 | 1.3 | 0.8 |
| Max (nA) | 12.1 | 7.6 | 7.2 |
| Knee Flexion/Extension | |||
| Mean ± s.d. (nA) | 3.9 ± 2.4 | 2.0 ± 1.9 | 2.0 ± 1.5 |
| Min (nA) | 0.0 | 0.6 | 0.0 |
| Max (nA) | 9.7 | 5.5 | 4.9 |
| Hip Flexion/Extension | |||
| Mean ± s.d. (nA) | 2.6 ± 1.5 | 0.9 ± 0.9 | 5.0 ± 2.6 |
| Min (nA) | 0.0 | 0.0 | 1.2 |
| Max (nA) | 5.2 | 2.8 | 7.7 |
| WL1/WL2 | (n = 12) | (n = 12) | |
| Mean ± s.d. (nA) | 8.7 ± 3.5 | 3.5 ± 1.9 | 5.3 ± 3.9 |
| Min (nA) | 4.7 | 1.2 | 1.0 |
| Max (nA) | 17.2 | 6.5 | 14.0 |
In the spinalized condition, the ΔPT was not significantly different between the depolarized and hyperpolarized condition (paired t test, P = 0.05) and the ΔPTs were subsequently averaged.
Spinalization widens MRRFs
With the cord intact and tonic monoaminergic drive, data from this study show that the MRRF of ankle and knee extensor motoneurones is relatively focused. However, electrical connectivity studies have demonstrated polysynaptic pathways that cross multiple spinal segments from both muscle and cutaneous afferents and contribute to whole limb coordination, especially in acutely spinalized preparations (Eccles & Lundberg, 1959; Lundberg et al. 1987). Human and animal studies show that often following cord injury, local muscle stretch or cutaneous stimulation will result in a whole limb reflex response suggesting alterations in motoneuronal receptive field (Bennett et al. 2004; Onushko & Schmit, 2007).
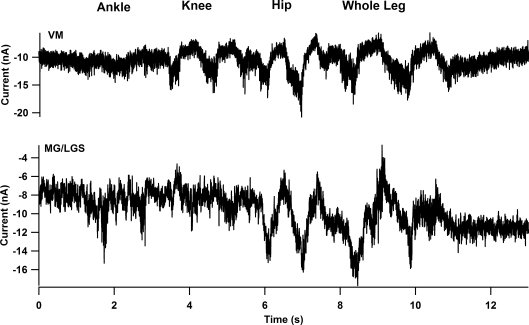
As with the cord-intact sample, each cell's MRRF was assessed from ΔPT amplitudes for each movement. Figure 7 shows the ΔPT amplitudes for all cells for each motoneurone sample in the spinalized condition. For the MG/LGS cells in the intact cord experiments, ankle ΔPT amplitudes were significantly larger than knee and hip ΔPT (see Figs 4 and 5). However, following acute spinalization, the mean hip ΔPT (5.0 ± 2.6 nA) was larger than the mean ankle ΔPT (3.7 ± 2.5 nA), but the difference did not reach statistical significance (ANOVA, Bonferroni's correction, P = 0.04). Both ankle and hip ΔPT were significantly larger than knee ΔPT (ANOVA, Bonferroni's correction, P = 0.013). The lower panel in Fig. 8 shows the large movement-related IN modulation of an MG/LGS motoneurone to hip movements. Overall, the spinalized hip ΔPT was found to be significantly greater than the intact cord hip ΔPT measured in the hyperpolarized condition (ANOVA, Bonferroni's correction, P = 0.0001), but less than the depolarized hip ΔPT (ANOVA, Bonferroni's correction P = 0.02). It appears that following spinalization, ankle motoneurones broaden their receptive field through an increase in hip ΔPT. As with the intact condition, the WLΔPT (6.1 ± 4.6 nA) was less than the total ΔPT (10.5 ± 5.1 nA) due to the summation of individual ankle, knee and hip ΔPT values (paired t test, P = 0.0007). When PICs are small, excitation and inhibition in motoneurones usually sum nearly linearly (Powers & Binder, 2000), supporting the suggestion above that the primary source of this nonlinear summation is biomechanical.
Figure 7. The ΔPT for each joint movement for all MG/LGS and VM cells in the decerebrate, spinalized condition.
A, the ΔPT for MG/LGS (n = 15). On average, the order of ΔPT amplitude for MG/LGS cells was WL > ankle = hip > knee. This represented an increase in the average hip ΔPT as compared to the cord-intact sample (hyperpolarized voltage condition, ANOVA, Bonferroni's correction, P = 0.0001). (Note in only 2/12 cells (dashed lines) was hip ΔPT < knee ΔPT.) B, the ΔPT for VM cells (n = 10). The overall pattern of the ΔPT amplitude was WL > knee = hip > ankle. As with the MG/LGS cells, this represented an increase in the hip IN as compared to the cord-intact sample (ANOVA, Bonferroni's correction, P < 0.002).
Figure 8. Acute spinalization resulted in the widening of the MRRF for MG/LGS and VM cells.
The panels show the current traces of individual cells from the hyperpolarized voltage condition. In contrast with their MRRF in the intact cord condition, both the VM (top panel) and MG (bottom panel) show an increased modulation to hip movements. Note in the MG cell example, the modulation of IN due to hip movements is equal to if not larger than the IN modulation due to ankle movement.
A comparison of the net +ΔPT and −ΔPT between the intact and spinalized samples revealed differences during the hip movements. The spinalized −ΔPT was greater as compared to the intact hyperpolarized −ΔPT (t test, P = 0.015) and the +ΔPT was also larger compared to the intact depolarized and hyperpolarized +ΔPTs (t test, P = 0.002). These results suggest that the increase in movement-related hip IN seen in the spinalized condition is due to an increase in both −ΔPT and +ΔPT, with a greater overall contribution from +ΔPT. Since the increased +ΔPT is not likely to be due to PICs or from excitatory brainstem drive, the additional +ΔPT may be due to a decrease in tonic descending inhibition of excitatory polysynaptic pathways from the hip to the ankle.
To examine if the widening of receptive fields is limited to ankle extensor motoneurones, the movement protocol was also repeated in VM cells following acute spinalization. Similar to the adaptations in MG/LGS MRRF following spinalization, VM cells also showed an increase in hip ΔPT (Fig. 7B) as compared to intact condition (P < 0.002). Mean knee ΔPT was larger than hip ΔPT (3.2 ± 1 nA and 2.5 ± 1.5 nA, respectively), but this did not reach statistical significance (paired t test, P = 0.13). Both the knee and hip ΔPT values were larger than the ankle ΔPT (paired t test, P = 0.001). Possible mechanisms for adaptations in MRRF seen in the MG/LGS and VM motoneurones are considered in the discussion.
Cutaneous afferents have a limited contribution to the MRRF following acute spinalization
Both muscle and cutaneous afferents which utilize polysynaptic pathways could be responsible for the significant changes in hip ΔPT seen in the VM and MG/LGS cells seen after acute spinalization (Eccles & Lundberg, 1959). However, it was shown previously that in the decerebrate, intact cord preparation the PIC amplitude of ankle extensors varied with static joint position and cutaneous denervation did not alter the modulation pattern (Hyngstrom et al. 2007). In the present study, however, it is possible that cutaneous input might have a larger effect on motoneuronal IN due to changes in the levels of excitability in interneuronal circuitry secondary to both spinalization as well as the comparatively more dynamic movement protocol. If this hypothesis is true, then diminishing cutaneous input might result in MRRFs that resemble the cord-intact condition, i.e. focused.
To examine this hypothesis, the same movement protocols were repeated in five MG/LGS cells and five VM cells with the cord spinalized and the lower limb denervated (skin separated from the muscle, see Methods). It was found that the overall MRRF pattern was not different from the spinalized, cutaneous intact condition. Like in the spinalized cutaneous intact condition, MG/LGS cells' ankle and hip flexion ΔPT were greater than knee ΔPT (ANOVA, Bonferroni's correction, P = 0.01), but not from each other (ANOVA, Bonferroni's correction, P = 0.52). VM cells also maintained the spinalized cutaneous intact MRRF pattern with knee and hip ΔPT being significantly greater than ankle ΔPT (ANOVA, Bonferroni's correction, P = 0.01) but not from each other (ANOVA, Bonferroni's correction P = 0.44). These result suggest that the change in the MRRF for VM and MG/LGS cells is related to muscle afferents with polysynaptic connections within the cord such as the group II afferents (cf. Lundberg et al. 1987).
Discussion
Our results demonstrate that in the intact cord, the IN generated by limb movements was significantly enhanced when the cell was depolarized and PICs were active (up to 3-fold). Despite this amplification, the presence of PICs did not alter the overall pattern of each cell's MRRF. The MRRFs for both the ankle extensors MG and LGS and the knee extensor VM were dominated by input generated from rotation of the joint that produced the largest change in length of the innervated muscle. Thus, MG and LGS received the most input from ankle rotation and VM from knee rotation. These focused MRRFs are consistent with the pattern of excitation and disynaptic inhibition from muscle spindle Ia afferents (see below). It should be emphasized, however, that hip rotations had a small but significant effect, which may reflect the convergent input from muscle spindle group II afferents (see below). We have previously demonstrated that Ia reciprocal inhibition effectively suppresses the dendritic PIC and may provide a focused, local inhibitory pathway to oppose the diffuse descending modulation from brainstem monoaminergic inputs. We proposed that Ia reciprocal inhibition could ‘sculpt’ individual joint motions from a background of diffuse excitatory neuromodulation. The relatively focused MRRFs seen in the present movements results would allow Ia reciprocal inhibition to fulfil this role without undue interference from sensory input from other joints.
When descending drive was removed via acute spinalization, the MRRFs for both the ankle extensors and VM were broadened, in the sense that input from hip rotation increased markedly. In fact, the IN from hip rotation became as large as the IN from ankle (MG, LGS) or knee rotation (VM), suggesting that in this state, group II effects are as large as group Ia actions (see below).
Choice of motor pools studied
There are over 30 muscles in the cat hindlimb and our results apply only to the two extensor motor pools studied. These pools were chosen for two reasons: (1) extensor motor pools are known to have strong PICs, whereas excitability in flexor pools in the intact cord decerebrate may be substantially less (Hounsgaard et al. 1988); and (2) access to the muscle nerves could be achieved without extensive surgery, allowing sensory inflow to be as natural as possible. Results for the muscles acting at the hip, which often have complex mechanical actions at more than one joint, are especially difficult to predict and will require additional study.
Joint rotations evoked synaptic currents large enough to shape motor output
In the decerebrate preparation, the current-to-frequency gain for repetitive firing is about 2 spikes nA−1 s−1 (Lee et al. 2003). In the depolarized condition, where ankle ΔPT values were ∼7 nA and whole limb ΔPT values were ∼9 nA, a functionally significant change in firing would be predicted to be generated by these movements. For example, 14–18 spikes s−1 could modulate an S unit through nearly all of its force range and an FR unit through 30–50% (Kernell et al. 1983; Botterman et al. 1986). Although smaller in amplitude, the ankle motoneurone ΔPT values for the hip and knee in the depolarized condition could contribute to the cell's total rate modulation when combined with ankle movement. These estimates assume that the PIC is activated in the voltage range traversed by the membrane potential during repetitive firing, which is likely to be the case (Lee & Heckman, 1998a,b; Harvey et al. 2005). The PIC approximately doubled the ΔPT values (hyperpolarized data being about half of depolarized) and thus the PIC is likely to enhance the cell's probability of repetitively firing to functionally relevant inputs. PICs are likely to exist in the soma as well as in the dendrites (Manuel et al. 2007; Moritz et al. 2007). If the cell were not voltage clamped it would be reasonable to expect that somatic PICs would also affect the gain of the ΔPT values. In addition, monoaminergic input to the motoneurone also includes hyperpolarization of spike threshold (Krawitz et al. 2001), a reduction in the spike afterhyperpolarization, and facilitation of the subthreshold H-current (Powers & Binder, 2001). All of these effects would further contribute to the impact of the ΔPT values measured here on motor output (see for example the dynamic clamp studies of Manuel et al. 2006, 2007), but, like the dendritic PIC, would probably not affect the form of the MRRF.
Biomechanics of pretibial and triceps surae muscles contribute to the MRRF
If Ia excitation and reciprocal inhibition dominate MRRFs, then the differences in ΔPT seen during movement at different joints (Fig. 4) should be consistent with the relative changes in agonist and antagonist muscle lengths induced by the joint rotations. Again using the muscle length model developed by Burkholder & Nichols (2004), we compared normalized ΔPT of ankle extensor motoneurones during knee flexion/extension to normalized changes in muscle lengths of MG. During knee Fl/Ext, the change in MG length was 77% of the length change during ankle Fl/Ext. The ΔPT values of MG motoneurones for knee Fl/Ext compared to ankle Fl/Ext were smaller than predicted by these relative length changes: 56% for the depolarized state and 53% for hyperpolarized levels. This analysis suggests that muscle length and its effect on Ia excitation of MG and LGS is not sufficient to account for the MRRF of MG/LGS motoneurones. The explanation of this discrepancy may, however, lie with Ia reciprocal inhibition from antagonists. MG/LGS motoneurones primarily receive Ia inhibition from TA and EDL (Nichols et al. 1999; Nichols & Cope, 2001). TA does not cross the knee, and although EDL does have an insertion on the femur in the cat, its moment arm is small and the model did not predict significant changes in muscle length for EDL during knee movements. Thus, knee rotation markedly changed the lengths of MG and LGS but not their antagonists TA and EDL. In other words, ankle rotation combined Ia excitation and reciprocal inhibition; knee rotation lacked significant modulation from reciprocal inhibition. These results are consistent with effects primarily from muscle spindle Ia afferents and their monosynaptic and disynaptic connections to ankle extensors.
For VM, a MRRF dominated by knee movement is also consistent with muscle length changes activating primarily Ia afferents in agonists and antagonists. The slight modulation from the hip may reflect Ia reciprocal inhibition from the antagonist knee flexors, which also cross the hip joint. Thus, when the spinal cord is intact, the MRRFs of MG, LGS, VM and VL are reasonably consistent with the known patterns of Ia excitation and reciprocal inhibition.
Widening of the movement-related receptive field
In MG/LGS and VM cells, we were able to show a widening of MRRF following acute spinalization. Specifically, both cell groups saw an increase in ΔPT from hip flexion/extension that was not primarily due to cutaneous afferents. In the case of MG/LGS, there was a relative increase in the excitatory IN caused by hip flexion. It is not likely to originate from group Ia connections as there appear to be no monosynaptic connections between the hip extensors and ankle extensors (Eccles et al. 1957; Eccles & Lundberg, 1958; Nichols et al. 1999). Instead the hip contribution is more likely to originate from group II pathways which have whole limb connectivity through polysynaptic pathways (Jankowska, 1992). Although originally considered part of the flexion reflex afferent (FRA) system (Eccles & Lundberg, 1959), EPSPs in extensors have been shown to exist (Lundberg et al. 1987; Jankowska, 1992). We suggest that higher threshold afferents in groups II and III do not participate as these are not activated strongly by the midrange joint rotations used here (Cleland et al. 1990), though contribution in the spinalized state cannot be ruled out.
Work examining pathways associated with locomotion has shown the importance of stretch of hip musculature (Kriellaars et al. 1994; Hiebert et al. 1996) and may indicate a general flow of information from proximal to distal MNs. Our results support this, by revealing significant hip on to knee and ankle pathways when the cord was intact. Although these pathways were only strong following spinalization for our passive movements, they could be utilized in the intact cord state by either a decrease in monoaminergic drive or a facilitation of these convergent pathways via cortical commands or via the locomotor central pattern generator.
Implications for pathology seen after spinal cord injury
Following spinal cord injury, MNs may lose supraspinal control over converging afferent pathways, which would result in uncontrolled widening of motoneurones MRRF and unwanted whole limb reflex responses. Experiments in chronic spinal cord injury in humans and animals have shown evidence for whole limb reflex responses to local stimuli (Schmit et al. 2002; Bennett et al. 2004; Gorassini et al. 2004; Onushko & Schmit, 2007). Although limited to the sagittal plane, our study is the first to provide direct quantification of widened MRRFs in MNs following spinalization as a result of enhanced muscle afferent transmission. Future studies which address the regulation of MRRF patterns, either through monoaminergic or cortical pathways, could result in better management of whole limb spasms seen following spinal cord injury.
Acknowledgments
The authors would like to thank Jack Miller for his assistance with the surgical preparations and data collection. We also thank Thomas Sandercock for his work in adapting the muscle model for our purposes. This study was funded by the US National Institute of Health NINDS NS034282 (C.J.H.) and US National Research Service Award Pre-doctoral Fellowship F31NS048757-03 (to A.S.H.).
References
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol. 2002;508:219–226. doi: 10.1007/978-1-4615-0713-0_27. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998a;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998b;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Binder MD, Robinson FR, Powers RK. Distribution of effective synaptic currents in cat triceps surae motoneurons. VI. Contralateral pyramidal tract. J Neurophysiol. 1998;80:241–248. doi: 10.1152/jn.1998.80.1.241. [DOI] [PubMed] [Google Scholar]
- Björklund A, Skagerberg G. Descending monoaminergic projections to the spinal cord. In: Sjolund B, Bjorklund A, editors. Brain Stem Control of Spinal Mechanisms. Amsterdam: Elsevier. Biomedical Press; 1982. pp. 55–88. [Google Scholar]
- Botterman BR, Iwamoto GA, Gonyea WJ. Gradation of isometric tension by different activation rates in motor units of cat flexor carpi radialis muscle. J Neurophysiol. 1986;56:494–506. doi: 10.1152/jn.1986.56.2.494. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Nichols TR. Three-dimensional model of the feline hindlimb. J Morphol. 2004;261:118–129. doi: 10.1002/jmor.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland CL, Hayward L, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular free nerve endings. J Neurophysiol. 1990;64:1319–1330. doi: 10.1152/jn.1990.64.4.1319. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in α-motoneurones induced by intravenous injection of L-DOPA and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958;144:271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic CaV1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol. 2005;94:3961–3974. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–361. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe REW. Spinal motoneurons: synaptic inputs and receptor organization. In: Cope TC, editor. Motor Biology of the Spinal Cord. London: CRC press; 2001. pp. 21–46. [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Reinking RM, Stuart DG. The cat step cycle: hindlimb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973;141:1–42. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Grande G, Armstrong S, Neuber-Hess M, Rose PK. Distribution of contacts from vestibulospinal axons on the dendrites of splenius motoneurons. J Comp Neurol. 2005;491:339–351. doi: 10.1002/cne.20699. [DOI] [PubMed] [Google Scholar]
- Grande G, Bui TV, Rose PK. Effect of localized innervation of the dendritic trees of feline motoneurons on the amplification of synaptic input: a computational study. J Physiol. 2007a;583:611–630. doi: 10.1113/jphysiol.2007.134999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G, Bui TV, Rose PK. Estimates of the location of L-type Ca2+ channels in motoneurons of different sizes: a computational study. J Neurophysiol. 2007b;97:4023–4035. doi: 10.1152/jn.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol. 2005;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Analysis of effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat. J Neurophysiol. 1988;60:1946–1966. doi: 10.1152/jn.1988.60.6.1946. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman C, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending modulation, focused local inhibition. J Physiol. 2007 doi: 10.1113/jphysiol.2007.145078. in press. doi 10.1113/jphysiol.2007.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH. Advances in measuring active dendritic currents in spinal motoneurons in vivo. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. London: CRC Press; 2001. pp. 89–106. [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol. 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Heckman CJ. Summation of excitatory and inhibitory synaptic inputs by motoneurons with highly active dendrites. J Neurophysiol. 2008 doi: 10.1152/jn.01253.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci. 2007;10:363–369. doi: 10.1038/nn1852. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA. Relation between isometric force and stimulus rate in cat's hindlimb motor units of different twitch contraction time. Exp Brain Res. 1983;50:220–227. doi: 10.1007/BF00239186. [DOI] [PubMed] [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol. 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ. Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol. 2003;90:3617–3624. doi: 10.1152/jn.00521.2003. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998a;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998b;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol. 2003;89:27–39. doi: 10.1152/jn.00137.2002. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. The afterhyperpolarization conductance exerts the same control over the gain and variability of motoneurone firing in anaesthetized cats. J Physiol. 2006;576:873–886. doi: 10.1113/jphysiol.2006.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. Resonant or not, two amplification modes of proprioceptive inputs by persistent inward currents in spinal motoneurons. J Neurosci. 2007;27:12977–12988. doi: 10.1523/JNEUROSCI.3299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian muscle receptors and their central actions. In: Davson H, Greenfield ADM, Whittam R, Brindley GS, editors. Monographs of the Physiological Society. London: Edward Arnold Ltd; 1972. pp. 1–630. [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol. 2007;98:1042–1047. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC. The organization of distributed proprioceptive feedback in the chronic spinal cat. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. London: CRC Press; 2001. pp. 305–326. [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Ex Sport Sci Rev. 1999;27:255–284. [PubMed] [Google Scholar]
- Onushko T, Schmit BD. Reflex response to imposed bilateral hip oscillations in human spinal cord injury. J Neurophysiol. 2007;98:1849–1861. doi: 10.1152/jn.00461.2007. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol. 2000;83:483–500. doi: 10.1152/jn.2000.83.1.483. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit BD, Benz EN, Rymer WZ. Afferent mechanisms for the reflex response to imposed ankle movement in chronic spinal cord injury. Exp Brain Res. 2002;145:40–49. doi: 10.1007/s00221-002-1080-2. [DOI] [PubMed] [Google Scholar]